Abstract
Previous reports have drawn attention to persistently decreased platelet counts among liver donors. We hypothesized an etiologic association between altered platelet counts and post-donation splenomegaly and sought to explore this relationship. This study analyzed de-identified CT/MR scans of 388 donors from 9 A2ALL centers read at a central computational image analysis lab. Resulting liver and spleen volumes were correlated with time-matched clinical lab values. Pre-donation liver volumes varied twofold in healthy subjects, even when normalized by body surface area (BSA) (range: 522 – 1887cc/m2, N=346). At 3 months post-donation liver volumes were, on average, 79% of pre-donation volumes (IQR: 73-86%, N=165) and approached 88% at 1 year (IQR: 80-93%, N=75). The mean spleen volume pre-donation was 245 cc (N=346). Spleen volumes greater than 100% of pre-donation volume occurred in 92% of donors at 3 months (N=165) and 88% at 1 year post-donation (N=75). We sought to develop a standard spleen volume (SSV) model to predict “normal” spleen volumes in donors pre-donation and found that decreased platelet counts, younger age, higher pre-donation liver volume, higher hemoglobin and higher BSA predicted a larger spleen volume (N=344, R2=0.52). When applied to post-donation values some large volumes were under predicted by the SSV model. Models developed on the reduced sample of post-donation volumes yielded smaller under-predictions. These findings confirm previous observations of thrombocytopenia associated with splenomegaly post-donation. The results of the SSV model suggest the biology of this phenomenon is complex. This merits further long term mechanistic studies of liver donors with investigation into the role of other factors such as thrombopoietin, and exposure to viral infections to better understand the evolution of spleen volume after liver donation.
Keywords: Hepatectomy, Infection, Liver Function, Splenomegaly, Survival, Thrombopoietin
Introduction and aims
Although living donor liver transplantation (LDLT) has a high success rate (1,2) and increases access to transplantation (2), concerns for donor safety and the technical complexity of the recipient operation have greatly limited expansion of the procedure in Western countries. Although LDLT is the principal donor source for liver transplantation in Asian countries (3,4), LDLT has accounted for less than 5% of procedures performed annually in the United States in the past 5 years. Unfortunately, the limited use of LDLT persists despite demonstration of safety in the donors and efficacy in the recipients. There is compelling survival benefit for the recipient in choosing LDLT (5) even in patients with MELD as low as 10 (6), and the morbidity of donation in a large multicenter trial of LDLT (7) was acceptable as previously observed in single center studies.
Despite apparent pre-existing good health and early clinical recovery of most donors, abnormal laboratory tests were noted in some subjects at least one year after donation. We first reported a persistent decline in platelet counts through one year after donation in a subset of liver donors from a single center in 2004 (8). This finding was subsequently observed in the much larger A2ALL cohort (9). In the 487 subjects in that cohort, platelet counts were significantly decreased at one year for the population as a whole and below the lower limit of normal in 7% of 327 subjects with normal lab values pre-donation who were 1 year or more post-donation. In a sub-study of quantitative liver function of A2ALL donors, we noted that splenomegaly in some subjects corresponded to decreased platelet counts (10).
In the current study, we sought to determine whether abnormal lab tests after donation were associated with splenomegaly or volumetric alterations in the liver by examining the relationship between liver and spleen volumes and the evolution of laboratory tests up to one year after donation. Because spleen size is associated with platelet counts, and may be an indicator of portal hypertension, we sought to characterize “normal” spleen volume prior to donation and changes after donor partial hepatectomy. In addition, we sought to identify predictors of abnormal laboratory tests and to identify a subset of subjects at risk for marked splenomegaly after donation.
Methods
The Adult-to-Adult Living Donor Liver Transplantation Cohort Study (A2ALL) is an observational study at 9 US centers that was conducted to evaluate the efficacy of LDLT in adult recipients and to characterize the impact of donation on healthy subjects. CT/MR scans were collected on 388 subjects who underwent donor hepatectomy between July 1998 and May 2010. Demographic and clinical variables were collected both prospectively and retrospectively with follow-up to 11 years post-donation. Imaging and analysis: CT/MR scans pre-donation, at three months (M3) and one year (Y1) after donation were collected as available from the study sites, de-identified and transmitted using AG Mednet's system (AG Mednet, Inc. Boston, MA) to a central Computational Image Analysis lab at Columbia University Medical Center (11, 12).
Images were coded to permit merging with clinical information. Liver and spleen volumes were calculated using a proprietary, organ-generic algorithm developed by the Computational Image Analysis lab (13, 14). Scan-based volumes were correlated with clinical and laboratory features at corresponding times.
Statistical methods
We used means, standard deviations (SD), ranges, box plots, and percentages to describe donor characteristics by time point. Correlation coefficients and scatter plots were used to assess relationships between corresponding variables at different time points as well as different variables at the same time point. Spaghetti plots were used to assess within-person trends in variables over time.
Linear regression was used to model pre-donation spleen volume as a function of laboratory and patient characteristics. Variables tested in the model included body surface area (BSA), height, weight, body mass index, age, gender, pre-donation liver volume, platelet count, white blood cell count and hemoglobin. Model fit was assessed using R-squared, leverage and influence diagnostics, and residual plots. Variable selection was performed using the method of best subsets. Logistic regression was used to test predictors of pre-donation spleen volumes greater than 400 cc. All analyses were performed using SAS version 9.2 (SAS Institute, Inc., Cary, NC).
Results
Donor characteristics are summarized in Table 1. The gender distribution was nearly equal and the majority of donors were white (92.5%) and non-Latino (86.3%). Height ranged from 134.6 to 195.6 cm and weight from 43.1-135.0 kg. Although mean body mass index was 26.3, the range was substantial including subjects in the overweight and even obese categories (IQR: 23.3-28.8, range: 16.4-42.4). Only 25 subjects (6.4%) were left lobe donors.
Table 1.
Characteristics of 388 living liver donors
| Characteristic | N | Mean (Standard Deviation) or % | Range |
|---|---|---|---|
| Age (years) | 388 | 37.8 (10.4) | 18.2 – 62.7 |
| Sex | |||
| Male | 189 | 48.7 | |
| Female | 199 | 51.3 | |
| Ethnicity | |||
| Hispanic | 53 | 13.7 | |
| Non-Hispanic | 335 | 86.3 | |
| Race | |||
| White | 359 | 92.5 | |
| African American | 11 | 2.8 | |
| Asian | 6 | 1.5 | |
| Other | 12 | 3.1 | |
| Height (cm) | 388 | 171.8 (10.2) | 134.6 – 195.6 |
| Weight (kg) | 388 | 77.9 (14.8) | 43.1 – 135.0 |
| Body mass index (kg/m2) | 388 | 26.3 (3.9) | 16.4 – 42.4 |
| Left lobe donor | 25 | 6.4 |
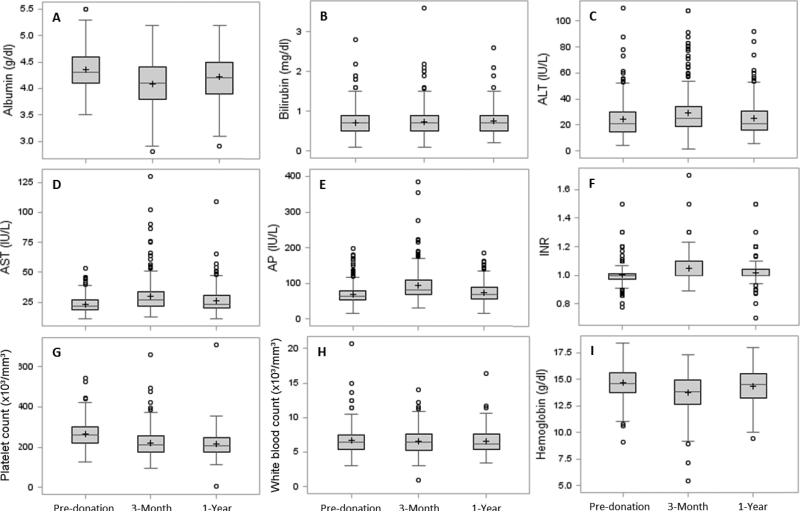
Table 2 summarizes the laboratory and organ volume data pre-donation, M3, and Y1. Several laboratory tests of liver function (albumin, bilirubin, AST, ALT, alkaline phosphatase, and international normalized ratio) were significantly different from pre-donation at M3 and/or Y1, although the changes were small and generally remained in the normal range. Similarly, alterations in the hematologic profiles were seen for hemoglobin, (although Y1 levels returned to values very close to pre-donation), and platelet counts, (which declined at M3 and remained significantly lower than pre-donation at Y1 [p<0.0001]). A subset of patients (8.9%) had platelet counts below the lower limit of normal (150×103/mm3) as observed in our previous reports. Graphical representations of the laboratory tests are depicted in Figure 1. Some of these had skewed distributions.
Table 2.
Donor lab values and liver and spleen volumes by time point (pre-donation, and post-donation at 3 months and 1 year)
| Mean (SD) Range N | Pre-Donation | 3-Month | 1-Year |
|---|---|---|---|
|
Lab values at each time point
| |||
| Albumin (g/dl) | 4.4 (0.4) | 4.1 (0.4)* | 4.2 (0.4) * |
| 3.5 – 5.5 | 2.8 – 5.2 | 2.9 – 5.2 | |
| 387 | 265 | 192 | |
| Bilirubin (mg/dl) | 0.7 (0.3) | 0.7 (0.4) | 0.8 (0.3)* |
| 0.1 – 2.8 | 0.1 – 3.6 | 0.2 – 2.6 | |
| 388 | 272 | 197 | |
| ALT (IU/L) | 24.4 (12.5) | 29.5 (16.5) * | 25.2 (13.5) |
| 4.0 – 110.0 | 1.5 – 108.0 | 6.0 – 92.0 | |
| 388 | 272 | 195 | |
| AST (IU/L) | 23.3 (6.6) | 29.9 (13.9) * | 26.1 (11.0) * |
| 11.0 – 53.0 | 13.0 – 130.0 | 11.0 – 109.0 | |
| 387 | 272 | 197 | |
| AP (IU/L) | 68.4 (24.8) | 93.8 (42.9) * | 74.1 (26.3) * |
| 15.0 – 197.0 | 30.0 – 385.0 | 16.0 – 186.0 | |
| 388 | 271 | 196 | |
| INR | 1.00 (0.08) | 1.05 (0.09) * | 1.02 (0.09) * |
| 0.78 – 1.50 | 0.89 – 1.70 | 0.70 – 1.50 | |
| 382 | 254 | 186 | |
| Platelet count (×103/mm3) | 264.3 (63.0) | 221.6 (67.6) * | 214.4 (63.7) * |
| 126.0 – 543.0 | 94.0 – 660.0 | 3.6 – 708.0 | |
| 387 | 270 | 192 | |
| White blood count (×103/mm3) | 6.6 (1.8) | 6.6 (1.7) | 6.6 (1.7) |
| 3.1 – 20.7 | 0.9 – 14.0 | 3.4 – 16.4 | |
| 387 | 269 | 194 | |
| Hemoglobin (g/dl) | 14.6 (1.4) | 13.7 (1.8) * | 14.4 (1.5) * |
| 9.1 – 18.4 | 5.4 – 17.3 | 9.4 – 18.0 | |
| 387 | 268 | 192 | |
|
Volumes at each time point | |||
| Liver volume (cc) | 1601.0 (327.3) | 1241.2 (257.1) * | 1440.5 (274.0) * |
| 863.8 – 3250.8 | 790.0 – 2024.1 | 936.3 – 2122.0 | |
| 346 | 182 | 90 | |
| Spleen volume (cc) | 245.6 (107.3) | 314.3 (136.4) * | 323.6 (181.4) * |
| 67.1 – 774.6 | 77.6 – 842.2 | 82.5 – 1171.7 | |
| 346 | 182 | 90 | |
| Liver/Spleen Ratio | 7.4 (2.8) | 4.6 (1.8)* | 5.5 (2.6)* |
| 2.5 – 22.8 | 1.7 – 11.2 | 1.2 – 15.9 | |
| 346 | 182 | 90 | |
Significantly different from pre-donation (p<0.05) by paired t-test
Figure 1.
Distributions of lab values shown as box plots by time points. The box spans from the first to the third quartile, and shows the median (line crossing the box) and mean (+). Whiskers extend to the farthest data point within 1.5*interquartile range from the box ends, with all outlying points shown individually as circles.
Results below are based on all available data. Due to incomplete follow-up for many donors, we have repeated all analyses below restricted to right lobe donors with complete volume data (pre-donation, 3-month and 1-year, N=48). Only one left lobe donor had complete volume data and was excluded from these analyses. All results were similar to the results presented below, taking the smaller sample size into account. These analyses are available in the supplementary materials (supplementary table S1, corresponding to Table 3, and figures S1-S6).
Table 3.
Linear regression models predicting spleen volumes (cc)
| Model A: Pre-donation Spleen Volume (N=344, R2=0.52) | ||
|---|---|---|
| Variable | Parameter Estimate | P-value |
| Pre-donation liver volume (per 100 cc) | 11.14 | <0.001 |
| Platelet count at evaluation (× 50,000/mm3) | −19.07 | <0.001 |
| Body Surface Area | 115.22 | <0.001 |
| Hemoglobin at evaluation (g/dl) | 11.64 | <0.001 |
| Donor age at evaluation (per 10 years) | −13.23 | <0.001 |
| Model B: Post-donation M3 Spleen Volume (N=167, R2=0.40) | ||
|---|---|---|
| Variable | Parameter Estimate | P-value |
| Post-donation M3 liver volume (per 100 cc) | 16.43 | <0.001 |
| Platelet count at evaluation (× 50,000/mm3) | −28.46 | <0.001 |
| Gender (ref: male) | 77.03 | <0.001 |
| Model C: Post-donation Y1 Spleen Volume (N=75, R2=0.42) | ||
|---|---|---|
| Variable | Parameter Estimate | P-value |
| Post-donation Y1 liver volume (per 100 cc) | 17.64 | <0.001 |
| Platelet count at evaluation (× 50,000/mm3) | −18.01 | 0.04 |
| Hemoglobin (g/dl) | 32.75 | <0.001 |
Liver and spleen volumes over time
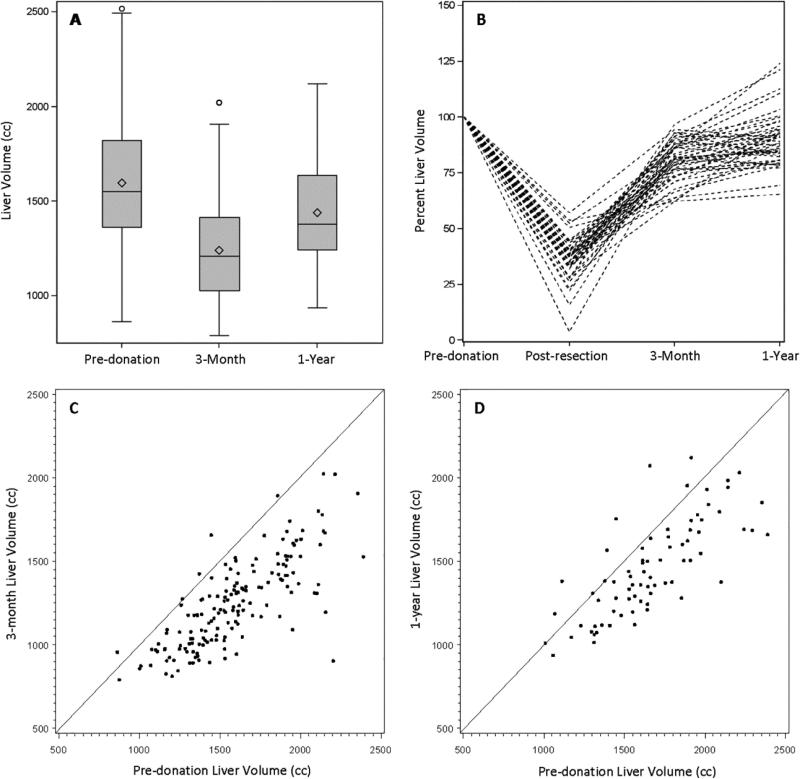
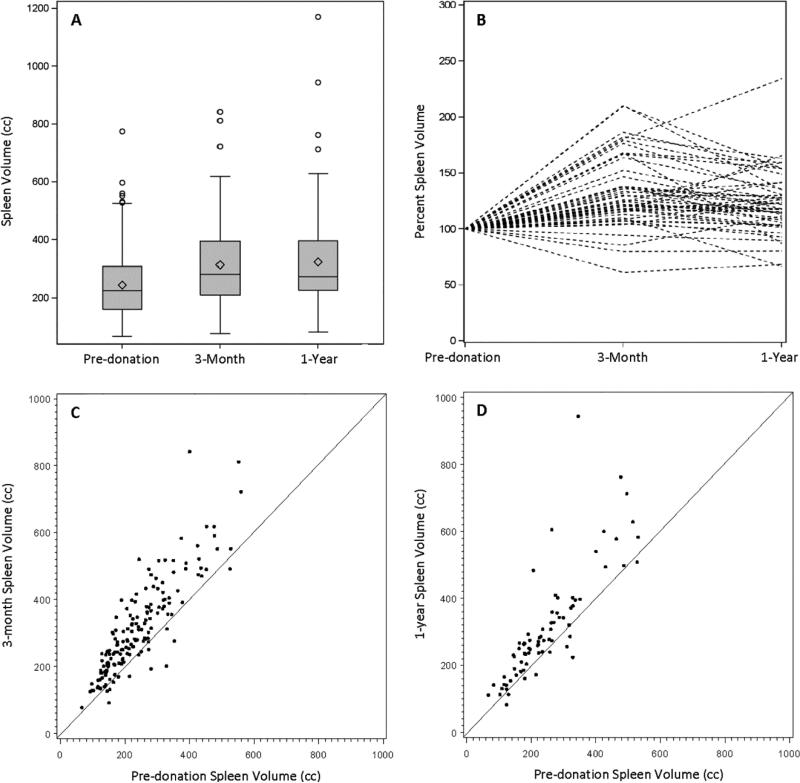
The mean pre-donation total liver volume was 1601 cc (Table 2), averaging 2.0% of the donor's body weight (range 1.4-5.2%). There were 363 right lobe and 25 left lobe donors (6.4%). The mean right lobe volume was 1067cc and mean left lobe volume was 599 cc (67% and 33% of total liver volume, respectively). The mean ± standard deviation of pre-donation spleen volumes were 245 ±107 cc; these volumes substantially increased at M3 and Y1 (paired t-tests; M3: N=165, Y1: N=75; p<0.0001 for both). The ratio of liver volume to spleen volume was 7.4 pre-donation, 4.6 at M3 and 5.5 at Y1; the latter two were significantly lower than pre-donation (paired t-tests; M3: N=165, Y1: N=75; p<0.0001 for both).
In Figure 2, panel A depicts liver volumes using box plots at pre-donation, M3 and Y1. Liver volumes were 79% of pre-donation volume at M3 (N=165) and approached 88% of the pre-donation volume at Y1 (N=75). Liver volumes pre-donation were highly variable. Even when normalized by BSA, liver volumes varied twofold in these healthy subjects; this pattern was observed at all time points (range in cc pre-donation: 522 – 1887; M3: 428 – 932; Y1: 542 – 980). In panel B, the spaghetti plot demonstrates the course of liver volumes through resection and regeneration as a percentage of the donor's pre-donation liver volume for individual subjects who had all necessary measurements (N=46). The comparisons in liver volume between pre-donation and post-donation time points are shown individually in panels C and D. At both M3 (panel C, N=165) and Y1 (panel D, N=75), the majority of livers were smaller than pre-donation.
Figure 2.
Liver volumes pre-donation, post-resection (panel B only), and post-donation at 3 months and 1 year. Volumes are shown as (A) box plots by time point (pre-donation: N=346, 3-month: N=182, 1-year: N=90), (B) a spaghetti plot showing volumes over time as a percent of pre-donation volumes for donors with complete data at all 4 time points (N=46), and as scatter plots of (C) 3 month volume (N=165) and (D) 1 year volume (N=75) plotted against pre-donation volumes, with a diagonal identity line.
With respect to the spleen volumes (Figure 3), mean spleen volume pre-donation was 245 ± 107 cc and ranged from 67-775 cc (median: 226, IQR: 161-310) (panel A). Even when normalized by BSA, the range was substantial (40–396 cc/ m2), although larger subjects generally had larger spleens (r=0.52, p<0.0001). The spaghetti plot (panel B) among donors who had volumes at all three time points (N=49) demonstrates the course of the spleen volume over time with a range as high as 234% of pre-donation volume at Y1. The majority of subjects had spleen volumes greater than 100% of pre-donation volume at M3 and Y1. Similarly, panels C (N=165) and D (N=75) show the increase in spleen volumes over time.
Figure 3.
Spleen volumes pre-donation and post-donation at 3 months and 1 year. Volumes are shown as (A) box plots by time point (pre-donation: N=346, 3-month: N=182, 1-year: N=90), (B) a spaghetti plot showing volumes over time as a percent of pre-donation volume for donors with complete data at all 3 time points (N=49), and as scatter plots of (C) 3 month volume (N=165) and (D) 1 year volume (N=75) plotted against pre-donation volumes, with a diagonal identity line.
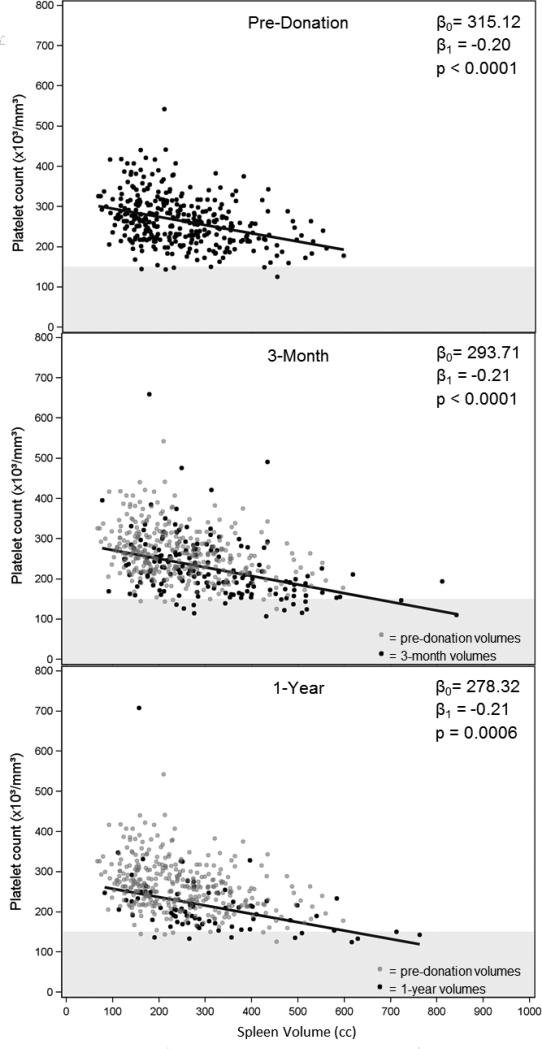
The relationship between spleen size and platelet counts (Figure 4)
Figure 4.
Scatter plots of platelet counts vs. spleen volumes at 3 time points. On the 3-month and 1-year plots, the pre-donation values are plotted as gray dots (N=345) behind the post-donation values (black dots; 3-month: N=167, 1-year: N=76). Regression lines are shown, and the intercepts (β0), slopes (β1) and p-values are given. Values below the lower limit of normal for platelet counts (150) are shown in the gray horizontal band.
There was a highly significant negative association between platelet count and spleen volume at all time points (panel A – pre-donation, panel B – M3, panel C – Y1) with a relatively consistent slope but decreasing intercepts over time. The pre-donation values are superimposed on panels B and C to show the comparison of post-donation to pre-donation data.
Development of a model for the prediction of standard spleen volume (SSV)
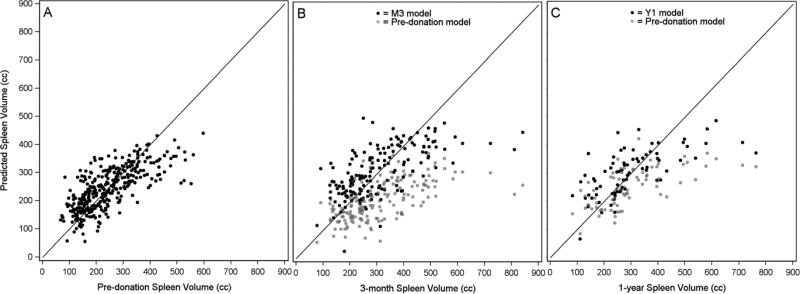
We sought to develop a model for the prediction of the “normal” spleen volume in healthy donors pre-donation (Table 3, Model A). Up to 14 variables were tested in model selection. The best model included 5 significant variables; larger spleen size was predicted by larger pre-donation liver volume, lower platelet counts, larger BSA, higher hemoglobin, and younger donor age. With an R2 value of 0.52 the model performed well for most observed spleen volumes but the fit suffered in a small group of observed large spleens where the model consistently under predicted the volumes. A logistic model was fit to predict the probability of pre-donation spleen volumes greater than 400 cc to further investigate the outliers in the linear regression model but the significant predictors were BSA and platelet counts, which were already seen as predictors of larger spleen volumes in the SSV.
Models for spleen volume over time
First we assessed the applicability of the SSV model to post-donation spleen volumes. Figure 5 presents predicted vs. observed spleen volumes using predictions from the pre-donation model. At M3 we found that the model consistently under-estimated the spleen volumes across all observed volumes. At Y1 under prediction continued, but only in the larger observed spleen volumes. To investigate if other variables might be important for predicting spleen volumes after liver hepatectomy that were not seen pre-donation, we developed models to predict post-donation spleen volumes at both M3 and Y1 (Table 3, Models B (N=167) and C (N=75), respectively). Although sample sizes in both post-donation models were smaller than at pre-donation, lower platelets and larger liver volume were still seen as significant predictors of larger spleen volumes. Lower platelets predicted even larger spleens at M3 than seen in the models at other time points (based on parameter estimates in the three models); similarly, larger liver volumes predicted slightly larger spleen volumes in both post-donation models compared to the pre-donation model. The Y1 model also showed that higher hemoglobin predicted larger spleen volumes, with nearly three times the magnitude as seen in the pre-donation model. At M3 males were predicted to have larger spleen volumes. Neither age at donation nor BSA were significant predictors in either post-donation model. Figure 5 also presents predicted vs. observed spleen volumes using predictions from the post-donation models. As seen in the application of the pre-donation model to post-donation volumes, the post-donation models show the under prediction of the observed large spleen volumes. This suggests persistent splenomegaly post-donation occurs in a variety of donors and changes in such spleen volumes cannot be completely explained through the application of linear models using patient demographics and lab values alone. Portal vein thrombosis, although of interest, could not be tested because only one case was reported for the donors in this analysis.
Figure 5.
Predicted vs. observed spleen volumes by time point. Panel A shows pre-donation predicted values calculated using the pre-donation spleen volume model (N=345). In Panels B (N=167) and C (N=75) the black dots show post-donation predicted values using the 3-month (M3) and 1-year (Y1) spleen volume models, respectively, and the light gray dots show predicted values calculated based on the pre-donation spleen volume model.
Effect of donated liver lobe on spleen volume
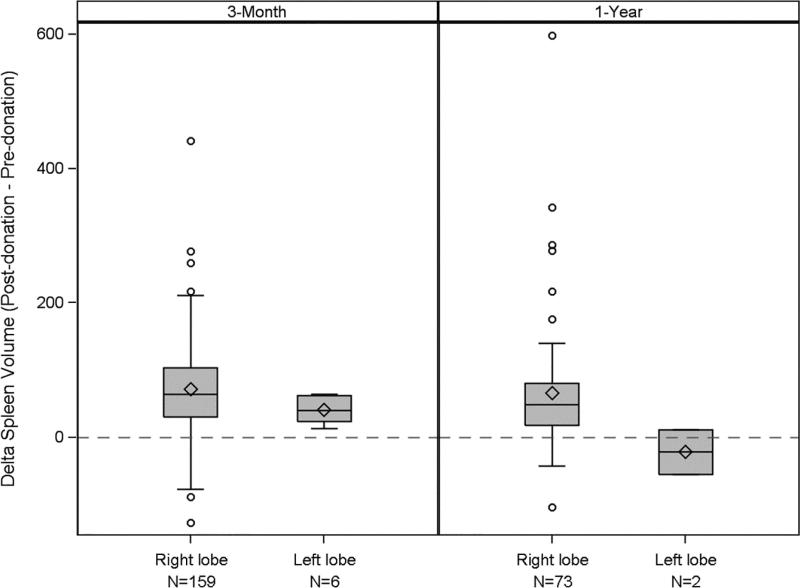
Because right hepatectomy involves resection of more than half of the liver resulting in a smaller remnant liver, we hypothesized that the impact of donation on spleen size might be different between right lobe and left lobe donors. We present the changes in spleen volume between pre- and post-donation for right and left lobe donors in Figure 6. At M3 the average change in spleen volume (δ=M3 – pre-donation) was significantly different between right and left lobe donors (right lobe mean δ=72 cc, left lobe mean δ =41 cc, p=0.01, N=165). At Y1 (δ=Y1 – pre-donation), the left lobe effect was even more pronounced (right lobe mean δ=66 cc, left lobe mean δ=−22 cc, p=0.2, N=75), although power was limited with only 2 left lobe donors. While the samples are small, these observations are consistent with the possibility that the extent of hepatic resection is a contributor to the risk of splenomegaly after donation.
Figure 6.
Spleen volume changes from pre- to post-donation: a comparison between right and left lobe donors at 3 months (p=0.01) and 1 year (p=0.20). (Note small numbers of left lobe donors limit statistical power.) The box spans from the first to the third quartile, and shows the median (line crossing the box) and mean (+). Whiskers extend to the farthest data point within 1.5*interquartile range from the box ends, with all outlying points shown individually as circles.
Long term laboratory abnormalities
We examined laboratory abnormalities beyond one year, and found among the 11 patients with abnormal platelet counts in either M3 or Y1 who had at least 1 measurement between year 2 and year 4, 8 had abnormal platelet counts at least one time during year 2 to year 4, indicating persistence of this condition in a subset of patients. The incidence of complications recorded for donors, however, was not different for the 12 donors with spleen volumes greater than 500 cc at Y1 (p=0.81).
Discussion
This study demonstrates that thrombocytopenia observed after live donor hepatectomy is highly correlated with persistent changes in spleen size. Increase in spleen size was observed in nearly all subjects after hepatectomy with a subset in the abnormal range. Our efforts to develop a predictive model for spleen size were complicated by the presence of a subset of subjects skewed toward larger spleen sizes both before and after liver donation, indicating that the biology of this is not subject to simplistic interpretation. Our SSV modeling identified a number of highly significant predictive variables for standard spleen volumes. A population of outliers emerged when this proposed standard spleen volume equation was applied to post-donation measures. Further, separate models developed in the post-donation setting on a smaller sample of donors were not able to identify any predictors to sufficiently model these large spleens either. The clinical significance of these findings will require long term study of donors with particular attention to these subjects.
Understanding the significance of persistent splenomegaly after donation is complicated by the paucity of study of spleen size in the literature. Pozo et al (15) characterized the spectrum of conditions associated with splenomegaly ranging from infections to malignancy. Perhaps more relevant to the population of potential donors, clinically apparent splenomegaly was identified in 2.5% of healthy college freshmen (16), none of whom developed clinical disease in over a decade of follow-up. Of the pathologic conditions associated with splenomegaly, infections and hematologic disorders figure prominently. We observed in the donor population that spleen size is critically related to hematologic parameters even in healthy subjects. That said, most of the published data on spleen size are from the hematology literature and generally did not take advantage of 3-D volumetrics based on more advanced interpretation of integrated sequences from cross-sectional imaging.
Our efforts to develop a predictive model for spleen size included liver size as well as a variety of hematologic parameters. The importance of liver size in our model can be attributed to the intrinsic relationship of blood flow between the liver and the spleen. In a recent cohort of patients with congenital hepatic fibrosis, a highly significant correlation between spleen size and platelet count was observed (17). Interestingly, however, the authors found no correlation between hepatic function and platelets or spleen size, similar to the findings in the current study, though perhaps albumin is too superficial a marker of hepatic function to fully explore this relationship. In addition to its role as an immune regulator and hematologic modulator, the spleen has been implicated as contributor to capacitance in the cardiovascular system (18), and the splanchnic circulation is a well known regulator of intravascular volume. Clearly, the complexities of these functions are well beyond the capacity of our study to investigate. Nonetheless, alterations of both liver volumes and spleen volumes over time are worth investigating on a more mechanistic level.
The relationship between the regenerating liver and the spleen is the core issue in the current study, as the subjects selected for donation are clinically healthy and are screened for a broad spectrum of health conditions. In addition to the simple parameters available in the ordinary donor workup, there is likely to be a relationship between spleen size and previous exposure to common viruses such as EBV, though the variability of such screening in the donor population did not permit us to address this issue. Surprisingly little has been reported on the issue of spleen size though the question has been addressed in ultrasonographic studies of athletes with mononucleosis (19). Our data reaffirmed that liver volume is not restored to pre-donation size in the majority of subjects at one year despite normalization of laboratory tests of liver function consistent with Pomfret's early report and recent publication from a Korean group with a large cohort of right lobe donations (9, 20, 21).
While portal hypertension may influence spleen size in extreme instances, the relationship between the not fully regenerated liver and the spleen may also affect platelet counts due to decreased levels of thrombopoietin (22). This hormone has been implicated in platelet development and is decreased in the presence of liver disease (22-24). In LDLT, Nagasako et al. demonstrated that low platelet counts were correlated with decreased levels of thrombopoietin on day 7 after liver donation (25). Thrombopoietin has not been studied long term in liver donors, and in light of our findings further mechanistic studies are warranted. The overall examination of platelet counts in our patients indicates that, although counts are significantly lower over time in all subjects, very few fall outside of normal range. This is similar to the issue of liver regeneration, although the livers at one year are, on average, 88% of pre-donation, none of the subjects have clinically evident functional impairments. The Everson study cited earlier (10) included corresponding quantitative liver function assessment that might yield better information if repeated with a larger number of patients.
Our data are dependent on the accuracy of cross-sectional imaging, which has been widely used in surgical planning in LDLT (26, 27). To overcome variability in volumetrics associated with center practices and techniques we collected all available scans and re-analyzed them using a central computational laboratory (9-14). In addition, liver and spleen volumes are not routinely assessed in clinical evaluation of patients with liver disease though this is clearly a useful adjunct in characterizing the course of recovery after liver surgery. Our data demonstrate wide variability of liver and spleen size in healthy subjects. While peripheral to the main aims of this paper, we caution against the rigid use of equations of standard liver volume in surgical decision-making in hepatectomy. Although it is sometimes necessary to have an objective norm, clinician should be aware of variation among individuals.
The most important practical question raised by this study is the clinical significance of persistent abnormalities of liver volume, spleen volume and platelet counts after donation. Although we only have volumetric data for a quarter of our subjects at one year, we have essentially complete data for the subjects' laboratory values, and the correlation of platelet counts with volume data are consistent over time. Clinical follow-up of right lobe donors is now well over a decade in many centers, and few subjects with chronic disease have been identified. However, we feel strongly that the small subset of donors who are outliers with respect to organ volumes and laboratory results merit close long term follow-up.
Strengths of the current study include the large number of subjects and the multicenter collection of experience that best reflects the diversity of practice and experience. Thus, this report is a valuable complement to the single-center reports on this issue. Weaknesses include the lack of complete follow-up with an increasing number of missing scans with time after donation. Furthermore, analysis of correlative mechanistic data will be essential to better understanding the physiology of splenomegaly after donation. Finally, the small number of left lobe donors with complete follow-up has provided some confirmation but prevents us from fully addressing the argument made by Makuuchi et al (3) that left lobe donors might fare better than right lobe donors due to the larger residual liver volume and less portal hypertension (28).
In conclusion, we have confirmed that the persistent thrombocytopenia observed in living liver donors is associated with splenomegaly that is persistent up to one year after donation. However, abnormalities outside the normal range are limited to a small number of subjects. The variability of liver and spleen volumes pre-donation is re-iterated and reminds us that the biologic complexity bedevils the creation of rigid formulas for surgical planning. Nonetheless, the extreme outliers clearly need close follow-up to ascertain whether these findings are harbingers of subclinical disease that may evolve. This study is clearly hypothesis generating, and two issues need to be studied: first the role of regulatory hormones such as ADH and thrombopoietin, which might be altered after donation and affect spleen size and platelets, and secondly the need to extend follow-up on subjects much longer after donation to ascertain the effects of donation on long term health.
Supplementary Material
Acknowledgement
The following individuals were instrumental in the planning and conduct of this study at each of the participating institutions:
Columbia University Medical Center, New York, NY (DK62483): PI: Jean C. Emond, MD; Co-Is: Robert S. Brown, Jr., MD, MPH, James Guarrera, MD, FACS, Martin R. Prince, MD, PhD, Benjamin Samstein, MD, Elizabeth Verna, MD, MS; Study Coordinators: Taruna Chawla, MD, Scott Heese, MPH, Theresa Lukose, PharmD, Rudina Odeh-Ramadan, PharmD, Jonah Zaretsky, BS.
Northwestern University, Chicago, IL (DK62467): PI: Michael M.I. Abecassis, MD, MBA; Co-Is: Talia Baker, MD, Laura M. Kulik, MD, Daniela P. Ladner, MD; Study Coordinator: Patrice Al-Saden, RN, CCRC.
University of California Los Angeles, Los Angeles, CA (DK62496): PI: Johnny C. Hong, MD; Co-I: Ronald W. Busuttil, MD, PhD; Study Coordinator: Janet Mooney, RN, BSN.
University of California San Francisco, San Francisco, CA (DK62444): PI: Chris E. Freise, MD, FACS; Co-I: Norah A. Terrault, MD, MPH; Study Coordinator: Dulce MacLeod, RN. University of Colorado, Aurora, CO (DK62536): PI: James R. Burton, Jr., MD; Co-Is: Gregory T. Everson, MD, FACP, Igal Kam, MD, James Trotter, MD; Study Coordinators: Carlos Garcia, RN, BS, Anastasia Krajec, RN.
University of Michigan Health System, Ann Arbor, MI (DK62498): PI: Robert M. Merion, MD, FACS; DCC Staff: Mary Akagi, MS, CCRP, Douglas R. Armstrong, BSN, MS, Abby Brithinee, BA, Margaret Hill-Callahan, BS, LSW, Lisa Holloway, BS, CCRC, Terese A. Howell, BS, CCRC, Brenda W. Gillespie, PhD, Beth Golden, BScN, Anna S.F. Lok, MD, Monique Lowe, MSI, Akinlolu O. Ojo, MD, PhD, Samia Shaw, AAIT, Abigail Smith, MS, Robert A. Wolfe, PhD. University of North Carolina, Chapel Hill, NC (DK62505): PI: Paul H. Hayashi, MD, MPH; Study Coordinator: Tracy Russell, MA.
University of Pennsylvania, Philadelphia, PA (DK62494): PI: Abraham Shaked, MD, PhD; Co-Is: Kim M. Olthoff, MD, FACS, K. Rajender Reddy, MD, Mark A. Rosen, MD, PhD; Study Coordinators: Brian Conboy, PA, MBA, Mary Kaminski, PA-C, Debra McCorriston, RN, Mary Shaw, RN, BBA.
University of Virginia, Charlottesville, VA (DK62484): PI: Carl L. Berg, MD; Co-I: Timothy L. Pruett, MD; Study Coordinator: Jaye Davis, RN.
Virginia Commonwealth University - Medical College of Virginia, Richmond, VA (DK62531): PI: Robert A. Fisher, MD, FACS; Co-Is: Martha K. Behnke, PhD, Adrian Cotterell, MD, FACS, Ann Fulcher, MD, Pamela M. Kimball, PhD, HCLD, Mary E. Olbrisch, PhD, ABPP, Marc P. Posner, MD, FACS, Mark A. Reimers, PhD, Amit Sharma, MD, R. Todd Stravitz, MD, FACP; Study Coordinators: April Ashworth, RN, BSN, Joanne Davis, RN, Sarah Hubbard, Andrea Lassiter, BS, Luke Wolfe, MS.
National Institute of Diabetes and Digestive and Kidney Diseases, Division of Digestive Diseases and Nutrition, Bethesda, MD: Edward Doo, MD, James E. Everhart, MD, MPH, Jay H. Hoofnagle, MD, Stephen James, MD, Patricia R. Robuck, PhD, Leonard B. Seeff, MD, Averell H. Sherker, MD, FRCPC, Rebecca J. Torrance, RN, MS.
Grants and Financial Support:
This study was presented in part at the 63rd annual meeting of the American Association for the Study of Liver Diseases, Boston, MA, November 9-13, 2012.
This study was supported by the National Institute of Diabetes & Digestive & Kidney Diseases through cooperative agreements (grants U01-DK62444, U01-DK62467, U01-DK62483, U01-DK62484, U01-DK62494, U01-DK62496, U01-DK62498, U01-DK62505, U01-DK62531, and U01-DK62536). Additional support was provided by Health Resources and Services Administration (HRSA), and the American Society of Transplant Surgeons (ASTS).
Abbreviations
- A2ALL
Adult-to-Adult Living Donor Liver Transplantation Cohort Study
- BSA
body surface area
- LDLT
living donor liver transplantation
- M3
Month 3
- SD
standard deviation
- SSV
standard spleen volume
- Y1
Year 1
Footnotes
Conflicts of Interest:
The authors have no conflicts to disclose.
References
- 1.Olthoff KM, Merion RM, Ghobrial RM, Abecassis MM, Fair JH, Fisher RA, et al. Outcomes of 385 adult-to-adult living donor liver transplant recipients: a report from the A2ALL Consortium. Ann Surg. 2005;242:314–23. doi: 10.1097/01.sla.0000179646.37145.ef. discussion 23-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shiffman ML, Brown RS, Jr., Olthoff KM, Everson G, Miller C, Siegler M, Hoofnagle JH. Living donor liver transplantation: summary of a conference at The National Institutes of Health. Liver Transpl. 2002;8:174–88. doi: 10.1053/jlts.2002.30981. [DOI] [PubMed] [Google Scholar]
- 3.Makuuchi M, Miller CM, Olthoff K, Schwartz M. Adult-adult living donor liver transplantation. J Gastrointest Surg. 2004;8:303–12. doi: 10.1016/j.gassur.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Moon DB, Lee SG, Hwang S, Kim KH, Ahn CS, Ha TY, et al. Toward more than 400 liver transplantations a year at a single center. Transplant Proc. 2013;45:1937–41. doi: 10.1016/j.transproceed.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 5.Berg CL, Gillespie BW, Merion RM, Brown RS, Jr, Abecassis MM, Trotter JF, et al. Improvement in survival associated with adult-to-adult living donor liver transplantation. Gastroenterology. 2007;133:1806–13. doi: 10.1053/j.gastro.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berg CL, Merion RM, Shearon TH, Olthoff KM, Brown RS, Jr, Baker TB, et al. Liver transplant recipient survival benefit with living donation in the model for endstage liver disease allocation era. Hepatology. 2011;54:1313–21. doi: 10.1002/hep.24494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abecassis MM, Fisher RA, Olthoff KM, Freise CE, Rodrigo DR, Samstein B, et al. Complications of living donor hepatic lobectomy--a comprehensive report. Am J Transplant. 2012;12:1208–17. doi: 10.1111/j.1600-6143.2011.03972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rudow DL, Brown RS, Jr., Emond JC, Marratta D, Bellemare S, Kinkhabwala M. One-year morbidity after donor right hepatectomy. Liver Transpl. 2004;10:1428–31. doi: 10.1002/lt.20280. [DOI] [PubMed] [Google Scholar]
- 9.Trotter JF, Gillespie BW, Terrault NA, Abecassis MM, Merion RM, Brown RS, Jr, et al. Laboratory test results after living liver donation in the adult-to-adult living donor liver transplantation cohort study. Liver Transpl. 2011;17:409–17. doi: 10.1002/lt.22246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Everson GT, Hoefs JC, Niemann CU, Olthoff KM, Dupuis R, Lauriski S, et al. Functional elements associated with hepatic regeneration in living donors after right hepatic lobectomy. Liver Transpl. 2013;19:292–304. doi: 10.1002/lt.23592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo X, Zhao B, Schwartz L. Computer-aided tumor segmentation using local region-based active contours. Columbia University Invention Report IR #290 [Google Scholar]
- 12.Guo X, Zhao B, Schwartz L. Techniques for segmentation of organs and tumors and objects. 2014 Jun; US Patent (pending) PCT/US13/36166.
- 13.Liu F, Zhao B, Kijewski PK, Wang L, Schwartz LH. Liver segmentation for CT images using GVF snake. Med Phys. 2005;32:3699–706. doi: 10.1118/1.2132573. [DOI] [PubMed] [Google Scholar]
- 14.Tan Y, Guo P, Mann H, Marley SE, Juanita Scott ML, Schwartz LH, et al. Assessing the effect of CT slice interval on unidimensional, bidimensional and volumetric measurements of solid tumours. Cancer Imaging. 2012;12:497–505. doi: 10.1102/1470-7330.2012.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pozo AL, Godfrey EM, Bowles KM. Splenomegaly: investigation, diagnosis and management. Blood Rev. 2009;23:105–11. doi: 10.1016/j.blre.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Ebaugh FG, Jr., McIntyre OR. Palpable spleens: ten-year follow-up. Ann Intern Med. 1979;90:130–1. doi: 10.7326/0003-4819-90-1-130_2. [DOI] [PubMed] [Google Scholar]
- 17.Gunay-Aygun M, Font-Montgomery E, Lukose L, Tuchman Gerstein M, Piwnica-Worms K, Choyke P, et al. Characteristics of congenital hepatic fibrosis in a large cohort of patients with autosomal recessive polycystic kidney disease. Gastroenterology. 2013;144:112–21. e2. doi: 10.1053/j.gastro.2012.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamza SM, Kaufman S. Role of spleen in integrated control of splanchnic vascular tone: physiology and pathophysiology. Can J Pharmacol. 2009;87:1–7. doi: 10.1139/Y08-103. [DOI] [PubMed] [Google Scholar]
- 19.Hosey RG, Kriss V, Uhl TL, DiFiori J, Hecht S, Wen DY. Ultrasonographic evaluation of splenic enlargement in athletes with acute infectious mononucleosis. Br J Sports Med. 2008;42:974–7. doi: 10.1136/bjsm.2008.050807. [DOI] [PubMed] [Google Scholar]
- 20.Kim SJ, Na GH, Choi HJ, You Y, Kim DG. Effect of donor right hepatectomy on splenic volume and platelet count for living donor liver transplantation. JGastrointest Surg. 2013;17:1576–83. doi: 10.1007/s11605-013-2219-0. [DOI] [PubMed] [Google Scholar]
- 21.Pomfret EA, Pomposelli JJ, Gordon FD, Erbay N, Lyn Price L, Lewis WD, Jenkins RL. Liver regeneration and surgical outcome in donors of right-lobe liver grafts. Transplantation. 2003;76:5–10. doi: 10.1097/01.TP.0000079064.08263.8E. [DOI] [PubMed] [Google Scholar]
- 22.Ikura Y, Ohsawa M, Okada M, Iwai Y, Wakasa K. The significance of platelet consumption in the development of thrombocytopenia in patients with cirrhosis. Am J Med Sci. 2013;346:199–203. doi: 10.1097/MAJ.0b013e31826e364d. [DOI] [PubMed] [Google Scholar]
- 23.Peck-Radosavljevic M, Zacherl J, Meng YG, Pidlich J, Lipinski E, Längle F, et al. Is inadequate thrombopoietin production a major cause of thrombocytopenia in cirrhosis of the liver? J Hepatol. 1997;27:127–31. doi: 10.1016/s0168-8278(97)80291-7. [DOI] [PubMed] [Google Scholar]
- 24.Jelkmann W. The role of the liver in the production of thrombopoietin compared with erythropoietin. Eur JGastroenterol Hepatol. 2001;13:791–801. doi: 10.1097/00042737-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Nagasako Y, Jin MB, Miyazaki H, Nakayama M, Shimamura T, Furukawa H, et al. Thrombopoietin in postoperative thrombocytopenia following living donor hepatectomy. Liver Transplan. 2006;12:435–9. doi: 10.1002/lt.20608. [DOI] [PubMed] [Google Scholar]
- 26.Krupski G, Rogiers X, Nicolas V, Maas R, Malagó M, Broelsch CE, Bücheler E. Computed tomography versus magnetic resonance imaging--aided volumetry of the left lateral segment before living related liver donation: a case report. Liver Transplan Surg. 1996;2:388–90. doi: 10.1002/lt.500020510. [DOI] [PubMed] [Google Scholar]
- 27.Higashiyama H, Yamaguchi T, Mori K, Nakano Y, Yokoyama T, Takeuchi T, et al. Graft size assessment by preoperative computed tomography in living related partial liver transplantation. Br JSurg. 1993;80:489–92. doi: 10.1002/bjs.1800800429. [DOI] [PubMed] [Google Scholar]
- 28.Ishizawa T, Sugawara Y, Hasegawa K, Ikeda M, Tamura S, Makuuchi M. Extent of hepatectomy on splenic hypertrophy and platelet count in live liver donors. Clin Transplant. 2006;20:234–8. doi: 10.1111/j.1399-0012.2005.00474.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.