Abstract
Background
Heterogeneity in response to treatment of pulmonary arterial hypertension (PAH) is a major challenge to improving outcome in this disease. Although vasodilator responsive PAH (VR-PAH) accounts for a minority of cases, VR-PAH has a pronounced response to calcium channel blockers and better survival than non-responsive PAH (VN-PAH). We hypothesized that VR-PAH has a different molecular etiology from VN-PAH that can be detected in the peripheral blood.
Methods and Results
Microarrays of cultured lymphocytes from VR-PAH and VN-PAH patients followed at Vanderbilt University were performed with quantitative PCR performed on peripheral blood for the 25 most different genes. We developed a decision tree to identify VR-PAH patients based on the results with validation in a second VR-PAH cohort from the University of Chicago. We found broad differences in gene expression patterns on microarray analysis including cell-cell adhesion factors, cytoskeletal and rho/GTPase genes. 13/25 genes tested in whole blood were significantly different: EPDR1, DSG2, SCD5, P2RY5, MGAT5, RHOQ, UCHL1, ZNF652, RALGPS2, TPD52, MKNL1, RAPGEF2 and PIAS1. Seven decision trees were built using expression levels of two genes as the primary genes: DSG2, a desmosomal cadherin involved in Wnt/β-catenin signaling, and RHOQ, which encodes a cytoskeletal protein involved in insulin-mediated signaling. These trees correctly identified 5/5 VR-PAH in the validation cohort.
Conclusions
VR-PAH and VN-PAH can be differentiated using RNA expression patterns in peripheral blood. These differences may reflect different molecular etiologies of the two PAH phenotypes. This biomarker methodology may identify PAH patients that have a favorable treatment response.
Keywords: Pulmonary arterial hypertension, biomarker, vasodilator responsive, treatment
Introduction
Although pulmonary arterial hypertension (PAH) is a devastating disease with high mortality despite advanced therapies1, 2, a subset of patients characterized by acute vasodilator responsiveness has excellent survival3. Patients who have an acute drop in mean pulmonary artery pressure of at least 10mmHg to less than 40mmHg with preserved cardiac output in response to vasodilators such as inhaled nitric oxide are defined as having vasodilator-responsive PAH (VR-PAH) and their survival is measured in decades as opposed to months for non-responsive PAH patients (VN-PAH) 3. Moreover, VR-PAH patients can be safely treated with calcium channel blocker therapy as opposed to more expensive medications4.
VR-PAH is an infrequent sub-phenotype of PAH and most commonly found in patients with idiopathic PAH3, 5. Patients with VR-PAH are identified at the time of diagnostic right heart catheterization (RHC) during acute challenge with vasodilators such as nitric oxide, adenosine and prostacyclin6. It has been noted that a positive vasodilator response can be elicited with many vasodilators such as nitric oxide, adenosine, hydralazine and calcium channel blockers. The multiple pathways that these drugs affect suggest an underlying, but as yet unidentified mechanism that is shared among VR-PAH patients. Identification of molecular markers associated with VR-PAH may provide insight into disease mechanism or serve as biomarkers of this important sub-phenotype of PAH.
We hypothesized that VR-PAH has a different molecular signature than VN-PAH and that this difference can be exploited to differentiate VR-PAH using peripheral blood samples. To test this hypothesis we performed microarray on cultured lymphocytes from VR-PAH and VN-PAH and identified the genes that displayed the greatest intergroup differences in expression. We confirmed the differences using whole blood-isolated RNA. We developed a PCR-based prediction tool and validated it in the peripheral blood of an independent validation cohort of VR-PAH.
Methods
Approval was obtained from the Vanderbilt University Intuitional Review Board (protocol 9401) and written informed consent was obtained from all research subjects. As part of this protocol, all patients with PAH were offered enrollment into the Vanderbilt Pulmonary Hypertension Research Cohort (VPHRC) as previously described7. Patients were generally enrolled within one year of their fist visit at Vanderbilt University Medical Center. This cohort is enriched in heritable PAH and family members were enrolled at the time of their referral to our cohort as previously described8. In these experiments only idiopathic and heritable PAH patients with available, high-quality peripheral blood lymphocytes and/or high quality RNA samples were included for analysis. Healthy controls were free from known cardiovascular disease (n=229). In the validation cohort, patients followed at the University of Chicago with a diagnosis of idiopathic PAH (SR) who agreed to participate in research were offered entry between August 2012 and March 2014. Patients consenting and providing blood samples were included in the analysis.
Definition of vasodilator-responsive PAH
PAH was defined according to standard criteria10. Patients were diagnosed with idiopathic or heritable PAH by experienced clinicians (IMR, ARH) and did not have evidence of connective tissue disease, liver disease or congenital heart disease on history, physical examination, laboratory or serologic testing, echocardiography or chest imaging. At the time of initial RHC all patients underwent vasodilator testing using inhaled nitric oxide at up to 40ppm as previously described11. VR-PAH diagnosis required a drop in mean pulmonary artery pressure (mPAP) of at least 10mmhg to <40mmHg with preserved cardiac output. VN-PAH patients met hemodynamic criteria for PAH, but did not for vasodilator responsiveness. VR-PAH patients were further required to have at least one year of therapy with calcium channel blockers. Because some patients could not tolerate CCB therapy5, phosphodiesterase 5 inhibitor therapy was occasionally added at the discretion of the treating physician. Patients that received endothelin receptor antagonist or prostaglandin therapy were not included as use of these medications was generally reserved for patients failing calcium channel blocker therapy clinically in our practice and suggested non-classic VR-PAH phenotype. Thirty two idiopathic and heritable VN-PAH patients were used as comparators as well as 22 healthy controls. Some of the data from these disease and healthy controls have previously been published9. One patient in the idiopathic PAH group in this publication was identified as VR-PAH and included in this new analysis in the VR-PAH group. All of these patients were recruited from the Vanderbilt site. Microarray was re-performed using the same platform on 2 randomly selected VN-PAH samples to confirm reproducibility over time in cultured lymphocytes.
Cultured lymphocytes
Lymphocytes culture was performed as previously described12. Briefly, EDTA anticoagulated venous blood was collected from patients. Lymphocytes were isolated and exposed to Epstein-Barr Virus (EBV) to induce cell immortalization. Two mL blood was diluted with 2 mL PBS, layered on top of 3 mL of Lymphocyte Separation Medium (MP Biomedicals) and centrifuged for 10 minutes at 1,000×g at room temperature. Using a Pasteur pipet, the lymphocytes were removed from the serum/Lympho Sep Media interface, washed in 10 mL PBS and then resuspended in 3 mL lymphoblast media (RPMI 1640 media containing L-glutamine, and 20% fetal bovine serum) containing 2μg/mL cyclosporine. The lymphocytes were then infected with 3 mL Epstein-Barr virus (EBV) and transferred to a T-25 vent capped flask. The cells were incubated at 37°C/5% CO2 and fed weekly with lymphoblast media + cyclosporine until signs of growth occurred which occurred generally in six weeks.
Microarray
RNA was isolated from lymphocytes using a Qiagen RNeasy mini kit (Valencia, Calif.). First and second strand complementary DNA was synthesized using standard techniques. Biotin-labeled antisense complementary RNA was produced by an in vitro transcription reaction. Human Genome U133 Plus 2.0 microarrays (Affymetrix, Foster City, Calif.) were hybridized with 20 μg cRNA. Target hybridization, washing, staining, and scanning probe arrays were done following an Affymetrix GeneChip Expression Analysis Manual. All array results have been submitted to the NCBI gene expression and hybridization array data repository (GEO, www.ncbi.nlm.nih.gov/geo/), as series (pending).
PCR confirmation in whole blood
Whole blood was collected in PAXgene tubes (BD 762165, Switzerland) at the time of enrollment and frozen within 24 hours of collection. RNA was isolated using the PAXgene blood RNA kit (BD 762164, Switzerland). First strand cDNA was made using QuantiTect® Reverse Transcription Kit (Qiagen) from 1ug total RNA. Quantitative real-time PCR was performed using a total reaction volume of 25 μL, containing 5 μL of diluted cDNA, 12.5 μL SYBR Green Supermix (Applied Biosystems Foster City, CA) and 0.03 μL of each oligonucleotide primer (250 μM). PCR was carried out in a StepOnePlus Real Time PCR System (Applied Biosystems) using 40 cycles of 95°C for 15 seconds followed by 60°C for 1 minute with a ten minute 95°C initial soak. Each measurement was made in triplicate and expressed relative to the detection of the standard hypoxanthine guanine phosphoribosyl transferase (HPRT). A priori we planned to perform PCR for the 25 genes that had the greatest intergroup expression difference, as identified by microarray. PCR was performed for the primer sets of these genes: HPRT, EPDR1, DSG2, SCD5, P2RY5, MGAT5, RHOQ, UCHL1, ZNF652, RALGPS2, TPD2, MKNL1, RAPGEF2, PIAS1, ARRB2, MBNL1, PDE7, CLEC2B, EPS8, FNBP1, STX16, DAPK1, SLC33A1, B4GALT5, ZNF207, FOXN3 (Integrated DNA Technologies IDT® Coralville, Iowa).
Array Analysis
The open source software, R2.13/Bioconductor2.8, was utilized for microarray analyses. Preprocessing of all cell files was carried out using the RMA (Robust Multi-array Average) algorithm, in which raw intensity values are background corrected, log2 transformed, and then quantile normalized. This was followed by duplicate probe removal to retain probes with higher interquartile range (IQR). Of 56,613 probe sets, 13,741 had an average expression in any group of over 7 (log base 2 units), and 5212 of these had a range of expression from maximum to minimum values of over 30%. These were used for an undirected principle components analysis (PCA). Finally, the 5212 probe list was sorted by the absolute value of the log-transformed difference between the responder and the non-responder group, with the sum of the standard deviations of each group subtracted. This returns a list sorted by minimum confidence interval fold change. The top 75 genes were used for a heat map (with hierarchical clustering), and the top 25 non sex-related were used for validation as biomarkers.
For hierarchical clustering, rows were standardized by subtracting mean and dividing by standard deviation; correlation was used as the distance metric, using the centroid linkage method. Analysis of enriched gene function groups was performed using the 2010 release of Webgestalt, using the hypergeometric test for enrichment of wither Gene Ontology consortium categories or KEGG pathways. PCA and hierarchical clustering were performed in JMP 10, a subset of SAS.
Statistical Analysis
Continuous variables of demographic data are reported as mean ± standard deviation. The Wilcoxon rank-sum test was used to compare differences in continuous variables between VR-PAH and VN-PAH groups. Paired measurements were compared using the Wilcoxon signed-rank test. Categorical variables were compared between groups using the chi-square test or Fisher exact test. A p value of < 0.05 was considered statistically significant. We used Wilcoxon rank-sum test to compare peripheral blood gene expression between VR-PAH and VN-PAH groups. To control the false discovery rate (FDR), the nominal p-values were corrected for multiple testing of genes. Genes that were believed to be differentially expressed at an FDR of 5% were further used to build decision trees to discriminate between VR-PAH and VN-PAH13. We used rpart package and explored all the trees that can perfectly separate VR-PAH from VN-PAH with two splits. Statistical analyses were performed using Prism 5.0 software (Graph Pad Software Inc, La Jolla, CA) and R 3.0.1.
Results
Eight patients with VR-PAH were identified from the Vanderbilt cohort (Table 1). Functional class was unknown in one child, one patient was class II, four were class III and two were class IV. All were alive at the time of analysis. Invasive hemodynamic primary data were available for 7/8 VR-PAH patients. The single VR-PAH patient without primary data for analysis underwent diagnostic RHC 18 years prior to enrollment at a different center. Data on the 32 VN-PAH patients are presented in Table 1. There were no significant differences in demographic characteristics between the two patient groups with the exception of PAH etiology and gender. There was a significantly higher proportion of HPAH in VN-PAH compared with VR-PAH (p=0.01), in keeping with prior published reports14. There was a strong female predominance in the VN-PAH cohort (30/32) and all of the VR-PAH patients were female, which is typical of PAH1, 15. Of the idiopathic VN-PAH from Vanderbilt, none have BMPR2 mutation, however one did have a mutation in KCNK316. While three HPAH patients included were from the same kindred, all the remaining patients were unrelated. All patients with VR-PAH patients were treated with calcium channel blockers (diltiazem = 1, nifedipine = 3, amlodipine = 5, one patient was treated sequentially with two) and two were also treated with phosphodiesterase type 5 inhibitors due to limited tolerance of high dose calcium channel blockers (gingival hyperplasia = 1, peripheral edema = 1). No VR-PAH patients were treated with any other class of PAH-directed therapy. Hemodynamics were different between VR-PAH and VN-PAH with significantly higher mean pulmonary arterial pressure and pulmonary vascular resistance in VN-PAH compared with VR-PAH (p<0.05). While nitric oxide had no significant effect on VN-PAH, patients with VR-PAH had significant decrease in mean pulmonary arterial pressure and pulmonary vascular resistance (p<0.05) with unchanged cardiac output. No patients in the VR-PAH cohort have died, while 11/32 VN-PAH patients have died at the time of data analysis.
Table 1.
Characteristics of vasodilator-responsive and non-responsive PAH patients
| Vasodilator-Responsive PAH (n=8) | Vasodilator Non-Responsive PAH (n=32) | |||
|---|---|---|---|---|
|
| ||||
| Age at diagnosis (years, mean ± SD) | 27.33 ± 12.3 | 36.9 ± 17.7 | ||
|
| ||||
| Female, number | 8 | 30 | ||
|
| ||||
| PAH Sub-Type | ||||
| Idiopathic | 8 | 17 | ||
| Heritable | 0 | 15 | ||
|
| ||||
| Follow Up Duration (years, mean ± SD) | 8.1 ± 6.2 | 9.6 ± 4.6 | ||
|
| ||||
| Hemodynamics at Diagnosis | Baseline (n=7) | Nitric Oxide (n=7) | Baseline (n=24) | Nitric Oxide (n=14) |
| RAP (mmHg) | 3.8 ± 3.8 | 3.2 ± 3.0 | 7.8 ± 4.5 | 7.9 ± 4.2 |
| mPAP (mmHg) | 45.9 ± 9.2 | 23.6 ± 5.8* | 59.9 ± 14.7* | 51.2 ± 14.4 |
| PWP (mmHg) | 6.1 ± 2.5 | 5.1 ± 3.7 | 9.7 ± 4.4 | 8.9 ± 4.6 |
| CI (L/min/m2) | 2.8 ± 1.4 | 3.3 ± 1.6 | 2.2 ± 0.7 | 2.2 ± 0.7 |
| PVR (Wood Units) | 9.5 ± 3.9 | 4.2 ± 2.0* | 15.7 ± 6.3* | 12.4 ± 5.7 |
CCB = calcium channel blocker, PDE5I = phosphodiesterase type 5 inhibitor. RAP=right atrial pressure, mPAP = mean pulmonary artery pressure, PWP = pulmonary wedge pressure, CI = cardiac index, PVR = pulmonary vascular resistance,
p<0.05 vs. VR-PAH baseline.
Microarray Analysis
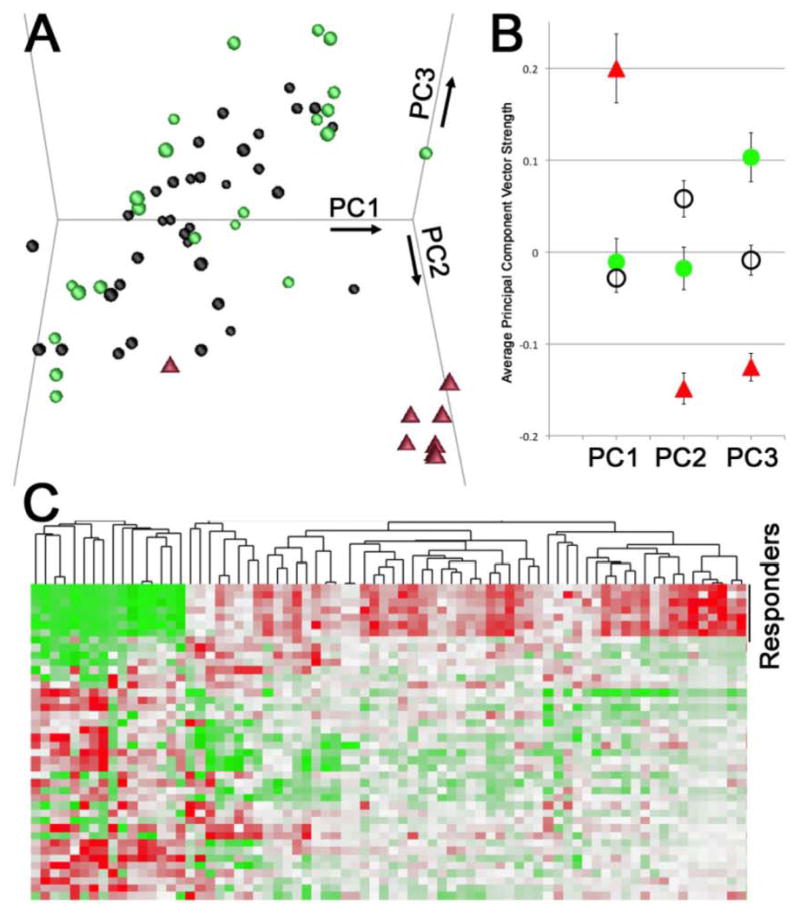
To test the hypothesis that a different molecular etiology of VR-PAH from VN-PAH could be detected in cultured lymphocytes, we performed microarray on VR-PAH, VN-PAH and controls. We performed principal component analysis (PCA) and found that VR-PAH patients were significantly different from VN-PAH in each of the first three principal components (Figure 1A, 1B). Each principal component corresponds to a list of genes that are roughly coregulated, with the first principal component (PC1) being the cluster of genes that explains the largest part of the variance across samples, PC2 being the gene group explaining the next most variance, et cetera. The analysis was performed without identifying gene groups a priori; the grouping of the patient samples is thus a natural result of gene expression differences, rather than the result of selection. PC1 consists of genes that are upregulated in VR-PAH, but not changed between VN-PAH and controls; PC2 consists of genes upregulated in VN-PAH but downregulated in VR-PAH; and PC3 consists of genes downregulated in both VR-PAH and VN-PAH. A heat map of the 75 probe sets most significantly changed between VR-PAH and VN-PAH groups is shown in Figure 1C. These data demonstrate that expression patterns in lymphocytes cultured ex vivo and without drug exposure maintained different expression patterns in VR-PAH compared with VN-PAH.
Figure 1.
Principle Components Analysis (PCA) in VR-PAH. (A) Plot of the 3 strongest principal components, representing 14%, 11%, and 7% respectively of the total variability among the 64 arrays, shows strong separation of the calcium channel responders (red triangles) compared to non-responsive PAH (black circles) or healthy controls (green circles). (B) Each of the first three principal components separates calcium channel responders (red triangles) from both non-responsive PAH patients (black circles) and from healthy controls (p<.01 by ANOVA with post-hoc t-test). (C) Heat map of expression of 75 genes differentially expressed in calcium channel responders (top group) compared to non-responders (bottom group). Each column corresponds to a gene, while each row corresponds to a patient; green corresponds to high expression, and red to low expression. Normalization has been performed on each gene such that the range and the average are the same across genes.
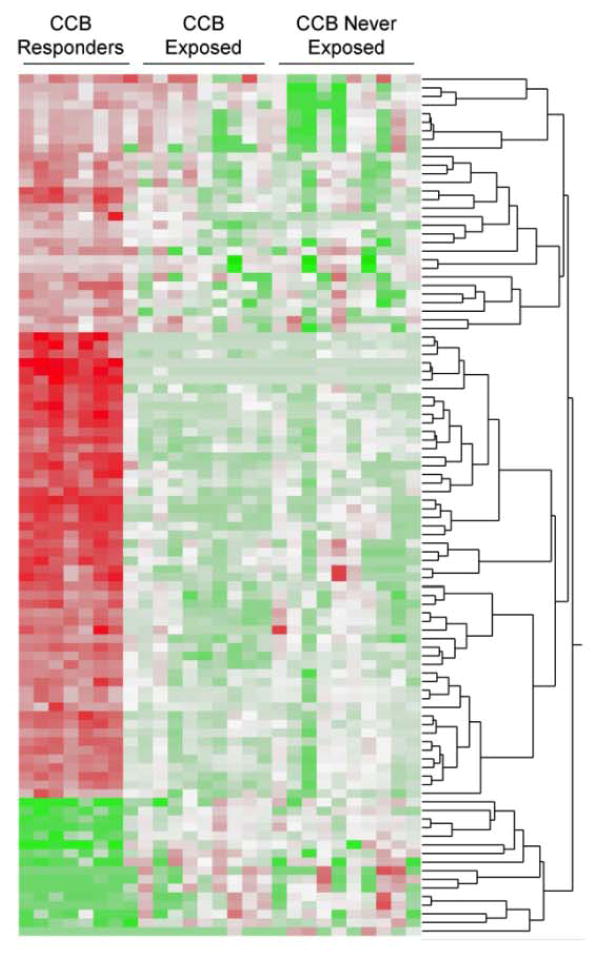
To test the hypothesis that there might be epigenetic effects of calcium channel blocker exposure on cultured lymphocytes despite ex vivo culture for several weeks, we examined the VN-PAH group for exposure to calcium channel blockers. We included only patients followed at Vanderbilt with medication histories prior to enrollment (n=19). Of these, 9 had been exposed to calcium channel blockers at the time of enrollment. Separation of microarray data by never- or ever-exposure to calcium channel blockers did not show any differences that were attributable to this medication class and the differences from the VR-PAH persisted (Figure 2). Thus an epigenetic effect of calcium channel blockers was unlikely to account for the observed differences of VR-PAH and VN-PAH.
Figure 2.
Heatmap of microarray data in 19 VN-PAH patients with medication histories followed at Vanderbilt. 9/19 had prior exposure to calcium channel blockers prior to enrollment. Differences between VR-PAH and VN-PAH persisted after segregation of VN-PAH by ever- or never-exposure to calcium channel blocker medication.
For PCR follow up, we focused on comparisons between VR-PAH and VN-PAH, as the goal was to identify VR-PAH in patients with PAH, but unknown vasoreactivity. After exclusion of two genes ascribed to sex differences in the cohorts (SOX9 (EntrezGene 6662) and STEAP1 (EntrezGene 26872)), we selected the 25 most significantly differently expressed named genes between VR-PAH and VN-PAH. Genes selected for PCR in peripheral blood are presented in Table 2. In broad gene ontogeny categories, there were eight genes related to Rho GTPases or cytoskeleton, three transcription factors, three cell-cell adhesion factors and 11 miscellaneous genes. Of note, four genes were calcium-dependent (DAPK1, DSG2, EPDR1, TPD52) and one was activated by adenosine (P2RY5).
Table 2.
Genes selected for PCR in peripheral blood
| Gene | EntrezGene | Fold Change VR-PAH vs. VN-PAH | Gene description | Gene Ontology Group |
|---|---|---|---|---|
| ARRB2: Arrestin, beta 2 | 409 | 2.02 | desensitize g protein coupled receptors dampening responses | Miscellaneous |
| B4GALT5: UDP-Gal:betaGlcNAc beta 1,4-galactosyltransferase, polypeptide 5 | 9334 | 3.17 | biosynthesis of glycoconjugates | Miscellaneous |
| CHES1: Checkpoint suppressor 1 | 1112 | 2.507 | transcription factor | Transcription factor |
| CLEC2B: C-type lectin domain family 2, member B | 9976 | 1.86 | cell-cell adhesion | Cell-cell adhesion |
| DAPK1: death-associated protein kinase 1 | 1612 | −2.23 | positive mediator of apoptosis; calmodulin-dependent | Miscellaneous |
| DSG2: Desmoglein 2 | 1829 | −2.66 | cell-cell adhesion, calcium binding transmembrane glycoprotein | Cell-cell adhesion |
| EPDR1: ependymin related protein 1 (zebrafish) | 54749 | −1.85 | calcium-dependent cell-cell adhesion | Cell-cell adhesion |
| EPS8: epidermal growth factor receptor pathway substrate 8 | 2059 | −1.98 | EGFR pathway member, similar to Ras and Rac and growth-factor mediated actin remodeling | Rho GTPase/cytoskeleton |
| FNBP1: Formin binding protein 1 | 23048 | 1.97 | formin-binding protein family, contains Rho family protein-binding domain | Rho GTPase/cytoskeleton |
| MBNL1: Muscleblind-like (Drosophila) | 4154 | 1.85 | associated with myotonic dystrophy, results in alternative splicing of proteins | Miscellaneous |
| MGAT5: Mannosyl (alpha-1,6-)-glycoprotein beta-1,6-N-acetyl-glucosaminyltransferase | 4249 | 1.96 | enzyme involved in regulation of biosynthesis of glycoprotein oligosaccharides; alters migratory behavior of cells | Miscellaneous |
| MKLN1: Muskelin 1, intracellular mediator containing kelch motifs | 4289 | 2.10 | mediator of cell spreading, cytoskeletal responses to TSP1 | Rho GTPase/cytoskeleton |
| P2RY5: purinergic receptor P2Y, G-protein coupled, 5 | 10161 | 2.03 | G protein coupled receptor, activated by adenosine and uridine | Rho GTPase/cytoskeleton |
| PDE7A: phosphodiesterase 7A | 5150 | 2.51 | phosphodiesterase | Miscellaneous |
| PIAS1: Protein inhibitor of activated STAT, 1 | 8554 | 1.84 | protein inhibitor of STAT-1, related to spermatogenesis, interacts with androgen receptor | Miscellaneous |
| RALGPS2: Ral GEF with PH domain and SH3 binding motif 2 | 55103 | 1.83 | ras small GTPase, poorly characterized | Rho GTPase/cytoskeleton |
| RAPGEF2: Rap guanine nucleotide exchange factor (GEF) 2 | 9693 | 1.89 | member of RAS-subfamily of GTPases | Rho GTPase/cytoskeleton |
| RHOQ: Ras homolog gene family, member Q | 23433 | 2.37 | small GTPase, cytoskeleton regulation | Rho GTPase/cytoskeleton |
| SCD5: stearoyl-CoA desaturase 5 | 79966 | 2.45 | ER membrane protein catalyzes monounsaturated fatty acids from saturated fatty acids | Miscellaneous |
| SLC33A1: Solute carrier family 33 (acetyl-CoA transporter), member 1 | 9197 | 2.29 | required for o-acetylated gangliosides; associated with spastic paraplegia | Miscellaneous |
| STX16: syntaxin 16 | 8675 | 2.19 | target for v-SNARES allowing for vesicle docking/fusion on membrane | Rho GTPase/cytoskeleton |
| TPD52: Tumor protein D52 | 7163 | 1.86 | tumor protein, expressed in exocrine cells, regulates Ca+ dependent protein secretion | Miscellaneous |
| UCHL1: ubiquitin carboxyl-terminal esterase L1 (ubiquitin thiolesterase) | 7345 | −2.29 | thiol protease hydrolyzes peptide bond on ubiquitin | Miscellaneous |
| ZNF207: zinc finger protein 207 | 7756 | 2.41 | zinc finger gene | Transcription factor |
| ZNF652: zinc finger protein 652 | 22834 | 2.06 | zinc finger protein | Transcription factor |
Peripheral Blood Gene Expression
Because isolation and culture of peripheral lymphocytes is not feasible for routine use, we sought to confirm our microarray results in peripheral blood without specific isolation of a blood compartment. We hypothesized that whole blood RNA expression would reflect the findings from microarray analysis of cultured lymphocytes that were quantitatively greatest, but might not detect the more subtle differences that can be observed in lymphocytes. We identified 8 VR-PAH and 13 VN-PAH patients with available whole blood collected via venipuncture using RNA preservation tubes and isolated RNA. qPCR was performed for the genes in Table 2. After correction for multiple comparisons, we found statistically different expression in 13/25 genes at a FDR level of 5% as listed in Table 3. Of the genes statistically different between the two groups, five were related to rho GTPase and cytoskeleton, two were cell-cell adhesion factors, two were developmental genes, one transcription factor and three were of miscellaneous function. Three of these genes were known to be calcium dependent or activated by adenosine (EPDR1, DSG2 and TPD52).
Table 3.
Differences in peripheral blood gene expression levels between VN-PAH and VR-PAH
| Gene | Raw P-value | Corrected P-value (FDR) |
|---|---|---|
| EPDR1 | 0.0002 | 0.00049 |
| DSG2 | 0.00004 | 0.00049 |
| SCD5 | 0.00007 | 0.00049 |
| P2RY5 | 0.00012 | 0.00049 |
| MGAT5 | 0.00012 | 0.00049 |
| RHOQ | 0.00012 | 0.00049 |
| UCHL1 | 0.00029 | 0.00105 |
| ZNF652 | 0.00044 | 0.00138 |
| RALGPS2 | 0.00134 | 0.00138 |
| TPD52 | 0.00245 | 0.00634 |
| MKNL1 | 0.00596 | 0.01241 |
| RAPGEF2 | 0.00596 | 0.01241 |
| PIAS1 | 0.00994 | 0.01911 |
| ARRB2 | 0.05349 | 0.09551 |
| MBNL1 | 0.07560 | 0.12600 |
| PDE7 | 0.10419 | 0.16280 |
| CLEC2B | 0.21026 | 0.29203 |
| EPS8 | 0.21026 | 0.29203 |
| FNBP1 | 0.23813 | 0.31333 |
| STX16 | 0.26846 | 0.33557 |
| DAPK1 | 0.30113 | 0.35848 |
| SLC33A1 | 0.41367 | 0.47008 |
| B4GALT5 | 0.54663 | 0.59416 |
| ZNF207 | 0.64520 | 0.67208 |
| FOXN3 | 0.91553 | 0.91553 |
FDR = false discovery rate
Validation in an External Cohort
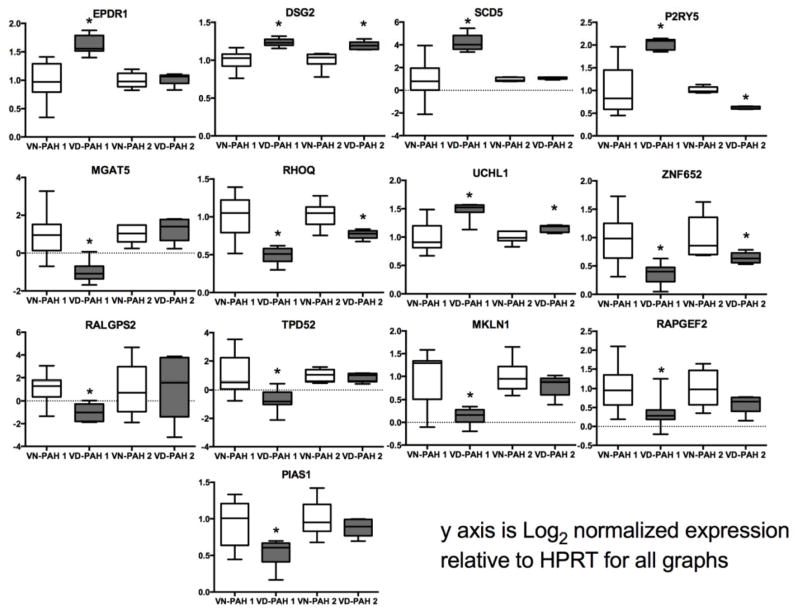
We sought to validate our whole blood RNA expression findings in a second cohort of patients followed at the University of Chicago (Supplemental Table 1). Whole blood from five patients with confirmed long-term response to calcium channel blockers and 6 VN-PAH patients recruited separately was collected in RNA isolation tubes and sent to our center. RNA was isolated and PCR performed for the 25 most differentially expressed genes found in the discovery cohort. Figure 3 and Supplemental Table 2 show qPCR results of the 13 significantly different genes from the primary analysis in the Vanderbilt cohort in both our cohort and the University of Chicago and control patients. In general there was broad concordance in findings of differentially expressed genes in the two VR-PAH cohorts, but lower numbers in the University of Chicago cohort may have reduced statistical power in that group. Gene expression was suppressed in VR-PAH compared with VN-PAH in 8/13 genes and only upregulated in 5/13 genes compared with VN-PAH.
Figure 3.
Whole blood qPCR of genes differentially expressed in microarray analysis. qPCR of whole blood was performed in controls, VN-PAH and VR-PAH. VR-PAH 1 and VN-PAH 1 represent the Vanderbilt cohort, VR-PAH 2 and VN-PAH 2 represent the University of Chicago cohort. *p<0.05 by Kruskall-Wallis with Dunn’s multiple comparisons test.
Development and Validation of a Peripheral Blood-Based RNA Expression Model to Detect VR-PAH
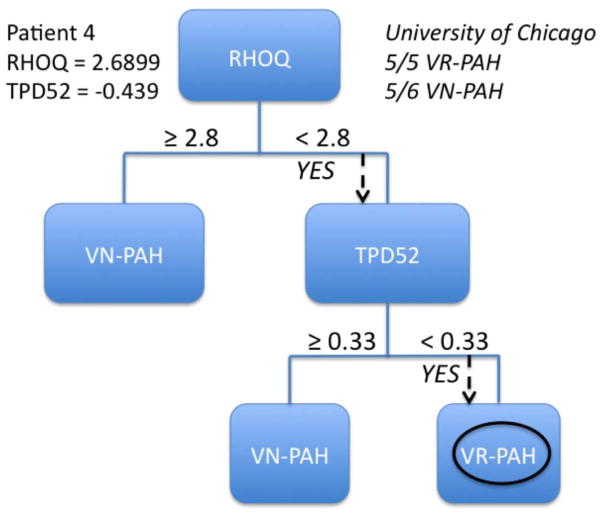
We sought to use the differentially expressed genes identified above to develop a peripheral blood qPCR test to differentiate VR-PAH from VN-PAH. We thus constructed decision trees using expression levels of significantly differently expressed genes to discriminate between the two types of PAH. The University of Chicago cohort was used to validate the strength of the proposed decision trees. We found that no more than two genes were required to distinguish VR-PAH from VN-PAH. DSG2, EPDR1, SCD5, MGAT5, or RHOQ could serve as the primary decision point. The secondary gene could include one of the genes in the primary decision if not used or P2RY5, ZNF652, RALGPS2, MKNL1, RAPFEF2, TPD52, PIAS1. Decision trees with a primary gene of DSG2 and RHOQ correctly identified 5/5 VR-PAH in the validation cohort, however the combination of RHOQ ≥ 2.8 cycles different from HPRT and TPD52 ≥ 0.33 cycles different from HPRT was able to segregate 5/6 VN-PAH correctly as well in the validation cohort (Figure 4). Additional combinations of two genes that successfully identify 5/5 VR-PAH patients in the validation cohort are presented in Table 4. These data demonstrate that mRNA expression patterns in peripheral blood of selected genes can be used to differentiate VN-PAH from VR-PAH.
Figure 4.
Example of a decision tree to differentiate VR-PAH from VN-PAH. The figure shows an example of a decision tree based on the primary gene RHOQ and TPD52 as the secondary gene. Numbers shown within the tree are for the performance of the tree in the Vanderbilt cohort. In the upper right is the performance of the decision tree in the University of Chicago validation cohort. Below the tree is a demonstration of how the tree would be performed in one patient from VR-PAH.
Table 4.
Gene combinations successfully identifying VR-PAH patients
| Primary Gene Expression Level | Secondary Gene Expression Level | External Validation Accuracy | |
|---|---|---|---|
| VR-PAH | VN-PAH | ||
| RHOQ ≥ 2.8 | TPD52 ≥ 0.33 | 5/5 | 5/6 |
| DSG2 ≥ −7.8 | P2RY5 ≥ −9.9 | 5/5 | 1/6 |
| ZNF652 ≥ 1.0 | 5/5 | 1/6 | |
| RALGPS2 < 1.5 | 5/5 | 1/6 | |
| TPD52 ≥ −0.39 | 5/5 | 0/6 | |
| PIAS1 ≥ 1.2 | 5/5 | 1/6 | |
| UCHL1 ≥ −8.5 | 5/5 | 1/6 | |
Expression levels are cycle number relative to HPRT.
Discussion
We have used ex vivo cultured lymphocytes from VR- and VN-PAH patients to identify genes differentially expressed in these two distinct PAH treatment phenotypes. We then used these expression differences to develop and validate a whole blood RNA expression-based profile that distinguishes VR-PAH from VN-PAH. These data show that VR-PAH and VN-PAH likely have a different molecular etiology and the resultant gene expression differences can be reliably detected in the peripheral blood. These studies serve as proof of the concept that PAH treatment response phenotypes may be identified in peripheral blood and these markers could be used prospectively to predict response to therapy, so-called personalized medicine. Extension of this approach to other classes of PAH therapies may be useful.
Treatment of PAH has advanced significantly in the past several decades with many recent publications showing improved survival compared with historical controls2, 17, 18. Nonetheless, there remains substantial morbidity and mortality; delay of therapy and inability to identify appropriate therapy remain important challenges for practitioners treating PAH19. We used VR-PAH as a readily recognized, clinically distinct phenotype with improved survival (5-year survival 98% vs. 48%3) and well-defined response to therapy3, 5 as a tool to show proof of the concept that peripheral blood RNA expression can identify patients likely to respond to specific PAH-directed therapies. If validated prospectively, these profiles may become the first companion diagnostic test for identifying the most appropriate treatment of PAH. Availability of a blood test would also help assure that VR-PAH patients are not missed, which can have tragic consequences.
Use of cultured lymphocytes in development of potential qPCR targets provided several advantages in this study. First, they were cultured outside of the patients for several weeks, thus removing any potential influence of calcium channel blockers as demonstrated in Figure 2, PAH directed therapy or other environmental effects. Second, cultured lymphocytes remove much of the well-described variability in whole blood RNA expression profiles that may reflect day to day variability in inflammation, dietary or environmental exposures. Thus they can provide greater insight into more subtle underlying molecular differences in patient phenotypes and this has been used as an advantage of these cell lines in other medical diseases20. We found significant differences in gene expression profiles using microarray analysis in VR-PAH and VN-PAH that required two principal components to fully separate the groups. Major gene ontology groups that were different included cytoskeletal/Rho GTPase genes, cell-cell adhesion genes, transcription factors and developmental pathways, pathways that are known to be associated with PAH. The identification of several calcium-dependent genes in the most differentially expressed group using an unbiased, discovery-based approach could not be attributed to drug effect given the long culture period of the lymphocytes outside of drug exposure and may suggest that alterations in calcium-dependent cell-cell adhesion plays a role in VR-PAH.
We used an unbiased discovery approach to identify differentially expressed genes in cultured lymphocytes and did identify several gene ontology groups previously published to play a role in PAH. Specifically, cytoskeletal genes, ion channels and developmental pathways have been well described to play a role in human and animal models of PAH9, 16, 21, 22. VR-PAH patients were markedly different from VN-PAH patients in these pathways, some to greater degree than others, but all appeared to be different. This may lend insight into a different molecular etiology of VR-PAH. The identification of several calcium- and adenosine-dependent genes may be purely coincidence or may reflect the potent effect of many vasodilators in the treatment of VR-PAH. Although this study did not explore how the differentially expressed genes may contribute to phenotypes of PAH treatment, the strong differences in several genes’ expression is provocative. DSG2 or desmoglein-2 is a desmosomal cadherin that is known to be highly expressed in cardiac tissue 23–25 and has been shown to underlie some cases of arrhythmogenic right ventricular dysplasia23, 24, 26. Although the mechanism by which DSG2 mutations causes this disease are presently incompletely understood, alterations in Wnt/β-catenin signaling may play a role27, and this pathway is similarly implicated in idiopathic and heritable PAH28. RHOQ also strongly differentiated VR-PAH from VN-PAH. This cytoskeletal gene facilitates protein transport and has been shown to be important in insulin-mediated signaling29, another pathway of importance in PAH30–32. Of note, only EPDR1, UCHL1, DSG2, SCD5 and P2RY5 expression levels were elevated in VR-PAH compared with VN-PAH, and gene expression levels were more commonly suppressed in VR-PAH which may reflect underlying disease mechanism. Of note, the methodology we used to identify these genes used an unbiased approach and did not identify many genes previously found to be important in pathogenesis of PAH33–35. This methodology does not discredit their role in pulmonary hypertension, but this approach did not identify these genes to be the within the top 25 most differentially expressed. If these molecules are functionally important in VR-PAH, they may explain how several different compounds can produce vasodilation in this phenotype.
Despite these and other potential genes of interest, further study is warranted to determine if the differentially expressed genes identified here play a role in disease pathogenesis or are simply serving as biomarkers of disease phenotype. In either case, their markedly different expression in VR-PAH and VN-PAH may be exploited to segregate the two treatment phenotypes in peripheral blood. Multiple genes were identified with differential expression in the two phenotypes, implying that the two treatment phenotypes are quite disparate on a molecular level. Prospective validation will require refinement of cut off mRNA expression levels through collection of a statistically appropriate number of newly diagnosed VR-PAH patients through multiple participating centers.
This study has some limitations. The numbers of VR-PAH are low for several reasons. First, in order to be most stringent in definition of phenotype, we excluded any patient being treated with endothelin receptor antagonists or prostacyclins as these patients may have a different, or intermediate phenotype. This left us with only female patients, and these results may not be applicable to male patients. Second, VR-PAH accounts for 5–10% of all idiopathic PAH which is itself very rare. The use of a second, separate cohort for validation tested in a different batch adds strength to the findings, however validation in larger cohorts will be useful for further confirmation of findings. Microarray in peripheral lymphocytes by definition can only detect genes expressed in lymphocytes. Thus there may be other, important genes in different tissue types. Nonetheless, we were able to detect clear differences in PAH treatment phenotype in our cultured lymphocytes, including cell-cell adhesion factors. This study was designed to identify a peripheral blood signature of VR-PAH, but cannot by nature lend insight into how the differentially expressed genes may or may not contribute to disease pathogenesis in each treatment phenotype. Further study will be needed to understand the mechanistic implications of the identified genes.
In conclusion, we have identified an RNA expression profile in peripheral blood that can identify VR-PAH patients from VN-PAH patients. These data may serve as a proof of concept that peripheral blood RNA expression profiles can be used to identify optimal drug treatment in PAH. Further study is warranted to determine if this experimental model may be applied to understanding mechanisms of forms of pulmonary hypertension (WHO Groups 2–5) or whether it may be used to identify optimal treatment a priori in PAH.
Supplementary Material
Acknowledgments
Funding Sources: The project was supported by CTSA award No. UL1TR000445 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health., NIH 1 P01 HL 108800 (Hemnes, Newman) and 1 K08 HL 093363 (Hemnes), NIH-RO1-HL071115, 1RC1HL099462 (Archer).
Footnotes
Disclosures: ARH, AWT, SLA, SR, CY, HN, NP, MF, LW, IMR, EDA, JHN and JW report no conflicts of interest.
References
- 1.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, Dromer C, Pison C, Reynaud-Gaubert M, Haloun A, Laurent M, Hachulla E, Simonneau G. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006;173:1023–30. doi: 10.1164/rccm.200510-1668OC. [DOI] [PubMed] [Google Scholar]
- 2.Benza RL, Miller DP, Gomberg-Maitland M, Frantz RP, Foreman AJ, Coffey CS, Frost A, Barst RJ, Badesch DB, Elliott CG, Liou TG, McGoon MD. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL) Circulation. 2010;122:164–72. doi: 10.1161/CIRCULATIONAHA.109.898122. [DOI] [PubMed] [Google Scholar]
- 3.Sitbon O, Humbert M, Jais X, Ioos V, Hamid AM, Provencher S, Garcia G, Parent F, Herve P, Simonneau G. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation. 2005;111:3105–11. doi: 10.1161/CIRCULATIONAHA.104.488486. [DOI] [PubMed] [Google Scholar]
- 4.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, Rubin LJ, Tapson VF, Varga J. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53:1573–619. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Rich S, Brundage BH. High-dose calcium channel-blocking therapy for primary pulmonary hypertension: evidence for long-term reduction in pulmonary arterial pressure and regression of right ventricular hypertrophy. Circulation. 1987;76:135–41. doi: 10.1161/01.cir.76.1.135. [DOI] [PubMed] [Google Scholar]
- 6.Hemnes AR, Forfia PR, Champion HC. Assessment of pulmonary vasculature and right heart by invasive haemodynamics and echocardiography. Int J Clin Pract Suppl. 2009:4–19. doi: 10.1111/j.1742-1241.2009.02110.x. [DOI] [PubMed] [Google Scholar]
- 7.Brittain EL, Pugh ME, Wheeler LA, Robbins IM, Loyd JE, Newman JH, Austin ED, Hemnes AR. Prostanoids But Not Oral Therapies Improve Right Ventricular Function in Pulmonary Arterial Hypertension. JACC Heart failure. 2013;1:300–307. doi: 10.1016/j.jchf.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brittain EL, Pugh ME, Wheeler LA, Robbins IM, Loyd JE, Newman JH, Larkin EK, Austin ED, Hemnes AR. Shorter survival in familial versus idiopathic pulmonary arterial hypertension is associated with hemodynamic markers of impaired right ventricular function. Pulm Circ. 2013;3:589–98. doi: 10.1086/674326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Austin ED, Menon S, Hemnes AR, Robinson LR, Talati M, Fox KL, Cogan JD, Hamid R, Hedges LK, Robbins I, Lane K, Newman JH, Loyd JE, West J. Idiopathic and heritable PAH perturb common molecular pathways, correlated with increased MSX1 expression. Pulm Circ. 2011;1:389–98. doi: 10.4103/2045-8932.87308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, Elliott CG, Gaine SP, Gladwin MT, Jing ZC, Krowka MJ, Langleben D, Nakanishi N, Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54:S43–54. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 11.Robbins IM, Newman JH, Johnson RF, Hemnes AR, Fremont RD, Piana RN, Zhao DX, Byrne DW. Association of the metabolic syndrome with pulmonary venous hypertension. Chest. 2009;136:31–6. doi: 10.1378/chest.08-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.West J, Cogan J, Geraci M, Robinson L, Newman J, Phillips JA, Lane K, Meyrick B, Loyd J. Gene expression in BMPR2 mutation carriers with and without evidence of pulmonary arterial hypertension suggests pathways relevant to disease penetrance. BMC Med Genomics. 2008;1:45. doi: 10.1186/1755-8794-1-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benjamini YH. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B. 1995;57:289–300. [Google Scholar]
- 14.Elliott CG, Glissmeyer EW, Havlena GT, Carlquist J, McKinney JT, Rich S, McGoon MD, Scholand MB, Kim M, Jensen RL, Schmidt JW, Ward K. Relationship of BMPR2 mutations to vasoreactivity in pulmonary arterial hypertension. Circulation. 2006;113:2509–15. doi: 10.1161/CIRCULATIONAHA.105.601930. [DOI] [PubMed] [Google Scholar]
- 15.Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, Barst RJ, Benza RL, Liou TG, Turner M, Giles S, Feldkircher K, Miller DP, McGoon MD. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest. 2010;137:376–87. doi: 10.1378/chest.09-1140. [DOI] [PubMed] [Google Scholar]
- 16.Ma L, Roman-Campos D, Austin ED, Eyries M, Sampson KS, Soubrier F, Germain M, Tregouet DA, Borczuk A, Rosenzweig EB, Girerd B, Montani D, Humbert M, Loyd JE, Kass RS, Chung WK. A novel channelopathy in pulmonary arterial hypertension. N Engl J Med. 2013;369:351–61. doi: 10.1056/NEJMoa1211097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, Dromer C, Pison C, Reynaud-Gaubert M, Haloun A, Laurent M, Hachulla E, Cottin V, Degano B, Jais X, Montani D, Souza R, Simonneau G. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation. 2010;122:156–63. doi: 10.1161/CIRCULATIONAHA.109.911818. [DOI] [PubMed] [Google Scholar]
- 18.D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115:343–9. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 19.Deano RC, Glassner-Kolmin C, Rubenfire M, Frost A, Visovatti S, McLaughlin VV, Gomberg-Maitland M. Referral of patients with pulmonary hypertension diagnoses to tertiary pulmonary hypertension centers: the multicenter RePHerral study. JAMA Intern Med. 2013;173:887–93. doi: 10.1001/jamainternmed.2013.319. [DOI] [PubMed] [Google Scholar]
- 20.Welsh M, Mangravite L, Medina MW, Tantisira K, Zhang W, Huang RS, McLeod H, Dolan ME. Pharmacogenomic discovery using cell-based models. Pharmacol Rev. 2009;61:413–29. doi: 10.1124/pr.109.001461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson JA, Hemnes AR, Perrien DS, Schuster M, Robinson LJ, Gladson S, Loibner H, Bai S, Blackwell TR, Tada Y, Harral JW, Talati M, Lane KB, Fagan KA, West J. Cytoskeletal defects in Bmpr2-associated pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol. 2012;302:L474–84. doi: 10.1152/ajplung.00202.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrell NW, Adnot S, Archer SL, Dupuis J, Jones PL, MacLean MR, McMurtry IF, Stenmark KR, Thistlethwaite PA, Weissmann N, Yuan JX, Weir EK. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S20–31. doi: 10.1016/j.jacc.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Awad MM, Dalal D, Cho E, Amat-Alarcon N, James C, Tichnell C, Tucker A, Russell SD, Bluemke DA, Dietz HC, Calkins H, Judge DP. DSG2 mutations contribute to arrhythmogenic right ventricular dysplasia/cardiomyopathy. Am J Hum Genet. 2006;79:136–42. doi: 10.1086/504393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pilichou K, Nava A, Basso C, Beffagna G, Bauce B, Lorenzon A, Frigo G, Vettori A, Valente M, Towbin J, Thiene G, Danieli GA, Rampazzo A. Mutations in desmoglein-2 gene are associated with arrhythmogenic right ventricular cardiomyopathy. Circulation. 2006;113:1171–9. doi: 10.1161/CIRCULATIONAHA.105.583674. [DOI] [PubMed] [Google Scholar]
- 25.Schwarz MA, Owaribe K, Kartenbeck J, Franke WW. Desmosomes and hemidesmosomes: constitutive molecular components. Annu Rev Cell Biol. 1990;6:461–91. doi: 10.1146/annurev.cb.06.110190.002333. [DOI] [PubMed] [Google Scholar]
- 26.Awad MM, Calkins H, Judge DP. Mechanisms of disease: molecular genetics of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Nat Clin Pract Cardiovasc Med. 2008;5:258–67. doi: 10.1038/ncpcardio1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia-Gras E, Lombardi R, Giocondo MJ, Willerson JT, Schneider MD, Khoury DS, Marian AJ. Suppression of canonical Wnt/beta-catenin signaling by nuclear plakoglobin recapitulates phenotype of arrhythmogenic right ventricular cardiomyopathy. J Clin Invest. 2006;116:2012–21. doi: 10.1172/JCI27751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chow K, Fessel JP, Kaoriihida S, Schmidt EP, Gaskill C, Alvarez D, Graham B, Harrison DG, Wagner DH, Jr, Nozik-Grayck E, West JD, Klemm DJ, Majka SM. Dysfunctional resident lung mesenchymal stem cells contribute to pulmonary microvascular remodeling. Pulm Circ. 2013;3:31–49. doi: 10.4103/2045-8932.109912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chunqiu Hou J, Pessin JE. Lipid Raft targeting of the TC10 amino terminal domain is responsible for disruption of adipocyte cortical actin. Mol Biol Cell. 2003;14:3578–91. doi: 10.1091/mbc.E03-01-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pugh ME, Robbins IM, Rice TW, West J, Newman JH, Hemnes AR. Unrecognized glucose intolerance is common in pulmonary arterial hypertension. J Heart Lung Transplant. 2011;30:904–11. doi: 10.1016/j.healun.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.West J, Niswender KD, Johnson JA, Pugh ME, Gleaves L, Fessel JP, Hemnes AR. A potential role for insulin resistance in experimental pulmonary hypertension. Eur Respir J. 2013;41:861–71. doi: 10.1183/09031936.00030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hansmann G, de Jesus Perez VA, Alastalo TP, Alvira CM, Guignabert C, Bekker JM, Schellong S, Urashima T, Wang L, Morrell NW, Rabinovitch M. An antiproliferative BMP-2/PPARgamma/apoE axis in human and murine SMCs and its role in pulmonary hypertension. J Clin Invest. 2008;118:1846–57. doi: 10.1172/JCI32503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Archer SL, Gomberg-Maitland M, Maitland ML, Rich S, Garcia JG, Weir EK. Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1alpha-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. Am J Physiol Heart Circ Physiol. 2008;294:H570–8. doi: 10.1152/ajpheart.01324.2007. [DOI] [PubMed] [Google Scholar]
- 34.Bonnet S, Michelakis ED, Porter CJ, Andrade-Navarro MA, Thebaud B, Haromy A, Harry G, Moudgil R, McMurtry MS, Weir EK, Archer SL. An abnormal mitochondrial-hypoxia inducible factor-1alpha-Kv channel pathway disrupts oxygen sensing and triggers pulmonary arterial hypertension in fawn hooded rats: similarities to human pulmonary arterial hypertension. Circulation. 2006;113:2630–41. doi: 10.1161/CIRCULATIONAHA.105.609008. [DOI] [PubMed] [Google Scholar]
- 35.Hong Z, Smith AJ, Archer SL, Wu XC, Nelson DP, Peterson D, Johnson G, Weir EK. Pergolide is an inhibitor of voltage-gated potassium channels, including Kv1.5, and causes pulmonary vasoconstriction. Circulation. 2005;112:1494–9. doi: 10.1161/CIRCULATIONAHA.105.556704. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.