Abstract
Preclinical research in the field of nanomedicine continues to produce a steady stream of new nanoparticles with unique capabilities and complex properties. With improvements come promising treatments for diseases, with the ultimate goal of clinical translation and better patient outcomes compared to current standards of care. Here, we outline engineering considerations for nanomedicines, with respect to design criteria, targeting and stimuli-triggered drug release strategies. General properties, clinical relevance and current research advances of various nanomedicines are discussed in light of how these will realize their potential and shape the future of the field.
Introduction
Nanomedical engineering involves the manipulation of matter in the size range of 1–1000 nm for medical applications. With the concurrent rise of the fields of biomedical engineering and nanotechnology, the intersected field of nanomedical engineering has grown remarkably in the past decades.1 Nanomedicine refers more specifically to medicines with nanoscale size, properties or features that are administered for patient benefit. There are numerous types of nanomedicines, but they generally can be categorized as therapeutic agents, medical imaging agents, or carriers for drug delivery. Nanomedicines also hold potential for disease detection and diagnosis.2 Nanomedical engineering seeks to rationally design and develop these, often with emphasis on size, shape, degradation and surface properties. An understanding of the biological properties of both the target tissue and the physiological route that must be travelled by the nanoparticles to reach that tissue is beneficial.
A large research focus of nanomedicines has been placed on cancer treatments3, although there have also been intensive research efforts spent on other health problems including cardiovascular disease4 and respiratory diseases5 amongst many others6. Cancer chemotherapy drugs are somewhat unique in that they are often intended to be toxic. Traditional small-molecule cancer therapeutics such as doxorubicin, gemcitabine, fluorouracil, cisplatin, paclitaxel suffer from limited selectivity between tumor and healthy tissues, leading to considerable side effects. Compared with the free drugs, nanoparticulate formulations frequently exert less systemic toxicity due to their reduced volumes of distribution which decreases drug access to critical organs like the heart and kidney. In nanoparticulate form, the drug does not pass as easily through fenestrations in the blood vessels of those organs. Nanoparticles can also increase the deposition of drugs in tumors due to the enhanced permeability and retention (EPR) effect which takes advantage of leaky tumor microvasculature and a lack of developed lymphatic draining system.7 It has also been demonstrated that nanoparticles are able to overcome biological barriers in the case of multidrug resistance, a phenomena in which small molecule drugs are pumped out of cancer cell membranes by protein efflux pumps after patients have undergone multiple rounds of chemotherapy.8
Often, nanoparticle formulations are designed to solve simple problems. When hydrophobic drugs cannot be dissolved in water, nanoparticulate formulations are considered because the alternative is to dissolve the drugs with surfactants or non-aqueous solvents for administration.9 Several successful nanomedicine formulations have become clinically relevant just by solving the problems of more straightforward drug formulations (Box 1). Introduction of a nanoparticulate system adds complexity that makes reproducible drug formulation and also safety regulation more difficult.10 Thus, a strong advantage compared to existing standards of care should be apparent for new nanomedicines to hope to make it to the clinic. This is especially the case for the more exotic and complex types of nanomedicines that are in preclinical evaluation.11
Mitigating toxicity of existing chemotherapies.
One of the keys to the success of two well-known nanomedicines is their ability to reduce the toxicity of therapeutic agents. The liposomal doxorubicin formulation Doxil has been successful more for its ability to reduce the cardiotoxicity associated with the use of free doxorubicin than for its therapeutic effects. Cardiotoxicity is the main dose-limiting factor of doxorubicin and is significantly reduced by nanoparticle encapsulation. In this case, the nanoparticles serve to protect certain organs from accumulation of the chemotherapeutic agent. Abraxane, a nanoparticulate form of paclitaxel bound to human albumin also demonstrates the ability of nanoparticles to reduce toxicity. In this case the toxicity is not of the drug but of the excipients used to solubilize the drug. paclitaxel is a poorly soluble hydrophobic drug with a propensity for aggregation and in order for it to be administered it needs to be solubilized with surfactants such as Cremophor EL. However, this surfactant can induce severe allergic reactions and limits the dosing of the paclitaxel. The development of Abraxane eliminates the toxicities associated with the delivery vehicle. In both cases the use of nanoparticles serves to reduce adverse effects associated with an existing therapy.
NANOPARTICLE DESIGN STRATEGIES
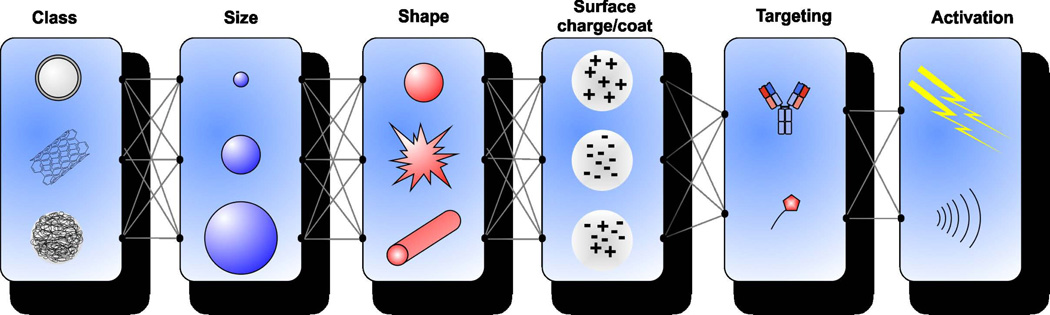
General nanoparticle design principles are useful to consider for nanomedicines. Nanoparticle size, shape, surface chemistry and composition are all key criteria which influence nanosystem behavior in biological contexts12. As shown in Figure 1, these numerous variables lead to a virtually endless combination of potential nanoparticles that could be developed and therefore a semi-rational approach is required.
Figure 1.
Combinatorial criteria to be considered when designing nanoparticles. These include the class of nanoparticle, size, shape surface charge, targeting, and activation mechanism. Each factor affects the efficiency. Class determines the basic properties of the particle. Size, shape, surface charge, and targeting generally affect pharmacokinetics and biodistribution. Activation can provide improved distribution or bioavailability of encapsulated drugs.
Size affects the behavior of nanoparticles in the body (Figure 2). It has been shown that nanoparticles smaller than 30 nm can move into systemic circulation following administration into the lungs.13 After nanoscale materials enter circulation in the blood stream, if they are small enough to pass through the glomerular basement membranes within the kidney, they will leave circulation through renal clearance into urine. Administration of quantum dots with hydrodynamic diameters of less than 5.5 nm resulted in rapid and efficient renal clearance.14 Renal clearance of nanoparticles is attractive because the introduced nanomaterials enter and leave the body, mitigating many long term safety concerns. However, renal clearance typically occurs too rapidly to enable enough accumulation of the nanoparticles into target tissues and therefore most administered nanomedicines avoid this effect. On the other hand, if nanoparticles are too large, they are also rapidly cleared from circulation. As blood passes through interendothelial cell slits of the spleen, nanoparticles that are over 200 nm in diameter get trapped and are rapidly cleared from circulation.15 This is clearly affected by the deformability of the materials, since 8 micron-sized red blood cells pass through these slits constantly and remain in circulation for months.
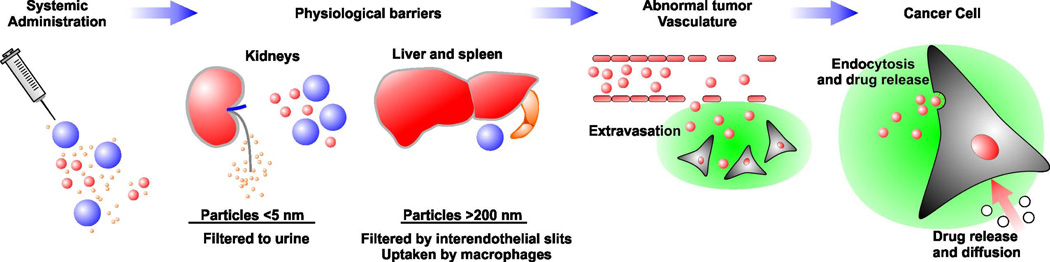
Figure 2.
In vivo fate of nanoparticles following systemic administration. Small nanoparticles can be cleared by the kidneys whereas larger nanoparticles can be cleared by the liver and spleen. Nanoparticles then extravasate into the tumor tissue due to the large fenestrations in the tumor vasculature. Extravasated nanoparticles deliver drugs to target cells through endocytosis or through the breakdown of the nanoparticles and release of the drug.
For nanoparticles to be uptaken into solid tumors, size is also a critical factor. However, there can be variability depending on the type of tumor and since most studies have been restricted to animal models, there is less certainty on the topic. Following extravasation from blood vessels, it has been shown that nanoparticles that are less than 60 nm in diameter can better navigate the collagen-containing extracellular matrix and more deeply penetrate the bulk of the tumor.16 It has also been shown that compared to larger ones, gold nanoparticles of 20 nm diameter can more easily diffuse out of tumors and are less likely to be retained.17
While the size of nanoparticles has been examined extensively, there has been somewhat less emphasis on their shape until recently. This is because for the most part, traditional nanoparticles used in vivo such as liposomes, polymeric nanoparticles, and proteins are roughly spherical in shape. The hydrodynamic radius, which is a standard measure of nanoparticle size, presumes this. However, several non-spherical nanoparticles such as gold nanorods and carbon nanotubes as well as other structures with high aspect ratios are now being investigated. For example, PEGylated filomicelles, which are long worm-like micelles, circulated for one week after intravenous injection in rodents, which is a much longer than typical spherical particles.18 They also could be used for delivery of paclitaxel to tumors. Quasi-one-dimensional single-wall carbon nanotubes (SWNTs) have shown high tumor accumulation, with their nanoscale shape and flexibility likely contributing.19 Magnetic iron oxide nanoworms also demonstrated that unique shapes and structures can result in improved in vivo behavior20.
In general, forming nanoparticles into complex shapes can be technically challenging. New advances in top-down nanofabrication using particle replication in nonwetting templates have opened the door for opportunities to explore nanoparticles with shapes that otherwise would be difficult to create.21 Another approach makes use of viruses from nature, which exhibit a wonderful diversity of shapes and there have been efforts to use viral nanoparticles both as nanocarriers as well as templates for assembling other nanoparticles.22 DNA nanotechnology is another area with great potential for designing nanoparticulate shapes of virtually any desired form using a bottom-up self-assembly approach23 and these are now increasingly being used for in vivo experimentation.24 Figure 3 shows some of the exotic shapes that are possible for nanomedicine. Based on the recent progress in developing biocompatible nanoparticles with more complex shapes, the relationships between nanoparticle shape and in vivo behaviors will be more clearly elucidated in coming years.
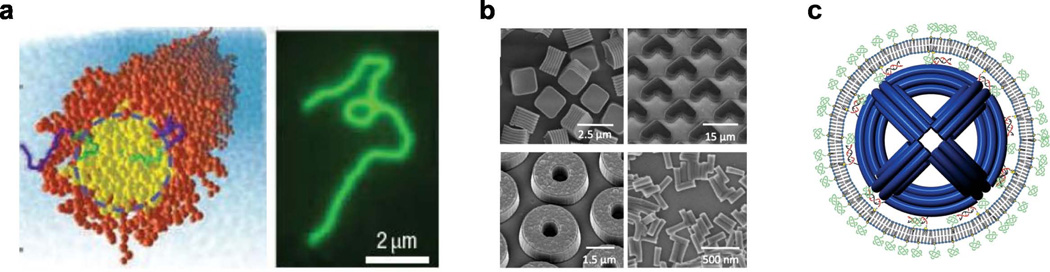
Figure 3.
a) Filomicelles; self-assembled di-block copolymers yellow/green indicates hydrophobic polymer center orange/blue indicates hydrophilic polymer (left), fluorescence imaging of a single filomicelle showing its long size. b) SEM images of particles produced by PRINT technology; cubic particles, hydrogel boomerangs, hydrogel toroids, and hydrogel rods. c) Schematic of liposome enclosed DNA nano-octahedron (DNO). The liposomes are fused to the DNO through DNA lipid complexes which bind the liposome bilayer to the DNO. This system uses the pegylated liposomes to function as a viral like capsid shell to protect the nanoparticle. Reprinted with permission from references18,21,24. Respective copyrights: 2007, Macmillan Publishers; 2011, John Wiley and Sons; and 2014, American Chemical Society.
Surface charge is another important factor that should be considered when designing nanoparticles. Since the luminal surface of vascular endothelium is negatively charged, positively charged molecules tend to have a higher transvascular transport efficiency compared with neutral or negatively charged molecules of similar sizes.25 This suggests that using cationic surface charges may enhance delivery of nanoparticles in the blood vessels of the target tissues. However, positively charged molecules will bind non-specifically to all blood vessel walls and therefore have a faster clearance rate compared to neutral or negatively charged molecules, which may counterbalance delivery advantages to target tissues26. Furthermore, cationic lipids and polymers, which can be easily used to confer a cationic nature upon nanoparticles of interest, pose concerns with respect to their safety.27 It has been shown that strong positive or negative charges lead to rapid clearance of nanoparticles, whereas a slight negative charges leads to a lesser amount of phagocytosis.28 Recently, zwitterionic charge coatings, which contains a mix of both positive and negative ionic charges have been shown to improve nanoparticle circulation times.29,30
Upon administration into the blood stream, nanoparticles immediately become coated with a protein corona during the opsonization process. The surface chemistry of the nanoparticle plays a critical role in determining the corona composition, which can rapidly flag the nanoparticles for removal.31 Coating nanoparticles with a protective layer of hydrophilic polyethylene glycol (PEG) has become standard practice for modifying surface chemistry.32 PEG coatings for nanoparticle surface modification are widely commercially available, may improve the solubility of the nanoparticles and in many cases have been shown to provide a degree of protection from rapid liver clearance by the reticular endothelial system.33 However, there has been some controversy about mild immunogenic properties of the synthetic polymer.34 Functionally, PEGylation does not confer circulation time to nanoparticles anywhere close to that of many native blood components. Alternative approaches, such as coating nanoparticles with red blood cell membranes have been proposed.35 Figure 4 shows an illustrative schematic diagram of such a process.
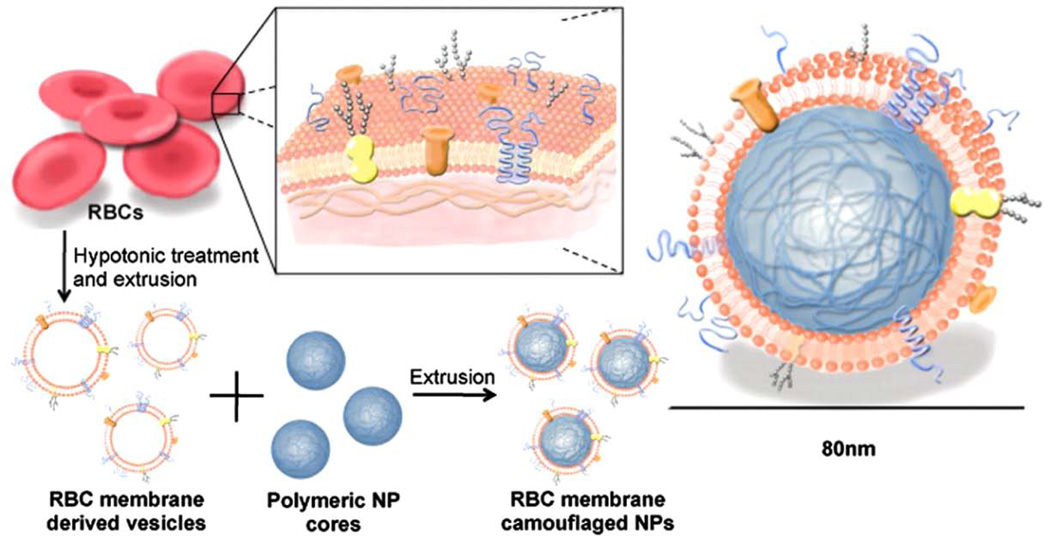
Figure 4.
Red blood cell (RBC)-membrane-coated polymeric nanoparticles. RBC membranes are isolated from the intracellular contents. The isolated RBC membranes are then fused to the polymeric nanoparticles with the aim of creating particles with increased circulation time. Reprinted with permission from reference35. Copyright 2008, National Academy of Sciences.
For drug nanocarriers, an additional consideration is that drugs must be made bioavailable in the target tissues. If the drug is immediately released from the nanocarrier following administration, the nanocarrier serves only as a solubilizing vehicle, which in some cases is sufficient. If the drug is released too slowly from the nanocarrier it may not be able to reach its molecular targets to exert any effect. Thus, an appropriate and defined release mechanism is desired. Biocompatible polymeric nanoparticles have been extensively developed for that purpose and can tune the release rate of drugs from the carrier with exquisite control.36 Other triggered release mechanisms can be achieved through designing nanoparticle which are responsive to certain environmental conditions such as pH, or through external stimuli such as heat.
The perfect nanoparticle would be able to target diseased locations without accumulating in healthy tissue. In practice, nanomedicines are far from this ideal. Like free drugs, nanoparticles which are developed to deliver drugs will induce side effects due to drug accumulation in healthy organs. Removal of the particles from the blood due to opsonization and uptake by the reticuloendothelial system is a major barrier.37 Additionally, the serum proteins that adsorb to nanoparticles when they are introduced in the body may significantly interfere with targeting or triggered release approaches.
Another challenge is one of bioavailability. That is having the drug in a form which can be toxic to the target cells. While nanoparticles can accumulate with large drug doses in some tumors, the drug often remains within the nanoparticles in the extracellular space outside of the cells. This is a challenge that may be overcome by a triggering mechanism which can release the drug from the nanoparticle at the target site or by engineering the nanoparticles to be more efficiently up-taken by cells. It is these barriers that many researchers aim to overcome when designing nanoparticles.
TARGETING MECHANISMS
Passive tumor targeting based on the EPR effect takes advantage of the abnormal vasculature and lack of lymphatic system in tumor tissue. Vessels in tumors are irregularly shaped and leaky due to the rapid growth of tumors, resulting in abnormal blood supply. In tumor vasculature, the size of the gap between leaky endothelial cells is in the range of 100–780 nm depending on the type of tumor38 as opposed to 5–10 nm in healthy vasculature.39 It seems likely that even with improvements, passive targeting will still only achieve a relatively modest proportion of drug deposition into tumor tissues, with most of the drug being taken up by other organs. While improved passive targeting strategies may greatly enhance drug efficacy, the concept of a “homing missile” that can specifically deliver nanoparticulate drug payloads to target sites remains a highly appealing yet elusive goal.
Active targeting involves functionalization of the surface of nanoparticles with receptor-specific agents such as small ligands, aptamers, peptides, and antibodies.40 Ideally, the molecular target should be overexpressed in the tissue of interest and minimally expressed in non-targeted tissue as well as possess a rapid internalization rate.41 Active targeting strategies were initially expected to deposit more drug in the targeted tissues and reduce off-target effects. A few targeted therapeutic agents have been clinically approved, with many currently undergoing clinical trials. Table 1 gives some examples of targeted therapeutic agents. However, numerous studies have shown that active nanoparticulate targeting strategies fail to dramatically increase the concentration of drugs in tumors and, in many cases, the biodistribution of the therapeutic is barely changed.42,43 This growing evidence suggests that the main factor that determines the accumulation of drugs in tumors is the EPR effect. However, it is important to note that in a number of cases active targeting has shown enhanced anti-tumor efficacy, including drug-loaded immunoliposomes.44 The mechanism for this enhanced efficacy often stems from altered and improved cellular internalization. Despite the challenges of active targeting, it is worth describing several commonly-used active targeting strategies due to their great potential.
Table 1.
Some examples of targeted therapeutic agents
| Name | Nanoplatform/agent | Status | Indications |
|---|---|---|---|
| Aurimune(CYT-6091) | Colloidal gold nanoparticles/rhTNF | Phase II | Solid tumors |
| BIND-014 | PMSA-targeted polymer nanoparticle containing docetaxel | Phase II | Non-small cell lung cancer, Prostate cancer |
| Cyclosert(CALAA-01) | Cyclodextrin siRNA | Phase Ib | Solid tumors |
| MBP-426 | Transferrin-targeted oxaliplatin | Phase IIa | Gastric and esophageal adenocarcinoma |
| Mylotarg | Anti-CD33-calicheamicin conjugate | Approved then withdrawn | Refractory acute myelogenous leukemia |
| Ontak | Interleukin 2 targeted diphtheria toxin fragment | Approved in 2008 | Cutaneous T-cell lymphoma (CTCL) |
| Rexin-G | Targeting retroviral vector microRNA-122 | Phase III (USA) | Sarcoma, osteosarcoma, pancreatic cancer, and other solid tumor |
| SGT-53 | Transferrin-targeted liposomes for p53 gene therapy | Phase I | Various cancers |
| CRLX101(IT-101) | Cyclodextrin camptothecin formulation | Phase II | Various cancers |
Integrins, which comprise a large family of membrane-bound dimer proteins, are expressed on the blood vessels of tissues affected by vascular disorders, angiogenesis, wounds, and other conditions.45,46 The tripeptide motif of arginine-glycine-aspartic acid (RGD) binds to αvβ3-integrins that are expressed in newly forming vasculature and has been used extensively in targeting applications. For example, drug-loaded, RGD-conjugated polymers and liposomes have been shown to target tumor angiogenic vasculature with higher biodistribution and efficacy than the untargeted versions.47,48
Epidermal growth factor receptors (EGFR) are transmembrane proteins that are expressed in many cancers and promote solid tumor growth.49 EGFR is a family of receptors that comprises four members including EGFR, human epidermal growth factor receptor-2(HER-2), HER-3 and HER-4. HER-2, the second member of the EGFR family, has been extensively researched for antibody-targeting drug delivery. For instance, immunoliposomes were developed combining anti-HER2 monoclonal antibodies (mAbs) with the pharmacokinetics of sterically stabilized liposomes.44 The enhanced anti-tumor efficacy primarily occurred through improved cellular internalization, as opposed to better overall tumor biodistribution.
Transferrin, an iron-carrying blood plasma glycoprotein, has been conjugated to nanoparticles to enhance cellular internalization via endocytosis.50 The transferrin receptor is expressed in many types of tumors. For instance, CALAA-01 is a cyclodextrin-based, transferrin-targeted nanoparticle that has progressed to human clinical trials for siRNA delivery.51 Similar to EGFR-targeting, administration of transferrin-targeted gold nanoparticles showed that the targeting agent does not change nanoparticle biodistribution, but enhances intracellular delivery.52
Folate receptors are overexpressed in approximately 40 % of human cancers and are one of the most intensively investigated targeting ligands.53 Folate is an important metabolite for nucleobase synthesis. Conjugation of carboxylic acid of folic acid does not prevent recognition by the folate receptor, which has enabled many different targeting approaches. Folate targeted, doxorubicin-loaded polymeric micelles were shown to be more effective than untargeted ones and also modestly enhanced tumor biodistribution.54 60 nm paclitaxel and folate-conjugated nanoparticles did not deliver significantly more drug to tumors but efficacy was enhanced compared to non-targeted nanoparticles.55
NANOCARRIERS
Research in developing nanoparticles as delivery vehicles and imaging agents are ever increasing. Among the most commonly used nanoparticles are liposomes, polymer-drug conjugates, polymeric nanoparticles, micelles, carbon nanotubes, and quantum dots. Some nanoparticles have been successfully clinically applied and many more are currently being clinically evaluated. Table 2 lists representative nanomedicines that are currently approved. Table 3 lists some representative nanomedicines currently in clinical trials. Note that monoclonal antibodies are not included in these lists, although they could be considered to be a nanomedicine based on their size. It is likely that next generation nanoparticles will build upon the existing foundation of current generation of nanoparticles that have progressed to the clinic.
Table 2.
Some examples of nanomedicines on the market
| Name | Nanomedicine | Status | Indications |
|---|---|---|---|
| Abraxane | Albumin-bound paclitaxel nanoparticles | Approved in 2005 | Metastatic breast and pancreatic cancer |
| Ambisome | Liposomal amphotericin B | Approved in 1997 | Fungal infections |
| Daunoxome | Liposomal daunorubicin | Approved in 1996 | Kaposi's sarcoma |
| DepoDur | Liposomal morphine | Approved in 2004 | Post-surgical pain relief |
| Doxil | Liposomal doxorubicin | Approved in 1995 | Ovarian cancer, Kaposi's Sarcoma |
| Genexol-PM | Polymeric micelles with paclitaxel | Available in Asian countries | Breast and lung cancers |
| Myocet | Liposomal doxorubicin (no PEGylation) | Available in Canada and Europe | Metastatic breast cancer |
| Neulasta | PEG-granulocyte colony-stimulating factor(PEG-CSF) | Approved in 2002 | Febrile neutropenia |
| Oncaspar | PEG-L-asparaginase | Approved in 1994 | Lymphocytic leukemia, Non-Hodgkin's lymphoma |
| PEGASYS | PEG-interferon α2a | Approved in 2002 | Hepatitis C |
| PEGIntron | PEG-interferon α2b | Approved in 2001 | Hepatitis C |
| Visudyne | Liposomal verteporfin | Approved in 2000 | Age-related macular degeneration |
Table 3.
Some examples of nanomedicines undergoing clinical trials
| Product/agent | Nanoplatform/agent | Status | Indications |
|---|---|---|---|
| Combidex | Iron oxide nanoparticles | Phase III | Tumor imaging |
| CPX-1 | Liposomal Irinotecan:Floxuridine | Phase II | Colorectal cancer |
| CPX-351 | Liposoma cytarabine and daunorubicin | Phase III | Acute myeloid leukemia |
| CRLX101(IT-101) | Cyclodextrin NPs/Camptothecin | Phase II | Various cancers |
| S-CKD602 | PEGylated liposomal belotecan | Phase II | Various cancers |
| SP1049C | P-glycoprotein targeting pluronic (poloxamer) micelle formulation of doxorubicin | Phase III | Various cancers |
| VivaGel(SPL7013) | Lysine-based dendrimer | Phase III | Topical microbicide for prevention of HIV and HSV |
| ThermoDox | Heat-triggered liposomal Dox | Phase III | Breast cancer, primary liver cancer |
| LiPlaCis | PLA2 triggered liposomal cisplatin | Phase I stopped | Various cancers |
Liposomes
Liposomes are commonly used as nanomedicines due to their biocompatibility from being formed from lipids that are already found in the body. They are spherical vesicles composed of lipid bilayers which surround an aqueous core. They were first described by British haematologist Alec D Bangham in 1965.56 Liposomes can be used as carriers for administration of pharmaceutical drugs, with the hydrophilic drugs encapsulated in the aqueous core and hydrophobic drugs retained within the bilayers. As of 2012, 12 liposomal drugs have been approved and many more are in clinical trials. The first generation of liposomes had short circulation time due to rapid clearance by the reticuloendothelial (RES) system. RES is a part of the immune system and consists of phagocytic cells such as monocytes and macrophages located in liver, spleen, lymph nodes and bone marrow57. Incorporation of lipid-anchored PEG derivatives prolongs the circulating half-life of liposomes58. PEGylated liposomes, also named “stealth liposomes”, can reduce the uptake by RES because the long hydrophilic PEG chains act as a steric brush to suppress the clearance by RES. Liposomes have been examined in many different ways, including assessing the effects of size, dose, and surface charge on pharmacokinetic parameters and anti-tumor efficacy.59 In addition to liposomes, lipid-based micelle-like nanoparticles are viable carriers for therapeutic and imaging agents.60,61 One interesting approach is to use or mimic naturally-occurring lipoproteins nanoparticles for anti-cancer applications.62
Doxil, approved in 1995, was one of the first nano-drugs approved by United States food and drug administration (FDA) for treatment of HIV-related Kaposi’s sarcoma and was subsequently approved for the treatment of platinum-resistant ovarian cancer and multiple myeloma. Doxil exemplifies long-circulating and stable PEGylated liposomes that use active loading driven by a transmembrane ammonium sulfate gradient to stably incorporate the doxorubicin (loading efficacy higher than 90%).63 AmBisome is a unilamellar liposomal amphotericin B preparation for the systemic treatment of fungal infections.64 The liposomal formulation has prolonged circulation time after intravenous administration. AmBisome effectively reduced amphotericin B associated nephrotoxicity without loss in efficacy.65 DepoDur, approved by FDA in 2004, is an extended release multivesicular liposomal epidural morphine. The main advantage for DepoDur is its extended release property which reduces the frequency of dosing and more consistent serum concentrations. DepoDur has been increasingly used for treating acute postoperative pain without the use of infusions.66
A novel class of liposomes called porphysomes comprising of lipids which are made up of a single fatty acid side chain and a porphyrin group are recently developed.67 Porphyrins have a long history of use as theranostic agents.68 These liposomes can be assembled using different lipid compositions and tailored to different purposes. Amongst these uses are photothermal therapy (PTT)69, photodynamic therapy (PDT)70 and biophotonic imaging.71 Porphysomes have been shown to be effective at curing tumors as a PTT agent where the intact nanoparticles are used to generate heat. In contrast, they are also used as PDT agents in which the dissociated porphyrins are used to generate singlet oxygen. The ability of porphyrins to chelate various metals makes them suitable for use as contrast agents for magnetic resonance,72 and radionuclide imaging.73,74 They have also been shown to be excellent photoacoustic imaging contrast agents71 due to their high NIR absorption and high fluorescence quenching that results in conversion of absorbed light into heat, which is required to generate photoacoustic signals. It has also been show that porphyrin-lipid containing liposomes can entrap anticancer drugs and release them upon exposure to NIR irradiation.75 This is shown in Figure 5.
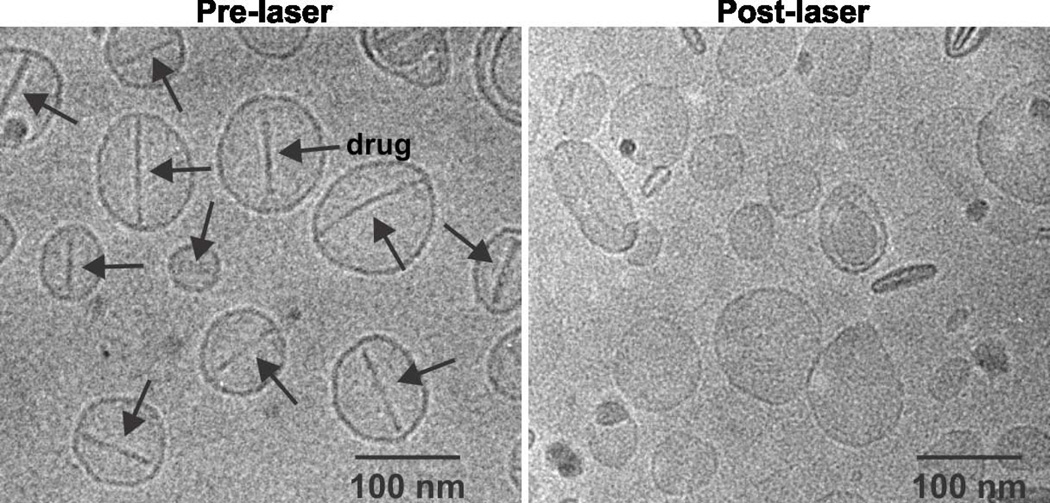
Figure 5.
Cryo-transmissino microscopy images of Dox–PoP-liposomes before and after light irradiation. Arrows indicate the presence of doxorubicin sulfate crystals. While the crystals are present in the “before” images they are not in the after images indicating dissolution of the crystals and release of the drug under light irradiation with minimal effect on the morphology of the nanoparticles. Reprinted with permission from reference 114. Copyright 2014, Macmillan Publishers.
Polymer-conjugated drugs
Polymer-drug conjugates form a well-established and clinically-successful class of nanomedicine. PEG, N-(2-Hydroxypropyl) methacrylamide (HPMA), polyglutamate (PGA) and dextrans are amongst the most frequently used hydrophilic polymers. Therapeutic proteins and small drugs can be conjugated with the hydrophilic polymers to increase circulation time, reduce immunogenicity, and enhance the therapeutic efficacy of the original drug. Polymer drug conjugate also have increased drug deposition in tumor compared with free drug due to the EPR effect. Currently there are several polymer-drug conjugates approved by FDA, especially protein therapeutics including PEG-asparaginase76, PEG-interferon α2a77, PEG-interferon α2b78, PEG-granulocyte colony-stimulating factor(PEG-CSF)79.
HPMA has been extensively studied. Typically, HPMA copolymers can be designed to be biodegradable by conjugation with a short linker peptide that can be degraded by the lysosomal thioldependent protease cathepsin. Many HPMA copolymer drug conjugates are in clinical trials including HPMA copolymer-doxorubicin80–82, HPMA copolymer-doxorubicin-galactosamine83, and HPMA copolymer-paclitaxel.84 Among them, HPMA copolymer-doxorubicin-galactosamine is notable because it contains galactosamine which can promote liver-targeting, through binding to the hepatic asialoglycoprotein receptor (ASGPR), which is highly expressed in normal hepatocytes. This is useful as targeting specific diseased organs could allow for the reduction of cytotoxcity to other organs and healthy tissue. However, in early clinical trials while targeting to the liver was observed, significant preferential uptake in the tumor was not. Polyglutamate and dextran conjugates such as polyglutamate-paclitaxel85, polyglutamate-camptothecin86, and dextran-doxorubicin87 have entered clinical trials.
Polymeric nanoparticles
Polymeric nanoparticles are developed by polymers encapsulating drugs into the polymer matrix. The most frequently used polymers used to formulate polymeric nanoparticles are poly (lactic-co-glycolic acid (PLGA), polylactides(PLA) and polycaprolactone(PCL) due to their biodegradability and biocompatibility. The release rate can be tuned from days to months by changing the ratio between lactide and glycolide. Several polymeric nanoparticles are on the market. Genexol-PM is a polymeric formulation of paclitaxel.88,89 Paclitaxel is effective for a wide range of cancers. However, due to its hydrophobicity, paclitaxel requires the use of solubilizing agents such as Cremophor EL which can cause serious hypersensitivity reactions and compromises the therapeutic value of paclitaxel. Polymeric paclitaxel addressed this issue and was approved in Asia for treatment of breast and lung cancers. Considering that polypeptides are polymers, Abraxane could be considered another polymeric paclitaxel formulation approved by FDA for treatment of breast cancer in 2005. It is a 130 nm, detergent-free and consists only of paclitaxel bound to human albumin, thus avoiding the toxicity and immunogenicity concerns of Cremophor. Binding between paclitaxel and albumin is noncovalent and reversible and allows for rapid release of the drug in vivo.90
BIND-01491 is a PEGylated, PLGA-based targeted polymeric nanoparticle formulation of docetaxel. It has completed phase I clinical trial and recently entered phase II clinical trial for non-small cell lung cancer, prostate cancer and metastatic castration-resistant prostate cancer. BIND-014 physically entraps docetaxel and is targeted to prostate-specific membrane antigen (PSMA) expressed on prostate cancer cells and the vasculature of most non-prostate solid tumors. Clinical trials have indicated that the BIND-014 is safe and has strong anti-tumor activity.
Pluronics are large, mass-produced triblock copolymers of polypropylene oxide and PEG that are relatively well defined. SP1049C is a pluronic micelle formulation of doxorubicin that targets P-glycoprotein. Doxorubicin is noncovalently incorporated into micelles with the pluronic block copolymers (Pluronic L61 and Pluronic F127). Pluronic L61 has been shown to enhance drug uptake in multi-drug resistant (MDR) cells with high expression of P-glycoprotein.92,93 The depletion of ATP is significant as the mechanisms responsible for multidrug resistance are energy dependent.
A family of novel pluronic nanoparticles called nanonaps was recently developed that are self-assembled with extremely hydrophobic naphthlocyanines (Nc) dyes.94 Unlike conventional micelles, nanonaps are kinetically stable and form frozen micelles that can be purified and concentrated to high dye concentrations. They withstood the harsh conditions in gastrointestinal (GI) tract and safely passed through it without systemic absorption, demonstrating that they can be used for safe gastrointestinal imaging. It is likely that this approach can be applied for forming frozen drug micelles.
Dendrimers
Dendrimers are a relatively new class of polymeric materials. Different from the polymeric nanoparticles which are formed from linear polymers, dendrimers are highly branched macromolecules with a high degree of surface functionality and versatility. Dendrimers have well-defined chemical structure and can vary in size from 5–100 nm. Drugs can be covalently conjugated to the surface of the dendrimers or physically entrapped in the interior of the core.95 Most dendrimers used for drug delivery are covalently conjugated with drugs to create a precisely-defined nanomedicine, which is the fundamental advantage of dendrimers. Additionally, drugs that are physically entrapped into dendrimer cores can easily leak out when exposed to biological fluids before they reach the intended sites. Dendrimers can be conjugated with many different functional moieties such as imaging agents and targeting moieties in addition to drugs due to their highly multivalent surface area.
The manufacturing process of dendrimers involves a series of repetitive steps starting with a central initiator core. Each growth step represents a new generation of polymer with a larger molecular diameter. Poly(amidoamine) (PAMAM), polypeptide and polyester can be built into dendrimers.96 Among them, PAMAM dendrimer is the most extensively investigated dendrimer. Surface modified PAMAM dendrimers are generally non-immunogenic, water soluble and possess terminal modifiable amine functional groups.
VivaGel is a topical dendrimeric microbicide for prevention of HIV.97 A phase I clinical trial demonstrated that VivaGel was generally safe and well tolerated.98 VivaGel contains a highly charged polyanionic surface to attach to targets on viruses, preventing virus attachment and/or absorption thus prevent infection.
Inorganic nanoparticles
Whereas most of the nanoparticles that have been translated to the clinic have been of organic nature, the field of nanotechnology has shown most interest in inorganic materials due to their fascinating optical and physical properties. Many of these have been explored for use as nanomedicines with some examples of progression to early-stage clinical trials. Safety is a concern since many inorganic nanoparticles are formed from heavy metal ions with known toxicities and the particles may also be non-degradable and persistent in the body.99
Gold nanoparticles are versatile with a wide range of applications from use as delivery vectors, imaging agent, and photothermal therapeutic agents.100 Gold nanoparticles comprise an inert gold core and a surface that is readily modified via sulphur-gold linkages. Depending on their shape, gold nanoparticles exhibit plasmon-resonance that converts NIR light to heat, and can be engineered to remotely trigger drug release101 and have been used for photothermal ablation of cancer.102 Varying shape and size allow for tunable properties such as absorbance, which can be tailored to specific applications such as photothermal therapy for which the use of particles with absorbance in the near infrared range of the spectrum is desired.103
Gold nanoparticles have been used for targeted drug applications. Recombinant human tumor necrosis factor alpha was bound to the surface of PEGylated colloidal gold particles. Pre-clinical tests showed rapid tumor accumulation following intravenous injection, with little accumulation in the liver and spleen, likely due to the small size (27 nm) and RES-avoiding properties.104 With promising pre-clinical results it progressed to clinical trials under the name Aurimune (CYT-6091).105 Phase I clinical trial results indicated that CYT-6091 was well tolerated and show preferential uptake at the tumor site.105 Currently CYT-6091 is undergoing phase II clinical trials.
Iron oxide magnetic nanoparticles have excellent biocompatibility and have been approved for use as imaging agents. Iron oxide nanoparticles generally have a core-shell structure, an iron oxide core composed of magnetite or maghemite, a hydrophilic shell, usually composed of starch, PVA or dextrin. They typically exhibit superaramagnetism. Magnetic nanoparticles have been used as contrast agents for magnetic resonance imaging (MRI) and as heat mediators for cancer hyperthermia therapy.106 Magnetic iron oxide nanoworms are elongated, dextran-coated particles composed of a linear aggregate of 5–10 iron oxides nanoparticles (50–80 nm).20 Different from the spherical shaped nanoparticles that exhibit a high uptake by phagocytes, nanoworms with a linear shape revealed a lower uptake by phagocytes and have long circulating half-lives of 18 hours. The elongated structure of the nanoworms also enhances the net magnetization and magnetic resonance contrast.
Quantum dots are nanometer-sized fluorescent semiconductor nanocrystals and can be modified to be water soluble and biocompatible.107 Quantum dots are well known for their wide range of excitation spectra and narrow, symmetrical and tunable emission spectra.108 They have been extensively studied for bio-imaging due to their superior brightness and photostability compared to organic dyes.109 They can be conjugated with many biological targets including antibodies, proteins and nucleic acids for immunohistochemistry and in situ hybridization.110 It has been shown that RGD-labeled quantum dots can effectively be used for cancer imaging in vivo.107
Carbon nanotubes (CNT) are emerging as a unique drug delivery system. Carbon nanotubes are members of the fullerene family. SWNT and multiple-walled carbon nanotubes are the two main types of carbon nanotubes (MWNT). SWNT are composed of a single layer graphite sheet while MWNT possess several graphite concentric layers. Most SWNTs have a diameter of approximately 1 nm while the length can vary to several hundreds or thousands of nanometers.15 For MWNTs, the diameter varies from 1.5 to 100 nm with a length that generally ranges from 1 to 50 µm. Carbon nanotubes are insoluble in water but can be made to dissolve by covalent or noncovalent functionalization. Their hydrophobicity also enables simple drug-loading via adsorption of drugs. CNTs demonstrate high tensile strength, excellent chemical and thermal stability, electrical and optical properties which make them intriguing nanomaterials for a wide range of applications including the use of carbon nanotubes as ion channel blockers111, nanovectors for the delivery of therapeutics112 and biosensors.113 As CNT are relativity new to biotechnology, much about them remains to be studied especially their long-term safety and biocompatibility.114
STIMULATED DRUG RELEASE
There are at least two types of stimulated drug release; environmentally-triggered release and externally-triggered release. Environmental release occurs when local stimuli, such as pH causes the nanoparticles to release their contents. Externally-triggered release occurs when an external stimulus, such as applied heat or light induces release of entrapped contents. Both concepts allow for the release of drug at a target site, the main difference being that external release mechanisms offer more control, for on-demand release. However, external release mechanisms also are significantly more difficult to implement are limited to treating localized conditions such as a problematic primary tumor as opposed to the metastatic disease.
pH triggering
pH-triggered release can be subdivided into three categories, orally deliverable drugs, tissue level mechanisms, and cellular level mechanisms.115 In the case of orally delivered drugs, the goal is often to encapsulate the drugs so they pass through the acidic conditions of the stomach without degradation, and then release into the higher pH environments of the duodenum, and other parts of the gastrointestinal (GI) tract. In this case drug release is achieved by pH dependent swelling, dissolution, or changes in surface charge.116 One potential application for this system is through oral administration of insulin loaded nanoparticles. Orally administered insulin loaded polymer-based nanoparticles have been shown to be able to protect their contents through the stomach and deliver them into the intestines117. However, getting the insulin from the intestines into the blood stream remains a challenge as enzymes in the intestines will degrade the nanoparticles and insulin as well. Additionally, the doses required to reduce blood glucose levels for orally administered insulin are significantly higher than required for injected insulin (30–100 IU/kg vs 1 IU/kg)118.
Tissue level mechanisms are related to the Warburg effect, whereby the tumor environment exhibits a pH value of 0.5–1 lower than physiologically normal tissues.119,120 Due to hypoxic conditions, tumor cells switch to anaerobic respiration and generate excessive lactic acid which causes the more acidic conditions. Nanoparticles are engineered to become destabilized and release their drug content at this reduced pH120–122. This may be achieved by designing nanoparticles such as polymeric micelles which dissociate under the mild acidic conditions of the tumor environment122, or pH induced swelling115.
In cellular level mechanisms, release of the drug occurs after the nanoparticles have been uptaken by cells. Following endocytosis, the nanoparticle are subjected to an acidic pH environments of 5–6.5 in endosomes and 4–5 in lysosomes123. Drug release is achieved similarly to the other pH mechanisms with release being induced by swelling, dissolution or acid induced bond cleavage of the carrier as well as destabilization of the endosomal membranes.120,121 pH sensitive liposomes have also been developed extensively by the Szoka group that are activated during endocytic uptake based on changes in charge that occur in the acidifying endosomal and lysosomal environments.124
It is possible for multiple pH targeting strategies to be used concurrently. TAT peptide-based micelles are an example. They are polymer based micelles to which are connected a PEG conjugated TAT complex. This complex at physiological pH (7.4) is shielded by formation of a complex with a copolymer of PEG and poly(methacryloyl sulfadimethoxine) (PSD). Under the mild acidic conditions of the tumor environment the PSD shielding complex dissociates leaving the TAT exposed.125 TAT, a HIV derived non-specific cell penetrating peptide, increases the uptake of the micelles through endocytosis. Following endocytosis, the micelles disintegrate in the low pH environment, releasing entrapped doxorubicin within the endosomes. This system has been shown to suppress tumor growth in mice.126
Enzymatic triggering
Enzymatic degradable nanoparticles work by releasing their encapsulated contents when exposed to the enzymes found at the target site. These delivery systems can be designed to be responsive to many different enzymes. This approach has been applied extensively to functional imaging probes.127,128 For example, nanoparticles made with peptide linkages may be degraded by proteases while those made with phospholipids can be degraded by lipases129. This system has the potential to induce minimal activation while the nanoparticles are in circulation in the blood. However, as many enzymes can be found in both healthy and diseased cells, the use of enzymes must be complemented by a specific targeting strategy or the use of enzymes which are present at greater levels in the diseased cells.120,129,130
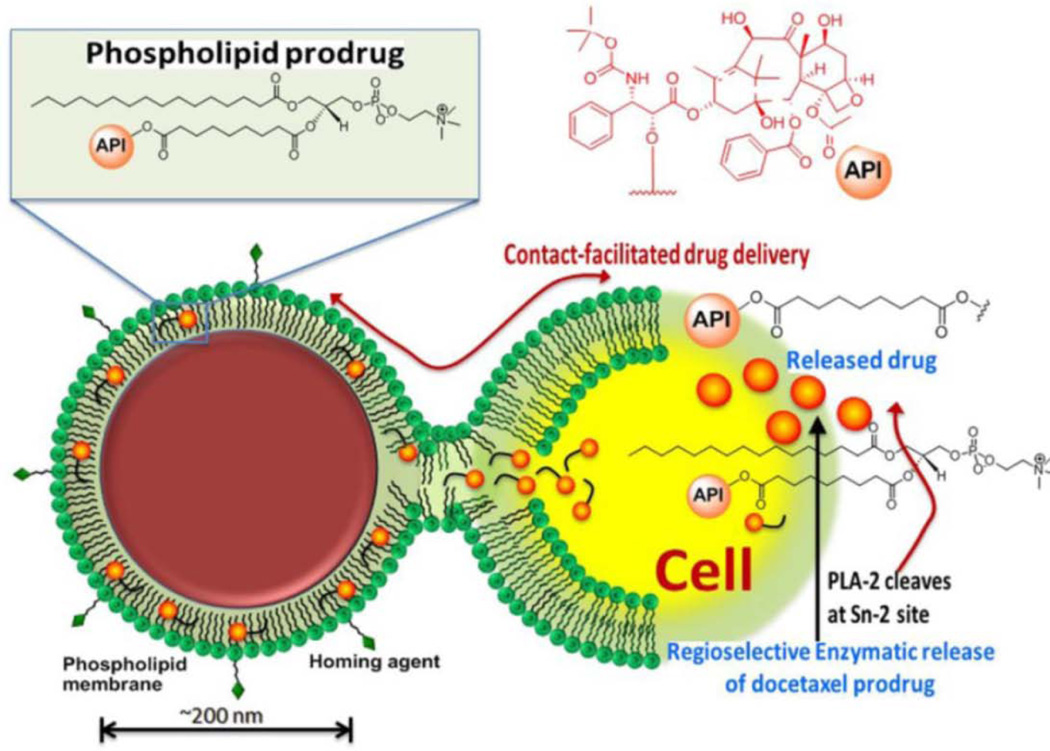
An example of the application of this mechanism is liposomes designed to be degraded by secretory phospholipase a2 (sPLA2). sPLA2 is a lipid hydrolyzing enzyme which is prevalent in the extracellular space of tumors. The responsiveness of liposomes to sPLA2 can by adjusted by altering the lipid composition. Cisplatin loaded sPLA2 responsive liposomes were shown to effectively suppress tumor growth in nude mouse xenographs131. Hydrophobic drugs may also be conjugated directly to the hydroxyl group normally occupied by the lipid fatty acid sidechain. At the target tissue, lipases may then cleave and liberate the drug. Phospholipid-fused porphyrins132, mycotoxins133, and taxanes have all been assembled into nanoparticles for this lipase activated mechanism134. This concept is illustrated in Figure 6, with a docetaxel-phospholipid prodrug.
Figure 6.
Schematic of lipase-cleavable docetaxel prodrug concept. In this system, a lipophilic enzymatically cleavable prodrug is entrapped in the phospholipid layer of the nanoparticle. The nanoparticle is targeted to cells through contact facilitated drug delivery where the phospholipid layer of the nanoparticle fuses with the cell membrane. The prodrug is then transferred into the cell where in undergoes enzymatic cleavage. Reprinted with permission from reference134. Copyright 2014, Ivyspring.
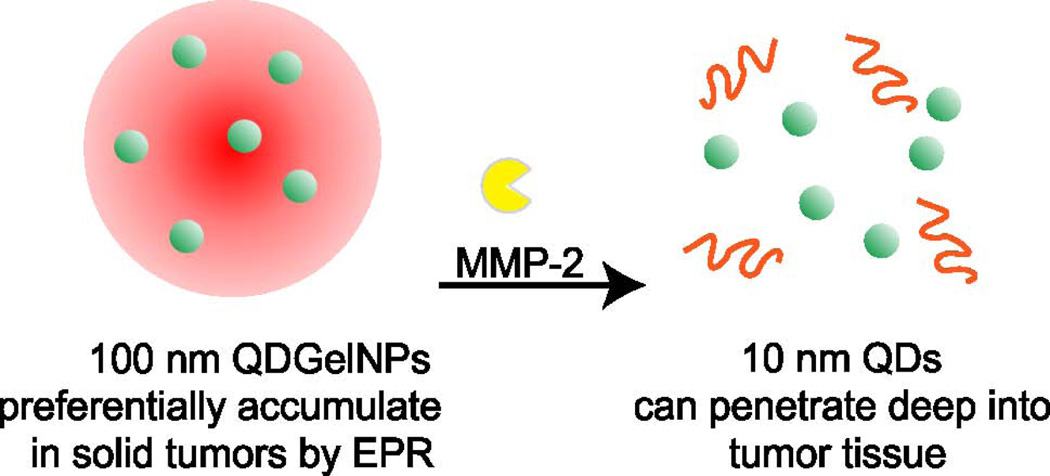
Another family of enzymes that are linked to cancers are the matrix metalloproteinases (MMPs). These proteases degrade the extracellular matrix, thus enabling the spread of tumor cells. One interesting approach developed 100 nm nanoparticles that themselves contained smaller nanoparticles that could be released upon cleavage of the larger nanoparticle by MMP-2.135 This concept is shown in Figure 7. In this way, the larger nanoparticle can effectively accumulate in the tumor via the EPR effect and upon proteolytic cleavage the smaller nanoparticles are released and can deeply penetrate the tumor.
Figure 7.
Protease-activated drug delivery. Multistage quantum dot gelatin nanoparticles (QDGelNPs) experience a size reduction through cleavage of their gelatin scaffold by MMP-2, a protease which is highly expressed in tumors. Reprinted with permission from reference135. Copyright 2011, National Academy of Sciences.
Heat triggering
Heat-triggered release typically involves heating drug encapsulated nanoparticles such as liposomes or polymer-based nanoparticles to a point at which entrapped drug becomes released. Generally these involves the use of an external heat source to induce a change in the nanoparticle which makes them permeable136,137. In the case of liposomes, for example, heating above a critical transition temperature, causes the liposome bilayer to change from a ridged crystalline phase to a more fluid liquid crystalline phase138. While this is often achieved by applying an external source of heat, other techniques have been developed which uses alternative external stimuli such as magnetic fields, and light irradiation, along with entrapped nanoparticles capable of generating heat139,140. In order for these systems to be clinically applicable they need to meet two key requirements, first the drug should be release quickly upon application of the stimuli; Second release should occur at temperature slightly above body temperature (39–40 °C) which is considered to be mild hyperthermia since at more elevated temperatures vascular shutdown occurs.141,142
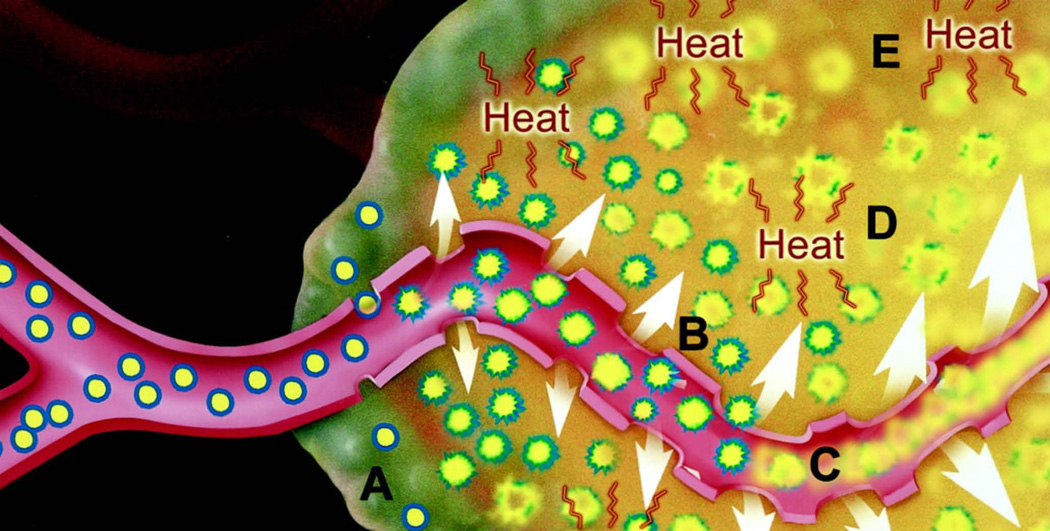
Of these techniques, the use of an external heat source with a special liposomal formulation, Thermodox, has been successful in advancing through phase III human clinical trials. Thermodox is a liposomal formulation of doxorubicin with rapid release of the drug under mild hyperthermia conditions. It is currently being clinically studied for the treatment of colorectal, breast, and liver cancers with one phase III clinical trial for primary liver cancer having been completed143,144. In addition to drug release from the liposomes at elevated temperatures, Thermodox seeks to take advantage of the therapeutic effects of hyperthermia itself. This includes increasing blood flow and tumor vessel permeability to nanoparticles142. In the clinical trials the liposomes were combined with radiofrequency ablation therapy, which itself kills tumors by heating them to elevated temperatures. In these trials the goal was for the liposomes to treat the cancer cells on the perimeter of the ablation zone where the temperature would be high enough to induce release but not sufficient to kill the cells on its own145. Figure 8 shows a schematic representation of the Thermodox activation mechanism.
Figure 8.
Heat-triggered drug release. Specially-designed Thermodox liposomes extravasate into the tumor through pores in leaky tumor blood vessels (A). Hyperthermia increases the blood vessel pore sizes (B). Hyperthermia triggers drug release from the liposomes in both the tumor blood vessels (C) and the tumor tissue (D). Hyperthermia itself can also be toxic to cancer cells (E). Reprinted with permission from reference 78. Copyright 2000, American Association for Cancer Research.
Magnetic triggering
Magnetic triggered release can be achieved by two methods; through the use of heat generating particles such as iron oxide, or through mechanical mechanisms. Though a few mechanical mechanisms have been demonstrated these have not been as well studied as the heat based mechanisms. A mechanism has been demonstrated in which release from nanospheres is induced by a high-frequency magnetic field (HFMF) which cause vibrations rupturing the shell of the particle.140,146The heat-triggered mechanisms involve entrapping heat generating particles in a thermoresponsive nanoparticle. Upon the application of an external magnetic field the particles generate heat which induces drug release from the nanoparticles.147–150 This system works similarly to the heat-triggered release system above except that the source of the heat is localized to the nanoparticles.
This has been demonstrated with magnetoliposomes, in which iron oxide particles were entrapped within liposomes and loaded with doxorubicin147. In this case, liposomes with a release temperature of 42°C were used and maximum drug release was achieved after 6 minutes. Heating of the bulk solution was minimal, though dependent on the concentrations used. While this demonstrates release can occur in the absence of significant heating, heating can also be beneficial. Heat based treatments in which magnetic nanoparticles are used to induce hyperthermia are currently being clinically evaluated.151
Ultrasonic triggering
Ultrasound has been shown to be able to release the contents from nanoparticles. This is achieved typically due to cavitation induced under ultrasound irradiation152–155, though ultrasound heat mediated release mechanisms also exist.156 In this system the ultrasound causes the formation of vapor bubbles which permeabilize the nanoparticle, allowing the entrapped drug to be released. One advantage of this system is that ultrasound is noninvasive, however, it can also cause cellular damage.154,155
This has been shown to be effective in vivo with cisplatin loaded liposomes and low frequency ultrasound (LFUS). In this study a stealth formulation of cisplatin liposomes which have been shown to suffer from poor bioavailability due to slow release kinetics were used with LFUS to treat C26 tumors on the footpad of BALB/c mice. The results showed that the combination of the liposomes and LFUS improves the effectives of the liposomes due to the increase of the bioavailability of the liposomes157.
Light triggering
Many nanotechnology-based mechanisms involving light activation have been developed.158 Photochemical mechanisms and heat related mechanisms are the two main categories. Photochemical mechanisms involve light induced chemical reactions which lead to the permeblization of the nanoparticles. These include reactions such as photooxidation, photoisomerization, and photocleavage. The heat related mechanisms work similarly to the magnetic-triggered release in that light sensitive heat generating particles such as gold nanoparticles are entrapped within the nanoparticles. When the nanoparticles are treated with light, the heat generating particles generate heat and causes release of the entrapped contents due to thermally induced permeability. More novel methods for lights-triggered release have also been shown. For example, the use of gold nanoparticle tethered liposomes has been shown to release the contents through cavation similar to the ultrasound mechanism101, and the use of channel proteins embedded within liposomes which open upon laser irradiation159.
For light-triggered release to be viable clinically, the wavelengths of light used would optimally be in the near infrared range (NIR) of the spectrum as this is the most biologically compatible range. There are two primary reasons for this, first NIR light provides better tissue penetration than ultraviolet light (UV) on the other end of the spectrum. Secondly UV light poses phototoxity to healthy tissue, therefore may not be safe. In addition, many photochemical mechanisms also tend to produce toxic reactive species, making them unlikely to be widely used. Methods which rely on photo-physical mechanisms which are activated in the NIR range are appealing since they may not have as many potential phototoxicity risks.139,160,161
Conclusion
The unique properties of carefully designed nanomedicines hold potential for the treatment of diseases. The goal of nanomedical engineering is to develop nanoparticles that migrate to where they are intended to go and exert therapeutic effect there. This may be achieved by minimizing removal from the body by physiological barriers and immune system. Currently, the nanomedicines that have been clinically approved generally are formed from relatively simple, rather than complex formulations. However, the potential payoff of targeted and triggered delivery is high enough to warrant development of more advanced systems. In addition to innovating new and potentially revolutionary materials and approaches for nanomedicines, it is imperative for the success of the field that future works focus on determining how to improve quantitative therapeutic biodistribution and bioavailability to target tissues. Collective and quantitative data is required to better elucidate which strategies hold the most potential for further research investment. From a clinical perspective, it is expected that nanomedical engineering will bring an increasing number of unique treatments into early stage clinical trials for evaluation with hopes of better disease treatments and outcomes.
ACKNOWLEDGMENTS
This work was made possible with support from the National Institutes of Health (R01EB017270 and DP5OD017898) and the Korean Ministry of Science, ICT and Future Planning (IT Consilience Creative Program, NIPA-2013-H0203-13-1001).
References
- 1.Kim BYS, Rutka JT, Chan WCW. Nanomedicine. N Engl J Med. 2010;363:2434–2443. doi: 10.1056/NEJMra0912273. [DOI] [PubMed] [Google Scholar]
- 2.Agasti SS, Rana S, Park M-H, Kim CK, You C-C, Rotello VM. Nanoparticles for detection and diagnosis. Adv Drug Deliv Rev. 2010;62:316–328. doi: 10.1016/j.addr.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 4.Mulder WJM, Jaffer FA, Fayad ZA, Nahrendorf M. Imaging and Nanomedicine in Inflammatory Atherosclerosis. Sci Transl Med. 2014;6:239sr1–239sr1. doi: 10.1126/scitranslmed.3005101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pison U, Welte T, Giersig M, Groneberg DA. Nanomedicine for respiratory diseases. Eur J Pharmacol. 2006;533:341–350. doi: 10.1016/j.ejphar.2005.12.068. [DOI] [PubMed] [Google Scholar]
- 6.Wagner V, Dullaart A, Bock A-K, Zweck A. The emerging nanomedicine landscape. Nat Biotechnol. 2006;24:1211–1217. doi: 10.1038/nbt1006-1211. [DOI] [PubMed] [Google Scholar]
- 7.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Controlled Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 8.Dong X, Mattingly CA, Tseng MT, Cho MJ, Liu Y, Adams VR, et al. Doxorubicin and Paclitaxel-Loaded Lipid-Based Nanoparticles Overcome Multidrug Resistance by Inhibiting P-Glycoprotein and Depleting ATP. Cancer Res. 2009;69:3918–3926. doi: 10.1158/0008-5472.CAN-08-2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strickley RG. Solubilizing Excipients in Oral and Injectable Formulations. Pharm Res. 2004;21:201–230. doi: 10.1023/b:pham.0000016235.32639.23. [DOI] [PubMed] [Google Scholar]
- 10.Hamburg MA. FDA’s Approach to Regulation of Products of Nanotechnology. Science. 2012;336:299–300. doi: 10.1126/science.1205441. [DOI] [PubMed] [Google Scholar]
- 11.Venditto VJ, Szoka FC., Jr Cancer nanomedicines: So many papers and so few drugs! Adv Drug Deliv Rev. 2013;65:80–88. doi: 10.1016/j.addr.2012.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albanese A, Tang PS, Chan WCW. The Effect of Nanoparticle Size, Shape, and Surface Chemistry on Biological Systems. Annu Rev Biomed Eng. 2012;14:1–16. doi: 10.1146/annurev-bioeng-071811-150124. [DOI] [PubMed] [Google Scholar]
- 13.Choi HS, Ashitate Y, Lee JH, Kim SH, Matsui A, Insin N, et al. Rapid translocation of nanoparticles from the lung airspaces to the body. Nat Biotechnol. 2010;28:1300–1303. doi: 10.1038/nbt.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soo Choi H, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, et al. Renal clearance of quantum dots. Nat Biotechnol. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moghimi SM, Hunter AC, Murray JC. Nanomedicine: current status and future prospects. FASEB J. 2005;19:311–330. doi: 10.1096/fj.04-2747rev. [DOI] [PubMed] [Google Scholar]
- 16.Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol. 2010;7:653–664. doi: 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perrault SD, Walkey C, Jennings T, Fischer HC, Chan WCW. Mediating Tumor Targeting Efficiency of Nanoparticles Through Design. Nano Lett. 2009;9:1909–1915. doi: 10.1021/nl900031y. [DOI] [PubMed] [Google Scholar]
- 18.Geng Y, Dalhaimer P, Cai S, Tsai R, Tewari M, Minko T, et al. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nanotechnol. 2007;2:249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Z, Cai W, He L, Nakayama N, Chen K, Sun X, et al. In vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in mice. Nat Nanotechnol. 2007;2:47–52. doi: 10.1038/nnano.2006.170. [DOI] [PubMed] [Google Scholar]
- 20.Park J-H, von Maltzahn G, Zhang L, Schwartz MP, Ruoslahti E, Bhatia SN, et al. Magnetic Iron Oxide Nanoworms for Tumor Targeting and Imaging. Adv Mater. 2008;20:1630–1635. doi: 10.1002/adma.200800004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, Byrne JD, Napier ME, DeSimone JM. More Effective Nanomedicines through Particle Design. Small. 2011;7:1919–1931. doi: 10.1002/smll.201100442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steinmetz NF. Viral nanoparticles as platforms for next-generation therapeutics and imaging devices. Nanomedicine Nanotechnol Biol Med. 2010;6:634–641. doi: 10.1016/j.nano.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pinheiro AV, Han D, Shih WM, Yan H. Challenges and opportunities for structural DNA nanotechnology. Nat Nanotechnol. 2011;6:763–772. doi: 10.1038/nnano.2011.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perrault SD, Shih WM. Virus-Inspired Membrane Encapsulation of DNA Nanostructures To Achieve In Vivo Stability. ACS Nano. 2014;8:5132–5140. doi: 10.1021/nn5011914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turner MR, Clough G, Michel CC. The effects of cationised ferritin and native ferritin upon the filtration coefficient of single frog capillaries. Evidence that proteins in the endothelial cell coat influence permeability. Microvasc Res. 1983;25:205–222. doi: 10.1016/0026-2862(83)90016-x. [DOI] [PubMed] [Google Scholar]
- 26.Dellian M, Yuan F, Trubetskoy VS, Torchilin VP, Jain RK. Vascular permeability in a human tumour xenograft: molecular charge dependence. Br J Cancer. 2000;82:1513–1518. doi: 10.1054/bjoc.1999.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lv H, Zhang S, Wang B, Cui S, Yan J. Toxicity of cationic lipids and cationic polymers in gene delivery. J Controlled Release. 2006;114:100–109. doi: 10.1016/j.jconrel.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 28.He C, Hu Y, Yin L, Tang C, Yin C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials. 2010;31:3657–3666. doi: 10.1016/j.biomaterials.2010.01.065. [DOI] [PubMed] [Google Scholar]
- 29.Liu X, Li H, Chen Y, Jin Q, Ren K, Ji J. Mixed-Charge Nanoparticles for Long Circulation, Low Reticuloendothelial System Clearance, and High Tumor Accumulation. Adv Healthc Mater. 2014 doi: 10.1002/adhm.201300617. n/a–n/a. [DOI] [PubMed] [Google Scholar]
- 30.Xiao W, Lin J, Li M, Ma Y, Chen Y, Zhang C, et al. Prolonged in vivo circulation time by zwitterionic modification of magnetite nanoparticles for blood pool contrast agents. Contrast Media Mol Imaging. 2012;7:320–327. doi: 10.1002/cmmi.501. [DOI] [PubMed] [Google Scholar]
- 31.Walkey CD, Chan WCW. Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chem Soc Rev. 2012;41:2780. doi: 10.1039/c1cs15233e. [DOI] [PubMed] [Google Scholar]
- 32.Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov. 2003;2:214–221. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 33.Owens DE, III, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm. 2006;307:93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 34.Schellekens H, Hennink WE, Brinks V. The Immunogenicity of Polyethylene Glycol: Facts and Fiction. Pharm Res. 2013;30:1729–1734. doi: 10.1007/s11095-013-1067-7. [DOI] [PubMed] [Google Scholar]
- 35.Hu C-MJ, Zhang L, Aryal S, Cheung C, Fang RH, Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci. 2011;108:10980–10985. doi: 10.1073/pnas.1106634108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hans ML, Lowman AM. Biodegradable nanoparticles for drug delivery and targeting. Curr Opin Solid State Mater Sci. 2002;6:319–327. [Google Scholar]
- 37.Moghimi SM, Hunter AC, Andresen TL. Factors Controlling Nanoparticle Pharmacokinetics: An Integrated Analysis and Perspective. Annu Rev Pharmacol Toxicol. 2012;52:481–503. doi: 10.1146/annurev-pharmtox-010611-134623. [DOI] [PubMed] [Google Scholar]
- 38.Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, et al. Regulation of transport pathways in tumor vessels: Role of tumor type and microenvironment. Proc Natl Acad Sci. 1998;95:4607–4612. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haley B, Frenkel E. Nanoparticles for drug delivery in cancer treatment. Urol Oncol Semin Orig Investig. 2008;26:57–64. doi: 10.1016/j.urolonc.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 40.Byrne JD, Betancourt T, Brannon-Peppas L. Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv Drug Deliv Rev. 2008;60:1615–1626. doi: 10.1016/j.addr.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 41.Allen TM. Ligand-targeted therapeutics in anticancer therapy. Nat Rev Cancer. 2002;2:750–763. doi: 10.1038/nrc903. [DOI] [PubMed] [Google Scholar]
- 42.Kirpotin DB, Drummond DC, Shao Y, Shalaby MR, Hong K, Nielsen UB, et al. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res. 2006;66:6732–6740. doi: 10.1158/0008-5472.CAN-05-4199. [DOI] [PubMed] [Google Scholar]
- 43.Pirollo KF, Chang EH. Does a targeting ligand influence nanoparticle tumor localization or uptake? Trends Biotechnol. 2008;26:552–558. doi: 10.1016/j.tibtech.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 44.Park JW, Hong K, Kirpotin DB, Colbern G, Shalaby R, Baselga J, et al. Anti-HER2 Immunoliposomes Enhanced Efficacy Attributable to Targeted Delivery. Clin Cancer Res. 2002;8:1172–1181. [PubMed] [Google Scholar]
- 45.Niu G. Why Integrin as a Primary Target for Imaging and Therapy. Theranostics. 2011;1:30–47. doi: 10.7150/thno/v01p0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruoslahti E. Rgd and Other Recognition Sequences for Integrins. Annu Rev Cell Dev Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 47.Mitra A, Mulholland J, Nan A, McNeill E, Ghandehari H, Line BR. Targeting tumor angiogenic vasculature using polymer–RGD conjugates. J Controlled Release. 2005;102:191–201. doi: 10.1016/j.jconrel.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 48.Dubey PK, Mishra V, Jain S, Mahor S, Vyas SP. Liposomes Modified with Cyclic RGD Peptide for Tumor Targeting. J Drug Target. 2004;12:257–264. doi: 10.1080/10611860410001728040. [DOI] [PubMed] [Google Scholar]
- 49.Nicholson RI, Gee JMW, Harper ME. EGFR and cancer prognosis. Eur J Cancer. 2001;37(Supplement 4):9–15. doi: 10.1016/s0959-8049(01)00231-3. [DOI] [PubMed] [Google Scholar]
- 50.Qian ZM, Li H, Sun H, Ho K. Targeted Drug Delivery via the Transferrin Receptor-Mediated Endocytosis Pathway. Pharmacol Rev. 2002;54:561–587. doi: 10.1124/pr.54.4.561. [DOI] [PubMed] [Google Scholar]
- 51.Davis ME, Zuckerman JE, Choi CHJ, Seligson D, Tolcher A, Alabi CA, et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464:1067–1070. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choi CHJ, Alabi CA, Webster P, Davis ME. Mechanism of active targeting in solid tumors with transferrin-containing gold nanoparticles. Proc Natl Acad Sci. 2010;107:1235–1240. doi: 10.1073/pnas.0914140107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Low PS, Kularatne SA. Folate-targeted therapeutic and imaging agents for cancer. Curr Opin Chem Biol. 2009;13:256–262. doi: 10.1016/j.cbpa.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 54.Yoo HS, Park TG. Folate receptor targeted biodegradable polymeric doxorubicin micelles. J Controlled Release. 2004;96:273–283. doi: 10.1016/j.jconrel.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 55.Wang X, Li J, Wang Y, Cho KJ, Kim G, Gjyrezi A, et al. HFT-T, a Targeting Nanoparticle, Enhances Specific Delivery of Paclitaxel to Folate Receptor-Positive Tumors. ACS Nano. 2009;3:3165–3174. doi: 10.1021/nn900649v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bangham AD, Horne RW. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J Mol Biol. 1964;8 doi: 10.1016/s0022-2836(64)80115-7. 660–IN10. [DOI] [PubMed] [Google Scholar]
- 57.Nie S. Understanding and overcoming major barriers in cancer nanomedicine. Nanomed. 2010;5:523–528. doi: 10.2217/nnm.10.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Klibanov AL, Maruyama K, Torchilin VP, Huang L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990;268:235–237. doi: 10.1016/0014-5793(90)81016-h. [DOI] [PubMed] [Google Scholar]
- 59.Drummond DC, Meyer O, Hong K, Kirpotin DB, Papahadjopoulos D. Optimizing Liposomes for Delivery of Chemotherapeutic Agents to Solid Tumors. Pharmacol Rev. 1999;51:691–744. [PubMed] [Google Scholar]
- 60.Constantinides PP, Chaubal MV, Shorr R. Advances in lipid nanodispersions for parenteral drug delivery and targeting. Adv Drug Deliv Rev. 2008;60:757–767. doi: 10.1016/j.addr.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 61.Mulder WJM, Strijkers GJ, van Tilborg GAF, Griffioen AW, Nicolay K. Lipid-based nanoparticles for contrast-enhanced MRI and molecular imaging. NMR Biomed. 2006;19:142–164. doi: 10.1002/nbm.1011. [DOI] [PubMed] [Google Scholar]
- 62.Ng KK, Lovell JF, Zheng G. Lipoprotein-Inspired Nanoparticles for Cancer Theranostics. Acc Chem Res. 2011;44:1105–1113. doi: 10.1021/ar200017e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barenholz Y. Doxil® — The first FDA-approved nano-drug: Lessons learned. J Controlled Release. 2012;160:117–134. doi: 10.1016/j.jconrel.2012.03.020. (Chezy) [DOI] [PubMed] [Google Scholar]
- 64.Boswell GW, Buell D, Bekersky I. AmBisome (Liposomal Amphotericin B): A Comparative Review. J Clin Pharmacol. 1998;38:583–592. doi: 10.1002/j.1552-4604.1998.tb04464.x. [DOI] [PubMed] [Google Scholar]
- 65.Fanos V, Cataldi L. Amphotericin B-Induced Nephrotoxicity: A Review. J Chemother. 2000;12:463–470. doi: 10.1179/joc.2000.12.6.463. [DOI] [PubMed] [Google Scholar]
- 66.Nagle PC, Gerancher JC. DepoDur® (extended-release epidural morphine): a review of an old drug in a new vehicle. Tech Reg Anesth Pain Manag. 2007;11:9–18. [Google Scholar]
- 67.Huynh E, Zheng G. Porphysome nanotechnology: A paradigm shift in lipid-based supramolecular structures. Nano Today. 2014;9:212–222. [Google Scholar]
- 68.Zhang Y, Lovell JF. Porphyrins as Theranostic Agents from Prehistoric to Modern Times. Theranostics. 2012;2:905–915. doi: 10.7150/thno.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jin CS, Lovell JF, Chen J, Zheng G. Ablation of Hypoxic Tumors with Dose-Equivalent Photothermal, but Not Photodynamic, Therapy Using a Nanostructured Porphyrin Assembly. ACS Nano. 2013;7:2541–2550. doi: 10.1021/nn3058642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jin CS, Cui L, Wang F, Chen J, Zheng G. Targeting-Triggered Porphysome Nanostructure Disruption for Activatable Photodynamic Therapy. Adv Healthc Mater. 2014 doi: 10.1002/adhm.201300651. n/a–n/a. [DOI] [PubMed] [Google Scholar]
- 71.Lovell JF, Jin CS, Huynh E, Jin H, Kim C, Rubinstein JL, et al. Porphysome nanovesicles generated by porphyrin bilayers for use as multimodal biophotonic contrast agents. Nat Mater. 2011;10:324–332. doi: 10.1038/nmat2986. [DOI] [PubMed] [Google Scholar]
- 72.MacDonald TD, Liu TW, Zheng G. An MRI-Sensitive, Non-Photobleachable Porphysome Photothermal Agent. Angew Chem Int Ed Engl. 2014;53:6956–6959. doi: 10.1002/anie.201400133. [DOI] [PubMed] [Google Scholar]
- 73.Liu TW, MacDonald TD, Jin CS, Gold JM, Bristow RG, Wilson BC, et al. Inherently Multimodal Nanoparticle-Driven Tracking and Real-Time Delineation of Orthotopic Prostate Tumors and Micrometastases. ACS Nano. 2013;7:4221–4232. doi: 10.1021/nn400669r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee J-H, Shao S, Cheng KT, Lovell JF, Paik CH. 99mTc-labeled porphyrin–lipid nanovesicles. J Liposome Res. 2014:1–6. doi: 10.3109/08982104.2014.932379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carter KA, Shao S, Hoopes MI, Luo D, Ahsan B, Grigoryants VM, et al. Porphyrin–phospholipid liposomes permeabilized by near-infrared light. Nat Commun. 2014;5 doi: 10.1038/ncomms4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fu CH, Sakamoto KM. PEG-asparaginase. Expert Opin Pharmacother. 2007;8:1977–1984. doi: 10.1517/14656566.8.12.1977. [DOI] [PubMed] [Google Scholar]
- 77.Rajender Reddy K, Modi MW, Pedder S. Use of peginterferon alfa-2a (40 KD) (Pegasys®) for the treatment of hepatitis C. Adv Drug Deliv Rev. 2002;54:571–586. doi: 10.1016/s0169-409x(02)00028-5. [DOI] [PubMed] [Google Scholar]
- 78.Wang Y-S, Youngster S, Grace M, Bausch J, Bordens R, Wyss DF. Structural and biological characterization of pegylated recombinant interferon alpha-2b and its therapeutic implications. Adv Drug Deliv Rev. 2002;54:547–570. doi: 10.1016/s0169-409x(02)00027-3. [DOI] [PubMed] [Google Scholar]
- 79.Molineux G. The Design and Development of Pegfilgrastim (PEG-rmetHuG-CSF, Neulasta®) Curr Pharm Des. 2004;10:1235–1244. doi: 10.2174/1381612043452613. [DOI] [PubMed] [Google Scholar]
- 80.Satchi R, Connors TA, Duncan R. PDEPT: polymer-directed enzyme prodrug therapy. Br J Cancer. 2001;85:1070–1076. doi: 10.1054/bjoc.2001.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Satchi-Fainaro R, Hailu H, Davies JW, Summerford C, Duncan R. PDEPT: Polymer-Directed Enzyme Prodrug Therapy. 2. HPMA Copolymer-β-lactamase and HPMA Copolymer-C-Dox as a Model Combination. Bioconjug Chem. 2003;14:797–804. doi: 10.1021/bc020091k. [DOI] [PubMed] [Google Scholar]
- 82.Vasey PA, Kaye SB, Morrison R, Twelves C, Wilson P, Duncan R, et al. Phase I Clinical and Pharmacokinetic Study of PK1 [N-(2-Hydroxypropyl)methacrylamide Copolymer Doxorubicin]: First Member of a New Class of Chemotherapeutic Agents—Drug-Polymer Conjugates. Clin Cancer Res. 1999;5:83–94. [PubMed] [Google Scholar]
- 83.Seymour LW, Ferry DR, Anderson D, Hesslewood S, Julyan PJ, Poyner R, et al. Hepatic Drug Targeting: Phase I Evaluation of Polymer-Bound Doxorubicin. J Clin Oncol. 2002;20:1668–1676. doi: 10.1200/JCO.2002.20.6.1668. [DOI] [PubMed] [Google Scholar]
- 84.Meerum Terwogt JM, ten Bokkel Huinink WW, Schellens JH, Schot M, Mandjes IA, Zurlo MG, et al. Phase I clinical and pharmacokinetic study of PNU166945, a novel water-soluble polymer-conjugated prodrug of paclitaxel. Anti-Cancer Drugs April 2001. 2001;12:315–323. doi: 10.1097/00001813-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 85.Singer JW. Paclitaxel poliglumex (XYOTAX™, CT-2103): A macromolecular taxane. J Controlled Release. 2005;109:120–126. doi: 10.1016/j.jconrel.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 86.Bhatt R, de Vries P, Tulinsky J, Bellamy G, Baker B, Singer JW, et al. Synthesis and in Vivo Antitumor Activity of Poly(l -glutamic acid) Conjugates of 20(S)-Camptothecin. J Med Chem. 2003;46:190–193. doi: 10.1021/jm020022r. [DOI] [PubMed] [Google Scholar]
- 87.Danhauser-Riedl S, Hausmann E, Schick H-D, Bender R, Dietzfelbinger H, Rastetter J, et al. Phase I clinical and pharmacokinetic trial of dextran conjugated doxorubicin (AD-70, DOX-OXD) Invest New Drugs. 1993;11:187–195. doi: 10.1007/BF00874153. [DOI] [PubMed] [Google Scholar]
- 88.Lee KS, Chung HC, Im SA, Park YH, Kim CS, Kim S-B, et al. Multicenter phase II trial of Genexol-PM, a Cremophor-free, polymeric micelle formulation of paclitaxel, in patients with metastatic breast cancer. Breast Cancer Res Treat. 2008;108:241–250. doi: 10.1007/s10549-007-9591-y. [DOI] [PubMed] [Google Scholar]
- 89.Lim WT, Tan EH, Toh CK, Hee SW, Leong SS, Ang PCS, et al. Phase I pharmacokinetic study of a weekly liposomal paclitaxel formulation (Genexol®-PM) in patients with solid tumors. Ann Oncol. 2010;21:382–388. doi: 10.1093/annonc/mdp315. [DOI] [PubMed] [Google Scholar]
- 90.Hawkins MJ, Soon-Shiong P, Desai N. Protein nanoparticles as drug carriers in clinical medicine. Adv Drug Deliv Rev. 2008;60:876–885. doi: 10.1016/j.addr.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 91.Hrkach J, Hoff DV, Ali MM, Andrianova E, Auer J, Campbell T, et al. Preclinical Development and Clinical Translation of a PSMA-Targeted Docetaxel Nanoparticle with a Differentiated Pharmacological Profile. Sci Transl Med. 2012;4:128ra39–128ra39. doi: 10.1126/scitranslmed.3003651. [DOI] [PubMed] [Google Scholar]
- 92.Venne A, Li S, Mandeville R, Kabanov A, Alakhov V. Hypersensitizing Effect of Pluronic L61 on Cytotoxic Activity, Transport, and Subcellular Distribution of Doxorubicin in Multiple Drug-resistant Cells. Cancer Res. 1996;56:3626–3629. [PubMed] [Google Scholar]
- 93.Batrakova EV, Li S, Elmquist WF, Miller DW, Alakhov VY, Kabanov AV. Mechanism of sensitization of MDR cancer cells by Pluronic block copolymers: Selective energy depletion. Br J Cancer. 2001;85:1987–1997. doi: 10.1054/bjoc.2001.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang Y, Jeon M, Rich LJ, Hong H, Geng J, Zhang Y, et al. Non-invasive multimodal functional imaging of the intestine with frozen micellar naphthalocyanines. Nat Nanotechnol. 2014;9:631–638. doi: 10.1038/nnano.2014.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nanjwade BK, Bechra HM, Derkar GK, Manvi FV, Nanjwade VK. Dendrimers: Emerging polymers for drug-delivery systems. Eur J Pharm Sci. 2009;38:185–196. doi: 10.1016/j.ejps.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 96.Lee CC, MacKay JA, Fréchet JMJ, Szoka FC. Designing dendrimers for biological applications. Nat Biotechnol. 2005;23:1517–1526. doi: 10.1038/nbt1171. [DOI] [PubMed] [Google Scholar]
- 97.Rupp R, Rosenthal SL, Stanberry LR. VivaGel™ (SPL7013 Gel): A candidate dendrimer - microbicide for the prevention of HIV and HSV infection. Int J Nanomedicine. 2007;2:561–566. [PMC free article] [PubMed] [Google Scholar]
- 98.Mcgowan I, Gomez K, Bruder K, Febo I, Chen BA, Richardson BA, et al. Phase 1 Randomized Trial of the Vaginal Safety and Acceptability of SPL7013 Gel (VivaGel®) in Sexually Active Young Women (MTN-004) AIDS Lond Engl. 2011;25:1057–1064. doi: 10.1097/QAD.0b013e328346bd3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Auffan M, Rose J, Bottero J-Y, Lowry GV, Jolivet J-P, Wiesner MR. Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat Nanotechnol. 2009;4:634–641. doi: 10.1038/nnano.2009.242. [DOI] [PubMed] [Google Scholar]
- 100.Giljohann DA, Seferos DS, Daniel WL, Massich MD, Patel PC, Mirkin CA. Gold Nanoparticles for Biology and Medicine. Angew Chem Int Ed. 2010;49:3280–3294. doi: 10.1002/anie.200904359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wu G, Mikhailovsky A, Khant HA, Fu C, Chiu W, Zasadzinski JA. Remotely Triggered Liposome Release by Near-Infrared Light Absorption via Hollow Gold Nanoshells. J Am Chem Soc. 2008;130:8175–8177. doi: 10.1021/ja802656d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huang X, El-Sayed IH, Qian W, El-Sayed MA. Cancer Cell Imaging and Photothermal Therapy in the Near-Infrared Region by Using Gold Nanorods. J Am Chem Soc. 2006;128:2115–2120. doi: 10.1021/ja057254a. [DOI] [PubMed] [Google Scholar]
- 103.Murphy CJ, Gole AM, Stone JW, Sisco PN, Alkilany AM, Goldsmith EC, et al. Gold Nanoparticles in Biology: Beyond Toxicity to Cellular Imaging. Acc Chem Res. 2008;41:1721–1730. doi: 10.1021/ar800035u. [DOI] [PubMed] [Google Scholar]
- 104.Paciotti GF, Myer L, Weinreich D, Goia D, Pavel N, McLaughlin RE, et al. Colloidal Gold: A Novel Nanoparticle Vector for Tumor Directed Drug Delivery. Drug Deliv. 2004;11:169–183. doi: 10.1080/10717540490433895. [DOI] [PubMed] [Google Scholar]
- 105.Libutti SK, Paciotti GF, Byrnes AA, Alexander HR, Gannon WE, Walker M, et al. Phase I and Pharmacokinetic Studies of CYT-6091, a Novel PEGylated Colloidal Gold-rhTNF Nanomedicine. Clin Cancer Res. 2010;16:6139–6149. doi: 10.1158/1078-0432.CCR-10-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ito A, Shinkai M, Honda H, Kobayashi T. Medical application of functionalized magnetic nanoparticles. J Biosci Bioeng. 2005;100:1–11. doi: 10.1263/jbb.100.1. [DOI] [PubMed] [Google Scholar]
- 107.Cai W, Shin D-W, Chen K, Gheysens O, Cao Q, Wang SX, et al. Peptide-labeled near-infrared quantum dots for imaging tumor vasculature in living subjects. Nano Lett. 2006;6:669–676. doi: 10.1021/nl052405t. [DOI] [PubMed] [Google Scholar]
- 108.Byers RJ, Hitchman ER. Quantum Dots Brighten Biological Imaging. Prog Histochem Cytochem. 2011;45:201–237. doi: 10.1016/j.proghi.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 109.Resch-Genger U, Grabolle M, Cavaliere-Jaricot S, Nitschke R, Nann T. Quantum dots versus organic dyes as fluorescent labels. Nat Methods. 2008;5:763–775. doi: 10.1038/nmeth.1248. [DOI] [PubMed] [Google Scholar]
- 110.Wu X, Liu H, Liu J, Haley KN, Treadway JA, Larson JP, et al. Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots. Nat Biotechnol. 2003;21:41–46. doi: 10.1038/nbt764. [DOI] [PubMed] [Google Scholar]
- 111.Park KH, Chhowalla M, Iqbal Z, Sesti F. Single-walled carbon nanotubes are a new class of ion channel blockers. J Biol Chem. 2003;278:50212–50216. doi: 10.1074/jbc.M310216200. [DOI] [PubMed] [Google Scholar]
- 112.Klumpp C, Kostarelos K, Prato M, Bianco A. Functionalized carbon nanotubes as emerging nanovectors for the delivery of therapeutics. Biochim Biophys Acta BBA - Biomembr. 2006;1758:404–412. doi: 10.1016/j.bbamem.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 113.Wang J. Carbon-Nanotube Based Electrochemical Biosensors: A Review. Electroanalysis. 2005;17:7–14. [Google Scholar]
- 114.Volder MFLD, Tawfick SH, Baughman RH, Hart AJ. Carbon Nanotubes: Present and Future Commercial Applications. Science. 2013;339:535–539. doi: 10.1126/science.1222453. [DOI] [PubMed] [Google Scholar]
- 115.Gao W, Chan JM, Farokhzad OC. pH-Responsive Nanoparticles for Drug Delivery. Mol Pharm. 2010;7:1913–1920. doi: 10.1021/mp100253e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Delie F, Blanco-Príeto MJ. Polymeric Particulates to Improve Oral Bioavailability of Peptide Drugs. Molecules. 2005;10:65–80. doi: 10.3390/10010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Damgé C, Maincent P, Ubrich N. Oral delivery of insulin associated to polymeric nanoparticles in diabetic rats. J Controlled Release. 2007;117:163–170. doi: 10.1016/j.jconrel.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 118.Chen M-C, Sonaje K, Chen K-J, Sung H-W. A review of the prospects for polymeric nanoparticle platforms in oral insulin delivery. Biomaterials. 2011;32:9826–9838. doi: 10.1016/j.biomaterials.2011.08.087. [DOI] [PubMed] [Google Scholar]
- 119.Hsu PP, Sabatini DM. Cancer Cell Metabolism: Warburg and Beyond. Cell. 2008;134:703–707. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 120.Kratz F, Müller IA, Ryppa C, Warnecke A. Prodrug strategies in anticancer chemotherapy. Chem Med Chem. 2008;3:20–53. doi: 10.1002/cmdc.200700159. [DOI] [PubMed] [Google Scholar]
- 121.Gao W, Chan JM, Farokhzad OC. pH-Responsive Nanoparticles for Drug Delivery. Mol Pharm. 2010;7:1913–1920. doi: 10.1021/mp100253e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lee ES, Bae YH. Recent progress in tumor pH targeting nanotechnology. J Control Release Off J Control Release Soc. 2008;132:164–170. doi: 10.1016/j.jconrel.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Meng F, Zhong Y, Cheng R, Deng C, Zhong Z. pH-sensitive polymeric nanoparticles for tumor-targeting doxorubicin delivery: concept and recent advances. Nanomed. 2014;9:487–499. doi: 10.2217/nnm.13.212. [DOI] [PubMed] [Google Scholar]
- 124.Chu C-J, Szoka FC. pH-Sensitive Liposomes. J Liposome Res. 1994;4:361–395. [Google Scholar]
- 125.Sethuraman VA, Bae YH. TAT peptide-based micelle system for potential active targeting of anti-cancer agents to acidic solid tumors. J Controlled Release. 2007;118:216–224. doi: 10.1016/j.jconrel.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lee ES, Gao Z, Kim D, Park K, Kwon IC, Bae YH. Super pH-sensitive multifunctional polymeric micelle for tumor pHe specific TAT exposure and multidrug resistance. J Controlled Release. 2008;129:228–236. doi: 10.1016/j.jconrel.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lovell Jf, Zheng G. Activatable Smart Probes For Molecular Optical Imaging And Therapy. J Innov Opt Health Sci. 2008;01:45–61. [Google Scholar]
- 128.Lovell JF, Liu TWB, Chen J, Zheng G. Activatable photosensitizers for imaging and therapy. Chem Rev. 2010;110:2839–2857. doi: 10.1021/cr900236h. [DOI] [PubMed] [Google Scholar]
- 129.De la Rica R, Aili D, Stevens MM. Enzyme-responsive nanoparticles for drug release and diagnostics. Adv Drug Deliv Rev. 2012;64:967–978. doi: 10.1016/j.addr.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 130.Delcea M, Möhwald H, Skirtach AG. Stimuli-responsive LbL capsules and nanoshells for drug delivery. Adv Drug Deliv Rev. 2011;63:730–747. doi: 10.1016/j.addr.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 131.Andresen TL, Jensen SS, Kaasgaard T, Jorgensen K. Triggered Activation and Release of Liposomal Prodrugs and Drugs in Cancer Tissue by Secretory Phospholipase A2. Curr Drug Deliv. 2005;2:353–362. doi: 10.2174/156720105774370203. [DOI] [PubMed] [Google Scholar]
- 132.Lovell JF, Jin CS, Huynh E, MacDonald TD, Cao W, Zheng G. Enzymatic Regioselection for the Synthesis and Biodegradation of Porphysome Nanovesicles. Angew Chem. 2012;124:2479–2483. doi: 10.1002/anie.201108280. [DOI] [PubMed] [Google Scholar]
- 133.Zhou H, Yan H, Senpan A, Wickline SA, Pan D, Lanza GM, et al. Suppression of inflammation in a mouse model of rheumatoid arthritis using targeted lipase-labile fumagillin prodrug nanoparticles. Biomaterials. 2012;33:8632–8640. doi: 10.1016/j.biomaterials.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pan D, Schmieder AH, Wang K, Yang X, Senpan A, Cui G, et al. Anti-angiogenesis therapy in the Vx2 rabbit cancer model with a lipase-cleavable Sn 2 taxane phospholipid prodrug using α(v)β3-targeted theranostic nanoparticles. Theranostics. 2014;4:565–578. doi: 10.7150/thno.7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wong C, Stylianopoulos T, Cui J, Martin J, Chauhan VP, Jiang W, et al. Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc Natl Acad Sci. 2011;108:2426–2431. doi: 10.1073/pnas.1018382108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yavlovich A, Singh A, Tarasov S, Capala J, Blumenthal R, Puri A. Design of liposomes containing photopolymerizable phospholipids for triggered release of contents. J Therm Anal Calorim. 2009;98:97–104. doi: 10.1007/s10973-009-0228-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bae YH, Okano T, Hsu R, Kim SW. Thermo-sensitive polymers as on-off switches for drug release. Makromol Chem Rapid Commun. 1987;8:481–485. [Google Scholar]
- 138.Yatvin MB, Weinstein JN, Dennis WH, Blumenthal R. Design of liposomes for enhanced local release of drugs by hyperthermia. Science. 1978;202:1290–1293. doi: 10.1126/science.364652. [DOI] [PubMed] [Google Scholar]
- 139.Fomina N, Sankaranarayanan J, Almutairi A. Photochemical mechanisms of light-triggered release from nanocarriers. Adv Drug Deliv Rev. 2012;64:1005–1020. doi: 10.1016/j.addr.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Brazel CS. Magnetothermally-responsive Nanomaterials: Combining Magnetic Nanostructures and Thermally-Sensitive Polymers for Triggered Drug Release. Pharm Res. 2009;26:644–656. doi: 10.1007/s11095-008-9773-2. [DOI] [PubMed] [Google Scholar]
- 141.Needham D, Anyarambhatla G, Kong G, Dewhirst MW. A new temperature-sensitive liposome for use with mild hyperthermia: characterization and testing in a human tumor xenograft model. Cancer Res. 2000;60:1197–1201. [PubMed] [Google Scholar]
- 142.Kong G, Anyarambhatla G, Petros WP, Braun RD, Colvin OM, Needham D, et al. Efficacy of Liposomes and Hyperthermia in a Human Tumor Xenograft Model: Importance of Triggered Drug Release. Cancer Res. 2000;60:6950–6957. [PubMed] [Google Scholar]
- 143.Miller AD. Lipid-Based Nanoparticles in Cancer Diagnosis and Therapy. J Drug Deliv. 2013;2013:e165981. doi: 10.1155/2013/165981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Poon RT, Borys N. Lyso-thermosensitive liposomal doxorubicin: a novel approach to enhance efficacy of thermal ablation of liver cancer. Expert Opin Pharmacother. 2009;10:333–343. doi: 10.1517/14656560802677874. [DOI] [PubMed] [Google Scholar]
- 145.Hong CW, Libutti SK, Wood BJ. Liposomal doxorubicin plus radiofrequency ablation for complete necrosis of a hepatocellular carcinoma. Curr Oncol. 2013;20:e274–e277. doi: 10.3747/co.20.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hu S-H, Chen S-Y, Liu D-M, Hsiao C-S. Core/Single-Crystal-Shell Nanospheres for Controlled Drug Release via a Magnetically Triggered Rupturing Mechanism. Adv Mater. 2008;20:2690–2695. doi: 10.1002/adma.200800193. [DOI] [PubMed] [Google Scholar]
- 147.Babincová M, Cicmanec P, Altanerová V, Altaner C, Babinec P. AC-magnetic field controlled drug release from magnetoliposomes: design of a method for site-specific chemotherapy. Bioelectrochemistry Amst Neth. 2002;55:17–19. doi: 10.1016/s1567-5394(01)00171-2. [DOI] [PubMed] [Google Scholar]
- 148.Amstad E, Kohlbrecher J, Müller E, Schweizer T, Textor M, Reimhult E. Triggered Release from Liposomes through Magnetic Actuation of Iron Oxide Nanoparticle Containing Membranes. Nano Lett. 2011;11:1664–1670. doi: 10.1021/nl2001499. [DOI] [PubMed] [Google Scholar]
- 149.Derfus AM, von Maltzahn G, Harris TJ, Duza T, Vecchio KS, Ruoslahti E, et al. Remotely Triggered Release from Magnetic Nanoparticles. Adv Mater. 2007;19:3932–3936. [Google Scholar]
- 150.Bringas E, Köysüren Ö, Quach DV, Mahmoudi M, Aznar E, Roehling JD, et al. Triggered release in lipid bilayer-capped mesoporous silica nanoparticles containing SPION using an alternating magnetic field. Chem Commun. 2012;48:5647–5649. doi: 10.1039/c2cc31563g. [DOI] [PubMed] [Google Scholar]
- 151.Kobayashi T. Cancer hyperthermia using magnetic nanoparticles. Biotechnol J. 2011;6:1342–1347. doi: 10.1002/biot.201100045. [DOI] [PubMed] [Google Scholar]
- 152.Kopechek JA, Abruzzo TM, Wang B, Chrzanowski SM, Smith DAB, Kee PH, et al. Ultrasound-Mediated Release of Hydrophilic and Lipophilic Agents From Echogenic Liposomes. J Ultrasound Med. 2008;27:1597–1606. doi: 10.7863/jum.2008.27.11.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.De Geest BG, Skirtach AG, Mamedov AA, Antipov AA, Kotov NA, De Smedt SC, et al. Ultrasound-Triggered Release from Multilayered Capsules. Small. 2007;3:804–808. doi: 10.1002/smll.200600441. [DOI] [PubMed] [Google Scholar]
- 154.Kim H-J, Matsuda H, Zhou H, Honma I. Ultrasound-Triggered Smart Drug Release from a Poly(dimethylsiloxane)– Mesoporous Silica Composite. Adv Mater. 2006;18:3083–3088. [Google Scholar]
- 155.Rapoport NY, Christensen DA, Fain HD, Barrows L, Gao Z. Ultrasound-triggered drug targeting of tumors in vitro and in vivo. Ultrasonics. 2004;42:943–950. doi: 10.1016/j.ultras.2004.01.087. [DOI] [PubMed] [Google Scholar]
- 156.Dromi S, Frenkel V, Luk A, Traughber B, Angstadt M, Bur M, et al. Pulsed-High Intensity Focused Ultrasound and Low Temperature–Sensitive Liposomes for Enhanced Targeted Drug Delivery and Antitumor Effect. Clin Cancer Res. 2007;13:2722–2727. doi: 10.1158/1078-0432.CCR-06-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Schroeder A, Honen R, Turjeman K, Gabizon A, Kost J, Barenholz Y. Ultrasound triggered release of cisplatin from liposomes in murine tumors. J Controlled Release. 2009;137:63–68. doi: 10.1016/j.jconrel.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 158.Tong R, Kohane DS. Shedding light on nanomedicine. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2012;4:638–662. doi: 10.1002/wnan.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Koçer A, Walko M, Meijberg W, Feringa BL. A Light-Actuated Nanovalve Derived from a Channel Protein. Science. 2005;309:755–758. doi: 10.1126/science.1114760. [DOI] [PubMed] [Google Scholar]
- 160.Leung SJ, Romanowski M. Light-Activated Content Release from Liposomes. Theranostics. 2012;2:1020–1036. doi: 10.7150/thno.4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yavlovich A, Smith B, Gupta K, Blumenthal R, Puri A. Light-sensitive lipid-based nanoparticles for drug delivery: design principles and future considerations for biological applications. Mol Membr Biol. 2010;27:364–381. doi: 10.3109/09687688.2010.507788. [DOI] [PMC free article] [PubMed] [Google Scholar]