Abstract
Background and Purpose
The relationships between cerebrovascular lesions visible on imaging and cognition are complex. We explored the possibility that cerebral cortical volume mediated the relationship.
Methods
1906 non-demented participants (59% women; 25% African-American; mean age 76.6 years) in the Atherosclerosis Risk in Communities (ARIC) study underwent cognitive assessments, risk factor assessments, and quantitative MR imaging for white matter hyperintensities (WMH) and infarcts. The Freesurfer imaging analysis pipeline was used to determine regional cerebral volumes. We examined associations of cognitive domain outcomes with cerebral volumes (hippocampus, and separate groups of posterior and frontal cortical regions of interest (ROI)) and cerebrovascular imaging features (presence of large or small cortical/subcortical infarcts and WMH volume). We performed mediation pathway analyses to assess the hypothesis that hippocampal and cortical volumes mediated associations between cerebrovascular imaging features and cognition.
Results
In unmediated analyses, WMH and infarcts were both associated with worse psychomotor speed/executive function (PS/EF). In mediation analyses, WMH and infarcts associations on PS/EF were significantly attenuated, but not abolished, by the inclusion of the posterior cortical ROI volume in the models, and the infarcts on PS/EF association was attenuated, but not abolished, by inclusion of the frontal cortical ROI volume.
Conclusions
Both WMH and infarcts were associated with cortical volume, and both lesions were also associated with cognitive performance, implying shared pathophysiological mechanisms. Although cross-sectional, our findings suggest that WMH and infarcts could be proxies for clinically covert processes that directly damage cortical regions. Microinfarcts are one candidate for such a clinically covert process.
Keywords: Magnetic resonance imaging, cerebral small vessel disease, white matter hyperintensities, cerebral infarction, cognition
The mechanisms by which cerebrovascular disease (CVD) causes cognitive impairment have been elusive. While the volume of infarcted tissue was an obvious initial candidate as a quantitative marker of pathology1, persons with clinically overt, large infarcts account for only a small fraction of cognitively impaired individuals with CVD2–5. The presence of even one visible lacunar infarct is associated with cognitive impairment or cognitive decline6–10, and the associations of white matter hyperintensities with cognition do not occur only with severe disease11–14. Because one or two lacunar infarcts or moderate WMH burden are themselves unlikely to be sufficient to damage enough cognitively eloquent grey matter or pathways, the associations of WMH and smaller infarcts with cognitive impairment imply that WMH or visible lacunes must be proxies for a more broadly distributed pathological process.
We had the opportunity to conduct a large-scale clinical and imaging cross-sectional analysis of non-demented individuals in the Atherosclerosis Risk in Communities (ARIC) Neurocognitive Study cohort. We tested the hypothesis that the cognitive consequences of WMH and smaller infarcts are mediated by another pathophysiological process, specifically variations in regional cerebral cortical volume. Regional cortical volumes are a measure of neuronal and synaptic structural integrity. Our analyses do not require us to specify how visible subcortical cerebrovascular lesions cause loss of cortical volume, but microinfarcts are the most plausible candidate mechanism5, 15–18.
METHODS
Participants
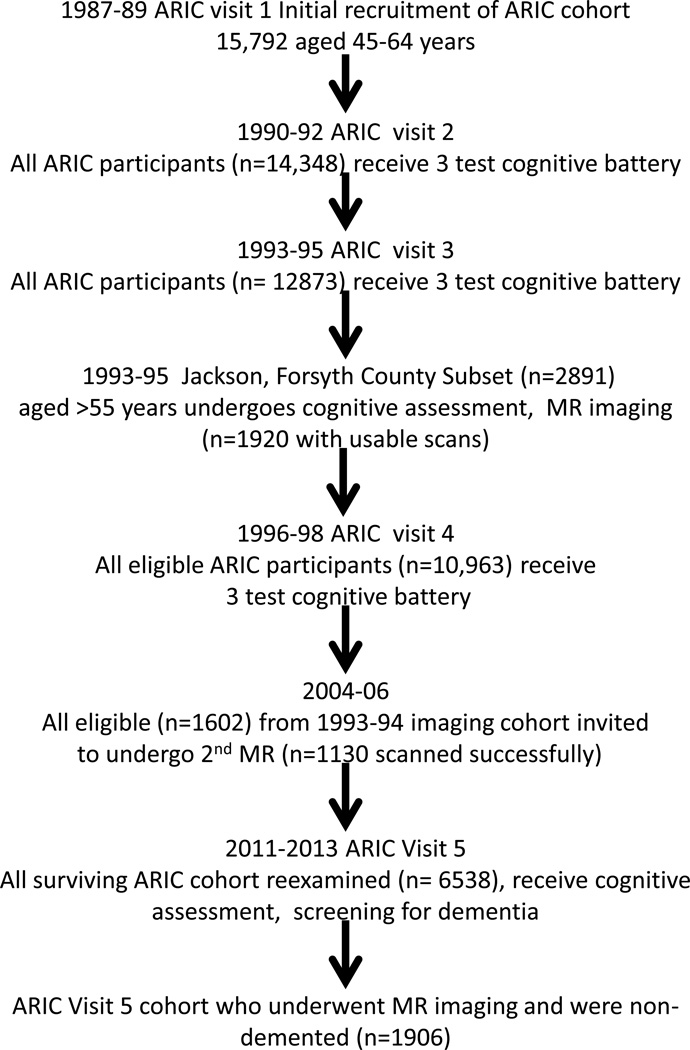
The ARIC study began with a 1987–89 baseline examination of cardiovascular risk factors in men and women aged 45–64 years who were representative of four US communities, Washington County MD, Forsyth County NC, Jackson MS, and suburban Minneapolis MN. See Figure 1 and Appendix for ARIC study flow.
Figure 1.
Time line of the ARIC study relevant to current analysis.
ARIC conducted a fifth examination (V5) between June, 2011 and August, 2013; institutional review boards of each ARIC center approved the protocol. Of 10,749 original ARIC cohort members alive at the start of V5 recruitment, 713 (6.6%) died prior to an examination, leaving 10,036 alive through August 2013. Of these, 6,538 (age 66–90) took part (5,918 full clinic exams, 228 abbreviated clinic exams, 392 home or care-facility exams). The overall V5 response rate was 65% (6,538/10,036).
A subset of ARIC V5 participants without contraindications were selected for a brain MRI: 1) all persons who had previous scans in 2004–6, 2) those with low cognitive test scores\declines on longitudinally-administered tests, and 3) an age-stratified random sample of the remaining individuals. Sampling fractions for the random sample were set for participants <80 and ≥80 years of age to approximate the age distribution of those selected from the cognitively suspect group and were modified slightly over the course of the study to achieve a goal of approximately 2000 total MRI scans. 74/1980 (4%) were excluded due to cognitive impairment sufficient to suspect dementia (Mini-Mental Status Examination (MMSE) scores, <21 if white and <19 if African-American).
Cognitive Assessments
Participants were administered a battery of neuropsychological tests. Standardized administration and scoring have been described previously with normative data from the ARIC cohort19. Cognitive domains included: Memory (Delayed Word Recall Test, Logical Memory immediate and delayed recall, and incidental Learning from the Wechsler Memory Scale-III), Psychomotor Speed/Executive Function (PS/EF) (Digit Symbol Substitution Test, Trail Making Test parts A and B and WAIS-R Digits Span Backwards), and Language (Letter fluency, Boston Naming Test, and Animal Naming). As previously reported, we constructed Z-scores for each domain by averaging the test scores within a domain, subtracting the domain mean and dividing by the domain standard deviation. A global composite Z-score was also derived from the three domain scores. The language domain Z-score lacked associations with imaging features in preliminary analyses and was not further examined in mediation analyses.
Vascular Risk Factors and APOE genotype
All participants also underwent an extensive evaluation of vascular risk factors at each ARIC visit20, 21 Medical histories for diabetes mellitus, hypertension, smoking and a history of stroke (through December 31, 2011) were used in the current analyses. APOE genotyping was performed using standard methods. (see Appendix for details.)
Imaging
MR scans were performed at each site on 3 Tesla Siemens (various models) scanners using a common set of sequences that included a 3D volumetric Magnetization Prepared Gradient Echo (MPRAGE) and a Fluid Attenuated Inversion Recovery (FLAIR) sequences. WMH burden was measured quantitatively using an algorithm developed at Mayo Clinic Rochester22, 23 and reported in cm3. WMH were defined as has been codified in recent guidelines24. All analyses involving WMH include total intracranial volume as a covariate. Freesurfer (version 5.1)25 was used to calculate regional cortical volumes, reported in cm3.
Brain infarcts were identified, counted and measured by a trained imaging technician and confirmed by radiologists (KK, CRJ) as previously described26. Cortical infarctions were characterized on FLAIR sequences as hyperintense lesions ≥ 10 mm (large) or 5–10 mm (small) in greatest dimension, extending to the cortical surface, that includes cortical grey matter and may include underlying white matter. Subcortical infarctions were characterized as hyperintense lesions with a dark center (≥ 3mm in diameter) seen in the white matter, infratentorial, and central gray/capsular regions, and distinguishable from perivascular spaces. Because the number of participants with multiple infarcts was low, we collapsed all infarct ratings into a new variable representing the presence of at least one infarct of any type, size or location, referred to as “infarcts.”
Using the Freesurfer atlas27, we prespecified 3 regions of interest (ROI’s) based on relevance to cerebrovascular disease or cognition. They were (1) the combined right and left hippocampal formations; (2) Posterior ROI: mean cortical volume of a group of regions that are part of the posterior default mode network28 and are associated with Alzheimer’s disease (AD)29 from both right and left hemispheres: hippocampus, parahippocampal gyrus, entorhinal cortex, inferior parietal lobule, precuneus and cuneus; and (3) Frontal ROI: mean cortical volume of regions in the frontal lobe from both right and left hemispheres: rostral/caudal anterior cingulate, rostral/caudal mid-frontal, lateral orbital frontal, medial orbital frontal, paracentral, pars opercularis, pars triangularis, precentral, superior frontal, and frontal pole. Frontal dysfunction has been specifically implicated in CVD30. All ROI volumes are expressed in cm3, and all models adjusted for total intracranial volume to account for differences in head size across participants.
Statistical Analyses
Primary analyses were conducted using general linear models. Potential nonlinear relationships were examined with lowess smooth curves and modeled using fractional polynomial and linear-spline formulations. Potential outlier effects were assessed with DFFITS for influential points, Cook’s D statistic, and graphical displays such as residual and added-variable plots.
ARIC participants were selected to receive an MRI under the probabilistic sampling plan described above. Sampling weights were derived as the product of inverse sampling fractions and the inverse probability of completing the exam to account for dropout/missingness. All models incorporated these probability sampling weights in order to represent the full ARIC visit 5 clinic cohort.
Scores from the Trail Making tests were first log-transformed and multiplied by −1 so that low scores across all tests indicated worse performance. For the Logical Memory tests, a single Z score was created as the average Z score for the immediate and delayed recall sections. Participants who were unable to complete any test due to cognitive impairment were assigned a Z score of −2 for that test.
White matter hyperintensity burden was positively skewed, therefore and we log2-transformed WMH volumes.
Nonlinearity diagnostics showed that associations between cognitive composites and volumetric measures were substantially stronger for participants with smaller versus larger volumes. We expressed this analytically using fully stratified models for structural association estimates, with cut-points of ≤6 mm3 for hippocampal ROIs, ≤60 mm3 for posterior cortical ROI’s and ≤150 mm3 for frontal cortical ROIs, and using fractional polynomial formulations for mediation estimates. Cut-point knots were found using maximum likelihood type approaches.
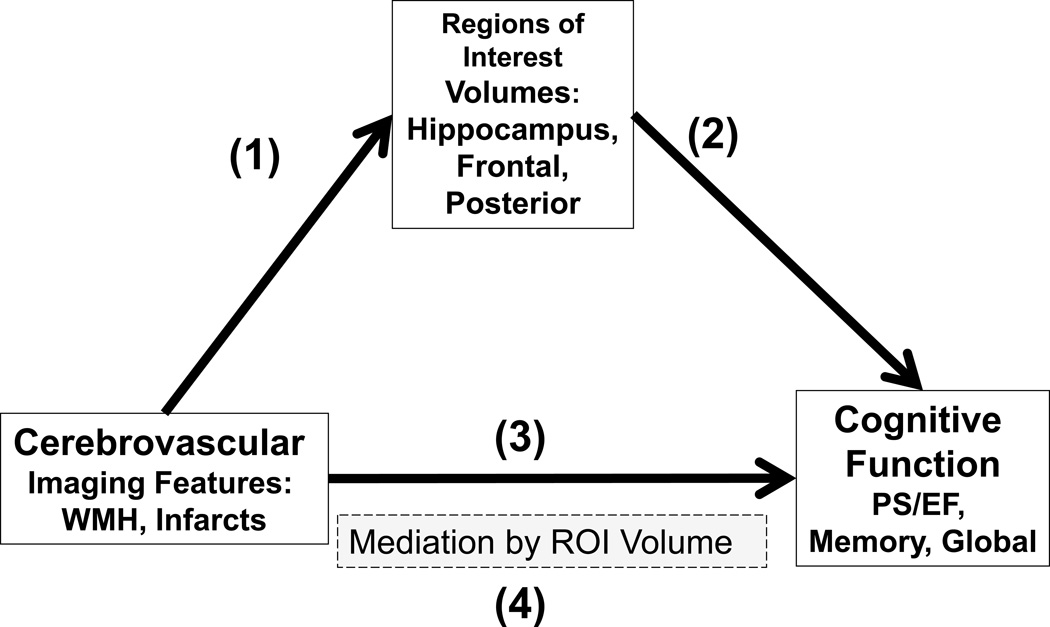
We used standard mediation pathway approaches31 to examine whether relationships between CVD imaging features (WMH and infarcts) and cognition (global, memory, PS/EF) potentially operated through regional volumetric paths (hippocampal, posterior and frontal ROI’s); see Figure 2. First, we examined relationships between CVD imaging features and ROI volumes. Second, we examined relationships between ROI volumes and cognition. Third, we examined relationships between CVD imaging features and cognition. Fourth, we assessed whether relationships between CVD imaging features and cognition were attenuated when additionally adjusting for ROI volumes. Formal mediation estimates were calculated from indirect effects (i.e. the difference between path #3: total effect and path #4: direct effect) using structural equation models32 with pathways specified as in Figure 2.
Figure 2.
Analysis model for mediation in ARIC Neurocognitive Study. With strong mediating influences, pathways (1) and (2) should be present, and the apparent pathway (3) should be attenuated when additionally adjusting for the potential mediator, as in pathway (4). Abbreviations: ROI=Brain regions of interest; WMH=white matter hyperintensities; INF= any subcortical or cortical infarction; ROI= Region of Interest; PS/EF = Psychomotor speed/executive function.
All models were adjusted for clinical and demographic variables including age, sex, race, education, history of diabetes, history of hypertension, history of alcohol use, history of smoking, APOE 4 genotype and total intracranial volume. Interaction terms were examined to assess potential modifying effects of sex and/or race; none were supported. Sensitivity analyses to examine stability of estimates were conducted examining adjustment model, nonlinearity threshold and sampling weight incorporation; similar results were found throughout.
RESULTS
Table 1 shows that the 1906 non-demented ARIC participants who underwent MR (mean age 75 (range 67–90) years, 60% women, 22% black) and the 4558 who did not were fairly similar. Vascular risk factors were common in the cohort, with 29% diabetic and 68% hypertensive. The APOE 4 allele frequency of 28% was in line with a typical non-demented population.
Table 1.
Participant Characteristics. ARIC Neurocognitive Study
| Participants with MR |
Participants without MR |
|||
|---|---|---|---|---|
| Type | Characteristic | N=1906 | N=4558 | |
| Demographic | Sex | Female | 2662 (58%) | 3580 (61%) |
| Race | Black | 986 (22%) | 1258 (21%) | |
| Age (yrs) | 75.55 (5.21) | 75.21 (5.21) | ||
| Education Level | < 11 years | 705 (15%) | 639 (11%) | |
| High School/Vocational | 1902 (42%) | 2408 (41%) | ||
| College + | 1942 (43%) | 2793 (48%) | ||
| Center | Forsyth | 947 (21%) | 1345 (23%) | |
| Jackson | 903 (20%) | 1131 (19%) | ||
| Minneapolis | 1458 (32%) | 1801 (31%) | ||
| Washington | 1250 (27%) | 1578 (27%) | ||
| Clinical | Diabetic | Yes | 1258 (29%) | 1576 (27%) |
| Hypertension | Yes | 3055 (68%) | 3883 (66%) | |
| Drinking | Current | 2097 (50%) | 3078 (53%) | |
| Former | 1249 (30%) | 1470 (25%) | ||
| Never | 843 (20%) | 1193 (20%) | ||
| Smoking | Current | 262 (7%) | 299 (5%) | |
| Former | 2025 (52%) | 2842 (49%) | ||
| Never | 1583 (41%) | 2409 (41%) | ||
|
MRI Cerebrovascular Imaging Features |
WMH Volume (cm3) | 17.29 (16.86) | ||
| WMH % of total WM volume | 3.88 (3.45) | |||
|
Large Cortical Infarct Frequency |
0 | 1820 (96%) | ||
| 1 | 55 (3%) | |||
| 2+ | 25 (1%) | |||
|
Small Cortical Infarct Frequency |
0 | 1770 (93%) | ||
| 1 | 108 (6%) | |||
| 2+ | 22 (1%) | |||
|
Subcortical Infarct Frequency |
0 | 1543 (81%) | ||
| 1 | 260 (14%) | |||
| 2+ | 97 (5%) | |||
|
Cognitive Measures |
Delayed Word Recall | 5.00 (1.87) | 5.31 (1.87) | |
| WAIS-r Digit Symbol Substitution | 36.37 (11.41) | 38.53 (12.19) | ||
| Letter Fluency | 33.01 (12.48) | 34.17 (11.73) | ||
| Mini-Mental State Examination | 27.39 (2.22) | 27.28 (3.35) | ||
|
ROI Volumes |
Hippocampal (cm3) | 6.89 (0.93) | ||
| Posterior ROI (cm3) | 59.07 (6.90) | |||
| Frontal ROI (cm3) | 150.21 (16.00) | |||
Cells contain N (%) for categorical and mean (sd) for continuous / semi-continuous variables
We first determined total adjusted associations between sets of cognitive composites, volumetric composites and the two CV imaging features (WMH and infarcts). For path #1 in Figure 2, we found associations between the volumetric measures, and both WMH and infarcts after adjusting for demographics, vascular risk factors and APOE genotype (Table 2). For example, each doubling of WMH burden (i.e. a one unit increase in log2(WMH cm3) volume was associated with a decrease in hippocampal ROI volume of 0.095 cm3, or 0.102 std units. Similarly, posterior cortical volumes decreased by 0.348 cm3 with doubling WMH burden, relationally about ½ the effect size of the hippocampal effect (0.050 std units); note that posterior volumes were around 10 times as large. All path #1 associations were significant, except for infarcts on frontal ROI volume, which was therefore excluded from mediation analyses.
Table 2.
Associations of Regions of Interest Volumes with Cardiovascular Imaging Features*. ARIC Neurocognitive Study.
| Cerebrovascular Imaging Feature (Predictor) |
Volumetric Region of Interest (Outcome) |
Association β p-value, (95% CI) | |
|---|---|---|---|
| Raw Scale Outcomes (cm3) |
Standardized Outcomes |
||
| White Matter Hyperintensities {log2(WMH)} |
Hippocampal | −0.095 p<0.001 (−0.133,−0.058) |
−0.102 p<0.001 (−0.143,−0.062) |
| Posterior | −0.348 p=0.001 (−0.561,−0.135) |
−0.050 p=0.001 (−0.081,−0.020) |
|
| Frontal | −0.953 p<0.001 (−1.443,−0.464) |
−0.060 p<0.001 (−0.090,−0.029) |
|
| Any Infarct {Large Cortical, Small Cortical or Subcortical} |
Hippocampal | −0.135 p=0.005 (−0.230,−0.040) |
−0.145 p=0.005 (−0.248,−0.043) |
| Posterior | −0.684 p=0.010 (−1.202,−0.165) |
−0.099 p=0.010 (−0.174,−0.024) |
|
| Frontal | −0.528 p=0.408 (−1.778,0.723) |
−0.033 p=0.408 (−0.111,0.045) |
|
Example interpretation: Each 1 unit increase in log2(WMH), (a doubling of WMH burden), was associated with a decrease in hippocampal ROI volume of 0.095 cm3, or 0.102 std volume units (std volumes constructed by subtracting the mean and dividing by the SD). Similarly, posterior cortical volumes decreased by 0.348 cm3 with doubling WMH burden, relationally about ½ the effect size of the hippocampal effect (0.050 std volume units), given that posterior volumes were around 10 times larger.
CI: Confidence Interval
For path #2 in Figure 2, adjusted models revealed nonlinear associations between volumetric and cognitive measures with threshold effects indicating larger associations for smaller vs larger volumes (Table 3). For example, each 1 cm3 increase from 4–6 cm3 in hippocampal ROI was associated with a 0.44 increase in the (standardized) global cognition measure, while increases from 6–10 cm3 showed little to no association. The posterior cortical ROI was associated with all three cognitive composites. The hippocampal ROI was associated with all three but only marginally for PS/EF. The frontal ROI was associated with PS/EF and global cognition marginally. Marginal and non-significant relationships were excluded from mediation analyses.
Table 3.
Associations of Cognitive Composite Scores with Region of Interest Volumes, stratified by ROI size. ARIC Neurocognitive Study.
| Volumetric Measures Standardized with p value, 95% Confidence Interval |
||||
|---|---|---|---|---|
| Cognitive Composite Outcome |
Hippocampal ROI | Posterior ROI | Frontal ROI | |
| Smaller Volumes* | Global Cognition |
0.44 p=0.001 (0.18,0.70) |
0.04 p<0.001 (0.02,0.05) |
0.01 p=0.049 (0.00,0.02) |
| Memory |
0.45 p<0.001 (0.20,0.70) |
0.03 p=0.002 (0.01,0.05) |
0.00 p=0.276 (−0.00,0.01) |
|
| PS/EF | 0.23 p=0.088 (−0.03,0.50) |
0.05 p<0.001 (0.03,0.07) |
0.02 p<0.001 (0.01,0.02) |
|
| Larger Volumes* | Global Cognition | 0.05 p=0.149 (−0.02,0.12) |
0.02 p=0.059 (−0.00,0.04) |
0.00 p=0.946 (−0.01,0.01) |
| Memory | 0.03 p=0.369 (−0.04,0.11) |
0.01 p=0.193 (−0.01,0.03) |
−0.00 p=0.914 (−0.01,0.01) |
|
| PS/EF |
0.09 p=0.024 (0.01,0.17) |
0.02 p=0.038 (0.00,0.03) |
−0.01 p=0.158 (−0.01,0.00) |
|
See Statistical Analysis, Methods for definition of size.
Example interpretation: Each 1 cm3 increase from 4–6 cm3 (the smaller volume) in the hippocampal ROI was associated with a 0.44 increase in the (standardized) global cognition measure
For path #3 in Figure 2, adjusted total associations between cerebrovascular imaging features and cognition were evaluated (Table 4: first column). Associations of PS/EF with both WMH and infarcts were supported (expected decrease of −0.061 PS/EF with each doubling of WMH burden), as well as a marginal association between the global composite and WMH. Marginal and non-significant relationships were excluded from mediation analyses.
Table 4.
Mediation Models of Associations of Cognition with Cerebrovascular Imaging Features: Effects of Region of Interest Volumes. Values for the mediations analyses are shown for relationships for which all bivariate associations were significant at p<0.01. ARIC Neurocognitive Study.
| Unmediated Associations Base Model (β) p-value 95% CI |
With Mediation by inclusion of Volumetric Measures Standardized β, p-value 95% Confidence Intervals |
|||||
|---|---|---|---|---|---|---|
| Cerebro- vascular Imaging Feature |
Cognitive Composite Outcome |
With Clinical & Demographic Adjustment |
Base Model + Posterior ROI |
Mediation estimate |
Base Model + Frontal ROI |
Mediation estimate |
|
White Matter Hyper- intensities {log2(WMH)} |
Global Cognition |
−0.046 p=0.037 (−0.090,−0.003) |
Not estimated; Path 3 unsupported | Not estimated; Path 3 unsupported | ||
| Memory | −0.027 p=0.274 (−0.076,0.021) |
Not estimated; Path 3 unsupported | Not estimated; Path 3 unsupported | |||
| PS/EF |
−0.061 p=0.001 (−0.098,−0.023) |
−0.050 p=0.008 (−0.088,−0.013) |
−0.010 p=0.003 (−0.017, −0.003) |
−0.055 p=0.004 (−0.092,−0.017) |
−0.006 p=0.032 (−0.011, 0.000) |
|
|
Any Infarct {Large Cortical, Small Cortical or Subcortical} |
Global Cognition |
−0.074 p=0.125 (−0.168,0.020) |
Not estimated; Path 3 unsupported | Not estimated; Path 3 unsupported | ||
| Memory | −0.050 p=0.358 (−0.156,0.056) |
Not estimated; Path 3 unsupported | Not estimated; Path 3 unsupported | |||
| PS/EF |
−0.141 p=0.006 (−0.241,−0.042) |
−0.119 p=0.020 (−0.220,−0.019) |
−0.017 p=0.011 (−0.030, −0.004) |
Not estimated; Path 3 unsupported | ||
Example Interpretation: Each 1 unit increase in log2(WMH), (a doubling of WMH burden) was associated with a decrease in the global cognition Z-score measure of 0.046 standard deviations
We also considered the relationship between WMH and infarcts and conducted mediation analyses of their individual relationships to PS/EF by the other feature. Although there was a slight attenuation of the association between infarcts and PS/EF (Table 4: standardized −.141) by the inclusion of WMH in the model, the association of infarcts and PS/EF remained significant (standardized = −.117 (−.218, −.015) p=0.025). There was no evidence for mediation of the WMH and PS/EF association by infarcts. Therefore, it was justifiable to consider the two features as being largely independent.
We considered mediation models (Figure 2, path #4), when all three bivariate associations were supported (paths #1–3). There were three, all related to PS/EF performance: (i) WMH association mediated by posterior cortical ROI; (ii) WMH association mediated by frontal ROI; and (iii) infarcts association mediated by frontal cortical ROI. We found evidence of some mediation effects for each of these (Table 4: mediation columns). For example, approximately 16% (−0.010/−0.061) of the association between WMH and PS/EF might be explained by a mediation pathway via effects of WMH on posterior cortical ROI volumes. Even after inclusion of the volumetric mediators, significant associations between PS/EF and the WMH and infarcts features remained.
DISCUSSION
In a biracial group of non-demented elderly individuals, cross-sectional mediation analyses showed that two cortical ROI’s – one representing posterior cortical regions that are part of the default mode network and another, a group of frontal regions – moderately mediated associations between WMH burden and a cognitive composite representing psychomotor speed and executive function (PS/EF). The association between infarcts, the variable representing the presence of any infarct, and the PS/EF composite was also moderately mediated by the posterior cortical ROI. These findings imply that some of the impact of WMH or infarcts burden on cognitive function may have been the result of mechanisms that these lesions share with pathological processes that affect cortical volume. Although the magnitude of the mediation effect was modest, our findings support the hypothesis that a widely distributed process beyond the visible lesions leads to cognitive impairment. Our results do not specifically implicate cortical microinfarcts as the lesion that links visible cerebrovascular disease and cognitive function, but microinfarcts are the prime prospect, in the absence of stronger candidates, for a diffuse microvascular process that affects isocortex.
In bivariate analyses, both CVD imaging features – WMH and infarcts – were associated with posterior cortical ROI volume. Only WMH were associated with frontal ROI volume. These associations themselves suggest shared pathophysiologic mechanisms, with mediation analyses substantially strengthening the argument by showing that the associations impacted cognition. Several prior reports have shown associations between brain volume and burden of CVD lesions longitudinally33, 34 and cross-sectionally35.
The conceptual model that motivated our analyses required some important predicates that were supported both by prior literature and our own findings. Paths #1, #2 and #3 in Figure 2 were required to show significant associations. WMH and infarcts were associated with cognition, specifically PS/EF, as observed by others7, 13, 36. Second, for all 3 of our ROI’s, cortical volume was associated with cognition, which has also been frequently observed37–42. Each ROI showed associations with cognition corresponding to expected cognitive-anatomic relationships: hippocampal volume was strongly associated with the memory composite, the posterior ROI showed similar associations with all 3 cognitive measures, and frontal ROI was associated with PS/EF. Third, there were associations, as observed by others, between WMH13, 43–45, infarcts6, 8, 46, 47 and cognition.
The motivation for conducting these analyses was based on the view that neither a small number of visible lacunar infarcts in various locations nor a modest amount of WMH seem sufficiently destructive to cause cognitive dysfunction. We sought evidence for another covert process that was linked pathophysiologically to the visible cerebrovascular lesions. Microinfarcts are the most attractive candidate lesion fitting that description48–52. Clinical-pathological studies17, 49 support an association between microinfarcts and brain volume loss, justifying our use of cortical volume as the indicator of the covert microscopic process.
There are methodological considerations that might have reduced our ability to demonstrate more robust mediation of the relationship between vascular imaging features and cognition by regional cortical volume. Many other regions, beyond the 3 we chose, were not explored, and our choice of cortical regions might have failed to include other salient regions for cerebrovascular disease. Microinfarcts are typically found in cortical regions at boundaries of major vascular territories49, 53. Both our posterior and frontal ROI’s include watershed territory, but also included non-watershed regions as well. That both our posterior and frontal cortical ROI’s mediated WMHPS/EF associations suggests that the underlying process affecting isocortex was not highly localized. Other important methodological factors include: 1) ours was a cross-sectional study; 2) our non-demented cohort represented robust survivors of the original sample, and 3) we imaged only a subset of ARIC visit 5 participants (though we utilized an inverse proportional weighting approach for representing the ARIC visit 5 clinic visit cohort as a whole).
Our findings support our hypothesis that cognitive function in the setting of imaging-visible cerebrovascular lesions is at least in part mediated by a process that affects cerebral cortical volume. However, the modest magnitude of the mediation effect should also prompt consideration of alternative mechanisms or explanations for the influence of WMH and infarcts directly on cognition. One obvious mechanism would be disconnection by white matter disease or subcortical infarcts of cortical-cortical or cortical-subcortical pathways. There is evidence from CADASIL that disconnection could occur as a result cerebrovascular disease54. Perhaps alterations in connectivity55 and structural changes in white matter pathways that are observed with diffusion tensor imaging but cannot be detected by FLAIR imaging56 are the critical underlying mechanisms that link observable WMH, visible infarcts and cognitive impairment. Not all studies detect unique contributions from diffusion tensor imaging57. However, just as the diffusion tensor imaging changes are dissociable from the white matter hyperintensity changes, perhaps the connectivity changes may be dissociable from diffusion tensor imaging changes.
To the best of our knowledge, no prior study has sought to explore how measures of brain volume mediate relationships between cerebrovascular lesions and cognition. One prior study58 found that WMH burden and brain atrophy measures displayed a synergistic interactive effect on declines in executive function. Another study that recruited patients with active large vessel vascular disease in brain, heart, or peripheral vasculature59 found an interaction between brain volume, infarcts and severe WMH for executive dysfunction. In the current analyses, we asked a different question: whether relationships between overt cerebrovascular disease and cognition were mediated by cortical volume. In finding such a relationship, it strengthens the argument that there is a link between overt and covert cerebrovascular disease and cognition.
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions. This is ARIC manuscript #2288.
Sources of funding
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Neurocognitive data is collected by U01 HL096812, HL096814, HL096899, HL096902, HL096917 with previous brain MRI examinations funded by R01-HL70825.
Dr. Knopman serves as Deputy Editor for Neurology®; serves on a Data Safety Monitoring Board for Lundbeck Pharmaceuticals and for the Dominantly Inherited Alzheimer’s Disease Treatment Unit. He receives research support from the NIH.
Kejal Kantarci serves on the data safety monitoring board for Pfizer Inc and Takeda Global Research & Development Center, Inc; and she is funded by the NIH and Minnesota Partnership for Biotechnology and Medical Genomics.
Clifford R. Jack, Jr. serves as a consultant for Janssen, Bristol-Meyer-Squibb, General Electric, Siemens, and Johnson and Johnson and is involved in clinical trials sponsored by Allon and Baxter, Inc. He receives research funding from the National Institutes of Health and the Alexander Family Alzheimer's Disease Research Professorship of the Mayo Foundation Family.
Footnotes
Statistical Analyses were performed by Michael E Griswold and Seth T. Lirette
Disclosures:
Michael E. Griswold -none
Seth T. Lirette -none
Rebecca F. Gottesman -none
A. Richey Sharrett -none
Jonathan Graff-Radford - none
Andrea L.C. Schneider -none
B. Gwen Windham - none
Laura H. Coker - none
Marilyn S. Albert - none
Thomas H. Mosley, Jr. - none
References
- 1.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 2.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 3.Sonnen JA, Santa Cruz K, Hemmy LS, Woltjer R, Leverenz JB, Montine KS, et al. Ecology of the aging human brain. Arch Neurol. 2011;68:1049–1056. doi: 10.1001/archneurol.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knopman D, Parisi JE, Boeve BF, Rocca WA, Cha RH, Apaydin H, et al. Vascular Dementia in a Population-based autopsy study. Arch Neurol. 2003;60:569–576. doi: 10.1001/archneur.60.4.569. [DOI] [PubMed] [Google Scholar]
- 5.Esiri MM, Wilcock GK, Morris JH. Neuropathological assessment of the lesions of significance in vascular dementia. J Neurol Neurosurg Psychiatry. 1997;63:749–753. doi: 10.1136/jnnp.63.6.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 7.Carey CL, Kramer JH, Josephson SA, Mungas D, Reed BR, Schuff N, et al. Subcortical lacunes are associated with executive dysfunction in cognitively normal elderly. Stroke. 2008;39:397–402. doi: 10.1161/STROKEAHA.107.491795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saczynski JS, Sigurdsson S, Jonsdottir MK, Eiriksdottir G, Jonsson PV, Garcia ME, et al. Cerebral infarcts and cognitive performance: importance of location and number of infarcts. Stroke. 2009;40:677–682. doi: 10.1161/STROKEAHA.108.530212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Flier WM, van Straaten EC, Barkhof F, Verdelho A, Madureira S, Pantoni L, et al. Small vessel disease and general cognitive function in nondisabled elderly: the LADIS study. Stroke. 2005;36:2116–2120. doi: 10.1161/01.STR.0000179092.59909.42. [DOI] [PubMed] [Google Scholar]
- 10.Jokinen H, Gouw AA, Madureira S, Ylikoski R, van Straaten EC, van der Flier WM, et al. Incident lacunes influence cognitive decline: the LADIS study. Neurology. 2011;76:1872–1878. doi: 10.1212/WNL.0b013e31821d752f. [DOI] [PubMed] [Google Scholar]
- 11.Carmichael O, Schwarz C, Drucker D, Fletcher E, Harvey D, Beckett L, et al. Longitudinal changes in white matter disease and cognition in the first year of the Alzheimer disease neuroimaging initiative. Arch Neurol. 2010;67:1370–1378. doi: 10.1001/archneurol.2010.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prins ND, van Dijk EJ, den Heijer T, Vermeer SE, Koudstaal PJ, Oudkerk M, et al. Cerebral white matter lesions and the risk of dementia. Arch Neurol. 2004;61:1531–1534. doi: 10.1001/archneur.61.10.1531. [DOI] [PubMed] [Google Scholar]
- 13.Murray ME, Senjem ML, Petersen RC, Hollman JH, Preboske GM, Weigand SD, et al. Functional impact of white matter hyperintensities in cognitively normal elderly subjects. Arch Neurol. 2010;67:1379–1385. doi: 10.1001/archneurol.2010.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith EE, Salat DH, Jeng J, McCreary CR, Fischl B, Schmahmann JD, et al. Correlations between MRI white matter lesion location and executive function and episodic memory. Neurology. 2011;76:1492–1499. doi: 10.1212/WNL.0b013e318217e7c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovari E, Gold G, Herrmann FR, Canuto A, Hof PR, Bouras C, et al. Cortical microinfarcts and demyelination affect cognition in cases at high risk for dementia. Neurology. 2007;68:927–931. doi: 10.1212/01.wnl.0000257094.10655.9a. [DOI] [PubMed] [Google Scholar]
- 16.Smith EE, Schneider JA, Wardlaw JM, Greenberg SM. Cerebral microinfarcts: the invisible lesions. Lancet Neurol. 2012;11:272–282. doi: 10.1016/S1474-4422(11)70307-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Launer LJ, Hughes TM, White LR. Microinfarcts, brain atrophy, and cognitive function: the Honolulu Asia Aging Study Autopsy Study. Ann Neurol. 2011;70:774–780. doi: 10.1002/ana.22520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White L. Brain lesions at autopsy in older Japanese-American men as related to cognitive impairment and dementia in the final years of life: a summary report from the Honolulu-Asia aging study. J Alzheimers Dis. 2009;18:713–725. doi: 10.3233/JAD-2009-1178. [DOI] [PubMed] [Google Scholar]
- 19.Schneider AL, Sharrett AR, Gottesman RF, Coresh J, Coker L, Wruck L, et al. Normative Data for 8 Neuropsychological Tests in Older Blacks and Whites From the Atherosclerosis Risk in Communities (ARIC) Study. [Accessed November 19, 2014];Alzheimer Dis Assoc Disord. 2014 doi: 10.1097/WAD.0000000000000042. [published online ahead of print April 22, 2014]. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=ovft&AN=00002093-900000000-99629&PDF=y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knopman DS, Mosley TH, Catellier DJ, Coker LH. Fourteen-year longitudinal study of vascular risk factors, APOE genotype, and cognition: the ARIC MRI Study. Alzheimers Dement. 2009;5:207–214. doi: 10.1016/j.jalz.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 21.Knopman DS, Penman AD, Catellier DJ, Coker LH, Shibata DK, Sharrett AR, et al. Vascular risk factors and longitudinal changes on brain MRI: The ARIC study. Neurology. 2011;76:1879–1885. doi: 10.1212/WNL.0b013e31821d753f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jack CR, Jr, O'Brien PC, Rettman DW, Shiung MM, Xu Y, Muthupillai R, et al. FLAIR histogram segmentation for measurement of leukoaraiosis volume. J Magn Reson Imaging. 2001;14:668–676. doi: 10.1002/jmri.10011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raz L, Jayachandran M, Tosakulwong N, Lesnick TG, Wille SM, Murphy MC, et al. Thrombogenic microvesicles and white matter hyperintensities in postmenopausal women. Neurology. 2013;80:911–918. doi: 10.1212/WNL.0b013e3182840c9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 26.Kantarci K, Weigand SD, Przybelski SA, Shiung MM, Whitwell JL, Negash S, et al. Risk of dementia in MCI: combined effect of cerebrovascular disease, volumetric MRI, and 1H MRS. Neurology. 2009;72:1519–1525. doi: 10.1212/WNL.0b013e3181a2e864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 28.Jones DT, Machulda MM, Vemuri P, McDade EM, Zeng G, Senjem ML, et al. Age-related changes in the default mode network are more advanced in Alzheimer disease. Neurology. 2011;77:1524–1531. doi: 10.1212/WNL.0b013e318233b33d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dickerson BC, Stoub TR, Shah RC, Sperling RA, Killiany RJ, Albert MS, et al. Alzheimer-signature MRI biomarker predicts AD dementia in cognitively normal adults. Neurology. 2011;76:1395–1402. doi: 10.1212/WNL.0b013e3182166e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuczynski B, Jagust W, Chui HC, Reed B. An inverse association of cardiovascular risk and frontal lobe glucose metabolism. Neurology. 2009;72:738–743. doi: 10.1212/01.wnl.0000343005.35498.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 32.Rabe-Hesketh S, Skrondal A, Pickles A. Generalized multilevel structural equation modeling. Psychometrika. 2004;69:167–190. [Google Scholar]
- 33.Kloppenborg RP, Nederkoorn PJ, Grool AM, Vincken KL, Mali WP, Vermeulen M, et al. Cerebral small-vessel disease and progression of brain atrophy: the SMART-MR study. Neurology. 2012;79:2029–2036. doi: 10.1212/WNL.0b013e3182749f02. [DOI] [PubMed] [Google Scholar]
- 34.Barnes J, Carmichael OT, Leung KK, Schwarz C, Ridgway GR, Bartlett JW, et al. Vascular and Alzheimer's disease markers independently predict brain atrophy rate in Alzheimer's Disease Neuroimaging Initiative controls. Neurobiol Aging. 2013;34:1996–2002. doi: 10.1016/j.neurobiolaging.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Appelman AP, Vincken KL, van der Graaf Y, Vlek AL, Witkamp TD, Mali WP, et al. White matter lesions and lacunar infarcts are independently and differently associated with brain atrophy: the SMART-MR study. Cerebrovasc Dis. 2010;29:28–35. doi: 10.1159/000255971. [DOI] [PubMed] [Google Scholar]
- 36.Thong JY, Hilal S, Wang Y, Soon HW, Dong Y, Collinson SL, et al. Association of silent lacunar infarct with brain atrophy and cognitive impairment. J Neurol Neurosurg Psychiatry. 2013;84:1219–1225. doi: 10.1136/jnnp-2013-305310. [DOI] [PubMed] [Google Scholar]
- 37.Mungas D, Jagust WJ, Reed BR, Kramer JH, Weiner MW, Schuff N, et al. MRI predictors of cognition in subcortical ischemic vascular disease and Alzheimer's disease. Neurology. 2001;57:2229–2235. doi: 10.1212/wnl.57.12.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.den Heijer T, Launer LJ, Prins ND, van Dijk EJ, Vermeer SE, Hofman A, et al. Association between blood pressure, white matter lesions, and atrophy of the medial temporal lobe. Neurology. 2005;64:263–267. doi: 10.1212/01.WNL.0000149641.55751.2E. [DOI] [PubMed] [Google Scholar]
- 39.Silbert LC, Quinn JF, Moore MM, Corbridge E, Ball MJ, Murdoch G, et al. Changes in premorbid brain volume predict Alzheimer's disease pathology. Neurology. 2003;61:487–492. doi: 10.1212/01.wnl.0000079053.77227.14. [DOI] [PubMed] [Google Scholar]
- 40.Jack CR, Jr, Shiung MM, Weigand SD, O'Brien PC, Gunter JL, Boeve BF, et al. Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology. 2005;65:1227–1231. doi: 10.1212/01.wnl.0000180958.22678.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mungas D, Harvey D, Reed BR, Jagust WJ, DeCarli C, Beckett L, et al. Longitudinal volumetric MRI change and rate of cognitive decline. Neurology. 2005;65:565–571. doi: 10.1212/01.wnl.0000172913.88973.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ridha BH, Anderson VM, Barnes J, Boyes RG, Price SL, Rossor MN, et al. Volumetric MRI and cognitive measures in Alzheimer disease : comparison of markers of progression. J Neurol. 2008;255:567–574. doi: 10.1007/s00415-008-0750-9. [DOI] [PubMed] [Google Scholar]
- 43.De Groot JC, De Leeuw FE, Oudkerk M, Van Gijn J, Hofman A, Jolles J, et al. Periventricular cerebral white matter lesions predict rate of cognitive decline. Ann Neurol. 2002;52:335–341. doi: 10.1002/ana.10294. [DOI] [PubMed] [Google Scholar]
- 44.Debette S, Beiser A, DeCarli C, Au R, Himali JJ, Kelly-Hayes M, et al. Association of MRI markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: the Framingham Offspring Study. Stroke. 2010;41:600–606. doi: 10.1161/STROKEAHA.109.570044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maillard P, Carmichael O, Fletcher E, Reed B, Mungas D, DeCarli C. Coevolution of white matter hyperintensities and cognition in the elderly. Neurology. 2012;79:442–448. doi: 10.1212/WNL.0b013e3182617136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schneider JA, Boyle PA, Arvanitakis Z, Bienias JL, Bennett DA. Subcortical infarcts, Alzheimer's disease pathology, and memory function in older persons. Ann Neurol. 2007;62:59–66. doi: 10.1002/ana.21142. [DOI] [PubMed] [Google Scholar]
- 47.Gold G, Kovari E, Herrmann FR, Canuto A, Hof PR, Michel JP, et al. Cognitive consequences of thalamic, basal ganglia, and deep white matter lacunes in brain aging and dementia. Stroke. 2005;36:1184–1188. doi: 10.1161/01.STR.0000166052.89772.b5. [DOI] [PubMed] [Google Scholar]
- 48.Longstreth WT, Jr, Sonnen JA, Koepsell TD, Kukull WA, Larson EB, Montine TJ. Associations between microinfarcts and other macroscopic vascular findings on neuropathologic examination in 2 databases. Alzheimer Dis Assoc Disord. 2009;23:291–294. doi: 10.1097/WAD.0b013e318199fc7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raman MR, Preboske GM, Przybelski SA, Gunter JL, Senjem ML, Vemuri P, et al. Antemortem MRI findings associated with microinfarcts at autopsy. Neurology. 2014;82:1951–1958. doi: 10.1212/WNL.0000000000000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Young VG, Halliday GM, Kril JJ. Neuropathologic correlates of white matter hyperintensities. Neurology. 2008;71:804–811. doi: 10.1212/01.wnl.0000319691.50117.54. [DOI] [PubMed] [Google Scholar]
- 51.Murray ME, Vemuri P, Preboske GM, Murphy MC, Schweitzer KJ, Parisi JE, et al. A quantitative postmortem MRI design sensitive to white matter hyperintensity differences and their relationship with underlying pathology. J Neuropathol Exp Neurol. 2012;71:1113–1122. doi: 10.1097/NEN.0b013e318277387e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schneider JA, Wilson RS, Cochran EJ, Bienias JL, Arnold SE, Evans DA, et al. Relation of cerebral infarctions to dementia and cognitive function in older persons. Neurology. 2003;60:1082–1088. doi: 10.1212/01.wnl.0000055863.87435.b2. [DOI] [PubMed] [Google Scholar]
- 53.Brundel M, de Bresser J, van Dillen JJ, Kappelle LJ, Biessels GJ. Cerebral microinfarcts: a systematic review of neuropathological studies. J Cereb Blood Flow Metab. 2012;32:425–436. doi: 10.1038/jcbfm.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duering M, Righart R, Csanadi E, Jouvent E, Herve D, Chabriat H, et al. Incident subcortical infarcts induce focal thinning in connected cortical regions. Neurology. 2012;79:2025–2028. doi: 10.1212/WNL.0b013e3182749f39. [DOI] [PubMed] [Google Scholar]
- 55.Lawrence AJ, Chung AW, Morris RG, Markus HS, Barrick TR. Structural network efficiency is associated with cognitive impairment in small-vessel disease. Neurology. 2014;83:304–311. doi: 10.1212/WNL.0000000000000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lawrence AJ, Patel B, Morris RG, MacKinnon AD, Rich PM, Barrick TR, et al. Mechanisms of cognitive impairment in cerebral small vessel disease: multimodal MRI results from the St George's cognition and neuroimaging in stroke (SCANS) study. PLoS One. 2013;8:e61014. doi: 10.1371/journal.pone.0061014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Norden AG, van Uden IW, de Laat KF, van Dijk EJ, de Leeuw FE. Cognitive function in small vessel disease: the additional value of diffusion tensor imaging to conventional magnetic resonance imaging: the RUN DMC study. J Alzheimers Dis. 2012;32:667–676. doi: 10.3233/JAD-2012-120784. [DOI] [PubMed] [Google Scholar]
- 58.Jokinen H, Lipsanen J, Schmidt R, Fazekas F, Gouw AA, van der Flier WM, et al. Brain atrophy accelerates cognitive decline in cerebral small vessel disease: the LADIS study. Neurology. 2012;78:1785–1792. doi: 10.1212/WNL.0b013e3182583070. [DOI] [PubMed] [Google Scholar]
- 59.Muller M, Appelman AP, van der Graaf Y, Vincken KL, Mali WP, Geerlings MI. Brain atrophy and cognition: interaction with cerebrovascular pathology? Neurobiol Aging. 2011;32:885–893. doi: 10.1016/j.neurobiolaging.2009.05.005. [DOI] [PubMed] [Google Scholar]