Abstract
Vesicating agents sulfur mustard (SM) and nitrogen mustard (NM) are reported to be easily absorbed by skin upon exposure causing severe cutaneous injury and blistering. Our studies show that topical exposure of NM (3.2 mg) onto SKH-1 hairless mouse skin, not only caused skin injury, but also led to significant body weight loss and 40–80 % mortality (120 h post-exposure), suggesting its systemic effects. Accordingly, further studies herein show that NM exposure initiated an increase in circulating white blood cells by 24 h (neutrophils, eosinophils and basophils) and thereafter a decrease (neutrophils, lymphocytes and monocytes). NM exposure also reduced both white and red pulp areas of the spleen. In the small intestine, NM exposure caused loss of membrane integrity of the surface epithelium, abnormal structure of glands and degeneration of villi. NM exposure also resulted in the dilation of glomerular capillaries of kidneys, and an increase in blood urea nitrogen/creatinine ratio. Our results here with NM are consistent with earlier reports that exposure to higher SM levels can cause damage to the hematopoietic system, and kidney, spleen and gastrointestinal tract toxicity. These outcomes will add to our understanding of the toxic effects of topical vesicant exposure, which might be helpful towards developing effective countermeasures against injuries from acute topical exposures.
Keywords: Nitrogen mustard, skin topical exposure, systemic toxicity, leucocytes, SKH-1 hairless mice
1. Introduction
Mustard agents were first developed in the 18th century but their use as chemical warfare agents was realized during World War I by the Germans (Blewett, 1986). The relative ease of synthesis and potent toxic manifestations as well as huge stockpiles of vesicants, make them one of the most feared potential agents of warfare and terrorism (Geraci, 2008, Saladi et al., 2006). From the variety of mustard agents developed, sulfur mustard (SM; 2, 2-dichloroethyl sulfide) has been most widely used as a warfare agent earning it the name, “King of war gases” (Blewett, 1986). SM, a vesicating agent, upon exposure causes debilitating effects to the respiratory tract, and ocular and dermal systems (Balali-Mood and Hefazi, 2005, 2006, Balali-Mood et al., 2005, Balali-Mood et al., 2008, Borak and Sidell, 1992, Davis and Aspera, 2001, Geraci, 2008, Ghabili et al., 2011, Ghanei and Harandi, 2007, Ghanei et al., 2010, Hefazi et al., 2006, Mansour Razavi et al., 2012, Petrali et al., 2000, Rowell et al., 2009, Saladi, Smith, 2006, Shohrati et al., 2007). The extent of injury by SM depends upon the concentration and duration of its exposure. At higher concentrations, it can cause damage to multiple organ systems (Kehe et al., 2008a). Subsequent systemic action of SM on the nervous, cardiac and digestive systems in humans and laboratory animals has also been reported (Dacre and Goldman, 1996). In severe cases of SM exposure, bronchitis, bronchopneumonia, intestinal lesions, leucopenia, and convulsions with systemic distress occur, all of which could result in death (Dacre and Goldman, 1996).
Nitrogen mustard [NM; bis (2-chloroethyl) methylamine (HN2)], an analogue of SM, was first developed as a chemical warfare agent during the 1930s; however, it has not been used in any military or other disputes (Papirmeister et al., 1985, Sharma et al., 2010b, Wang and Xia, 2007, Wormser et al., 2002). Both SM and NM belong to the category of vesicating agents and are believed to exhibit similar mechanism/s of toxicity (Saladi, Smith, 2006, Sharma et al., 2008, Ucar et al., 2007), which could be attributed to their high reactivity and alkylating properties, mainly due to the alkylation of DNA at N-7 position of guanine (Batal et al., 2014, Guainazzi et al., 2010, Rutman et al., 1969, Shukla et al., 2007) resulting in DNA strand breaks, and if unrepaired, this may eventually lead to cell death (Kehe et al., 2009a, Kehe et al., 2008b, Kehe and Szinicz, 2005, Malaviya et al., 2010). Alkylation is mediated by cyclic sulphonium ion in case of SM and cyclic immonium ion in case of NM (Price, 1958). These ions can react with organic and inorganic anions, amino groups, and sulphide groups in biological systems (Wheeler, 1962), causing severe damage even at very low concentrations. To study the cutaneous damage from SM, NM and other analogues, various animal models have been used (Chauhan et al., 1993, Cowan et al., 1993, Dabrowska et al., 1996, Dorandeu et al., 2011, Lomash et al., 2011, Lulla et al., 2013, Papirmeister, Gross, 1985, Riviere et al., 1995, Sharma, Vijayaraghavan, 2008, Wormser, 1991). However, at present, mechanistic aspects of the injury are still poorly understood and the lack of suitable models hampers the development and screening of effective therapeutic and prophylactic compounds.
In our studies aimed at examining the clinical and histopathological features, and molecular mechanisms of cutaneous lesions from topical vesicant exposure, we employed NM. NM has crosslinking property similar to SM, is commercially available and due to the containment facility required for SM, has been used by several groups to study vesicant--induced injury (Hardej and Billack, 2007, Lulla, Reznik, 2013, Pino and Billack, 2008, Sharma et al., 2010a). SKH-1 hairless mice, which has been found to be suitable for experiments with SM and SM analogues, was used as the animal model in this study These studies showed that NM exposure (3.2 mg/mouse) in mice led to severe cutaneous injury, and that NM-related clinical, histopathological, and immunohistochemical effects were similar to those observed following SM-exposure in humans and other animal models (Dorandeu, Taysse, 2011, Jain et al., 2011, Jain et al., 2014a, Pal et al., 2009, Tewari-Singh et al., 2011, Tewari-Singh et al., 2014a, Tewari-Singh et al., 2013, Tewari-Singh et al., 2014b, Tewari-Singh et al., 2009). Apart from skin injury, a single topical NM exposure at this concentration also resulted in 40–80% mortality and weight loss in mice suggesting systemic effects following its dorsal skin exposure. Hence, the objective of the present study was to examine the systemic toxic effects of NM following a single topical exposure in SKH-1 hairless mouse. Results from this study show that topical NM exposure induces toxic effects upon the hematopoietic system, spleen, small intestine and kidneys. Together, these pathological and toxic effects following cutaneous NM exposure could account for the high mortality observed with NM exposure.
2. Materials and Methods
2.1. Animals and NM exposure
SKH-1 hairless mice (male; 4–5 weeks of age), purchased from Charles River Labs (Wilmington, MA), were housed under standard conditions and acclimatized for a week before their use in experimental studies. The Institutional Animal Care and Use Committee (IACUC) of the University of Colorado Denver, CO, have approved the protocol for studies with mice reported in this article. In the study, 5–8 mice were assigned per group and their dorsal skin was topically exposed with either 200 μL acetone alone (vehicle control) or 3.2 mg NM [mechlorethamine hydrochloride; bis (2-chloroethyl) methylamine (HN2)] Sigma-Aldrich Chemical Co., St. Louis, MO) in 200 μL acetone, under a chemical and biological safety fume hood. This dose was selected based on reports of human and animal exposure to higher dose SM, as well as exposure studies with NM, showing skin injury, vesication, and both acute and chronic effects of SM (Firooz et al., 2011, Kehe and Szinicz, 2005, Smith et al., 1997). Acetone, was used as a vehicle for NM since it enhances the skin permeability to hydrophilic and amphipathic compounds, and has been successfully used as a vehicle for topical skin treatments (Dhanalakshmi et al., 2004, Tsai et al., 2001) It has been used as a vehicle for topical treatment of SM analog CEES as well as NM in our previous reported studies that have successfully established skin injury model of vesicant exposure (Jain, Tewari-Singh, 2014a, Tewari-Singh, Jain, 2013, Tewari-Singh, Jain, 2014b, Tewari-Singh, Rana, 2009).
2.2. Body weight and weight of organs
Following the above detailed control and NM exposures, mice were weighed daily. At the determined experimental time points (24, 72, and 120 h post exposure), mice were sacrificed by CO2 euthanasia followed by cervical dislocation, and dorsal skin, spleen, small intestine/gastrointestinal (GI) tract, kidney and liver were dissected out, weighed and either snap frozen in liquid nitrogen or fixed in 10% phosphate buffered formalin for Hematoxylin and Eosin (H&E) and other histostaining processes as reported earlier (Jain, Tewari-Singh, 2014a, Tewari-Singh, Jain, 2013, Tewari-Singh, Jain, 2014b).
2.3. Heart and breath rate measurements
After NM exposure, pulse oximeter (MouseOx Plus, Starr Life Sciences Corporation, Oakmont, PA, USA) was used to measure heart rate and breath rate of the mice at 24 h post exposure and every day thereafter until the end of the experiment. After 5–10 minutes of acclimatization and comfort, pulse oximeter clip was placed on the neck while holding the mice, and measurements were taken in triplicates for each mouse.
2.4. Hematological and biochemical analysis
Mice were euthanized at 24, 72, and 120 h post NM-exposure and blood was collected by cardiac puncture in BD vacutainer tubes (BD Biosciences; San Jose USA) containing sodium Heparin anticoagulant. Complete blood counts were taken on a Hemavet 950 Veterinary Hematology Analyzer (Drew Scientific, USA), at the Department of Pediatrics, School of Medicine, University of Colorado Denver. For complete blood count analysis, data was pooled from three sets of independent experiments with each set having 5–8 animals per group. Blood urea nitrogen (BUN) concentration in the serum was determined employing a colorimetric assay according to vendor’s protocol (K024-H1; Arbor Assays, Ann Arbor, MI, USA) by comparison of unknown samples to a standard curve generated with known concentrations of urea nitrogen. Serum creatinine was measured using the serum creatinine detection kit (KB02-H1; Arbor Assays, Ann Arbor, MI, USA) according to the vendor’s protocol. All samples were assayed in duplicate.
2.5. Histopathological evaluation
Formalin-fixed small intestine, liver, kidney and spleen samples were dehydrated in ascending concentration of ethanol, cleared in xylene, and embedded in paraffin (Triangle Biomedical Sciences, Durham, NC) as detailed earlier (Jain, Tewari-Singh, 2011, Tewari-Singh, Rana, 2009). Five micron sections were cut and processed for H&E staining and examined for NM-induced histopathological changes. In each case, histopathological changes were assessed in three sections from each animal using Axiovision Rel 4.5 software (Carl Zeiss, Inc. Germany). A minimum of three animals from one experiment were analyzed in each group except for NM exposed group (120 h), where only two mice survived.
2.6. Apoptotic cell death in the GI tract
Apoptotic cell death in the small intestine sections of control and NM exposed mice was analyzed using the DeadEnd Colorimetric terminal deoxynucleotidyl transferase (tdt)- mediated dUTP-biotin nick end labeling (TUNEL) system according to the manufacturer’s protocol as described earlier (Jain et al., 2011; Tewari-Singh et al., 2009). The brown colored TUNEL positive cells were quantified in 10 randomly selected fields/section at 400× magnification, and an apoptotic cell index was calculated as the number of apoptotic cells × 100 divided by total number of cells.
2.7. Statistical analysis
Data were analyzed using one-way analysis of variance (one-way ANOVA) to get the statistically significant difference in control versus treated groups, with Tukey or Bonferroni t-test for multiple comparisons (SigmaStat 2.03). Differences were considered significant if the p value was ≤ 0.05. Data are presented as the mean ± standard error of mean (SEM).
3. Results
3.1. Effect of topical NM exposure on body weight, breath rate and heart rate in mice
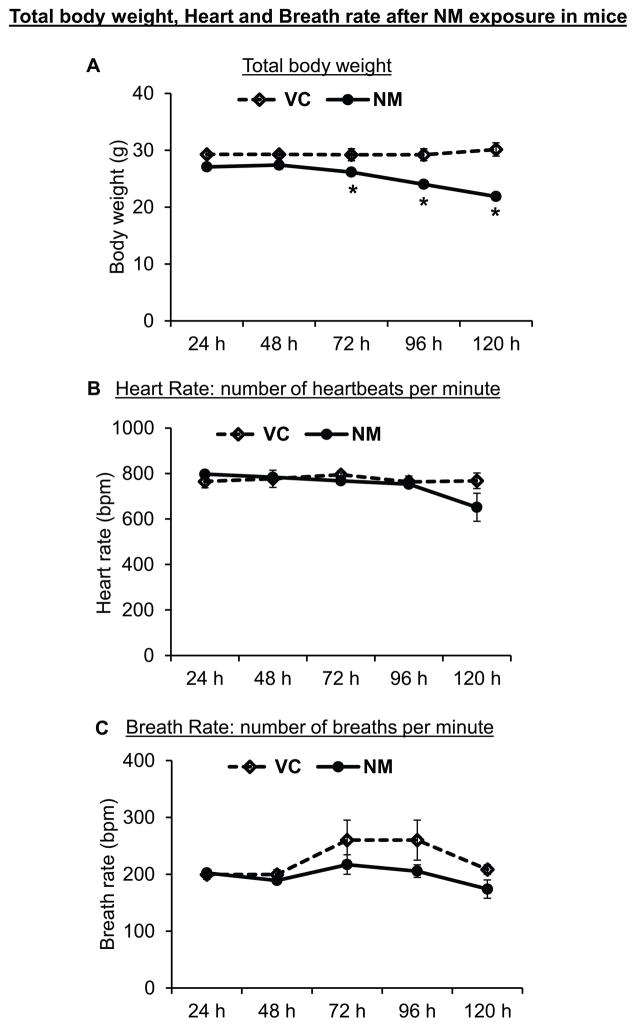
Following topical NM exposure, a generalized decrease in the apparent health of the mice was evident with a gradual decrease in the mean body weight, which became pronounced and attained statistical significance at 72 and 120 h after NM exposure (Fig 1A). At 120 h after exposure, a 20% decline in average body weight of NM-exposed mice was observed compared to vehicle control mice (mean body weight of control mice at 120 h was 27.07 ± 0.45 g compared to 21.86 ± 0.46 g for NM-exposed mice at this time point). To examine for a direct effect of NM on the lung or heart function, heart rate (heart beats per minute) and breath rate (number of breaths per minute) was measured following NM exposure; however, a significant decrease in their heart rate or breath rate was not observed (Fig 1B and C).
Figure 1. Topical application of NM on to the dorsal skin of SKH-1 hairless mice causes a decrease in body weight.
Dorsal skin of mice was exposed topically to either NM (3.2 mg) in 200 μL acetone or 200 μL of acetone alone. After exposure, the mice were weighed daily for up to 5 days post-exposure (A), and their heart rate (B) and breath rate (C) were recorded as detailed under ‘Materials and Methods’ section. VC, vehicle control (acetone); NM, nitrogen mustard-exposed mice. Data represent mean ± SEM of three-eight animals in each treatment group. *, p < 0.05.
3.2. Effect of topical NM exposure on blood leucocytes in mice
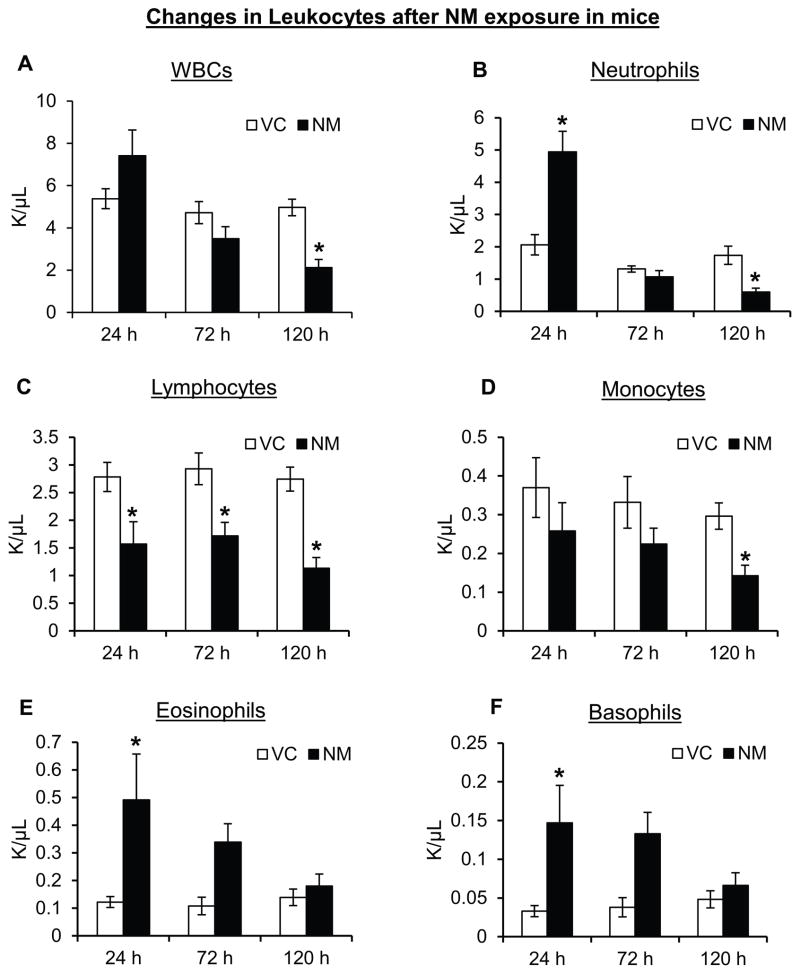
SM exposure at high dose in humans and laboratory animals is shown to damage hematopoietic tissues with progressive leucopenia (Dacre and Goldman, 1996). Therefore, blood analysis of mice following 24, 72 and 120 h of NM exposure was carried out to assess for possible hematological disturbances. Using a Hemavet 950 Veterinary Hematology Analyzer, total hemoglobin, hematocrit, platelets, red blood cells (RBCs) and white blood cells (WBCs) were analyzed. This analysis revealed that single topical exposure of NM led to a significant difference in WBCs. Initially, at early time point (24 h), a 1.4-fold increase in the total WBCs was observed, which was followed by a decrease at later time points (Fig. 2A). Furthermore, NM exposure also induced changes in different WBC types, with a significant increase (2.5-fold) in total neutrophil count at 24 h post-exposure, which was reversed by 120 h post-exposure where NM caused a significant decline (3-fold) in neutrophils (Fig. 2B). A significant decrease in the number of lymphocytes was also observed at all the study time points after NM exposure in these mice (Fig. 2C). The mean lymphocyte counts in the control mice at 24 h, 72 h and 120 h were 2.78 ± 0.26, 2.93 ± 0.29 and 2.74 ± 0.22 × 103/μL, as compared to 1.57 ± 0.40, 1.72 ± 0.24 and 1.13 ± 0.19 × 103/μL in NM-exposed mice, respectively (Fig. 2C). An NM-induced decrease in the total monocyte count was also observed at all the time points after NM exposure, with ~2-fold decrease at 120 h after exposure (Fig. 2D). Conversely, the number of eosinophils and basophils were found to be 4-fold and 5-fold higher, respectively, in NM-exposed mice compared to the control groups at 24 h post-exposure, which remained higher at 120 h and then dropped to control levels (Fig 2E and F).
Figure 2. Topical application of NM on to the dorsal skin of SKH-1 hairless mice causes changes in circulating leucocytes.
Dorsal skin of mice was exposed topically to either NM (3.2 mg) in 200 μL acetone or 200 μL of acetone alone, and after 24, 72 and 120 h of exposure, mice were sacrificed and blood was collected. Complete blood count was carried out using Hemavet as described in the ‘Materials and Methods’ section. The NM-induced changes observed in the blood leucocytes namely WBCs (A), neutrophils (B), lymphocytes (C), monocytes (D), eosinophils (E) and basophils (F) were recorded. VC, vehicle control (acetone); NM, nitrogen mustard-exposed mice. Data (from three experiments) represent mean ± SEM of five-eighteen animals in each treatment group. *, p < 0.05.
3.3. Effect of topical NM exposure on various organs in mice
3.3.1. Effect on the spleen
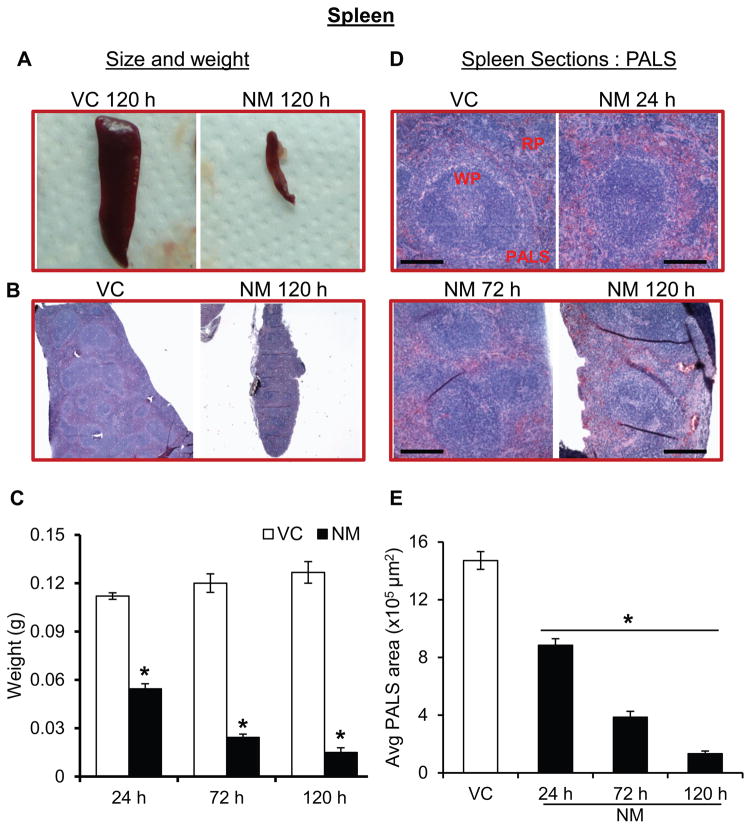
Since blood analysis from mice showed an NM-induced decrease in the circulating leucocytes (lymphocytes and monocytes), we examined the histology of the spleen. NM exposure caused a drastic time-dependent decrease in the size and weight of the spleen, (Fig 3A–C). At 24 h post-exposure, the spleen in NM-exposed mice was reduced by approximately half, and by 120 h, there was an 88% reduction in the weight of the spleen (Fig 3C). H&E staining of spleen sections from NM exposed mice showed that compared to control, there was a decrease in white pulp starting 24 h after NM exposure and that decrease was progressive until 120 h (Fig. 3D). A similar reduction in the amount of red pulp was also observed (Fig. 3D). Analysis of cross-sectional area of the periarterial lymphoid sheath (PALS), an indirect measurement of the lymphocytes present in the white pulp, indicated a progressive NM-induced time-dependent decrease until 120 h (Fig 3E). The average PALS area was reduced by 40%, 74%, and 90% after 24, 72, and 120 h of NM-exposure, respectively, compared to control mice (Fig. 3E).
Figure 3. Topical application of NM on to the dorsal skin of SKH-1 hairless mice causes toxic effects in the spleen.
Dorsal skin of mice was exposed topically to either NM (3.2 mg) in 200 μL acetone or 200 μL of acetone alone, and at 24, 72 and 120 h post-NM exposure, spleen was collected, weighted, processed, sectioned and subjected to H&E staining as detailed under ‘Materials and Methods’ section. NM-induced changes in the size of mice spleen (A and B), spleen weight (C) were recorded, and size and cross sectional area of PALS (D and E) were analyzed (representative pictures, D; quantification, E). VC, vehicle control (acetone); NM, nitrogen mustard-exposed mice; WP, white pulp; RP, red pulp; PALS, periarteriolar lymphoid sheath; red scale bar, 1 cm; black scale bar (histology images), 50 μm. Data represent mean ± SEM of three-eight animals in each treatment group. *, p < 0.05.
3.3.2. Effect on the GI tract
Severe SM exposure in humans has been shown to cause epigastric distress, anorexia, diarrhea and cachexia (Balali-Mood, 1986). The gastrointestinal (GI) tract of the mice was examined in detail. SM has been reported to cause damage to rapidly dividing cells in the body, and there is rapid division to constantly replace cells in the GI tract. H&E stained histology of intestinal sections from NM-exposed mice revealed inflammation and vacuolation (red arrows), edema, and cell death in the intestinal villi (Fig 4). A reduction in the number of Peyer’s patches was also observed (data not shown). Furthermore, NM exposure resulted in cells in the villi to become eosinophilic, and the villi demonstrated an increase in the number of apoptotic cells. NM exposure followed by recovery for 120 h resulted in a 60% increase in the edematous and vacuolated cells in the villi (Fig 4A and B). In addition, nuclei of the many cells in the villi appeared to be pyknotic, and a diffuse inflammatory response consisting predominantly of macrophages was also observed (Fig 4A and B). The lymphatics were grossly dilated and the lamina propria was thickened, indicating increased edema in the tissue (Fig. 4A). TUNEL staining of the GI tract sections from NM-exposed mice revealed significant NM-induced cell death in the GI tract compared to control mice (Fig 4C and D, green arrows). At 24 h post-NM exposure, the number of total dead cells was 4.5-fold higher compared to control mice, and at 120 h, NM-exposure caused an 8-fold increase in apoptotic cell death (Fig. 4D).
Figure 4. Topical application of NM on to the dorsal skin of SKH-1 hairless mice causes toxic effects in the small intestine.
Dorsal skin of mice was exposed topically to either NM (3.2 mg) in 200 μL acetone or 200 μL of acetone alone, and at 24, 72 and 120 h post-NM exposure, small intestine was collected, processed, sectioned and subjected to H&E/TUNEL staining as detailed under ‘Materials and Methods’ section. NM-induced histopathological changes (A and B), and apoptotic cell death (C and D) in the small intestine of mice was assessed (representative pictures, A and C) and quantified (B and D). VC, vehicle control (acetone); NM, nitrogen mustard-exposed mice; SM, smooth muscles; MM, muscularis mucosae; C, crypt; LP, lamina propria; V, villi; L, lumen; IG, intestinal glands; black arrow, columnar epithelium; red arrows, edematous and vacuolated villi; green arrows, TUNEL positive apoptotic cells; black scale bar, 100 μm. Data represents mean ± SEM of three-eight animals in each treatment group (except for NM 120 h where n=2). *, p < 0.05.
3.3.3. Effect on the kidneys
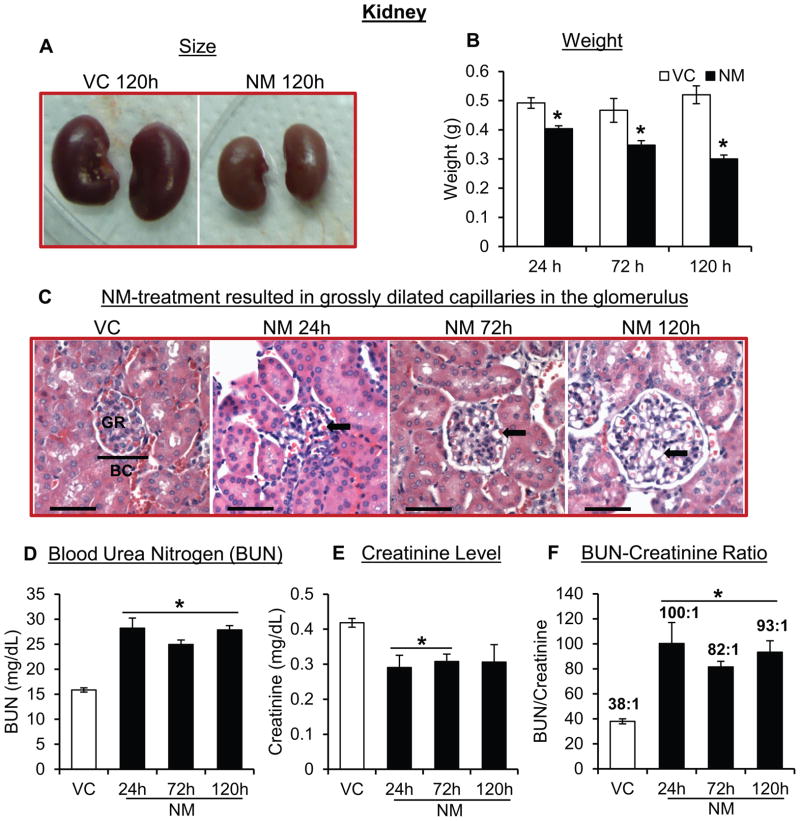
Kidneys were examined for possible toxic changes due to NM-exposure. Grossly, atrophy of the kidneys was observed (Fig 5A). There was a significant decrease in the kidney weight of mice following NM exposure. Following 24, 72 and 120 h of NM exposure, 18%, 25% and 42% decrease in the kidney weight was observed compared to control mice, respectively (Fig 5B). Microscopic analysis showed that the major effect was on the vasculature; in particular, we observed grossly dilated capillaries in the glomerulus of the NM-exposed mice (Fig 5C, black arrows). Whereas there was a slight NM effect on the proximal convoluted tubules, such changes were very minor at best and not obvious in case of distal convoluted tubules and collecting ducts. In addition to these glomerular histological changes, glomerular filtration rate was next assessed by measuring serum levels of BUN and creatinine. Significantly elevated levels of total BUN were observed in the serum of NM-exposed mice compared to control mice at all-time points post exposure. The average BUN level in control mice was 15.85 ± 0.42 mg/dL, while BUN levels 24 h, 72 h and 120 h after NM exposure were 28.19 ± 2.04, 24.94 ± 0.92 and 27.87 ± 0.85 mg/dL, respectively (Fig 5D). Serum creatinine levels in NM-exposed mice were significantly lower. The creatinine level in control mice was 0.42 ± 0.01 mg/dL compared to 0.29 ± 0.03, 0.31 ± 0.02 and 0.31 ± 0.05 mg/dL at 24 h, 72 h and 120 h post-NM exposure, respectively (Fig 5E). The BUN/Creatinine ratio in NM-exposed mice at 24 h was 2.6-fold higher in comparison with control mice (Fig 5F). A similar trend was also observed at 72 h and 120 h after NM exposure, where 2.1-fold and 2.4-fold increase in the BUN/Creatinine ratio, respectively, were observed (Fig 5F) suggesting a state of dehydration, and/or shock.
Figure 5. Topical application of NM on to the dorsal skin of SKH-1 hairless mice causes toxic effects in the kidneys.
Dorsal skin of mice was exposed topically to either NM (3.2 mg) in 200 μL acetone or 200 μL of acetone alone, and at 24, 72 and 120 h post-NM exposure, kidneys were collected, processed, sectioned and subjected to H&E staining as detailed under ‘Materials and Methods’ section. NM-induced changes in the size and weight were recorded (A and B), histopathology of the kidneys of mice was analyzed (representative pictures, C) and serum BUN levels (D), creatinine levels (E) and BUN/Creatinine ratios (F) were measured as detailed under the ‘Materials and Methods’ section. VC, vehicle control (acetone); NM, nitrogen mustard-exposed mice; GR, glomerulus; BC, bowman’s capsule; black arrows, dilated capillaries; red scale bar, 1 cm; black scale bar (histology images),100 μm. Data represent mean ± SEM of three-eight animals in each treatment group. *, p < 0.05.
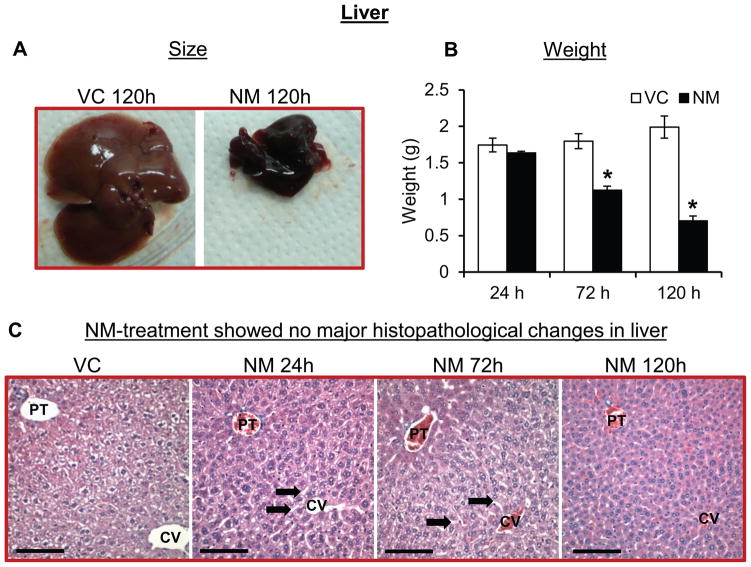
3.3.4. Effect on the liver
Histological analysis of liver was carried out to assess any toxic changes following NM exposure. Grossly, NM exposure caused a severe atrophy in the liver of mice (Fig. 6A). A decrease in the liver weight upon NM exposure was observed; following 120 h after exposure, there was a 65% reduction in liver weight compared to controls (Fig 6B). The liver was evaluated using all of the criteria proposed by Kleiner et al. (Kleiner et al., 2005) and as modified by Lanaspa et al. (Lanaspa et al., 2013). Histologically, there were no NM-related toxic effects, apart from a slight dilation in the sinusoids, in the liver sections from NM-treated mice (Fig. 6C). In particular, we did not observe any steatosis, inflammatory foci, or markers of cell injury or death. However, the hepatocytes appeared to have less cytoplasm indicating fluid loss.
Figure 6. Topical application of NM on to the dorsal skin of SKH-1 hairless mice causes decrease in size and weight of liver.
Dorsal skin of mice was exposed topically to either NM (3.2 mg) in 200 μL acetone or 200 μL of acetone alone. 24, 72 and 120 h post-NM exposure, liver was collected, processed, sectioned and subjected to H&E staining as detailed under ‘Materials and Methods’ section. NM-induced changes in the size and weight of the liver were recorded (A and B) and liver histopathology was analyzed (representative pictures, C). VC, vehicle control (acetone); NM, nitrogen mustard-exposed mice; PT, portal triad; CV, central vein; black arrows, dilated sinusoids; red scale bar, 1 cm; black scale bar (histology images), 100 μm. Data represent mean ± SEM of three-eight animals in each treatment group. *, p < 0.05.
4. Discussion
Exposure to mustard agents at higher doses is known to cause multiple complications including mortality. These agents can form monofunctional adducts as well as DNA interstrand crosslinks (Lawley and Brookes, 1967, Shahin et al., 2001), which are difficult to repair and lead to cell death of replicating cells (Kaina, 2003). Basal skin keratinocytes, bone marrow cells, gastrointestinal, corneal and tracheal epithelial, all have high cell turnover; therefore, these tissues are believed to be very susceptible to systemic mustard poisoning (Kehe et al., 2009b). Our published studies show that topical exposure to NM (3.2 mg/mouse), an analogue of SM, in SKH-1 mice caused cutaneous injuries comparable to those observed for humans and other animal models (Jain, Tewari-Singh, 2014a, Tewari-Singh, Jain, 2014b). In these studies, topical application of NM also caused a progressive decrease in the mouse body weight, followed by mortality. Therefore, in the present study, our aim was to identify possible systemic effects of NM that could explain the mortality following its topical exposure. Our data showed that apart from cutaneous injury, NM exposure also causes toxic hematological consequences, damage to the gastrointestinal tract and atrophy, and toxic effects in the spleen and kidneys of the mice. The systemic effects observed upon topical NM exposure could be attributed to its highly reactive and lipophilic nature; however, its percent absorption in the skin and in the systemic circulation need to be further assessed. It is reported for SM that when applied on human skin, about 80 % of it evaporates and of the remaining 20% that penetrates the skin, only 10–20% is fixed by macromolecules in the skin and the rest 80–90% is rapidly transported away by circulation (Chilcott et al., 2000, Kehe and Szinicz, 2005). However, it has to be noted that these studies were carried out using pure SM without any solvents. Though cutaneous absorption of NM and the percentage which enters the circulation is not known, NM is also reported to bind to cellular components in the skin (Renshaw, 1946). In this study, since NM was dissolved in acetone that facilitates its penetration, it might have less variation in the amount of NM absorbed in the skin tissue. Also, though the stability of NM in acetone needs further evaluation, there is one report where an ointment containing NM (10mg), Paraffin (50g) and 1 ml acetone was found to be stable till 40 days at 37°C (Cummings et al., 1993).
Similar to NM-caused decrease in weight loss in SKH-1 mice observed here, weight loss due to dermal SM and NM exposure in animals has also been reported (Dube et al., 1998, Kumar and Vijayaraghavan, 1997, Sharma et al., 2009). We hypothesize that the weight loss observed in these mice might be, in part, due to the injury caused by NM to the intestinal epithelium thereby hindering the absorption of the nutrients. Notably, a time-dependent increase in intestinal epithelial cell death (mainly in the villi) was observed upon NM exposure, and 60–70% of the total cells of the villi were highly edematous and vacuolated. Also, due to the necrosis and edematous damage to the villi of the small intestine, there could be loss of fluids and electrolytes (as has been reported for Iranian war veterans (Balali-Mood and Hefazi, 2006), resulting in severe dehydration in these mice. Exposure to SM in humans has been reported to cause GI tract irritation, with symptoms including nausea and vomiting, abdominal pain, bloody diarrhea, loss of appetite, cachexia, and dehydration (Balali-Mood, 1986, Balali-Mood and Hefazi, 2006).
NM exposure not only affected the intestinal epithelium but also caused significant changes in the circulating blood leucocytes. Hematological consequences following SM exposure have been documented (Anderson et al., 2006, Ghanei, 2004, Sharma, Vijayaraghavan, 2010b, Sugendran et al., 2013), but have not been well characterized for NM exposure. An increase in the total WBCs was observed at 24 h post-exposure, which is a sign of an inflammatory response; however, at later time points, a significant NM-related decrease in the total WBCs was observed. An early slight increase in the number of WBCs could be due to the fact that NM initially up regulates the production of inflammatory mediators, which result in an increase in the number of WBCs in the blood. An increased number of neutrophils at 24 h after NM exposure, indicating an inflammatory response due to toxic insult, is consistent with the reports from us and others where an increase in neutrophils and myeloperoxidase in the skin following SM and NM exposure is reported (Dacre and Goldman, 1996, Jain et al., 2014b, Wormser, 1991). At later time points (72 h and 120 h after NM-exposure), the number of WBCs; neutrophils, lymphocytes and monocytes decreased significantly.
SM exposure in humans and dermal exposure in mouse models has been shown to cause significant decreases in the weight of the spleen and histological changes in the thymus and spleen (Mehrdad et al., 2008, Pant and Vijayaraghavan, 1999, Sharma, Vijayaraghavan, 2010b, Sharma, Vijayaraghavan, 2009, Venkateswaran et al., 1994). A significant dose-related reduction in spleen cell number after intraperitoneal injection of SM has also been observed (Coutelier et al., 1991), and SM-induced damage to the lymph system and lymph node discoloration and spleen pathology were noted to be similar to that found in autopsies of SM victims (Alexander, 1947). In our studies, NM exposure caused a decrease in spleen size, weight and histopathological changes including decrease in both red and white pulp areas, which was quantified. Since the spleen purifies blood, stores monocytes that transform into macrophages, and is rich in lymphocytes; the NM-related spleen toxicity could cause decrease in these circulating leucocytes observed in mice. In Iranian war veterans, exposure to SM has been shown to impair both humoral as well as cellular immunity (Hassan et al., 2006, Mahmoudi et al., 2005). Furthermore, since the spleen is a major lymphoid reserve and is responsible for initiating immune response to pathogens, NM-induced damage to the spleen (decrease in the cross-sectional area of lymphoid follicles) could render the mice immunocompromised and thus prone to infections.
Although SM and NM toxicity in eye, respiratory tract and skin has been well characterized, there are only a few reports on the effect of SM/NM on the liver and kidneys (Dacre and Goldman, 1996). Topical exposure to SM has been shown to cause histopathological alterations and a rise in serum glutamic oxaloacetic transaminase (SGOT) and serum glutamic pyruvic transaminase (SGPT) in the guinea pig (Chauhan and Murty, 1997) indicating liver injury. Also, percutaneous application of SM and NM in mice has been shown to cause lesions including granulovascular degeneration with perinuclear clumping of the cytoplasm of hepatocytes and renal parenchymal cells (Sharma, Pant, 2010a). Congestion and hemorrhage has also been observed in per-cutaneously NM exposed mice liver (Sharma, Pant, 2010a). In the present study, we observed severe atrophy, discoloration and a decrease in weight of the liver after topical NM exposure. Apart from that, a slight dilation in the sinusoids at early time points and hepatocytes with lesser cytoplasm at later time point was also observed, with no other major histopathological changes. The lesser cytoplasm could be due to the fluid loss. A significant decrease in the weight of the kidneys and a slight pale discoloration was observed upon topical NM exposure. Histopathological analyses showed grossly dilated capillaries, suggesting some cell types (endothelial or mesangial cells) may be highly sensitive to NM exposure. Serum analysis of BUN and creatinine to access kidney function showed an elevated BUN levels and reduced creatinine levels after NM exposure. Reduced creatinine levels could be due to tissue catabolism or starvation. High BUN to creatinine ratio was observed in NM-exposed mice suggesting a state of pre-renal azotemia, which could be due to reduced blood flow to the kidneys because of dehydration, volume depletion or shock due to the toxic effect on other organs.
A recent report by Batal et al., has shown that upon cutaneous application, SM is able to diffuse through the skin and leads to DNA damage in brain, lungs, kidney, spleen and liver of SKH-1 mice (Batal, Boudry, 2014). This could contribute for the NM induced toxicity observed in various organs in the present study. Also, though systemic effects of topical application of SM and NM have been reported as discussed above in different mouse models, this study provides the quantitative assessments and pathological studies in a more relevant mouse model, which could be employed as useful biomarkers of systemic injury following topical vesicant exposure. SKH-1 hairless mouse, being unpigmented and immunocompetent, it is one of the most widely used mouse model in skin research as well as studies with mustard agents (Benavides et al., 2009, Dorandeu, Taysse, 2011, Jain, Tewari-Singh, 2014b, Kligman and Kligman, 1998). (Batal, Boudry, 2014, Batal et al., 2013, Jain, Tewari-Singh, 2014a, Tewari-Singh, Inturi, 2014a, Tewari-Singh, Jain, 2013, Tewari-Singh, Jain, 2014b).
In summary, here we report that a single topical NM exposure at a high (lethal) dose in a more useful SKH-1 hairless mouse model results in severe damage to the small intestine/GI tract, hematopoietic cells and spleen, kidneys, and changes in the circulating leucocytes. The finding of pre-renal azotemia is highly suggestive of hypovolemia and dehydration, which also likely contributed to the demise of these animals. Together, these toxic effects could account for the observed body weight loss and high mortality following topical NM exposure in mice. Detailed studies of systemic effects from topical vesicant exposure are warranted in future, which could be helpful in better understanding of vesicant-induced toxicity, and associated pathological effects and mechanisms for the development of broad-spectrum and superior therapeutics.
Acknowledgments
This work was supported by the Countermeasures Against Chemical Threats (CounterACT) Program, Office of the Director National Institutes of Health (OD) and the National Institute of Environmental Health Sciences (NIEHS), [Grant Number U54 ES015678]. The study sponsor (NIH) had no involvement in the study design; collection, analysis and interpretation of data; the writing of the manuscript; and the decision to submit the manuscript for publications.
Abbreviations
- BUN
blood urea nitrogen
- GI
gastrointestinal tract
- H&E
hematoxylin and eosin
- NM
nitrogen mustard
- PALS
periarterial lymphoid sheath
- SGOT
serum glutamic oxaloacetic transaminase
- SGPT
serum glutamic pyruvic transaminase
- SM
sulfur mustard
- TUNEL
Terminal deoxynucleotidyl transferase (tdt)- mediated dUTP-biotin nick end labeling
- WBCs
white blood cells
Footnotes
Conflict of interest
The authors state no conflict of interest.
References
- Alexander SF. Medical report of the Bari Harbor mustard casualties. Military Surgeon. 1947;101:1–17. [PubMed] [Google Scholar]
- Anderson DR, Holmes WW, Lee RB, Dalal SJ, Hurst CG, Maliner BI, et al. Sulfur mustard-induced neutropenia: treatment with granulocyte colony-stimulating factor. Military medicine. 2006;171:448–53. doi: 10.7205/milmed.171.5.448. [DOI] [PubMed] [Google Scholar]
- Balali-Mood M. Clinical and paraclinical findings in 233 patients with sulfur mustard poisoning. 1986. [Google Scholar]
- Balali-Mood M, Hefazi M. The pharmacology, toxicology, and medical treatment of sulphur mustard poisoning. Fundam Clin Pharmacol. 2005;19:297–315. doi: 10.1111/j.1472-8206.2005.00325.x. [DOI] [PubMed] [Google Scholar]
- Balali-Mood M, Hefazi M. Comparison of early and late toxic effects of sulfur mustard in Iranian veterans. Basic & clinical pharmacology & toxicology. 2006;99:273–82. doi: 10.1111/j.1742-7843.2006.pto_429.x. [DOI] [PubMed] [Google Scholar]
- Balali-Mood M, Hefazi M, Mahmoudi M, Jalali E, Attaran D, Maleki M, et al. Long-term complications of sulphur mustard poisoning in severely intoxicated Iranian veterans. Fundam Clin Pharmacol. 2005;19:713–21. doi: 10.1111/j.1472-8206.2005.00364.x. [DOI] [PubMed] [Google Scholar]
- Balali-Mood M, Mousavi S, Balali-Mood B. Chronic health effects of sulphur mustard exposure with special reference to Iranian veterans. Emerging health threats journal. 2008;1:e7. doi: 10.3134/ehtj.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batal M, Boudry I, Mouret S, Cléry-Barraud C, Wartelle J, Bérard I, et al. DNA damage in internal organs after cutaneous exposure to sulphur mustard. Toxicology and Applied Pharmacology. 2014;278:39–44. doi: 10.1016/j.taap.2014.04.003. [DOI] [PubMed] [Google Scholar]
- Batal M, Boudry I, Mouret S, Wartelle J, Emorine S, Bertoni M, et al. Temporal and spatial features of the formation of DNA adducts in sulfur mustard-exposed skin. Toxicology and applied pharmacology. 2013;273:644–50. doi: 10.1016/j.taap.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Benavides F, Oberyszyn TM, VanBuskirk AM, Reeve VE, Kusewitt DF. The hairless mouse in skin research. Journal of dermatological science. 2009;53:10–8. doi: 10.1016/j.jdermsci.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blewett W. Tactical weapons: is mustard still king. NBC Defense Technol Int. 1986;1:64–6. [Google Scholar]
- Borak J, Sidell FR. Agents of chemical warfare: sulfur mustard. Annals of emergency medicine. 1992;21:303–8. doi: 10.1016/s0196-0644(05)80892-3. [DOI] [PubMed] [Google Scholar]
- Chauhan RS, Murthy LVR, Pandey M. Histomorphometric Study of Animal Skin Exposed to Sulfur Mustard. Bulletin of environmental contamination and toxicology. 1993;51:138–45. doi: 10.1007/BF00201012. [DOI] [PubMed] [Google Scholar]
- Chauhan RS, Murty LVR. Effect of topically applied sulphur mustard on guinea pig liver. Journal of Applied Toxicology. 1997;17:415–9. doi: 10.1002/(sici)1099-1263(199711/12)17:6<415::aid-jat465>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Chilcott RP, Jenner J, Carrick W, Hotchkiss SAM, Rice P. Human skin absorption of bis-2-(chloroethyl)sulphide (sulphur mustard) in vitro. Journal of Applied Toxicology. 2000;20:349–55. doi: 10.1002/1099-1263(200009/10)20:5<349::AID-JAT713>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Coutelier JP, Lison D, Simon O, Willems J. Effect of sulfur mustard on murine lymphocytes. Toxicology letters. 1991;58:143–8. doi: 10.1016/0378-4274(91)90168-6. [DOI] [PubMed] [Google Scholar]
- Cowan FM, Yourick JJ, Hurst CG, Broomfield CA, Smith WJ. Sulfur mustard-increased proteolysis following in vitro and in vivo exposures. Cell biology and toxicology. 1993;9:269–77. doi: 10.1007/BF00755605. [DOI] [PubMed] [Google Scholar]
- Cummings J, MacLellan A, Langdon SJ, Smyth JF. The long term stability of mechlorethamine hydrochloride (nitrogen mustard) ointment measured by HPLC. The Journal of pharmacy and pharmacology. 1993;45:6–9. doi: 10.1111/j.2042-7158.1993.tb03669.x. [DOI] [PubMed] [Google Scholar]
- Dabrowska MI, Becks LL, Lelli JL, Jr, Levee MG, Hinshaw DB. Sulfur mustard induces apoptosis and necrosis in endothelial cells. Toxicology and applied pharmacology. 1996;141:568–83. doi: 10.1006/taap.1996.0324. [DOI] [PubMed] [Google Scholar]
- Dacre JC, Goldman M. Toxicology and pharmacology of the chemical warfare agent sulfur mustard. Pharmacol Rev. 1996;48:289–326. [PubMed] [Google Scholar]
- Davis KG, Aspera G. Exposure to liquid sulfur mustard. Annals of emergency medicine. 2001;37:653–6. doi: 10.1067/mem.2001.114322. [DOI] [PubMed] [Google Scholar]
- Dhanalakshmi S, Mallikarjuna GU, Singh RP, Agarwal R. Silibinin prevents ultraviolet radiation-caused skin damages in SKH-1 hairless mice via a decrease in thymine dimer positive cells and an up-regulation of p53-p21/Cip1 in epidermis. Carcinogenesis. 2004;25:1459–65. doi: 10.1093/carcin/bgh152. [DOI] [PubMed] [Google Scholar]
- Dorandeu F, Taysse L, Boudry I, Foquin A, Herodin F, Mathieu J, et al. Cutaneous challenge with chemical warfare agents in the SKH-1 hairless mouse. (I) Development of a model for screening studies in skin decontamination and protection. Human & experimental toxicology. 2011;30:470–90. doi: 10.1177/0960327110373615. [DOI] [PubMed] [Google Scholar]
- Dube SN, Husain K, Sugendran K, Vijayaraghavan R, Somani SM. Dose response of sulphur mustard: behavioral and toxic signs in rats. Indian J Physiol Pharmacol. 1998;42:389–94. [PubMed] [Google Scholar]
- Firooz A, Sadr B, Davoudi SM, Nassiri-Kashani M, Panahi Y, Dowlati Y. Long-term skin damage due to chemical weapon exposure. Cutaneous and Ocular Toxicology. 2011;30:64–8. doi: 10.3109/15569527.2010.529547. [DOI] [PubMed] [Google Scholar]
- Geraci MJ. Mustard gas: imminent danger or eminent threat? The Annals of pharmacotherapy. 2008;42:237–46. doi: 10.1345/aph.1K445. [DOI] [PubMed] [Google Scholar]
- Ghabili K, Agutter PS, Ghanei M, Ansarin K, Panahi Y, Shoja MM. Sulfur mustard toxicity: history, chemistry, pharmacokinetics, and pharmacodynamics. Critical reviews in toxicology. 2011;41:384–403. doi: 10.3109/10408444.2010.541224. [DOI] [PubMed] [Google Scholar]
- Ghanei M. Delayed haematological complications of mustard gas. Journal of applied toxicology: JAT. 2004;24:493–5. doi: 10.1002/jat.1006. [DOI] [PubMed] [Google Scholar]
- Ghanei M, Harandi AA. Long term consequences from exposure to sulfur mustard: a review. Inhal Toxicol. 2007;19:451–6. doi: 10.1080/08958370601174990. [DOI] [PubMed] [Google Scholar]
- Ghanei M, Poursaleh Z, Harandi AA, Emadi SE, Emadi SN. Acute and chronic effects of sulfur mustard on the skin: a comprehensive review. Cutaneous and ocular toxicology. 2010;29:269–77. doi: 10.3109/15569527.2010.511367. [DOI] [PubMed] [Google Scholar]
- Guainazzi A, Campbell AJ, Angelov T, Simmerling C, Scharer OD. Synthesis and molecular modeling of a nitrogen mustard DNA interstrand crosslink. Chemistry. 2010;16:12100–3. doi: 10.1002/chem.201002041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardej D, Billack B. Ebselen protects brain, skin, lung and blood cells from mechlorethamine toxicity. Toxicology & Industrial Health. 2007;23:209–21. doi: 10.1177/0748233707083541. [DOI] [PubMed] [Google Scholar]
- Hassan ZM, Ebtekar M, Ghanei M, Taghikhani M, Noori Daloii MR, Ghazanfari T. Immunobiological consequences of sulfur mustard contamination. Iran J Allergy Asthma Immunol. 2006;5:101–8. [PubMed] [Google Scholar]
- Hefazi M, Maleki M, Mahmoudi M, Tabatabaee A, Balali-Mood M. Delayed complications of sulfur mustard poisoning in the skin and the immune system of Iranian veterans 16–20 years after exposure. Int J Dermatol. 2006;45:1025–31. doi: 10.1111/j.1365-4632.2006.03020.x. [DOI] [PubMed] [Google Scholar]
- Jain AK, Tewari-Singh N, Gu M, Inturi S, White CW, Agarwal R. Sulfur mustard analog, 2-chloroethyl ethyl sulfide-induced skin injury involves DNA damage and induction of inflammatory mediators, in part via oxidative stress, in SKH-1 hairless mouse skin. Toxicology letters. 2011;205:293–301. doi: 10.1016/j.toxlet.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain AK, Tewari-Singh N, Inturi S, Orlicky DJ, White CW, Agarwal R. Histopathological and immunohistochemical evaluation of nitrogen mustard-induced cutaneous effects in SKH-1 hairless and C57BL/6 mice. Experimental and toxicologic pathology: official journal of the Gesellschaft fur Toxikologische Pathologie. 2014a;66:129–38. doi: 10.1016/j.etp.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain AK, Tewari-Singh N, Inturi S, Orlicky DJ, White CW, Agarwal R. Myeloperoxidase deficiency attenuates nitrogen mustard-induced skin injuries. Toxicology. 2014b;320C:25–33. doi: 10.1016/j.tox.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaina B. DNA damage-triggered apoptosis: critical role of DNA repair, double-strand breaks, cell proliferation and signaling. Biochemical pharmacology. 2003;66:1547–54. doi: 10.1016/s0006-2952(03)00510-0. [DOI] [PubMed] [Google Scholar]
- Kehe K, Balszuweit F, Emmler J, Kreppel H, Jochum M, Thiermann H. Sulfur mustard research--strategies for the development of improved medical therapy. Eplasty. 2008a;8:e32. [PMC free article] [PubMed] [Google Scholar]
- Kehe K, Balszuweit F, Steinritz D, Thiermann H. Molecular toxicology of sulfur mustard-induced cutaneous inflammation and blistering. Toxicology. 2009a;263:12–9. doi: 10.1016/j.tox.2009.01.019. [DOI] [PubMed] [Google Scholar]
- Kehe K, Raithel K, Kreppel H, Jochum M, Worek F, Thiermann H. Inhibition of poly(ADP-ribose) polymerase (PARP) influences the mode of sulfur mustard (SM)-induced cell death in HaCaT cells. Archives of toxicology. 2008b;82:461–70. doi: 10.1007/s00204-007-0265-7. [DOI] [PubMed] [Google Scholar]
- Kehe K, Szinicz L. Medical aspects of sulphur mustard poisoning. Toxicology. 2005;214:198–209. doi: 10.1016/j.tox.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Kehe K, Thiermann H, Balszuweit F, Eyer F, Steinritz D, Zilker T. Acute effects of sulfur mustard injury--Munich experiences. Toxicology. 2009b;263:3–8. doi: 10.1016/j.tox.2009.04.060. [DOI] [PubMed] [Google Scholar]
- Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- Kligman AM, Kligman LH. A hairless mouse model for assessing the chronic toxicity of topically applied chemicals. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 1998;36:867–78. doi: 10.1016/s0278-6915(98)00045-3. [DOI] [PubMed] [Google Scholar]
- Kumar O, Vijayaraghavan R. Effect on physiological variables & urinary metabolites following a single dermal application of sulphur mustard in rats. Defence Sci J. 1997;47:389–94. [Google Scholar]
- Lanaspa MA, Ishimoto T, Li N, Cicerchi C, Orlicky DJ, Ruzycki P, et al. Endogenous fructose production and metabolism in the liver contributes to the development of metabolic syndrome. Nat Commun. 2013:4. doi: 10.1038/ncomms3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawley PD, Brookes P. Interstrand cross-linking of DNA by difunctional alkylating agents. J Mol Biol. 1967;25:143–60. doi: 10.1016/0022-2836(67)90285-9. [DOI] [PubMed] [Google Scholar]
- Lomash V, Deb U, Rai R, Jadhav SE, Vijayaraghavan R, Pant SC. Designing of mouse model: a new approach for studying sulphur mustard-induced skin lesions. Burns: journal of the International Society for Burn Injuries. 2011;37:851–64. doi: 10.1016/j.burns.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Lulla A, Reznik S, Trombetta L, Billack B. Use of the mouse ear vesicant model to evaluate the effectiveness of ebselen as a countermeasure to the nitrogen mustard mechlorethamine. Journal of applied toxicology: JAT. 2013 doi: 10.1002/jat.2969. [DOI] [PubMed] [Google Scholar]
- Mahmoudi M, Hefazi M, Rastin M, Balali-Mood M. Long-term hematological and immunological complications of sulfur mustard poisoning in Iranian veterans. International immunopharmacology. 2005;5:1479–85. doi: 10.1016/j.intimp.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Malaviya R, Sunil VR, Cervelli J, Anderson DR, Holmes WW, Conti ML, et al. Inflammatory effects of inhaled sulfur mustard in rat lung. Toxicology and applied pharmacology. 2010;248:89–99. doi: 10.1016/j.taap.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour Razavi S, Salamati P, Saghafinia M, Abdollahi M. A review on delayed toxic effects of sulfur mustard in Iranian veterans. Daru: journal of Faculty of Pharmacy, Tehran University of Medical Sciences. 2012;20:51. doi: 10.1186/2008-2231-20-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrdad J, Bahadoran H, Asadi MH. Acute Effects of Sulphur Mustard Gas on the Number of Lymphocytes in the Rat’s Spleen. Int J Morphol. 2008;26:433–6. [Google Scholar]
- Pal A, Tewari-Singh N, Gu M, Agarwal C, Huang J, Day BJ, et al. Sulfur mustard analog induces oxidative stress and activates signaling cascades in the skin of SKH-1 hairless mice. Free radical biology & medicine. 2009;47:1640–51. doi: 10.1016/j.freeradbiomed.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pant SC, Vijayaraghavan R. Histomorphological and histochemical alterations following short-term inhalation exposure to sulfur mustard on visceral organs of mice. Biomedical and environmental sciences: BES. 1999;12:201–13. [PubMed] [Google Scholar]
- Papirmeister B, Gross CL, Meier HL, Petrali JP, Johnson JB. Molecular-Basis for Mustard-Induced Vesication. Fund Appl Toxicol. 1985;5:S134–S49. [PubMed] [Google Scholar]
- Petrali JP, Dick EJ, Brozetti JJ, Hamilton TA, Finger AV. Acute ocular effects of mustard gas: ultrastructural pathology and immunohistopathology of exposed rabbit cornea. Journal of applied toxicology: JAT. 2000;20 (Suppl 1):S173–5. doi: 10.1002/1099-1263(200012)20:1+<::aid-jat679>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Pino MA, Billack B. Reduction of vesicant toxicity by butylated hydroxyanisole in A-431 skin cells. Cutaneous and Ocular Toxicology. 2008;27:161–72. doi: 10.1080/15569520802092070. [DOI] [PubMed] [Google Scholar]
- Price CC. FUNDAMENTAL MECHANISMS OF ALKYLATION. Annals of the New York Academy of Sciences. 1958;68:663–8. doi: 10.1111/j.1749-6632.1958.tb42632.x. [DOI] [PubMed] [Google Scholar]
- Renshaw B. Mechanisms in production of cutaneous injuries by sulfur and nitrogen mustards. Washington, DC: Division 9, US Office of Scientific Research and Development, National Defense Research Commmittee; 1946. pp. 479–518. [Google Scholar]
- Riviere JE, Brooks JD, Williams PL, Monteiro-Riviere NA. Toxicokinetics of topical sulfur mustard penetration, disposition, and vascular toxicity in isolated perfused porcine skin. Toxicology and applied pharmacology. 1995;135:25–34. doi: 10.1006/taap.1995.1205. [DOI] [PubMed] [Google Scholar]
- Rowell M, Kehe K, Balszuweit F, Thiermann H. The chronic effects of sulfur mustard exposure. Toxicology. 2009;263:9–11. doi: 10.1016/j.tox.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Rutman RJ, Chun EH, Jones J. Observations on the mechanism of the alkylation reaction between nitrogen mustard and DNA. Biochimica et biophysica acta. 1969;174:663–73. doi: 10.1016/0005-2787(69)90295-0. [DOI] [PubMed] [Google Scholar]
- Saladi RN, Smith E, Persaud AN. Mustard: a potential agent of chemical warfare and terrorism. Clinical and experimental dermatology. 2006;31:1–5. doi: 10.1111/j.1365-2230.2005.01945.x. [DOI] [PubMed] [Google Scholar]
- Shahin S, Cullinane C, Gray PJ. Mitochondrial and nuclear DNA damage induced by sulphur mustard in keratinocytes. Chemico-biological interactions. 2001;138:231–45. doi: 10.1016/s0009-2797(01)00275-7. [DOI] [PubMed] [Google Scholar]
- Sharma M, Pant SC, Pant JC, Vijayaraghavan R. Nitrogen and sulphur mustard induced histopathological observations in mouse visceral organs. Journal of environmental biology/Academy of Environmental Biology, India. 2010a;31:891–905. [PubMed] [Google Scholar]
- Sharma M, Vijayaraghavan R, Agrawal OP. Comparative toxic effect of nitrogen mustards (HN-1, HN-2, and HN-3) and sulfur mustard on hematological and biochemical variables and their protection by DRDE-07 and its analogues. International journal of toxicology. 2010b;29:391–401. doi: 10.1177/1091581810365730. [DOI] [PubMed] [Google Scholar]
- Sharma M, Vijayaraghavan R, Ganesan K. Comparison of toxicity of selected mustard agents by percutaneous and subcutaneous routes. Indian journal of experimental biology. 2008;46:822–30. [PubMed] [Google Scholar]
- Sharma M, Vijayaraghavan R, Gautam A. DRDE-07 and its analogues as promising cytoprotectants to nitrogen mustard (HN-2)--an alkylating anticancer and chemical warfare agent. Toxicology letters. 2009;188:243–50. doi: 10.1016/j.toxlet.2009.04.015. [DOI] [PubMed] [Google Scholar]
- Shohrati M, Peyman M, Peyman A, Davoudi M, Ghanei M. Cutaneous and ocular late complications of sulfur mustard in Iranian veterans. Cutaneous and ocular toxicology. 2007;26:73–81. doi: 10.1080/15569520701212399. [DOI] [PubMed] [Google Scholar]
- Shukla PK, Mishra PC, Suhai S. Reactions of DNA bases with the anti-cancer nitrogen mustard mechlorethamine: A quantum chemical study. Chem Phys Lett. 2007;449:323–8. [Google Scholar]
- Smith KJ, Casillas R, Graham J, Skelton HG, Stemler F, Hackley BE., Jr Histopathologic features seen with different animal models following cutaneous sulfur mustard exposure. Journal of Dermatological Science. 1997;14:126–35. doi: 10.1016/s0923-1811(96)00560-9. [DOI] [PubMed] [Google Scholar]
- Sugendran K, Jeevaratnam K, Bhaskar ASB, Pant SC. Effect of Topically Applied Sulphur Mustard on Haematological Biochemical and Histological Parameters in Mice. 2013. [Google Scholar]
- Tewari-Singh N, Agarwal C, Huang J, Day BJ, White CW, Agarwal R. Efficacy of glutathione in ameliorating sulfur mustard analog-induced toxicity in cultured skin epidermal cells and in SKH-1 mouse skin in vivo. The Journal of pharmacology and experimental therapeutics. 2011;336:450–9. doi: 10.1124/jpet.110.173708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari-Singh N, Inturi S, Jain AK, Agarwal C, Orlicky DJ, White CW, et al. Catalytic antioxidant AEOL 10150 treatment ameliorates sulfur mustard analog 2-chloroethyl ethyl sulfide-associated cutaneous toxic effects. Free radical biology & medicine. 2014a;72:285–95. doi: 10.1016/j.freeradbiomed.2014.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari-Singh N, Jain AK, Inturi S, White CW, Agarwal R. Clinically-relevant cutaneous lesions by nitrogen mustard: useful biomarkers of vesicants skin injury in SKH-1 hairless and C57BL/6 mice. PloS one. 2013;8:e67557. doi: 10.1371/journal.pone.0067557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari-Singh N, Jain AK, Orlicky DJ, White CW, Agarwal R. Cutaneous injury-related structural changes and their progression following topical nitrogen mustard exposure in hairless and haired mice. PloS one. 2014b;9:e85402. doi: 10.1371/journal.pone.0085402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari-Singh N, Rana S, Gu M, Pal A, Orlicky DJ, White CW, et al. Inflammatory biomarkers of sulfur mustard analog 2-chloroethyl ethyl sulfide-induced skin injury in SKH-1 hairless mice. Toxicological sciences: an official journal of the Society of Toxicology. 2009;108:194–206. doi: 10.1093/toxsci/kfn261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai JC, Sheu HM, Hung PL, Cheng CL. Effect of barrier disruption by acetone treatment on the permeability of compounds with various lipophilicities: implications for the permeability of compromised skin. J Pharm Sci. 2001;90:1242–54. doi: 10.1002/jps.1077. [DOI] [PubMed] [Google Scholar]
- Ucar M, Korkmaz A, Reiter RJ, Yaren H, Oter S, Kurt B, et al. Melatonin alleviates lung damage induced by the chemical warfare agent nitrogen mustard. Toxicology letters. 2007;173:124–31. doi: 10.1016/j.toxlet.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Venkateswaran KS, Neeraja V, Sugendran K, Gopalan N, Vijayaraghavan R, Pant SC, et al. Dose-Dependent Effects on Lymphoid Organs Following a Single Dermal Application of Sulfur Mustard in Mice. Human & experimental toxicology. 1994;13:247–51. doi: 10.1177/096032719401300404. [DOI] [PubMed] [Google Scholar]
- Wang GQ, Xia ZF. Tissue injury by hot fluid containing nitrogen mustard. Burns: journal of the International Society for Burn Injuries. 2007;33:923–6. doi: 10.1016/j.burns.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Wheeler GP. Studies related to the mechanisms of action of cytotoxic alkylating agents: a review. Cancer research. 1962;22:651–88. [PubMed] [Google Scholar]
- Wormser U. Toxicology of Mustard Gas. Trends Pharmacol Sci. 1991;12:164–7. doi: 10.1016/0165-6147(91)90534-y. [DOI] [PubMed] [Google Scholar]
- Wormser U, Brodsky B, Reich R. Topical treatment with povidone iodine reduces nitrogen mustard-induced skin collagenolytic activity. Archives of toxicology. 2002;76:119–21. doi: 10.1007/s00204-001-0307-5. [DOI] [PubMed] [Google Scholar]