Abstract
Objective
To examine heart rate recovery (HRR) as an indicator of autonomic nervous system (ANS) dysfunction following maximal exercise testing in children and young adults with sickle cell anemia (SCA).
Study design
Recovery phase heart rate (HR) in the first 5 minutes following maximal exercise testing in 60 subjects with SCA and 30 matched controls without SCA was assessed. The difference between maximal HR and HR at both 1-minute (ΔHR1min) and 2-minute (ΔHR2min) recovery was our primary outcome.
Results
Compared with controls, subjects with SCA demonstrated significantly smaller mean ΔHR1min (23 bpm, 95% CI [20, 26] vs. 32 bpm, 95% CI [26, 37], p = 0.006) and ΔHR2min (39 bpm, 95% CI [36, 43] vs. 48 bpm, 95% CI [42, 53], p = 0.011). Subjects with SCA also showed smaller mean changes in HR from peak HR to 1 minute, from 1 minute to 2 minutes and from 2 through 5 minutes of recovery by repeated measures testing. In a multivariable regression model, older age was independently associated with smaller ΔHR1min in subjects with SCA. Cardiopulmonary fitness and hydroxyurea use, however, were not independent predictors of ΔHR1min.
Conclusions
Children with SCA demonstrate impaired HRR following maximal exercise. Reduced post-exercise HRR in SCA suggests impaired parasympathetic function, which may become progressively worse with age, in this population.
Keywords: Heart Rate Recovery, Sickle Cell Anemia, Cardiopulmonary Exercise Testing, Autonomic Function
Impaired heart rate recovery (HRR) following exercise, particularly at the 1 and 2-minute intervals, is an indicator of autonomic nervous system (ANS) dysfunction and represents a prognostic indicator of future cardiovascular morbidity and mortality in the general population (1, 2). A delayed decrease in heart rate measured in the first minute of recovery serves as a predictor of death independent of myocardial perfusion defects on nuclear imaging or heart rate response during exercise (1). Decreased exercise capacity also is associated with abnormal HRR in both men and women (1). Normal HRR at 1 minute post exercise has been shown to be vagally mediated, reflecting early parasympathetic reactivation rather than sympathetic withdrawal (3).
Cardiovascular ANS dysfunction, primarily impaired vagal function, also exists among individuals with both sickle cell anemia (SCA) and sickle cell trait. Sickle cell trait carriers exhibit altered sympatho-vagal balance both at rest and after exercise (4). In SCA, transient hypoxia produced changes in heart rate variability in young adults with SCA, but not in controls without SCA, indicating substantial parasympathetic withdrawal in response to oxygen desaturation (5). Moreover, individuals with SCA demonstrate poor cardiopulmonary fitness resulting in decreased physical functioning and reduced exercise capacity (6, 7). Potential causes for reduced exercise capacity in SCA include chronic anemia, cardiopulmonary disease, deconditioning and ongoing inflammation. However, the exact physiological mechanisms and factors responsible for exercise limitation in this population remain poorly understood. Whether or not autonomic dysfunction in patients with SCA is a function of low fitness is not known.
We hypothesized that following maximal cardiopulmonary exercise testing, children and young adults with SCA demonstrate reduced HRR compared with patients without SCA. We also hypothesized that reduced HRR is associated with poor cardiopulmonary fitness and other clinical variables in this cohort.
METHODS
We analyzed heart rate (HR) data obtained during the recovery phase of maximal cardiopulmonary exercise testing in a cohort of children and young adults with SCA enrolled in a prospective study aimed at characterizing the physiologic determinants of exercise limitation in this population. This study was approved by the Institutional Review Board of Ann & Robert H. Lurie Children’s Hospital of Chicago. Complete informed consent and assent were obtained where appropriate from all participants and their parents prior to initiating study activities.
Subjects were approached during routine visits and recruited from the Comprehensive Sickle Cell Program. We included subjects between 8 and 21 years old with hemoglobin SS or S−β0 thalassemia disease confirmed by routine hemoglobin analysis. Individuals receiving hydroxyurea therapy were eligible for participation. Subjects were excluded for the following: (1) participation in chronic transfusion program; (2) history of cardiovascular disease or significant chronic lung disease; or (3) history of lightheadedness, syncope or chest pain with physical exertion. Controls without SCA or sickle cell trait were recruited from siblings or relatives of subjects enrolled in this study, other patients seen in clinic or study advertisements placed in the clinic and hospital settings. Absence of SCA or trait was verified by hemoglobin analysis or confirmation of newborn state screen results. Controls were matched by age, sex and race.
All subjects and controls underwent incremental, symptom-limited, maximal cardiopulmonary exercise testing (CPET) at steady state. Steady state was defined by the absence of vaso-occlusive pain or other sickle cell related complication for at least 2 weeks or the absence of any transfusion for at least 3 months prior to testing. We followed a standard ramp cycle protocol (modified Godfrey protocol (8)) using an electronically braked VIAsprint 150P/200P cycle ergometer (CareFusion, USA). Initial workload and increments in workload every 1 minute (ie, work rate) were determined by subject height. Continuous breath-by-breath gas exchange and 12-lead electrocardiogram (ECG) data were monitored during exercise. Following a maximal exercise test, defined as reaching a Respiratory Exchange Ratio (RER) equal to or greater than 1.1, participants engaged in a 10-minute recovery phase. During recovery, each individual continued to pedal on the cycle ergometer without resistance at a cadence of 40 to 60 rpms for the first 3 minutes and then remained in a sitting position after getting off the cycle. Peak VO2 was determined by choosing the highest 20-second averaged, weight adjusted VO2 achieved during the last minute of exercise. Exercise testing was discontinued if the study participant requested to stop due to excessive fatigue, developed sickle cell related pain, experienced symptoms of severe shortness of breath, palpitations, cyanosis, dizziness, or exhibited significant decrease in oxygen saturation with worsening respiratory symptoms.
We analyzed tracings of post-exercise ECG data recorded over the first 5 minutes of the recovery period and measured HR at 1-minute intervals. We limited our analysis to the first 5 minutes of recovery given the lack of uniform data collection across subjects and controls after 5 minutes. Following standardized training, 2 research assistants used regular calipers to manually perform all measurements in leads II, V5, and V6. HR was determined by measuring the distance between consecutive RR intervals. Final HR values reflected the average of 3 independent measurements. HR reserve was calculated as the difference between peak HR and baseline HR.
Statistical Analyses
Summary statistics were used to report frequency and to evaluate distribution of all continuous data (SPSS v22.0, IBM). The difference between peak HR and HR at 1-minute (ΔHR1min) and 2 minutes (ΔHR2min) of recovery represented our primary outcome. Continuous variables were compared using Student t-test for independent samples between subjects and controls or ANOVA among subjects on hydroxyurea, subjects not on hydroxyurea and controls. Post hoc Bonferroni comparisons were performed for significant results found on ANOVA testing. Repeated measures ANOVA was used to evaluate group effect on HR change at 1 minute, from 1 to 2 minutes and through 5 minutes of recovery. We performed multivariable linear regression to assess the association between clinical variables and ΔHR1min in 2 separate models, one for subjects with SCA and controls without SCA combined and another for subjects with SCA only. Clinical variables included age, sex, peak VO2, baseline hemoglobin, baseline white blood cell (WBC) count and hydroxyurea use (subjects only). HRR time constants were calculated using standard exponential equations and displayed as mono-exponential curves. Results at 95% confidence interval with two-tailed, p values <0.05 were considered statistically significant.
RESULTS
Baseline and post-exercise HR data were assessed in 60 subjects with SCA and 30 matched controls without SCA. Post-exercise ECG data could not be interpreted due to excessive motion artifact in 2 subjects. Therefore, the final cohort consisted of 58 subjects (mean age 15.1 years, 95% CI [14.2, 16.0]) and 30 controls (mean age 14.5 years, 95% CI [13.3, 15.8]). In total, 30/58 (52%) and 15/30 (50%) of subjects and controls were male, respectively, and 22/58 (38%) subjects were receiving hydroxyurea treatment at the time of testing. Mean HR at baseline was significantly higher for subjects with SCA when compared with controls without SCA (78 bpm, 95% CI [76, 81] vs. 71 bpm, 95% CI [66, 76], p = 0.005) (Table I).
Table 1.
Characteristics and Heart Rate Recovery in Subjects and Controls
| Subjects (N=58) | Controls (N=20) | ||||||
|---|---|---|---|---|---|---|---|
| N | Mean | 95% CI | N | Mean | 95% CI | p valuea | |
|
|
|||||||
| Age (years) | 58 | 15.1 | 14.2, 16.0 | 30 | 14.5 | 13.3, 15.8 | 0.465 |
| Hemoglobin (g/dL) | 51 | 8.7 | 8.4, 9.1 | 28 | 12.9 | 12.4, 13.4 | <0.001 |
| Baseline HR (bpm) | 58 | 78 | 76, 81 | 30 | 71 | 66, 76 | 0.005 |
| Peak HR (bpm) | 58 | 177 | 174, 181 | 30 | 179 | 175, 184 | 0.467 |
| % Predicted HR achieved | 58 | 90 | 88, 92 | 30 | 91 | 88, 93 | 0.555 |
| HR Reserve (bpm) | 58 | 99 | 96, 103 | 30 | 109 | 103, 114 | 0.005 |
| ΔHR1 min (bpm) | 58 | 23 | 20, 26 | 28 | 32 | 26, 37 | 0.006 |
| ΔHR2 min (bpm) | 58 | 39 | 36, 43 | 28 | 48 | 42, 53 | 0.011 |
| ΔHR3 min (bpm) | 58 | 49 | 45, 53 | 28 | 60 | 54, 65 | 0.001 |
| ΔHR4 min (bpm) | 58 | 63 | 59, 67 | 29 | 71 | 66, 77 | 0.015 |
| ΔHR5 min (bpm) | 58 | 70 | 66, 73 | 29 | 77 | 72, 82 | 0.030 |
p value < 0.05 by Student T-Test for independent samples
We analyzed ECG tracings at the end of exercise and the beginning of the recovery phase to determine peak HR responses to maximal exercise testing. There was no significant difference in the mean peak HR achieved during maximal exercise in subjects with SCA versus controls without SCA (177 bpm, 95% CI [174, 181] vs. 179 bpm, 95% CI [175, 184], p = 0.457). However, subjects with SCA did demonstrate significantly lower mean HR reserve (99 bpm, 95% CI [95, 103] vs. 109 bpm, 95% CI [103, 114], p = 0.005), representing the difference between peak and baseline HR values.
We calculated HR from available ECG tracings at 1-minute intervals spanning the first 5 minutes of the recovery period following completion of the exercise phase of testing. Subjects with SCA demonstrated significantly slower reduction in HR following maximal exercise challenge when compared with controls. Significantly smaller mean ΔHR1min (23 bpm, 95% CI [20, 26] vs. 32 bpm, 95% CI [26, 37], p = 0.006) and ΔHR2min (39 bpm, 95% CI [36, 43] vs. 48 bpm, 95% CI [42, 53], p = 0.011) were observed in subjects versus controls. Mean change from peak HR throughout the remaining 5 minutes of recovery was also significantly smaller in subjects (Table I). Repeated measures ANOVA testing showed a significant between subjects effect on HRR. When compared with controls without SCA, subjects with SCA demonstrated smaller mean changes in HR from peak HR to 1 minute (p = 0.002), from 1 minute to 2 minutes (p = 0.005) as well as from 2 through 5 minutes (p = 0.008) of recovery.
We found that subjects with SCA on hydroxyurea had HRR values closer to those observed in controls. However, there was no difference in HRR at 1 minute in subjects by hydroxyurea status. When compared with controls without SCA, subjects with SCA not on hydroxyurea demonstrated a significantly smaller mean ΔHR1min (20 bpm, 95% CI [16, 25] vs. 32 bpm, 95% CI [26, 37], p = 0.002) but subjects with SCA on hydroxyurea did not (28 bpm, 95% CI [22, 33] vs. 32 bpm, 95% CI [26, 37], p = 0.879). Mean ΔHR1min was not significantly different in subjects on versus not on hydroxyurea therapy. The between subjects effect on HRR was influenced by hydroxyurea therapy for change in HR from peak HR to 1 minute only (p = 0.01).
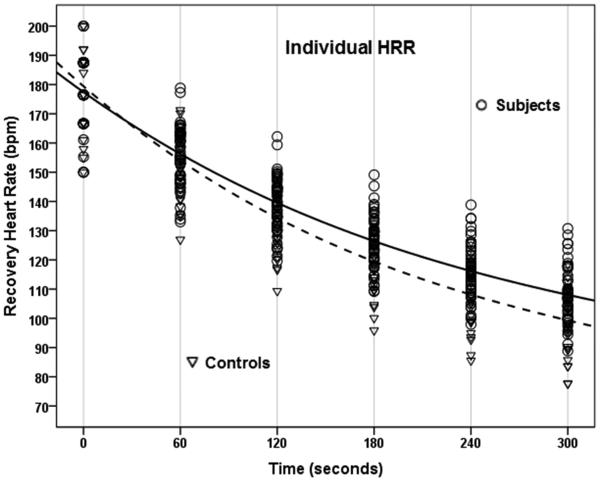
We also examined HRR by group as a function of time constant (T) values calculated using standard exponential equations and mono-exponential curve fitting. Time constants calculated over the first 5 minutes of recovery were greater in subjects with SCA (T = 128 sec, 95% CI [123, 134] vs. 109 sec, 95% CI [103, 116]), when compared with that observed in controls without SCA (Figure; available at www.jpeds.com).
Figure.
Calculated time constants demonstrate prolonged HRR over the first 5 minutes of recovery following exercise challenge in subjects (solid line) with SCA compared with controls (dotted line) (T = 128 sec, 95% CI [123, 134] vs. 109 sec, 95% CI [103, 116]). HRR curves are shown with individual (top) and aggregate (bottom) heart rate data.
We examined the relationship between ΔHR1min and clinical variables in our subjects with SCA and controls without SCA. We first performed bivariate regression with adjustment for age, sex, peak VO2, baseline hemoglobin and baseline WBC count separately to examine the influence of each variable on the difference in ΔHR1min between subjects and controls. The difference in ΔHR1min between subjects and controls remained significant after adjustment for age and sex. However, the effect of SCA on ΔHR1min was no longer present after adjustment for peak VO2, baseline hemoglobin or baseline WBC each, with peak VO2 exerting the greatest influence on the difference in ΔHR1min between subjects with SCA and controls without SCA (Table II; available at www.jpeds.com).
Table 2.
Change in effect of SCA on ΔHR1 min after adjustment for individual clinical variables
| Variable | β Coefficient | Standard Error | p valuea |
|---|---|---|---|
| Sickle Cell Anemia | 8.58 | 3.05 | 0.006 |
| Age (years) | 7.33 | 2.86 | 0.012 |
| Female Gender | 8.79 | 2.99 | 0.004 |
| Baseline Hemoglobin (g/dL) | 8.80 | 5.51 | 0.115 |
| Baseline WBC (×103/μL) | 5.16 | 3.46 | 0.140 |
| Peak VO2 (mL/kg/min) | 1.41 | 3.34 | 0.674 |
denotes change in p value associated with the effect of SCA on ΔHR1 min after adjustment for each variable
Combining these variables into a single multivariable regression model that included subjects with SCA and controls without SCA (adjusted R2 = 0.312), we found that only higher age and higher baseline WBC count were independent predictors of lower ΔHR1min (Table III). In a separate model for subjects with SCA only that included hydroxyurea therapy as an additional co-variate (adjusted R2 = 0.199), only higher age was an independent predictor of lower ΔHR1min (Table IV). There was no evidence for multicollinearity in either model, and no variance inflation factor above 4 was found to be associated with any variable.
Table 3.
Predictors of ΔHR1 min in subjects with SCA and controls without SCA
| Variable | β Coefficient | Standard Error | p value |
|---|---|---|---|
| Sickle Cell Anemia | 4.77 | 5.30 | 0.371 |
| Age (years) | − 1.42 | 0.48 | 0.004a |
| Female Sex | − 6.48 | 3.36 | 0.057 |
| Baseline Hemoglobin (g/dL) | − 0.18 | 1.01 | 0.856 |
| Baseline WBC (×103/μL) | − 1.08 | 0.46 | 0.021a |
| Peak VO2 (mL/kg/min) | 0.08 | 0.25 | 0.741 |
p value significant < 0.05
Table 4.
Predictors of ΔHR1 min in only subjects with SCA
| Variable | β Coefficient | Standard Error | p value |
|---|---|---|---|
| Age (years) | − 1.35 | 0.60 | 0.028a |
| Female Sex | − 3.11 | 4.13 | 0.455 |
| Baseline Hemoglobin (g/dL) | − 0.61 | 1.34 | 0.648 |
| Baseline WBC (×103/μL) | − 0.51 | 0.55 | 0.363 |
| Peak VO2 (mL/kg/min) | 0.23 | 0.34 | 0.504 |
| Hydroxyurea Use | 6.13 | 3.58 | 0.093 |
p value significant < 0.05
DISCUSSION
Exercise is associated with activation of the ANS, parasympathetic withdrawal and increased sympathetic activity. HRR in the period following acute physical exertion requires coordination of the parasympathetic and sympathetic arms of the ANS to ensure return of autonomic tone to baseline levels (9). Existing studies indicate that HRR in the first minute after exercise is primarily a function of parasympathetic reactivation, and HRR in the second minute and beyond requires the addition of sympathetic withdrawal to continued parasympathetic reactivation (10, 11). Whether or not derangements in parasympathetic reactivation and sympathetic withdrawal fully explain the reduced HRR observed at 1 and 2 minutes after exercise in our subjects with SCA, however, is not known. That reduced HRR is seen throughout the entire 5 minutes of recovery suggests the possibility of persistent abnormalities in the ANS after exercise, although it remains unclear if inadequate parasympathetic reactivation or sympathetic withdrawal represents the dominant problem after the first 2 minutes of recovery in SCA. Further studies to examine heart rate variability during exercise recovery would help to elucidate the specific contribution of ANS dysfunction to impaired HRR in SCA.
Whether or not reduced HRR predicts clinical events such as sudden cardiac death in SCA is not known. In our study, the mean decrement in HR at 1-minute of recovery following maximal exercise challenge was 23 bpm, which was significantly smaller than the mean decrement of 32 bpm observed in controls. Although HRR has not been previously examined in SCA, this notable difference in HRR is supported by studies that have examined autonomic function in other disease states. The mean ΔHR1min we observed in subjects with SCA is smaller than that reported for healthy children in several studies, in which ΔHR1min on average is in the middle to high 30 bpm range (12-14), but was similar to that measured in obese children or children with congenital heart disease following Fontan surgery (15, 16), a group known to have ANS dysfunction.
The etiology of blunted HRR in SCA is not known. A clearer understanding of HRR in SCA, however, is needed because there are compelling data that impaired HRR is associated with increased risk of cardiovascular-related mortality not only in the general population but also among individuals with cardiopulmonary risk factors conferred by heart failure and diabetes (17-19). Likewise, SCA is associated with development of cardiopulmonary disease with increasing age, ranging from pulmonary vascular disease to diastolic heart dysfunction (20, 21), which confers an increased risk of mortality. Sudden death, especially during vaso-occlusive pain episodes and acute chest syndrome, occurs not infrequently among adults with SCA, although the pathophysiologic mechanisms responsible for sudden death are poorly understood in this population (22, 23). No clinical, laboratory or physiologic predictors of sudden death to date have been established in SCA. Whether or not it may be attributed to ANS dysfunction or associated with reduced HRR after exercise in this population is not known. In adult studies, ΔHR1min values associated with highest risk of sudden death ranged from ≤ 12 to 25 bpm (1, 24). However, cutoffs values for HRR that predict cardiovascular events and sudden death in these studies cannot be extrapolated to a children with SCA, for which there are currently no standard definitions of abnormal HRR. The clinical relevancy of absolute cutoffs for ΔHR1min may differ among different populations, both healthy and with chronic disease, depending on the presence of other risk factors or underlying pathophysiology, respectively.
In the bivariate regression, lower peak VO2 best explained the difference in ΔHR1min observed between the 2 groups. This is not surprising because longitudinal studies demonstrate that higher levels of cardiopulmonary fitness and physical activity are associated with greater HRR after exercise in the general population (18). In contrast, risk factors for reduced fitness and physical activity, such as metabolic syndrome and cardiovascular disease, are associated with impaired HRR. However, peak VO2 was no longer independently associated with ΔHR1min when combined with all of the other variables in a multivariable regression model. In this model, only age and baseline WBC count remained independent predictors of ΔHR1min among subjects and controls. Because peak VO2 decreases with increasing age in the general population (14), the relationship between peak VO2 and ΔHR1min on bivariate regression may simply reflect the influence of age on ΔHR1min. The significant inverse relationship between WBC count and ΔHR1min is also supported by evidence suggesting inflammation may modulate ANS function and attenuate parasympathetic reactivation during HRR after exercise (25). Among subjects with SCA only, we found that older age alone represented a significant predictor of lower ΔHR1min in our multivariable model. Interestingly, hydroxyurea use was not independently related to HRR among subjects with SCA.
We did not directly examine heart rate variability during recovery, which would have provided a clearer picture of the contribution of parasympathetic re-activation and sympathetic withdrawal to HRR in our subjects with SCA. Thus, HRR should be considered only an indirect indicator of ANS function after exercise challenge. Although significant differences in ΔHR1min were evident between our subjects and controls, what degree of ANS impairment is clinically important in SCA is not clear. We also did not obtain routine echocardiography or pulmonary function testing in our subjects and thus, could not assess the impact of cardiopulmonary disease on HRR. Also, our data may have been subject to some degree of measurement error as all ECG readings were performed manually using calipers. Finally, that we did not find an independent effect of hydroxyurea on HRR among subjects with SCA may be due to our small sample size and incomplete assessment of adherence to therapy.
Our work provides further insight into the relationship among the ANS, cardiopulmonary fitness and clinical variables in SCA. Further studies are warranted to determine the influence of these physiologic findings on cardiovascular outcomes in this population.
Acknowledgments
Funded by the National Institutes of Health/National Heart, Lung, and Blood Institute (K23 HL094376).
Abbreviations
- HRR
heart rate recovery
- ANS
autonomic nervous system
- SCA
sickle cell anemia
- HR
heart rate
- WBC
white blood cell
- ECG
electrocardiogram
- CPET
cardiopulmonary exercise test
- CI
confidence interval
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
REFERENCES
- 1.Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341(18):1351–7. doi: 10.1056/NEJM199910283411804. [DOI] [PubMed] [Google Scholar]
- 2.Kokkinos P, Myers J, Doumas M, Faselis C, Pittaras A, Manolis A, et al. Heart rate recovery, exercise capacity, and mortality risk in male veterans. Eur J Prev Cardiol. 2012;19(2):177–84. doi: 10.1177/1741826711398432. [DOI] [PubMed] [Google Scholar]
- 3.Imai K, Sato H, Hori M, Kusuoka H, Ozaki H, Yokoyama H, et al. Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. J Am Coll Cardiol. 1994;24(6):1529–35. doi: 10.1016/0735-1097(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 4.Hedreville M, Barthelemy JC, Tripette J, Roche F, Hardy-Dessources MD, Pichot V, et al. Effects of strenuous exercise on autonomic nervous system activity in sickle cell trait carriers. Autonomic neuroscience : basic & clinical. 2008;143(1-2):68–72. doi: 10.1016/j.autneu.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Sangkatumvong S, Khoo MC, Kato R, Detterich JA, Bush A, Keens TG, et al. Peripheral vasoconstriction and abnormal parasympathetic response to sighs and transient hypoxia in sickle cell disease. Am J Respir Crit Care Med. 2011;184(4):474–81. doi: 10.1164/rccm.201103-0537OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connes P, Machado R, Hue O, Reid H. Exercise limitation, exercise testing and exercise recommendations in sickle cell anemia. Clin Hemorheol Microcirc. 2011;49(1-4):151–63. doi: 10.3233/CH-2011-1465. [DOI] [PubMed] [Google Scholar]
- 7.Callahan LA, Woods KF, Mensah GA, Ramsey LT, Barbeau P, Gutin B. Cardiopulmonary responses to exercise in women with sickle cell anemia. Am J Respir Crit Care Med. 2002;165(9):1309–16. doi: 10.1164/rccm.2002036. [DOI] [PubMed] [Google Scholar]
- 8.Godfrey S, Davies CT, Wozniak E, Barnes CA. Cardio-respiratory response to exercise in normal children. Clinical science. 1971;40(5):419–31. doi: 10.1042/cs0400419. [DOI] [PubMed] [Google Scholar]
- 9.Freeman JV, Dewey FE, Hadley DM, Myers J, Froelicher VF. Autonomic nervous system interaction with the cardiovascular system during exercise. Prog Cardiovasc Dis. 2006;48(5):342–62. doi: 10.1016/j.pcad.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Kannankeril PJ, Le FK, Kadish AH, Goldberger JJ. Parasympathetic effects on heart rate recovery after exercise. J Investig Med. 2004;52(6):394–401. doi: 10.1136/jim-52-06-34. [DOI] [PubMed] [Google Scholar]
- 11.Pierpont GL, Voth EJ. Assessing autonomic function by analysis of heart rate recovery from exercise in healthy subjects. Am J Cardiol. 2004;94(1):64–8. doi: 10.1016/j.amjcard.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 12.Laguna M, Aznar S, Lara MT, Lucia A, Ruiz JR. Heart rate recovery is associated with obesity traits and related cardiometabolic risk factors in children and adolescents. Nutr Metab Cardiovasc Dis. 2012 doi: 10.1016/j.numecd.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Singh TP, Evans S. Socioeconomic position and heart rate recovery after maximal exercise in children. Arch Pediatr Adolesc Med. 2010;164(5):479–84. doi: 10.1001/archpediatrics.2010.57. [DOI] [PubMed] [Google Scholar]
- 14.Singh TP, Rhodes J, Gauvreau K. Determinants of heart rate recovery following exercise in children. Med Sci Sports Exerc. 2008;40(4):601–5. doi: 10.1249/MSS.0b013e3181621ec4. [DOI] [PubMed] [Google Scholar]
- 15.Prado DM, Silva AG, Trombetta IC, Ribeiro MM, Guazzelli IC, Matos LN, et al. Exercise training associated with diet improves heart rate recovery and cardiac autonomic nervous system activity in obese children. Int J Sports Med. 2010;31(12):860–5. doi: 10.1055/s-0030-1267158. [DOI] [PubMed] [Google Scholar]
- 16.Singh TP, Gauvreau K, Rhodes J, Blume ED. Longitudinal changes in heart rate recovery after maximal exercise in pediatric heart transplant recipients: evidence of autonomic re-innervation? J Heart Lung Transplant. 2007;26(12):1306–12. doi: 10.1016/j.healun.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 17.Kriatselis CD, Nedios S, Kelle S, Helbig S, Gottwik M, von Bary C. Oxygen Kinetics and Heart Rate Response during Early Recovery from Exercise in Patients with Heart Failure. Cardiol Res Pract. 2012;2012:512857. doi: 10.1155/2012/512857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carnethon MR, Sternfeld B, Liu K, Jacobs DR, Jr., Schreiner PJ, Williams OD, et al. Correlates of heart rate recovery over 20 years in a healthy population sample. Med Sci Sports Exerc. 2012;44(2):273–9. doi: 10.1249/MSS.0b013e31822cb190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Georgoulias P, Demakopoulos N, Valotassiou V, Orfanakis A, Zaganides A, Tsougos I, et al. Long-term prognostic value of heart-rate recovery after treadmill testing in patients with diabetes mellitus. Int J Cardiol. 2009;134(1):67–74. doi: 10.1016/j.ijcard.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 20.Gladwin MT, Sachdev V, Jison ML, Shizukuda Y, Plehn JF, Minter K, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350(9):886–95. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- 21.Sachdev V, Machado RF, Shizukuda Y, Rao YN, Sidenko S, Ernst I, et al. Diastolic dysfunction is an independent risk factor for death in patients with sickle cell disease. J Am Coll Cardiol. 2007;49(4):472–9. doi: 10.1016/j.jacc.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manci EA, Culberson DE, Yang YM, Gardner TM, Powell R, Haynes J, Jr., et al. Causes of death in sickle cell disease: an autopsy study. Br J Haematol. 2003;123(2):359–65. doi: 10.1046/j.1365-2141.2003.04594.x. [DOI] [PubMed] [Google Scholar]
- 23.Romero Mestre JC, Hernandez A, Agramonte O, Hernandez P. Cardiovascular autonomic dysfunction in sickle cell anemia: a possible risk factor for sudden death? Clin Auton Res. 1997;7(3):121–5. doi: 10.1007/BF02308838. [DOI] [PubMed] [Google Scholar]
- 24.Jouven X, Empana JP, Schwartz PJ, Desnos M, Courbon D, Ducimetiere P. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med. 2005;352(19):1951–8. doi: 10.1056/NEJMoa043012. [DOI] [PubMed] [Google Scholar]
- 25.Jae SY, Ahn ES, Heffernan KS, Woods JA, Lee MK, Park WH, et al. Relation of heart rate recovery after exercise to C-reactive protein and white blood cell count. Am J Cardiol. 2007;99(5):707–10. doi: 10.1016/j.amjcard.2006.09.121. [DOI] [PubMed] [Google Scholar]