Abstract
Background and Purpose
A better understanding of the stroke risk factors in children with congenital heart disease (CHD) could inform stroke prevention strategies. We analyzed pediatric stroke associated with CHD in a large community-based, case-control study.
Methods
From 2.5 million children (< 20 years) enrolled in a Northern California integrated healthcare plan, we identified ischemic and hemorrhagic strokes and randomly selected age and facility-matched stroke-free controls (3 per case). We determined exposure to CHD (diagnosed prior to stroke) and used conditional logistic regression to analyze stroke risk.
Results
CHD was identified in 15/412 cases (4%) versus 7/1,236 controls (0.6%). Children (28 days – 20 years) with CHD had 19-fold (Odds Ratio [OR] 19; 95% Confidence Interval [CI] 4.2, 83) increased stroke risk compared to controls. History of CHD surgery was associated with >30-fold increased risk of stroke (OR 31; CI 4, 241 compared to controls). After excluding peri-operative strokes, a history of CHD surgery still increased childhood stroke risk (OR 13; CI 1.5, 114). The majority of children with stroke and CHD were outpatient at the time of stroke, and almost half the cases who underwent cardiac surgery had their stroke >5 years after the most recent procedure. An estimated 7% of ischemic and 2% of hemorrhagic childhood strokes in the population were attributable to CHD.
Conclusions
CHD is an important childhood stroke risk factor. Children who undergo CHD surgery remain at elevated risk outside of the peri-operative period, and would benefit from optimized long-term stroke prevention strategies.
Keywords: congenital heart disease, pediatric stroke, epidemiology
Introduction
The high frequency of children with CHD reported in pediatric stroke registries suggests that CHD is a strong risk factor for ischemic stroke.1–3 Cases of hemorrhagic stroke have also been reported in children with CHD.4, 5 However, the bulk of the CHD literature examining stroke focuses on specific scenarios which carry a high burden of neurologic injury, such as cardiac surgery,6, 7 cardiac catheterization,8 treatment with ventricular assist devices9 or extra-corporeal membrane oxygenation.10 Further, the length of follow-up in published studies is often limited in time, or specifically targets only the neonatal and infantile period.11–13 To design rational studies for long-term stroke prevention for children with CHD, a better understanding of the clinical factors that may influence CHD-associated stroke risk is needed. Community-based data that includes longer follow-up times, older children remote from surgery and hemorrhagic stroke outcomes could inform future stroke prevention studies.
The primary aim of this case-control study was to collect additional detailed cardiac data from children within a large, established, Northern California community-based cohort and measure the association of CHD with ischemic and hemorrhagic stroke. We hypothesized that clinical factors such as age at stroke (neonatal versus childhood), cyanotic or acyanotic heart lesion and history of a recent cardiac surgery may modify stroke risk, and secondarily aimed to evaluate these potential effect modifiers.
Methods
Study design and setting
We performed a case-control study of the association between CHD and stroke within the population of 2.5 million children (<20 years of age) enrolled in Kaiser Permanente Northern California (KPNC) from January 1993 to December 2007. KPNC is an integrated health care system that provides care to roughly one-third of the Northern California population. Cases were children with ischemic or hemorrhagic stroke. Three age and facility matched stroke-free controls per case were randomly selected. Medical records of the cases and controls were examined for the exposure: a CHD diagnosis made prior to the stroke. This study was approved by the institutional review boards of the Kaiser Foundation Research Institute (Oakland, CA) and the University of California, San Francisco; both institutional review boards waived the requirement for informed consent.
Case and control identification
Cases of symptomatic stroke and stroke-free controls were identified by methods described below and in prior reports.14, 15 Briefly, cases were ascertained through electronic searches of hospital discharge and outpatient diagnoses related to stroke, keyword searches of electronic head imaging reports, and cross-referencing with studies of cerebral palsy16 and perinatal arterial ischemic stroke17 utilizing a KPNC birth cohort. The inclusion criteria for stroke were: 1) documented clinical presentation consistent with stroke, such as a sudden onset focal neurologic deficit, headache, loss of consciousness or seizure; and 2) CT or MRI showing a focal infarct or intracerebral, subarachnoid and/or intraventricular hemorrhage in a location and of a maturity consistent with the neurologic signs and symptoms. Stroke was independently confirmed by two neurologists, with arbitration of disagreements by a third. Transient ischemic attacks, venous sinus thromboses, extra-axial hemorrhages and neonatal pure intra-ventricular hemorrhages were excluded. Cases were also excluded if the stroke occurred before enrollment in KPNC or outside the study period. Three controls per case were randomly selected by incidence density sampling from the remaining stroke-free children and matched by birth year, year of enrollment, and primary care facility.
Data abstraction and definitions
Using a standardized protocol, data were abstracted by a single pediatric nurse medical records analyst from all available KPNC medical records and out-of-plan facility records. Abstracted data included stroke presentation, type of heart lesion and medical and surgical interventions for CHD and were reviewed by a pediatric vascular neurologist to confirm. Exposure to CHD was defined as a diagnosis of CHD prior to the onset of stroke and documented in the medical record. Cyanotic heart disease included tetralogy of Fallot, transposition of the great arteries, total anomalous pulmonary venous return, truncus arteriosus, single ventricle or common ventricle, hypoplastic left heart syndrome, double outlet right ventricle and interrupted aortic arch. Acyanotic heart disease included atrial septal defect, ventricular septal defect, atrioventricular canal and coarctation of the aorta. Neonatal stroke occurred within the first 28 days of life. Childhood stroke occurred from 29 days to 20 years of life. Race was a dichotomous variable classified as either white or non-white by self-identification. Cardiac surgery was defined as cardiac surgery or catheterization at any time prior to stroke diagnosis or index date for controls (the date of the stroke in the matched case). Stroke or index date (for controls) that occurred in the 14 days after a cardiac surgery was considered peri-operative.
Data analysis
Stata 12, College Station, TX, was used for data analysis. Demographics and characteristics of stroke cases with and without CHD were compared using Chi-squared tests. To estimate relative risk, we calculated odds ratios (OR) with 95% confidence intervals (CI) using conditional or exact conditional logistic regression analyses to account for the pair-wise matching. We initially stratified ischemic and hemorrhagic stroke analyses to obtain separate risk estimates for these outcomes, but also performed additional analyses using a combined ischemic and hemorrhagic stroke outcome to provide risk estimates for any stroke. We tested for interaction between CHD and timing of stroke (neonatal versus childhood), using P<0.1 as a cutoff for interaction. The small number of CHD exposures did not allow further formal testing of interaction, but we report results of additional stratified analyses selected for clinical relevance. Multivariate analyses were adjusted for gender and race. We could not calculate OR for some variables because the risk factor did not occur in controls; for these we compared proportions using Fisher’s exact test. Mann-Whitney tests were used for non-parametric data. Except for interaction terms, alpha was set at 0.05.
Results
Descriptive characteristics of stroke cases and controls
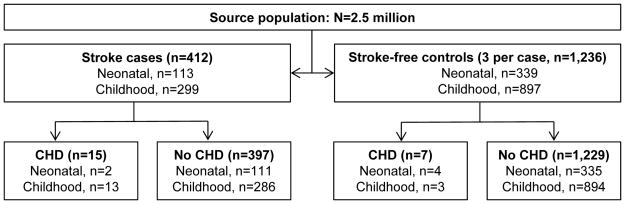
We identified 412 cases of stroke and 1,236 stroke-free controls (Figure 1). Among controls, we identified 7 with CHD, for an estimated CHD prevalence of 0.6% (CI 0.1%, 1%) among children in the stroke-free source population. CHD was identified among cases with both ischemic (11/216) and hemorrhagic (4/196) stroke. Among the children with stroke, those with CHD were younger and were less likely to present with a headache at stroke onset (Table 1). The majority (62%; CI 31, 92) of the non-neonatal cases with CHD were outpatients at the time of their stroke, although they were less likely to be outpatient than stroke cases without CHD. Among non-neonates with ischemic stroke, 55% (CI 46–65) were treated with an anti-thrombotic agent, which was not different in children with CHD compared to those without CHD.
Figure 1.
Stroke cases and controls with or without congenital heart disease identified from a population of children (<20 years of age) enrolled in Kaiser Permanente Northern California, 1993–2007.
Table 1.
Characteristics of children (<20 years) with ischemic or hemorrhagic stroke enrolled in KPNC, 1993–2007, stratified by presence of congenital heart disease.
| CHD n=15 n (%) |
No CHD n=397 n (%) |
P-value | |
|---|---|---|---|
| Demographics | |||
| Male sex | 11 (73) | 229 (58) | 0.2 |
| Non-white race | 6 (40) | 238 (60) | 0.1 |
| Neonatal stroke | 2 (13) | 111 (28) | 0.2 |
| Age of non-neonates, mean years (SD) | 7.4 (7.5) | 11.6 (6.3) | 0.02* |
| Ischemic stroke | 11 (73) | 205 (52) | 0.1 |
| Clinical presentation | |||
| Outpatient at time of stroke (non-neonates) | 8 (62) | 259 (91) | 0.001* |
| URI (prior 4 weeks, non-neonates) | 2 (15) | 56 (20) | 0.7 |
| Obesity (non-neonates) | 0 (0) | 8 (3) | 0.5 |
| Diabetes (non-neonates) | 0 (0) | 8 (3) | 0.5 |
| Headache at onset (non-neonates) | 1 (8) | 126 (46) | 0.01* |
| Hypertension | 0 (0) | 9 (2) | 0.6 |
| Seizure at onset | 8 (53) | 130 (34) | 0.1 |
| Vascular imaging performed | 6 (40) | 193 (49) | 0.5 |
| Abnormal vascular imaging † | 1 (17) | 103 (54) | 0.1 |
| Multiple etiologies for stroke | 0 (0) | 11 (3) | 0.5 |
| Length of stay, mean # days (SD) | 13.1 (13) | 13.5 (21) | 0.9 |
| Neurologic deficit at discharge (non-neonates) | 9 (69) | 162 (58) | 0.4 |
| Stroke Location‡ | |||
| Multifocal | 3 (20) | 82 (23) | 0.8 |
| Frontal | 4 (31) | 150 (43) | 0.4 |
| Temporal | 4 (33) | 106 (31) | 0.9 |
| Parietal | 4 (33) | 152 (45) | 0.4 |
| Occipital | 4 (31) | 72 (21) | 0.4 |
| Brainstem | 0 (0) | 20 (6) | 0.4 |
| Cerebellum | 1 (8) | 29 (8) | 0.9 |
| Laterality | |||
| Right | 4 (29) | 134 (37) | 0.5 |
| Left | 8 (57) | 171 (48) | 0.5 |
| Bilateral | 2 (15) | 65 (17) | 0.9 |
P<0.05;
if vascular imaging was performed;
stroke locations were not mutually exclusive, so percentages do not total to 100%.
Abbreviations: CHD = congenital heart disease, SD = Standard deviation, URI = upper respiratory infection.
Heart lesions in cases and controls were heterogeneous and included both cyanotic and acyanotic heart defects (supplemental Table). Cases and controls with were equally likely to receive their CHD diagnosis in the neonatal period (P=0.2). By the time of stroke or index date, children with CHD had undergone a mean of 1.7 surgeries (range 0–7). More cases with CHD had a cardiac surgery compared to controls (80% versus 57%, P=0.3). None of the children with CHD and hemorrhagic stroke had a known arterial ischemic stroke as a cause for the bleed, and only one of the four children with hemorrhagic stroke was on an anti-platelet or anti-coagulation at the time the bleed occurred. Four stroke cases and 5 controls had acquired cardiac disease but did not have CHD; these were not included in as CHD exposures in our analyses.
CHD is a risk factor for both hemorrhagic and ischemic stroke
In univariate stratified analyses, CHD was associated with both ischemic (OR 5.5, CI 2.0, 15) and hemorrhagic stroke (OR 13, CI 1.6, inf). In our model using a combined outcome for stroke of either type, we found a significant interaction between CHD and age (neonatal versus childhood) (P=0.03); that is, the effect of CHD on stroke risk was different depending on whether the patient was a child or neonate. CHD increased the point estimate of the OR for neonatal stroke, but the CI included null (OR 1.5, CI 0.3, 8.2). Among children >28 days, CHD increased stroke risk 19-fold (OR 19, CI 4.2, 83). Estimates of univariate OR were essentially unchanged after adjustment for gender and race (data not shown). The population attributable risk (the estimated proportion of stroke within a population that can be attributed to CHD) was 7% for childhood ischemic stroke and 2% for childhood hemorrhagic stroke.
Stratification of CHD risk for childhood stroke
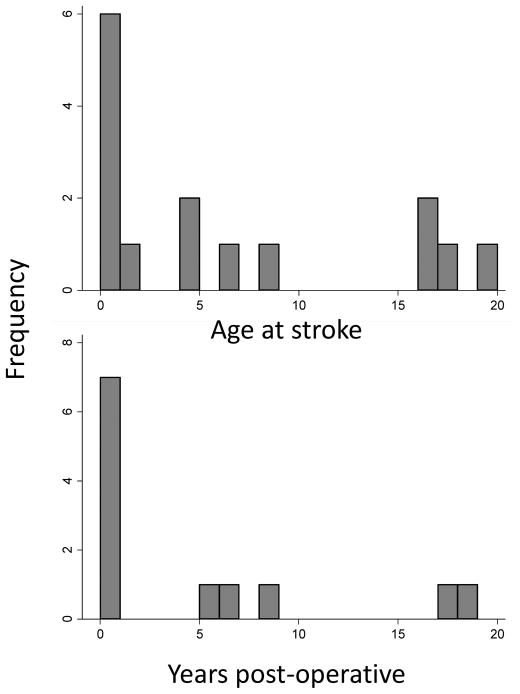
To examine clinically important subgroups, we conducted additional stratified analyses. Among children >28 days, both cyanotic and acyanotic CHD were associated with stroke (Table 2). Children with CHD and a history of cardiac surgery had the strongest association for stroke, with a 31-fold increased stroke risk compared to controls. In children with a history of cardiac surgery, the risk of stroke remained elevated by 13-fold compared to controls even after excluding cases of peri-operative stroke. In 5 of 12 cases who had a history of cardiac surgery, the stroke occurred years after their most recent procedure (Figure 2). Children with CHD who did not have cardiac surgery had a trend towards elevated stroke risk, but the broad confidence intervals included the null.
Table 2.
Stratified analyses of congenital heart disease as a risk factor for childhood stroke (occurring from age 29 days – 20 years) in a population enrolled in Kaiser Permanente Northern California, 1993–2007
| Cases, N=299 n (%) |
Controls, N=897 n (%) |
P-value | Unadjusted OR (95% CI)* |
|
|---|---|---|---|---|
| Cyanotic or Acyanotic | ||||
| Cyanotic heart disease | 7 (2) | 3 (0.3) | 0.003 | 7 (1.8, 27) |
| Acyanotic heart disease | 6(2) | 0 (0) | 0.0002 | n/a |
| Surgical repair (ever) | ||||
| CHD with repair | 11 (4) | 2 (0.2) | <0.0001 | 31 (4, 241) |
| CHD without repair | 2 (0.7) | 1 (0.1) | 0.2 | 6 (0.3, 66) |
| Time from surgery† | ||||
| Surgery, within prior 14 days | 6 (2) | 0 (0) | 0.0002 | n/a |
| Surgery, not in prior 14 days | 5 (2) | 2 (0.2) | 0.02 | 13 (1.5, 114) |
P-value for chi squared or Fisher exact;
Adjustment for gender and race did not change these associations.
Time from surgery to stroke (for cases) or index date (for controls).
Figure 2.
Among children with congenital heart disease and stroke (n=15), histograms demonstrating (A) age at the time of stroke; and (B) in those with a history of cardiac surgery (n=12), length of time from the most recent cardiac procedure to stroke.
Discussion
Improved medical care and advances in surgical techniques have increased life expectancy for children with complex CHD, but developmental delays, poorer academic achievement and behavior, and neurologic morbidity remain a challenge.18, 19 Stroke is a potentially preventable factor that may contribute to worsened neurodevelopmental outcome. Our study found that CHD is strongly associated with childhood stroke, with a 19-fold increased risk for ischemic or hemorrhagic stroke in children between the ages of 29 days and 20 years.
We found that the children with cardiac surgery for CHD were at greatest risk for stroke. Pediatric heart surgery, while life-saving, is complicated by a high proportion of thrombotic events20 and has been associated with a higher risk of delayed development and neurologic abnormalities.18, 21 Our study only included children with symptomatic strokes, but many of the radiographically identified peri-operative infarcts in children undergoing cardiopulmonary bypass for CHD are clinically silent in the acute period. Therefore, our study likely underestimates the full burden of insidious peri-operative neurologic injury, which may manifest as neuropsychological deficits later in life.
However, a history of cardiac surgery is also likely to be a marker for higher severity of CHD, which could itself convey a risk for stroke. In a series of children who had MRI performed after a cardiac operation with cardiopulmonary bypass, 10% demonstrated ischemic infarcts, with the timing thought to be pre-operative in about half.12 In our study, we found that stroke risk remained elevated in children who have surgical repair of CHD even when peri-operative strokes were excluded. Many of the children with CHD were outpatients at the time of their stroke, or suffered their stroke years after their last cardiac procedure. Elevated risk of stroke has also been reported in the growing population of adult CHD survivors.22, 23 These data underscore the biological risks of complex CHD aside from the procedural risks associated with surgery. Right to left shunting allows paradoxical embolism, and systemic venous thromboses in turn may be more likely because of increased systemic venous pressure, flow dynamics and propensity to coagulopathy.24 Stroke may be indirectly associated with CHD through underlying genetic syndromes featuring cerebrovascular abnormalities.25 Infective endocarditis, arrhythmias or depressed heart function associated with CHD could elevate stroke risk.
We estimated that 7% of childhood ischemic stroke could be attributed to CHD. Prior pediatric stroke series report much higher proportions of children with CHD. In the International Pediatric Stroke Study, 31% of children had an underlying cardiac disorder.3 In a large pediatric stroke series from the Great Ormond Hospital in London, 15% had CHD.1 Several factors may explain the discrepancy between these series and our study. Most importantly, the source population of our study is community-based rather than referral-based. A community-based study of Saudi children with stroke reported 4.8% children with CHD26, a proportion similar to our findings. Because we only included cases with CHD who were diagnosed before the stroke, any exposure misclassification should be similar for our cases and controls. Reassuringly, the prevalence of CHD in our controls (0.6%) is similar to that estimated in the general population (4–10 in 1000 live births, or 0.4%–1 %).27 Given these considerations, we believe the community-based nature of our study provides a relatively unbiased assessment of increased stroke risk in children with CHD.
We found that CHD was associated not only with ischemic, but also with hemorrhagic stroke. Magnetic resonance brain imaging demonstrating hemosiderin staining (suggesting prior brain hemorrhage) has been associated with poorer developmental outcome in children with repaired CHD.28 Further, the association of CHD with hemorrhagic stroke has important safety implications for anti-platelet and anti-coagulant medications. Despite this, hemorrhagic stroke risk related to CHD has previously received less attention than that for ischemic stroke, and few studies have examined this relationship directly.
Although prior reports have reported strokes in neonates with CHD,11, 29 we could not confirm an association of CHD and neonatal stroke. Despite our large population base and the advantages in power gained through using a case-control design, our study included only a small number of neonatal cases and controls with CHD, resulting in wide confidence intervals and limited precision of our risk estimates. Thus, it is possible, and even likely, that a larger study with a more precise estimate would demonstrate association of CHD and neonatal stroke.
Our study had important limitations. The small number of stroke cases and controls with a history of CHD resulted in wide confidence intervals and limited the precision of our risk estimates. We had too few cases to provide estimates of risk for specific procedures and devices. We did not include patent foramen ovale as a CHD exposure in our study because it is typically asymptomatic and we could not accurately assess the frequency in our controls. Finally, we could not completely avoid the possibility of spectrum bias if the types of CHD in children enrolled in Kaiser were systematically different than in other populations. This could happen, for example, if families of patients with more complex CHD systematically chose a different type of health insurance. Careful consideration must be taken before generalizing our findings to specific referral populations that may include more children with severe cardiac disease; our findings may underestimate effect for these groups.
Summary
CHD is associated with increased risk of both ischemic and hemorrhagic stroke in childhood. Children with complex CHD who require cardiac surgery are at the highest risk of stroke, and this risk remains elevated beyond the immediate post-operative period. Our study adds to the increasing weight of evidence that research to optimize peri-operative and long-term stroke prevention in children with complex CHD is needed. Future studies should include hemorrhagic stroke as an outcome to better understand its association with CHD and the safety of anti-platelets and anti-coagulants in this population. Finally, because many strokes among children with CHD occur outside of the hospital setting, providers should consider anticipatory guidance to help parents recognize signs of stroke in children with complex CHD who are at the greatest risk.
Supplementary Material
Acknowledgments
We thank Barbara Rowe for medical record data abstraction.
Sources of Funding: Research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke under award numbers K02 NS053883 (Dr. Fullerton) and 2K12NS001692-11 (Dr. Fox) and the National Center for Advancing Translational Sciences under Award Number KL2TR000143 (Dr. Fox).
Footnotes
Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Conflicts of Interest/Disclosures: none.
References
- 1.Ganesan V, Prengler M, McShane MA, Wade AM, Kirkham FJ. Investigation of risk factors in children with arterial ischemic stroke. Ann Neurol. 2003;53:167–173. doi: 10.1002/ana.10423. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman JL, Mack GK, Minich LL, Benedict SL, Heywood M, Stoddard GJ, et al. Failure to impact prevalence of arterial ischemic stroke in pediatric cardiac patients over three decades. Congenital heart disease. 2011;6:211–218. doi: 10.1111/j.1747-0803.2011.00510.x. [DOI] [PubMed] [Google Scholar]
- 3.Dowling MM, Hynan LS, Lo W, Licht DJ, McClure C, Yager JY, et al. International paediatric stroke study: Stroke associated with cardiac disorders. International journal of stroke: official journal of the International Stroke Society. 2013;8(Suppl A100):39–44. doi: 10.1111/j.1747-4949.2012.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carpenter J, Keating R, Weinstein S, Vezina G, Berger J, Bell MJ. Cerebral hemorrhage and vasospasm in a child with congenital heart disease. Neurocrit Care. 2008;8:276–279. doi: 10.1007/s12028-007-9005-3. [DOI] [PubMed] [Google Scholar]
- 5.Hudaoglu O, Kurul S, Cakmakci H, Men S, Yis U, Dirik E. Aorta coarctation presenting with intracranial aneurysm rupture. J Paediatr Child Health. 2006;42:477–479. doi: 10.1111/j.1440-1754.2006.00903.x. [DOI] [PubMed] [Google Scholar]
- 6.Domi T, Edgell DS, McCrindle BW, Williams WG, Chan AK, MacGregor DL, et al. Frequency, predictors, and neurologic outcomes of vaso-occlusive strokes associated with cardiac surgery in children. Pediatrics. 2008;122:1292–1298. doi: 10.1542/peds.2007-1459. [DOI] [PubMed] [Google Scholar]
- 7.Manlhiot C, Brandao LR, Kwok J, Kegel S, Menjak IB, Carew CL, et al. Thrombotic complications and thromboprophylaxis across all three stages of single ventricle heart palliation. J Pediatr. 2012;161:513–519. e513. doi: 10.1016/j.jpeds.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Liu XY, Wong V, Leung M. Neurologic complications due to catheterization. Pediatr Neurol. 2001;24:270–275. doi: 10.1016/s0887-8994(00)00272-1. [DOI] [PubMed] [Google Scholar]
- 9.Fraser CD, Jr, Jaquiss RD, Rosenthal DN, Humpl T, Canter CE, Blackstone EH, et al. Prospective trial of a pediatric ventricular assist device. N Engl J Med. 2012;367:532–541. doi: 10.1056/NEJMoa1014164. [DOI] [PubMed] [Google Scholar]
- 10.Almond CS, Singh TP, Gauvreau K, Piercey GE, Fynn-Thompson F, Rycus PT, et al. Extracorporeal membrane oxygenation for bridge to heart transplantation among children in the united states: Analysis of data from the organ procurement and transplant network and extracorporeal life support organization registry. Circulation. 2011;123:2975–2984. doi: 10.1161/CIRCULATIONAHA.110.991505. [DOI] [PubMed] [Google Scholar]
- 11.McQuillen PS, Barkovich AJ, Hamrick SE, Perez M, Ward P, Glidden DV, et al. Temporal and anatomic risk profile of brain injury with neonatal repair of congenital heart defects. Stroke; a journal of cerebral circulation. 2007;38:736–741. doi: 10.1161/01.STR.0000247941.41234.90. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Zimmerman RA, Jarvik GP, Nord AS, Clancy RR, Wernovsky G, et al. Perioperative stroke in infants undergoing open heart operations for congenital heart disease. Ann Thorac Surg. 2009;88:823–829. doi: 10.1016/j.athoracsur.2009.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beca J, Gunn JK, Coleman L, Hope A, Reed PW, Hunt RW, et al. New white matter brain injury after infant heart surgery is associated with diagnostic group and the use of circulatory arrest. Circulation. 2013;127:971–979. doi: 10.1161/CIRCULATIONAHA.112.001089. [DOI] [PubMed] [Google Scholar]
- 14.Fullerton HJ, Wu YW, Zhao S, Johnston SC. Risk of stroke in children: Ethnic and gender disparities. Neurology. 2003;61:189–194. doi: 10.1212/01.wnl.0000078894.79866.95. [DOI] [PubMed] [Google Scholar]
- 15.Fullerton HJ, Wu YW, Sidney S, Johnston SC. Risk of recurrent childhood arterial ischemic stroke in a population-based cohort: The importance of cerebrovascular imaging. Pediatrics. 2007;119:495–501. doi: 10.1542/peds.2006-2791. [DOI] [PubMed] [Google Scholar]
- 16.Wu YW, Croen LA, Shah SJ, Newman TB, Najjar DV. Cerebral palsy in a term population: Risk factors and neuroimaging findings. Pediatrics. 2006;118:690–697. doi: 10.1542/peds.2006-0278. [DOI] [PubMed] [Google Scholar]
- 17.Lee J, Croen LA, Backstrand KH, Yoshida CK, Henning LH, Lindan C, et al. Maternal and infant characteristics associated with perinatal arterial stroke in the infant. Jama. 2005;293:723–729. doi: 10.1001/jama.293.6.723. [DOI] [PubMed] [Google Scholar]
- 18.Bellinger DC, Wypij D, Rivkin MJ, DeMaso DR, Robertson RL, Jr, Dunbar-Masterson C, et al. Adolescents with d-transposition of the great arteries corrected with the arterial switch procedure: Neuropsychological assessment and structural brain imaging. Circulation. 2011;124:1361–1369. doi: 10.1161/CIRCULATIONAHA.111.026963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marino BS, Lipkin PH, Newburger JW, Peacock G, Gerdes M, Gaynor JW, et al. Neurodevelopmental outcomes in children with congenital heart disease: Evaluation and management: A scientific statement from the american heart association. Circulation. 2012;126:1143–1172. doi: 10.1161/CIR.0b013e318265ee8a. [DOI] [PubMed] [Google Scholar]
- 20.Manlhiot C, Menjak IB, Brandao LR, Gruenwald CE, Schwartz SM, Sivarajan VB, et al. Risk, clinical features, and outcomes of thrombosis associated with pediatric cardiac surgery. Circulation. 2011;124:1511–1519. doi: 10.1161/CIRCULATIONAHA.110.006304. [DOI] [PubMed] [Google Scholar]
- 21.Bellinger DC, Jonas RA, Rappaport LA, Wypij D, Wernovsky G, Kuban KC, et al. Developmental and neurologic status of children after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. N Engl J Med. 1995;332:549–555. doi: 10.1056/NEJM199503023320901. [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann A, Chockalingam P, Balint OH, Dadashev A, Dimopoulos K, Engel R, et al. Cerebrovascular accidents in adult patients with congenital heart disease. Heart. 2010;96:1223–1226. doi: 10.1136/hrt.2010.196147. [DOI] [PubMed] [Google Scholar]
- 23.Lin YS, Liu PH, Wu LS, Chen YM, Chang CJ, Chu PH. Major adverse cardiovascular events in adult congenital heart disease: A population-based follow-up study from taiwan. BMC cardiovascular disorders. 2014;14:38. doi: 10.1186/1471-2261-14-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giglia TM, Massicotte MP, Tweddell JS, Barst RJ, Bauman M, Erickson CC, et al. Prevention and treatment of thrombosis in pediatric and congenital heart disease: A scientific statement from the american heart association. Circulation. 2013;128:2622–2703. doi: 10.1161/01.cir.0000436140.77832.7a. [DOI] [PubMed] [Google Scholar]
- 25.Schievink WI, Mokri B, Piepgras DG, Gittenberger-de Groot AC. Intracranial aneurysms and cervicocephalic arterial dissections associated with congenital heart disease. Neurosurgery. 1996;39:685–689. doi: 10.1097/00006123-199610000-00006. discussion 689–690. [DOI] [PubMed] [Google Scholar]
- 26.Salih MA, Al-Jarallah AS, Abdel-Gader AG, Al-Jarallah AA, Al-Saadi MM, Kentab AY, et al. Cardiac diseases as a risk factor for stroke in saudi children. Saudi Med J. 2006;27(Suppl 1):S61–68. [PubMed] [Google Scholar]
- 27.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. American Heart Association Statistics C, Stroke Statistics S. Heart disease and stroke statistics--2014 update: A report from the american heart association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soul JS, Robertson RL, Wypij D, Bellinger DC, Visconti KJ, du Plessis AJ, et al. Subtle hemorrhagic brain injury is associated with neurodevelopmental impairment in infants with repaired congenital heart disease. J Thorac Cardiovasc Surg. 2009;138:374–381. doi: 10.1016/j.jtcvs.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirton A, Armstrong-Wells J, Chang T, Deveber G, Rivkin MJ, Hernandez M, et al. Symptomatic neonatal arterial ischemic stroke: The international pediatric stroke study. Pediatrics. 2011;128:e1402–1410. doi: 10.1542/peds.2011-1148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.