Abstract
Declarative memory (DM) impairments are reported in schizophrenia and in unaffected biological relatives of patients. However, the neural correlates of successful and unsuccessful encoding, mediated by the medial temporal lobe (MTL) memory system, and the influence of disease-related genetic liability remain under explored. This study employed an event-related functional MRI paradigm to compare activations for successfully and unsuccessfully encoded associative face-name stimuli between 26 schizophrenia patients (mean age: 33, 19m/7f), 30 controls (mean age: 29, 24m/6f) and 14 unaffected relatives of patients (mean age: 40, 5m/9f). Compared to controls or unaffected relatives, patients showed hyper-activations in ventral visual stream and temporo-parietal cortical association areas when contrasting successfully encoded events to fixation. Follow-up hippocampal regions-of-interest analysis revealed schizophrenia-related hyper-activations in the right anterior hippocampus during successful encoding; contrasting successful versus unsuccessful events produced schizophrenia-related hypo-activations in the left anterior hippocampus. Similar hippocampal hypo-activations were observed in unaffected relatives during successful versus unsuccessful encoding. Post-hoc analyses of hippocampal volume showed reductions in patients, but not in unaffected relatives compared to controls. Findings suggest that DM encoding deficits are attributable to both disease-specific and genetic liability factors that impact different components of the MTL memory system. Hyper-activations in temporo-occipital and parietal regions observed only in patients suggest the influence of disease-related factors. Regional hyper- and hypo-activations attributable to successful encoding occurring in both patients and unaffected relatives suggest the influence of schizophrenia-related genetic liability factors.
Keywords: hippocampus, fMRI, functional imaging, episodic memory, associative memory, medial temporal lobe
1. Introduction
Schizophrenia is characterized by a generalized cognitive impairment with pronounced deficits in memory and executive function (Ranganath et al., 2008; Reichenberg and Harvey, 2007). Specifically, patients with schizophrenia experience impairments in declarative memory (DM) (Aleman et al., 1999; Ranganath et al., 2008; Weiss and Heckers, 2001), which includes everyday memories of events (episodic memory) and facts (semantic memory) (Eichenbaum and Cohen, 2001). DM impairments are also reported in unaffected relatives of patients and increase with degree of biological relatedness, suggesting the involvement of schizophrenia genetic liability factors (Faraone et al., 2000; Whyte et al., 2005).
The hippocampus and medial temporal lobe (MTL) are essential for DM (Eichenbaum and Cohen, 2001). Prefrontal and posterior association regions also act to mediate memory processing (Sperling et al., 2010; Wang et al., 2010). Functional imaging studies of DM tasks in healthy subjects confirm MTL involvement and illustrate that regional activation is influenced by task characteristics, how information is learned, and whether encoding is successful (Buckner and Koutstaal, 1998; Preston et al., 2005).
DM relies on the successful encoding, storage, and retrieval of information. DM deficits in patients with schizophrenia and non-symptomatic relatives appear particularly attributable to encoding difficulties (Cirillo and Seidman, 2003). Since different network components contribute to the type and stage of DM processing (Brewer and Moghekar, 2002), encoding deficits may relate to dysfunctions confined to specific MTL regions and/or to disturbances in connected cortical regions. Although more frequently focused on attempted encoding, several DM studies have demonstrated altered neural activity in hippocampal, parahippocampal, and connected prefrontal regions in schizophrenia (Achim and Lepage, 2005; Heckers, 2001; Ragland et al., 2009). Fewer fMRI studies have examined DM in unaffected relatives (MacDonald et al., 2009), and none have dissociated disturbances in regional activity by examining encoding success for associative stimuli exclusively.
To identify the subcomponents of the MTL memory system affected by schizophrenia and disease-related genetic liability, we employed a validated event-related fMRI design (Sperling et al., 2003) to compare blood-oxygen-level-dependent (BOLD) responses for successful DM encoding in schizophrenia patients, first-degree unaffected biological relatives of patients, and community controls. The DM task, including novel associative face-name stimuli, is shown to elicit MTL and regionally specific hippocampal activations during successful encoding in controls. We hypothesized that patients would show differences in the magnitude of task-related brain activity in the MTL and associated cortical regions. Further, we predicted that relatives of patients, sharing approximately half of their genes with schizophrenia probands, would show intermediate abnormalities. Since successful encoding elicits greater neural activity in the anterior hippocampus (Sperling et al., 2003), hippocampal regions-of-interest (ROI) analyses were also conducted. Finally, post-hoc analysis of structural imaging data examined differences in hippocampal volumes across groups.
2. Methods
2.1. Subjects
Subjects included a sub-sample of participants enrolled in the University of California, Los Angeles (UCLA) Family Study (Nuechterlein et al., 2002; Yang et al., 2010; Yang et al., 2012). Community controls with demographics similar to schizophrenia probands were recruited using a survey research company. 70 participants completed fMRI scanning with good quality data, including 26 patients, 14 unaffected first-degree relatives of patients and 30 controls [Table 1]. Exclusion criteria included neurological disorders, mental retardation, and a history of drug or alcohol abuse.
Schizophrenia diagnosis was confirmed using the Structured Clinical Interview for DSM-IV – Patient version (SCID-I/P; (First M.B., 2002)) and informant information. Symptoms were assessed using the expanded 24-item Brief Psychiatric Rating Scale (BPRS; (Ventura et al., 2000). All patients were receiving standard antipsychotic medication (risperidone: n = 10, olanzapine: n = 4, aripiprazole: n = 5, clozapine: n = 2, quetiapine: n = 2, fluphenazine: n = 2, not reported: n = 2). Controls and unaffected relatives of patients were screened to exclude schizophrenia spectrum disorders using the Structured Clinical Interview for DSM-IV – Non Patient version (SCID-NP) and with the Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II; (First M.B., 1994)). The UCLA Institutional Review Board (IRB) approved all research procedures; informed written consent was obtained from all subjects.
2.2. Declarative memory task
The DM task, designed to dissociate changes in brain activity linked with attempted and successful encoding of face-name stimuli, included 455 color facial photographs varying in age, race, and gender selected from the National Institute of Standards and Technology, Facial Recognition Technology (FERET) database (Phillips et al., 1998). During scanning, each stimulus pair, a face with a unique and age-appropriate name, was presented once, intermixed with trials of visual fixation (.25 to 10 s) using a jittered event-related design. Temporal parameters for this task were identical to those of Sperling et al., 2003. Subjects viewed stimuli through MR compatible goggles during 5 separate runs, each including 140 time points and 91 novel face-name stimuli. To facilitate encoding, subjects indicated whether the name ‘fit’ the face simultaneously presented with a button press. Subjects were instructed to remember the face-name associations for a post-scan memory test.
2.3. Post-scan memory test
After scanning, a memory test that included the same face stimuli with a correct and incorrect name was administered. Subjects matched each face with its correct name and indicated whether they were “guessing”, “possibly correct”, “probably correct” or “definitely correct.” Correct “matches” and associated confidence ratings determined encoding success for events in subsequent fMRI analysis. However, since subjects applied this confidence scale subjectively, the 4 categories were re-binned into two categories, “high confidence” and “low confidence” based on responses definitely above chance. The three diagnostic risk groups did not differ with respect to the number of stimulus pairs rated with high or low confidence, F(2, 61) = .45, p =.63 or with respect to the number of stimulus pairs that were correctly rated with high, F(2, 61) = .12, p = .86 or low confidence F(2, 61) = .41, p = .66
2.4. Image acquisition
Functional T2*-weighted gradient echo, echo-planar images were acquired on a Siemens 3T Allegra system (Milwaukee, WI) at the UCLA Ahmanson-Lovelace Brain Mapping Center. To maximize in-plane resolution (3.1 mm x 3.1 mm) and minimize susceptibility artifacts within the hippocampus, acquisition included 26 slices (5 mm, interslice distance, 1 mm), positioned in the oblique coronal orientation (TR/TE: 2000/30 ms, flip angle: 90, matrix: 64 x 64; FOV: 200; total scan time: 23.5 minutes). A non-BOLD T2-weighted image acquired co-planar to the time series data (TR/TE: 5000/33 ms, flip angle: 90, matrix: 128 x 128; FOV: 200; scan time: 1.5 minutes) was collected on the same system. In addition, high-resolution T1-weighted MPRAGE scans (TR/TE: 1900/4.38 ms, TI = 1100; flip angle: 15; FOV: 256; matrix: 256 x 256; voxel size: 1 mm3; averages=4; total scan time=32.32 min) were acquired on a Siemens 1.5T Sonata scanner to facilitate multimodal within- and across-subject registration and estimate hippocampal volumes.
2.5. Data analysis
FMRI analysis followed the scheme outlined by Sperling et al. using FSL’s (www.fmrib.ox.ac.uk/fsl) fMRI Expert Analysis Tool (FEAT), version 5.98. Preprocessing included removal of non-brain tissue, motion correction, spatial smoothing (6 mm FWHM), denoising and high-pass filtering (70 s cut-off). All data was inspected and residual motion was controlled for using six rigid body movement parameters as regressors when modeling the BOLD response. FLIRT registered fMRI data across runs and with the T2 and T1-weighted images. For higher-level analyses, the T1-weighted images were registered to the MNI 152 average image.
FSL’s FILM (FMRIB's Improved Linear Model) compared: 1) all stimulus trials versus fixation to model attempted encoding (AE), 2) high-confidence correct trials versus fixation to model successful encoding (SE), and 3) high-confidence correct versus incorrect trials to model activations exclusive to successful encoding (ESE), i.e., separate from processes associated with the memory task in general. Group by contrast interactions were assessed for contrasts 2 and 3 above to establish differences in brain activation for SE and ESE [Fig. 1].
Figure 1.
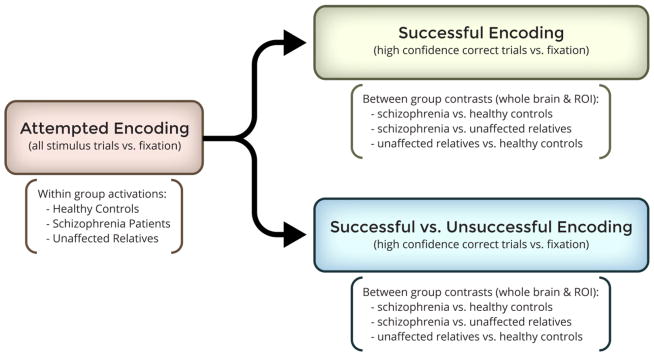
Diagram of the experimental design and fMRI contrasts included for study.
Mixed effects modeling, using FSL’s FLAME (FMRIB's Local Analysis of Mixed Effects), determined significant differences in regional activation between groups controlling for age gender, and post-scan memory performance using cluster correction and a z-threshold = 1.7, p < .05. For significance testing, cluster size inference based on Gaussian random field theory (Hayasaka and Nichols, 2003) compared 1) patients to controls, 2) relatives to controls, and 3) patients to relatives.
Since AE, SE, and ESE may lead to differential engagement of the hippocampus, anatomical ROIs were also used to examine focal hippocampal activations across groups. Hippocampal labels generated from the Harvard-Oxford probabilistic atlas were linearly registered to each subject’s functional data in each hemisphere and subsequently separated into anterior and posterior halves (bisecting midway across the longest oblique axis of the hippocampus) (Greicius et al., 2003) to visualize percent signal change within each segment.
Hippocampal volumes (including the subiculum) were estimated from each subject’s T1-weighted image using FreeSurfer’s image analysis suite (http://surfer.nmr.mgh.harvard.edu/). In brief, preprocessing of T1 data included to removal of non-brain tissue, intensity normalization and automated volumetric segmentation of the hippocampus using established and well-validated and documented procedures that make use of probabilistic information based on manually labeled training sets (Fischl et al., 2002; Fischl et al., 2004; Fischl, 2012). Each hippocampal segmentation was visually inspected and any small segmentation errors were corrected manually. A repeated measures ANCOVA was used to test for group differences in hippocampal volume.
3. Results
3.1. Demographic Variables and Performance
Table I includes demographic, clinical, and memory performance information by group. Patients and controls were similar in age, F(1, 54) = 2.86, p = .10, and gender, χ2 (1, 49) = .01, p = .94; all groups were of similar socioeconomic status, F(2, 61) = 2.59, p = .08. However, relatives were older than controls, (F(1, 42) = 10.33, p < .01, though not older than patients, p > .07. Relatives also differed in gender compared to patients and controls, (χ2(1, 43) = 8.33, p < .01 and χ2(1, 39) = 6.54, p = .01). Though performing above chance, patients showed poorer memory performance than controls, F(1, 48) = 7.94, p < .01. Relatives did not differ in performance from patients p > .7 or controls p > .09 [Table I]. Age, gender, and performance from the post-scan memory test were included as covariates in all fMRI analysis.
Table I.
Demographic and clinical characteristics of subjects
| Schizophrenia Patients (N = 26) | Patient Relatives (N = 14) | Community Controls (N = 30) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Mean | SD | Mean | SD | Mean | SD | |
|
| ||||||
|
Demographic Measures
| ||||||
| Age (Years)a | 33.38 | 9.0 | 39.6 | 11.8 | 29.3 | 9.0 |
| Current socioeconomic statusb | 35.5 | 15.1 | 47.9 | 21.3 | 44.8 | 18.4 |
| Years of educationb | 14.1 | 1.9 | 14.9 | 2.4 | 15.3 | 2.6 |
|
|
||||||
| Handedness (Non-dextral/Dextral)b | 1/26 | 4/10 | 2/28 | |||
|
|
||||||
| Gender (Male/Female)a | 19/7 | 5/9 | 24/6 | |||
|
| ||||||
|
DM performancea,c
| ||||||
| Percent correct | 68.1 | 6.1 | 69.0 | 6.8 | 70.6 | 7.1 |
|
| ||||||
|
Morphometric Measures (cm3)b
| ||||||
| Brain Volume | 1,421.81 | 141.62 | 1,324.44 | 151.57 | 1,427.77 | 141.55 |
| Left Hippocampal Volume | 4.41878 | .299180 | 4.66667 | .223482 | 4.58492 | .298868 |
| Right Hippocampal Volume | 4.23789 | .312493 | 4.43973 | .177229 | 4.45449 | .287219 |
|
| ||||||
|
Diagnostic Measures
| ||||||
| Duration of illness (Years)b | 9.92 | 8.39 | ||||
| BPRS total scoreb | 38.13 | 9.25 | ||||
| Withdrawalc | 1.7 | .70 | ||||
| Thinking disorderc | 1.6 | .72 | ||||
Patient relatives differed in age and gender with community controls and patients. Post-scan memory performance differed between patients and controls.
Handedness was estimated from a modified version of the Edinburgh Handedness Inventory [Oldfield, 1971] where participants with a laterality quotient of >0.7 were defined as dextral. Handedness Information was missing for one patient and one control. Current social economic status was derived from the Total Socioeconomic Index (TSEI; [Stevens and Cho, 1985]). Data for socioeconomic status and years of education was unavailable for 5 subjects, duration of illness data for 1 subject, BPRS scores for 3 subjects, and volumetric data for 1 subject.
BPRS scores were clustered into withdrawal (negative symptoms) and thinking disorder (positive symptoms) factor scores (Burger et al., 1997; Narr et al., 2009).
3.2. Attempted Encoding
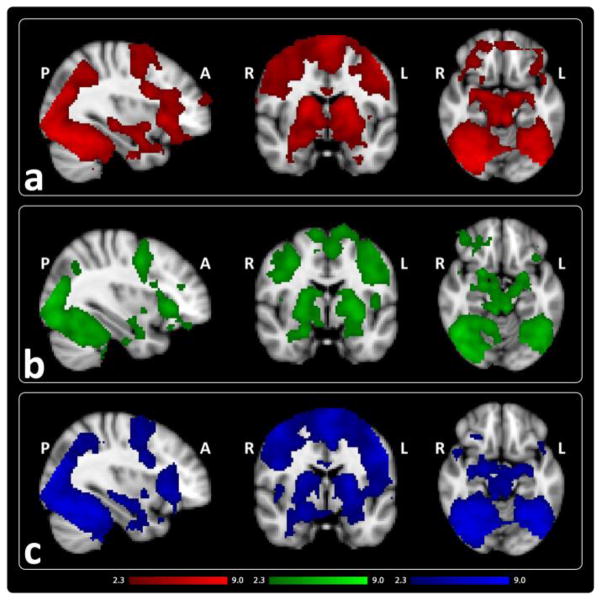
In line with previous findings (Sperling et al., 2003; Zeineh et al., 2003), AE was associated with activation in bilateral hippocampal, fusiform, ventral visual stream regions, thalamic, striatal, and lateral and ventral prefrontal regions within each group [Fig. 2, Table II].
Figure 2.
Mean activation for attempted encoding of associative face-name stimuli shown in a) controls (red) b) unaffected relatives of patients (green) and c) patients (blue) (z > 2.3, p < .05, corrected).
Table II.
Attempted Encoding (Within Group Peak Activations)
| Contrast | Group | Cortical Regiona | x | y | z | Z-score |
|---|---|---|---|---|---|---|
| Attempted Encoding (fixation vs. task) | Controls | 29% Lingual Gyrus, 28% Occipital Fusiform Gyrus | 14 | −82 | −12 | 9.10 |
|
| ||||||
| 75% Lingual Gyrus, 13% Intracalcarine Cortex | 0 | −76 | 0 | 9.09 | ||
|
| ||||||
| 67% Occipital Fusiform Gyrus, 9% Lingual Gyrus | 24 | −76 | −14 | 8.77 | ||
|
| ||||||
| 66% Temporal Occipital Fusiform Cortex, 7% Temporal Fusiform Cortex, 5% Inferior Temporal Gyrus | −36 | −50 | −22 | 8.57 | ||
|
| ||||||
| 29% Occipital Fusiform Gyrus, 6% Lateral Occipital Cortex, 5% Inferior Temporal Gyrus | 40 | −66 | −20 | 8.46 | ||
|
| ||||||
| 70% Temporal Occipital Fusiform Cortex | 38 | −50 | −22 | 8.45 | ||
|
| ||||||
| 35% Right Thalamus, 17% Right Hippocampus | 22 | −30 | −4 | 8.96 | ||
|
| ||||||
| 79% Brain-Stem | 8 | −32 | −4 | 8.25 | ||
|
| ||||||
| 9% Left Thalamus | −22 | −28 | −4 | 7.96 | ||
|
| ||||||
| 30% Inferior Frontal Gyrus, 18% Middle Frontal Gyrus | −40 | 18 | 24 | 7.09 | ||
|
| ||||||
| 63% Paracingulate Gyrus | 2 | 12 | 48 | 7.43 | ||
|
| ||||||
| 41% Precentral Gyrus, 23% Inferior Frontal Gyrus | 46 | 8 | 26 | 7.41 | ||
|
| ||||||
| Unaffected Relatives | 36% Inferior Temporal Gyrus 26% Lateral Occipital Cortex, 17% Temporal Occipital Fusiform Cortex | 48 | −60 | −16 | 7.07 | |
|
| ||||||
| 49% Lateral Occipital Cortex | 30 | −76 | 20 | 6.84 | ||
|
| ||||||
| 40% Lateral Occipital Cortex, 22% Occipital Fusiform Gyrus | 38 | −80 | −14 | 6.78 | ||
|
| ||||||
| 35% Lateral Occipital Cortex, 15% Occipital Pole, 15% Lateral Occipital Cortex | 36 | −86 | 4 | 6.59 | ||
|
| ||||||
| 74% Temporal Occipital Fusiform Cortex, 7% Inferior Temporal Gyrus | 40 | −48 | −22 | 6.56 | ||
|
| ||||||
| 30% Lingual Gyrus, 25% Occipital Fusiform Gyrus, 8% Occipital Pole | 14 | −86 | −10 | 6.39 | ||
|
| ||||||
| 69% Lateral Occipital Cortex | −46 | −82 | −4 | 7.26 | ||
|
| ||||||
| 35% Lateral Occipital Cortex, 16% Lateral Occipital Cortex, 13% Occipital Pole | −36 | −88 | 12 | 6.99 | ||
|
| ||||||
| 38% Inferior Temporal Gyrus, 20% Lateral Occipital Cortex, 14% Occipital Fusiform Gyrus, 5% Temporal Occipital Fusiform Cortex | −44 | −62 | −10 | 6.87 | ||
|
| ||||||
| 56% Temporal Occipital Fusiform Cortex, 16% Inferior Temporal Gyrus, 5% Occipital Fusiform Gyrus | −42 | −56 | −22 | 6.44 | ||
|
| ||||||
| 57% Lateral Occipital Cortex, 6% Lateral Occipital Cortex | −42 | −84 | 4 | 6.23 | ||
|
| ||||||
| Patients | 70% Temporal Occipital Fusiform Cortex, 6% Inferior Temporal Gyrus, 5% Occipital Fusiform Gyrus | −38 | −56 | −20 | 8.27 | |
|
| ||||||
| 57% Lateral Occipital Cortex, 12% Occipital Fusiform Gyrus | −46 | −70 | −12 | 8.24 | ||
|
| ||||||
| 41% Lingual Gyrus, 19% Occipital Fusiform Gyrus, 4% Occipital Pole | 12 | −84 | −10 | 8.24 | ||
|
| ||||||
| 68% Lateral Occipital Cortex | −44 | −80 | −6 | 8.08 | ||
|
| ||||||
| 48% Occipital Fusiform Gyrus, 11% Lateral Occipital Cortex, 6% Temporal Occipital Fusiform Cortex, 5% Inferior Temporal Gyrus | 40 | −66 | −18 | 8.06 | ||
|
| ||||||
| 54% Lateral Occipital Cortex | 30 | −78 | 20 | 8.03 | ||
|
| ||||||
| 42% Right Thalamus, 36% Right Hippocampus | 20 | −32 | −4 | 8.34 | ||
|
| ||||||
| 42% Precentral Gyrus, 19% Inferior Frontal Gyrus, 7% Middle Frontal Gyrus | −52 | 8 | 30 | 7.48 | ||
|
| ||||||
| 100% Left Thalamus | −8 | −20 | 6 | 7.15 | ||
|
| ||||||
| 72% Juxtapositional Lobule Cortex | −2 | 2 | 54 | 7.20 | ||
|
| ||||||
| 48% Precentral Gyrus, 8% Inferior Frontal Gyrus | 48 | 6 | 28 | 7.69 | ||
Cortical regions with probabilities < 5% were excluded.
3.3. Successful Encoding
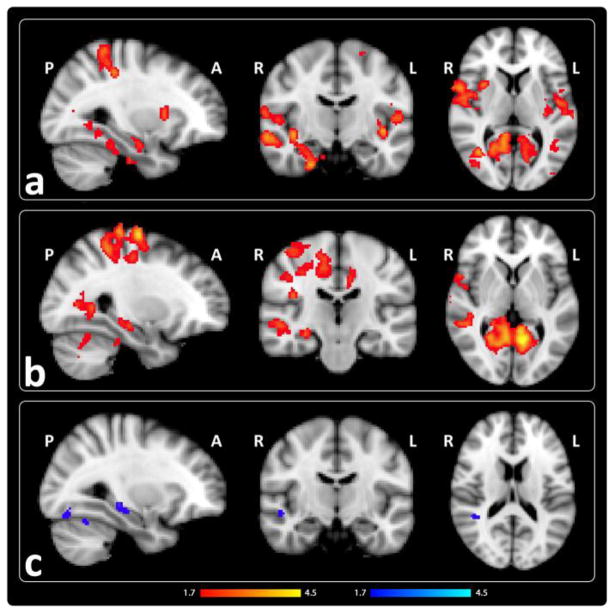
We examined SE by comparing activation during high-confidence correct trials versus fixation. Patients showed significant hyper-activations in bilateral ventral visual stream (fusiform and lingual gyri and medial and lateral occipital cortex), temporo-parietal association (precuneus and lateral superior and inferior parietal lobules) and sensorimotor areas compared to controls [Fig. 3a, Table III]. Though not identified as a peak activation, activity in the right anterior hippocampus was also greater in patients [Fig. 3a]. Schizophrenia-related hyper-activations were observed in similar regions when patients were compared to relatives [Fig. 3b, Table III]. Relatives showed decreased activation in the superior temporal gyrus and fusiform areas compared to controls [Fig. 3c, Table III].
Figure 3.
Successful encoding in patients is associated with increased activations in multiple brain areas. Regions showing increased activation in red and decreased activation in blue for high-confidence correct trials versus fixation in a) patients compared to controls, b) patients compared to unaffected relatives, and c) unaffected relatives compared to controls. (z > 1.7, p <.05, corrected).
Table III.
Successful Encoding (Peak Activations)
| Contrast | Cortical Regiona | Hemisphere | x | y | z | Z-score | |
|---|---|---|---|---|---|---|---|
| Successful Encoding (high-confidence correct vs. fixation) | Patients vs. Controls | 31% Cuneal Cortex, 27% Precuneus Cortex, 8% Supracalcarine Cortex, 6% Intracalcarine Cortex | Left | −12 | −72 | 22 | 4.35 |
|
| |||||||
| 40% Insular Cortex, 25% Planum Polare | Left | −42 | −10 | −6 | 4.20 | ||
|
| |||||||
| 35% Superior Parietal Lobule, 21% Postcentral Gyrus, 6% Supramarginal Gyrus | Left | −32 | −40 | 58 | 3.94 | ||
|
| |||||||
| 35% Cuneal Cortex, 29% Precuneus Cortex, 13% Supracalcarine Cortex | Right | 12 | −68 | 24 | 4.12 | ||
|
| |||||||
| 32% Precentral Gyrus, 5% Inferior Frontal Gyrus | Right | 64 | 8 | 10 | 4.04 | ||
|
| |||||||
| 20% Lateral Occipital Cortex | Right | 38 | −62 | 8 | 3.87 | ||
|
| |||||||
| Patients vs. Unaffected Relatives | 61% Lingual Gyrus, 13% Precuneus Cortex | Left | −10 | −58 | 2 | 4.60 | |
|
| |||||||
| 44% Superior Parietal Lobule, 18% Postcentral Gyrus, 5% Supramarginal Gyrus | Left | −30 | −42 | 64 | 3.92 | ||
|
| |||||||
| 42% Postcentral Gyrus, 7% Precentral Gyrus | Left | −24 | −34 | 58 | 3.62 | ||
|
| |||||||
| 38% Intracalcarine Cortex | Right | 22 | −68 | 8 | 4.29 | ||
|
| |||||||
| 43% Precentral Gyrus, 6% Superior Frontal Gyrus | Right | 28 | −12 | 70 | 4.03 | ||
|
| |||||||
| 52% Precentral Gyrus, 11% Middle Frontal Gyrus | Right | 44 | −4 | 60 | 4.01 | ||
|
| |||||||
| 40% Middle Temporal Gyrus, 36% Superior Temporal Gyrus | Right | 60 | −16 | −8 | 3.39 | ||
|
| |||||||
| Unaffected Relatives vs. Controls | 46% Superior Temporal Gyrus, 15% Middle Temporal Gyrus, 5% Supramarginal Gyrus | Right | 46 | −30 | 0 | 3.29 | |
|
| |||||||
| 28% Middle Temporal Gyrus, 10% Supramarginal Gyrus, 6% Middle Temporal Gyrus | Right | 46 | −40 | 4 | 3.11 | ||
|
| |||||||
| 23% Lingual Gyrus, 17% Occipital Fusiform Gyrus | Right | 12 | −74 | −14 | 3.16 | ||
Cortical regions with probabilities < 5% were excluded.
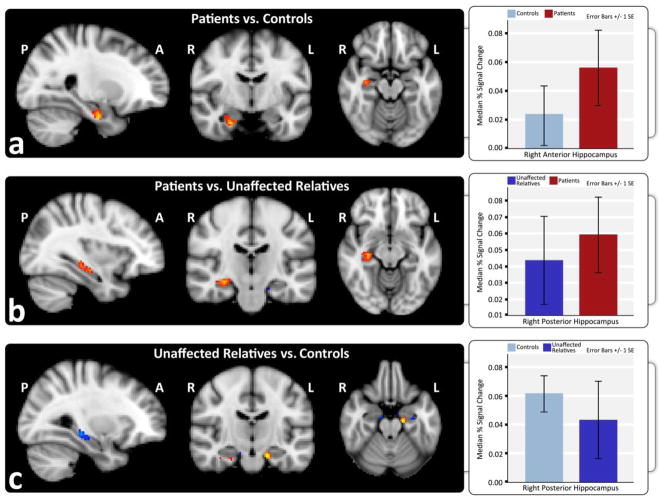
ROI analyses of SE revealed significant hyper-activation in the right anterior hippocampus in patients compared to controls [Fig. 4a] and in the right posterior hippocampus when compared to relatives [Fig. 4b]. Relatives showed decreased activation in the right posterior hippocampus compared to controls [Fig. 4c].
Figure 4.
Hippocampal ROI analysis shows successful encoding is associated with increased hippocampal activation in schizophrenia patients compared to controls and unaffected relatives. Increased activation is shown in red and decreased activation in blue for high-confidence correct trials versus fixation in a) patients compared to controls, b) patients compared to unaffected relatives, and c) unaffected relatives compared to controls. Graphs show the estimated median percent signal change within anatomically defined anterior and posterior hippocampal regions in each hemisphere for each diagnostic group.
3.4. Exclusive Successful Encoding
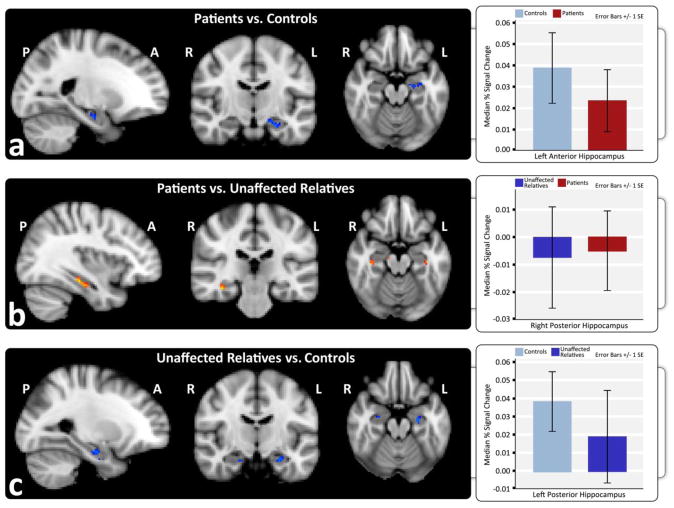
Whole brain analysis revealed no significant differences in brain activation between the three diagnostic risk groups for ESE. However, within ROIs, patients showed significant hypo-activation of the left anterior hippocampus compared to controls [Fig. 5a], but no difference from relatives [Fig. 5b]. Similar to patients, relatives showed hypo-activation in the left anterior hippocampus compared to controls [Fig. 5c].
Figure 5.
Retrieval success in exclusive successful encoding is associated with reductions in hippocampal activation in schizophrenia patients. Hippocampal ROIs showing increased activation in red and decreased activation in blue for high-confidence correct versus incorrect trials in a) patients compared to controls, b) patients compared to unaffected relatives, and c) unaffected relatives compared to controls. Graphs show the estimated median percent signal change within anatomically defined anterior and posterior hippocampal regions in each hemisphere for each diagnostic group.
3.5. Hippocampal Volume
Significant reductions in hippocampal volume were observed in patients compared to controls, F(1,49) = 5.01, p = .03, and relatives, F(1,33) = 4.35, p = .045, but not between relatives and controls, p > .59, covarying for age, gender, and brain volume [Table I, Fig. 6]. Though mean volumes were larger in the left versus the right hemisphere, there were no significant effects of asymmetry (p > .05)
Figure 6.
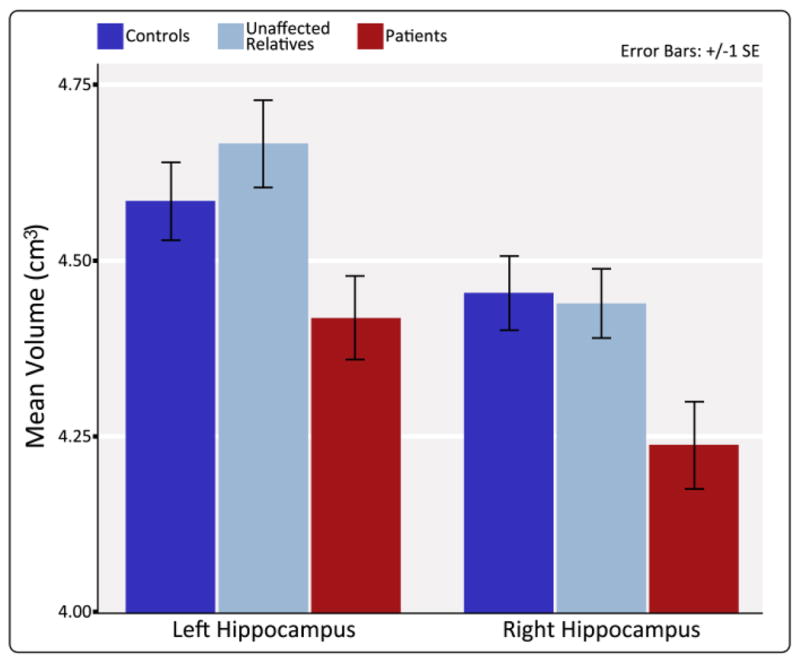
Schizophrenia patients exhibit significant decreases in left and right hippocampal volume compared to controls and unaffected relatives. Graph shows mean left and right hippocampal volumes for controls, unaffected relatives, and patients after correcting for sex and age and brain volume.
4. Discussion
Several novel findings emerged from whole brain and hippocampal ROI analysis of SE and ESE in schizophrenia patients, unaffected relatives and controls. While all groups showed similar activation in MTL and connected prefrontal and subcortical centers, SE was associated with increased activity in temporo-occipital (ventral visual stream) and parietal association areas in patients compared to the non-schizophrenia groups. In ROI analysis, the observed increased activity in the right anterior hippocampus of patients suggests the influence of disease-related effects. In contrast, during ESE, decreased activity observed in the left anterior hippocampus in both patients and relatives compared to controls suggests schizophrenia genetic liability effects.
Together these findings suggest that disease-related and genetically mediated alterations in circuitry both intrinsic and extrinsic to the MTL memory system contribute towards altered DM processing in schizophrenia. Differential hippocampal activity points to interacting processes. For example, increased hemodynamic response for SE observed in patients may indicate over-recruitment, lack of inhibition, more effortful and/or prolonged processing during SE (Kuperberg et al., 2007). Conversely, reduced activation of the left anterior hippocampus for ESE, suggest simultaneous under-recruitment of sub-regions that reflect a failure to organize information at the early stages of learning and lead to over compensation and hyper-activations in other components of the MTL circuit.
4.1. Medial Temporal Lobe
Several reviews indicate that DM impairments are pronounced in schizophrenia (Aleman et al., 1999; Heinrichs and Zakzanis, 1998) and occur in relatives of patients (Faraone et al., 2000; Toulopoulou et al., 2003). These observations together with postmortem and structural neuroimaging evidence suggest hippocampal and MTL function are central in the pathophysiology of schizophrenia (Harrison, 2004; Heckers, 2001). Still, few studies have focused on the neural processes underlying successful associative encoding (Ranganath et al., 2008). Partially in line with our findings, meta-analytic results show increased activation in parahippocampal regions during the encoding of episodic memories in schizophrenia (Ragland et al., 2009). The use of item-based rather than associative stimuli, which generally produce more robust hippocampal activation (Davachi and Wagner, 2002; Henson and Gagnepain, 2010), may account for sub-threshold hippocampal activity in patients and controls in prior studies.
In concordance with our results, an fMRI investigation of different encoding tasks demonstrated that schizophrenia patients activate hippocampal and overlying MTL regions during associative and successful memory encoding (Achim et al., 2007). However, patients also showed decreased activation within hippocampal and surrounding temporo-limbic regions during the encoding of arbitrary stimulus pairs. These findings indicate that although patients are able to recruit MTL regions, altered function in particular aspects of this system, including differential contributions of the hippocampus, occur during encoding. Evidence suggests the successful encoding of face-name pairs induces greater activity in anterior hippocampal regions (Sperling et al., 2003). By employing small volume correction, considered a more powerful approach for determining focal changes in hippocampal activation, (MacDonald et al., 2009), the present investigation showed schizophrenia-related hyper- and hypo-activations in the right and left anterior hippocampus for SE and ESE respectively. This suggests deficits and compensatory mechanisms in DM circuits, potentially lateralized for verbal and non-verbal processing of face-name pairs.
Hippocampal abnormalities and disturbances in episodic memory have been recognized as possible endophenotypes of schizophrenia genetic liability (Boos et al., 2007; Narr et al., 2002; Snitz et al., 2006; Toulopoulou et al., 2003). Though no published studies have examined associative encoding for successfully recalled events in unaffected relatives, an investigation of novel and repeated word-pair encoding - though addressing attempted encoding only - showed greater repetition suppression in bilateral anterior parahippocampal regions in relatives of patients (Thermenos et al., 2007) as partially consistent with our results.
In line with prior studies (Honea et al., 2005; Nelson et al., 1998; Wright et al., 2000), schizophrenia patients showed significantly smaller hippocampal volumes [Table I, Fig. 6]. Several studies support relationships between hippocampal structure and DM performance (Antonova et al., 2004; Herold et al., 2013; Thoma et al., 2009), suggesting altered activation may relate to abnormal macrostructure. The absence of reduced hippocampal volumes in relatives implies that abnormalities in hippocampal function can occur without observed differences in structure.
4.2. Cortical Association Regions
Prior schizophrenia studies have shown altered DM-related activation of prefrontal regions; meta-analytic results suggest largest effects in ventro- and dorso-lateral prefrontal cortex (Ragland et al., 2009). In this study, all groups showed activation in inferior prefrontal cortex during AE [Fig. 2] though group effects were mostly absent [Table III]. Since prefrontal recruitment may relate to higher-level processes including cognitive strategies used to facilitate encoding (Blumenfeld and Ranganath, 2007; Reber et al., 2002), the absence of robust differences in prefrontal regions may be a consequence of our study design, which did not manipulate encoding strategies or difficulty.
Patients showed increased activity in several other neocortical association areas compared to non-schizophrenia groups during SE. Specifically, hyper-activations in ventral visual stream, fusiform/lingual, and fusiform/parahippocampal regions, areas involved in the perception of faces and objects (Grill-Spector et al., 2004; Kanwisher and Yovel, 2006), may reflect impairments in tasks involving face discrimination (Pinkham et al., 2005; Whittaker et al., 2001). Other fMRI studies have shown fusiform dysfunction in schizophrenia during face processing (Quintana et al., 2003; Silverstein et al., 2010; Walther et al., 2009) that may represent deficits specific to configural processing at early stages of discrimination (Shin et al., 2008) and/or impairments of integration (Silverstein et al., 2010). Though prior studies also suggest impairments in face discrimination in unaffected relatives (Calkins et al., 2005), these effects were not observed in the current study.
Increased activation was also observed in parietal association areas including the precuneus and in superior temporal regions in patients compared to non-schizophrenia groups. These cortical association areas are reciprocally connected with the parahippocampus, with primary input to the hippocampus. These regions are involved in integrated perceptual processing (Eichenbaum and Cohen, 2001) and may contribute to conscious and effortful organization of information during encoding (Bearden et al., 2012). Reports of increased activation in parietal and superior temporal/insular regions have been observed in at least one prior study during SE in schizophrenia (Achim et al., 2007), suggesting DM processing relies on networks extrinsic to, but intricately connected with the MTL.
4.3. Limitations
Though negative symptoms may impact DM, prior evidence suggests that DM deficits in schizophrenia are independent of age, duration of illness and positive symptoms (Aleman et al., 1999; Bilder et al., 2000; Cirillo and Seidman, 2003; Goldberg and Weinberger, 1996). Since patients were relatively asymptomatic at assessment [Table I], we could not address these relationships. Prior evidence also suggests that antipsychotic medications have little impact on memory performance (Aleman et al., 1999; Gilbertson and van Kammen, 1997; Goldberg and Weinberger, 1996). Though influences of performance on the hemodynamic response are less clear, relatives who were not receiving medication also showed altered brain activity under particular task conditions. Finally, although gender and age were controlled for in all analyses, differences in age and smaller sample size of the relative group may have impacted our ability detect additional genetic liability effects with respect to regional brain activations or changes in hippocampal volume.
4.4. Conclusion
Altered brain activity during DM encoding in schizophrenia points to the involvement of both disease-specific and schizophrenia-related genetic liability factors. Results support that 1) DM encoding deficits impact different components of the MTL memory system and connected association regions, and 2) altered activity in the anterior hippocampi vary according to encoding success and genetic predisposition. DM has been shown as predictor of poor social and occupational functioning in schizophrenia (Bilder et al., 2000; Green et al., 2000). Thus, a better understanding of the underlying mechanisms may help direct efforts to improve social-vocational outcome. Due to the heritability of DM (Manns and Eichenbaum, 2006), findings in unaffected relatives suggest further clues regarding the genetic basis of schizophrenia.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) through grants MH73990 (KLN), MH49716 (KHN), MH66286 (KHN) and MH37705 (RA).
Role of the funding source:
The contents of this paper do not represent the official view of the National Institutes of Health who funded this study. The sponsors gave no conditions or requirements for the study and had no role in the preparation of the manuscript.
Abbreviations
- DM
declarative memory
- MTL
medial temporal lobe
- ROI
regions-of-interest
- AE
attempted encoding
- SE
successful encoding
- ESE
successful versus unsuccessful encoding for exclusive successful encoding
Footnotes
Author Contributions: All authors made substantive contributions the work presented in this paper. K.L.N. designed and implemented the study. R.P.W. supervised experimental design and methods. T.P. performed data analysis and drafted the manuscript. L.S.H. helped develop analytic tools, and with K.L.N. conducted data acquisition. S.J. and H.L. contributed to data analysis. All authors, including R.F.A and K.H.N., participated in data interpretation and commented on the manuscript.
Conflict of Interest:
The authors have no conflicts to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achim AM, Bertrand MC, Sutton H, Montoya A, Czechowska Y, Malla AK, Joober R, Pruessner JC, Lepage M. Selective abnormal modulation of hippocampal activity during memory formation in first-episode psychosis. Archives of General Psychiatry. 2007;64(9):999–1014. doi: 10.1001/archpsyc.64.9.999. [DOI] [PubMed] [Google Scholar]
- Achim AM, Lepage M. Episodic memory-related activation in schizophrenia: meta-analysis. Br J Psychiatry. 2005;187:500–509. doi: 10.1192/bjp.187.6.500. [DOI] [PubMed] [Google Scholar]
- Aleman A, Hijman R, de Haan EH, Kahn RS. Memory impairment in schizophrenia: a meta-analysis. Am J Psychiatry. 1999;156(9):1358–1366. doi: 10.1176/ajp.156.9.1358. [DOI] [PubMed] [Google Scholar]
- Antonova E, Sharma T, Morris R, Kumari V. The relationship between brain structure and neurocognition in schizophrenia: a selective review. Schizophrenia Research. 2004;70(2–3):117–145. doi: 10.1016/j.schres.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Bearden CE, Karlsgodt KH, Bachman P, van Erp TG, Winkler AM, Glahn DC. Genetic architecture of declarative memory: implications for complex illnesses. Neuroscientist. 2012;18(5):516–532. doi: 10.1177/1073858411415113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilder RM, Goldman RS, Robinson D, Reiter G, Bell L, Bates JA, Pappadopulos E, Willson DF, Alvir JM, Woerner MG, Geisler S, Kane JM, Lieberman JA. Neuropsychology of first-episode schizophrenia: initial characterization and clinical correlates. Am J Psychiatry. 2000;157(4):549–559. doi: 10.1176/appi.ajp.157.4.549. [DOI] [PubMed] [Google Scholar]
- Blumenfeld RS, Ranganath C. Prefrontal cortex and long-term memory encoding: an integrative review of findings from neuropsychology and neuroimaging. Neuroscientist. 2007;13(3):280–291. doi: 10.1177/1073858407299290. [DOI] [PubMed] [Google Scholar]
- Boos HB, Aleman A, Cahn W, Hulshoff Pol H, Kahn RS. Brain volumes in relatives of patients with schizophrenia: a meta-analysis. Archives of General Psychiatry. 2007;64(3):297–304. doi: 10.1001/archpsyc.64.3.297. [DOI] [PubMed] [Google Scholar]
- Brewer JB, Moghekar A. Imaging the medial temporal lobe: exploring new dimensions. Trends Cogn Sci. 2002;6(5):217–223. doi: 10.1016/s1364-6613(02)01881-8. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Koutstaal W. Functional neuroimaging studies of encoding, priming, and explicit memory retrieval. Proc Natl Acad Sci U S A. 1998;95(3):891–898. doi: 10.1073/pnas.95.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins ME, Gur RC, Ragland JD, Gur RE. Face recognition memory deficits and visual object memory performance in patients with schizophrenia and their relatives. The American journal of psychiatry. 2005;162(10):1963–1966. doi: 10.1176/appi.ajp.162.10.1963. [DOI] [PubMed] [Google Scholar]
- Cirillo MA, Seidman LJ. Verbal declarative memory dysfunction in schizophrenia: from clinical assessment to genetics and brain mechanisms. Neuropsychol Rev. 2003;13(2):43–77. doi: 10.1023/a:1023870821631. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Ryan J, Hunt C, Romine L, Wszalek T, Nash C. Hippocampal system and declarative (relational) memory: summarizing the data from functional neuroimaging studies. Hippocampus. 1999;9(1):83–98. doi: 10.1002/(SICI)1098-1063(1999)9:1<83::AID-HIPO9>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Davachi L, Wagner AD. Hippocampal contributions to episodic encoding: insights from relational and item-based learning. J Neurophysiol. 2002;88(2):982–990. doi: 10.1152/jn.2002.88.2.982. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. From conditioning to conscious recollection : memory systems of the brain. Oxford Univ; Upper Saddle River, NJ: 2001. [Google Scholar]
- Faraone SV, Seidman LJ, Kremen WS, Toomey R, Pepple JR, Tsuang MT. Neuropsychologic functioning among the nonpsychotic relatives of schizophrenic patients: The effect of genetic loading. Biol Psychiatry. 2000;48(2):120–126. doi: 10.1016/s0006-3223(99)00263-2. [DOI] [PubMed] [Google Scholar]
- First MB, SRL, Gibbon M, Williams JBW, Benjamin L. Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II) Biometrics Research Department, New York State Psychiatric Institute; New York: 1994. [Google Scholar]
- First MB, SRL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version, Patient Edition. Biometrics Research, New York State Psychiatric Insititute; New York: 2002. [Google Scholar]
- Fischl B. FreeSurfer. Neuroimage. 2012;62(2):774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole Brain Segmentation: Automated Labeling of Neuroanatomical Structures in the Human Brain. Neuron. 2002;33:341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, Dale AM. Sequence-Independent Segmentation of Magnetic Resonance Images. Neuroimage. 2004;23(Suppl 1):S69–84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Gilbertson MW, van Kammen DP. Recent and remote memory dissociation: medication effects and hippocampal function in schizophrenia. Biol Psychiatry. 1997;42(7):585–595. doi: 10.1016/S0006-3223(96)00435-0. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Weinberger DR. Effects of neuroleptic medications on the cognition of patients with schizophrenia: a review of recent studies. J Clin Psychiatry. 1996;57(Suppl 9):62–65. [PubMed] [Google Scholar]
- Grabner G, Janke AL, Budge MM, Smith D, Pruessner J, Collins DL. Symmetric atlasing and model based segmentation: an application to the hippocampus in older adults. Medical image computing and computer-assisted intervention : MICCAI. International Conference on Medical Image Computing and Computer-Assisted Intervention. 2006;9(Pt 2):58–66. doi: 10.1007/11866763_8. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Boyett-Anderson JM, Eliez S, Schatzberg AF, Reiss AL, Menon V. Regional analysis of hippocampal activation during memory encoding and retrieval: fMRI study. Hippocampus. 2003;13(1):164–174. doi: 10.1002/hipo.10064. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26(1):119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Knouf N, Kanwisher N. The fusiform face area subserves face perception, not generic within-category identification. Nat Neurosci. 2004;7(5):555–562. doi: 10.1038/nn1224. [DOI] [PubMed] [Google Scholar]
- Harrison PJ. The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology (Berl) 2004 doi: 10.1007/s00213-003-1761-y. [DOI] [PubMed] [Google Scholar]
- Hayasaka S, Nichols TE. Validating cluster size inference: random field and permutation methods. Neuroimage. 2003;20(4):2343–2356. doi: 10.1016/j.neuroimage.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Heckers S. Neuroimaging studies of the hippocampus in schizophrenia. Hippocampus. 2001;11(5):520–528. doi: 10.1002/hipo.1068. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12(3):426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Henson RN, Gagnepain P. Predictive, interactive multiple memory systems. Hippocampus. 2010;20(11):1315–1326. doi: 10.1002/hipo.20857. [DOI] [PubMed] [Google Scholar]
- Herold CJ, Lasser MM, Schmid LA, Seidl U, Kong L, Fellhauer I, Thomann PA, Essig M, Schroder J. Hippocampal volume reduction and autobiographical memory deficits in chronic schizophrenia. Psychiatry Res. 2013;211(3):189–194. doi: 10.1016/j.pscychresns.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry. 2005;162(12):2233–2245. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, Yovel G. The fusiform face area: a cortical region specialized for the perception of faces. Philos Trans R Soc Lond B Biol Sci. 2006;361(1476):2109–2128. doi: 10.1098/rstb.2006.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperberg GR, Deckersbach T, Holt DJ, Goff D, West WC. Increased temporal and prefrontal activity in response to semantic associations in schizophrenia. Archives of General Psychiatry. 2007;64(2):138–151. doi: 10.1001/archpsyc.64.2.138. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Thermenos HW, Barch DM, Seidman LJ. Imaging genetic liability to schizophrenia: systematic review of FMRI studies of patients' nonpsychotic relatives. Schizophr Bull. 2009;35(6):1142–1162. doi: 10.1093/schbul/sbn053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns JR, Eichenbaum H. Evolution of declarative memory. Hippocampus. 2006;16(9):795–808. doi: 10.1002/hipo.20205. [DOI] [PubMed] [Google Scholar]
- McGurk SR. The effects of clozapine on cognitive functioning in schizophrenia. J Clin Psychiatry. 1999;60(Suppl 12):24–29. [PubMed] [Google Scholar]
- Moser MB, Moser EI. Functional differentiation in the hippocampus. Hippocampus. 1998;8(6):608–619. doi: 10.1002/(SICI)1098-1063(1998)8:6<608::AID-HIPO3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Narr KL, van Erp TG, Cannon TD, Woods RP, Thompson PM, Jang S, Blanton R, Poutanen VP, Huttunen M, Lonnqvist J, Standerksjold-Nordenstam CG, Kaprio J, Mazziotta JC, Toga AW. A twin study of genetic contributions to hippocampal morphology in schizophrenia. Neurobiology of disease. 2002;11(1):83–95. doi: 10.1006/nbdi.2002.0548. [DOI] [PubMed] [Google Scholar]
- Nelson MD, Saykin AJ, Flashman LA, Riordan HJ. Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: A meta-analytic study. Archives of General Psychiatry. 1998;55(5):443–440. doi: 10.1001/archpsyc.55.5.433. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Asarnow RF, Subotnik KL, Fogelson DL, Payne DL, Kendler KS, Neale MC, Jacobson KC, Mintz J. The structure of schizotypy: relationships between neurocognitive and personality disorder features in relatives of schizophrenic patients in the UCLA Family Study. Schizophrenia Research. 2002;54(1–2):121–130. doi: 10.1016/s0920-9964(01)00359-0. [DOI] [PubMed] [Google Scholar]
- Phillips PJ, Wechsler H, Huang J, Rauss P. The FERET database and evaluation procedure for face-recognition algorithms. Image and Vision Computing. 1998;16(5):295–306. [Google Scholar]
- Pinkham A, Penn D, Wangelin B, Perkins D, Gerig G, Gu H, Lieberman J. Facial emotion perception and fusiform gyrus volume in first episode schizophrenia. Schizophrenia Research. 2005;79(2–3):341–343. doi: 10.1016/j.schres.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Preston AR, Shohamy D, Tamminga CA, Wagner AD. Hippocampal function, declarative memory, and schizophrenia: anatomic and functional neuroimaging considerations. Curr Neurol Neurosci Rep. 2005;5(4):249–256. doi: 10.1007/s11910-005-0067-3. [DOI] [PubMed] [Google Scholar]
- Quintana J, Wong T, Ortiz-Portillo E, Marder SR, Mazziotta JC. Right lateral fusiform gyrus dysfunction during facial information processing in schizophrenia. Biol Psychiatry. 2003;53(12):1099–1112. doi: 10.1016/s0006-3223(02)01784-5. [DOI] [PubMed] [Google Scholar]
- Ragland JD, Laird AR, Ranganath C, Blumenfeld RS, Gonzales SM, Glahn DC. Prefrontal activation deficits during episodic memory in schizophrenia. The American journal of psychiatry. 2009;166(8):863–874. doi: 10.1176/appi.ajp.2009.08091307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Minzenberg MJ, Ragland JD. The cognitive neuroscience of memory function and dysfunction in schizophrenia. Biol Psychiatry. 2008;64(1):18–25. doi: 10.1016/j.biopsych.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reber PJ, Siwiec RM, Gitelman DR, Parrish TB, Mesulam MM, Paller KA. Neural correlates of successful encoding identified using functional magnetic resonance imaging. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22(21):9541–9548. doi: 10.1523/JNEUROSCI.22-21-09541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, Harvey PD. Neuropsychological impairments in schizophrenia: Integration of performance-based and brain imaging findings. Psychol Bull. 2007;133(5):833–858. doi: 10.1037/0033-2909.133.5.833. [DOI] [PubMed] [Google Scholar]
- Shin YW, Na MH, Ha TH, Kang DH, Yoo SY, Kwon JS. Dysfunction in configural face processing in patients with schizophrenia. Schizophr Bull. 2008;34(3):538–543. doi: 10.1093/schbul/sbm118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein SM, All SD, Kasi R, Berten S, Essex B, Lathrop KL, Little DM. Increased fusiform area activation in schizophrenia during processing of spatial frequency-degraded faces, as revealed by fMRI. Psychol Med. 2010;40(7):1159–1169. doi: 10.1017/S0033291709991735. [DOI] [PubMed] [Google Scholar]
- Snitz BE, Macdonald AW, 3rd, Carter CS. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophr Bull. 2006;32(1):179–194. doi: 10.1093/schbul/sbi048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R, Chua E, Cocchiarella A, Rand-Giovannetti E, Poldrack R, Schacter DL, Albert M. Putting names to faces: successful encoding of associative memories activates the anterior hippocampal formation. Neuroimage. 2003;20(2):1400–1410. doi: 10.1016/S1053-8119(03)00391-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Dickerson BC, Pihlajamaki M, Vannini P, LaViolette PS, Vitolo OV, Hedden T, Becker JA, Rentz DM, Selkoe DJ, Johnson KA. Functional alterations in memory networks in early Alzheimer's disease. Neuromolecular Med. 2010;12(1):27–43. doi: 10.1007/s12017-009-8109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thermenos HW, Seidman LJ, Poldrack RA, Peace NK, Koch JK, Faraone SV, Tsuang MT. Elaborative verbal encoding and altered anterior parahippocampal activation in adolescents and young adults at genetic risk for schizophrenia using FMRI. Biol Psychiatry. 2007;61(4):564–574. doi: 10.1016/j.biopsych.2006.04.044. [DOI] [PubMed] [Google Scholar]
- Thoma RJ, Monnig M, Hanlon FM, Miller GA, Petropoulos H, Mayer AR, Yeo R, Euler M, Lysne P, Moses SN, Canive JM. Hippocampus volume and episodic memory in schizophrenia. J Int Neuropsychol Soc. 2009;15(2):182–195. doi: 10.1017/S1355617709090225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulopoulou T, Morris RG, Rabe-Hesketh S, Murray RM. Selectivity of verbal memory deficit in schizophrenic patients and their relatives. Am J Med Genet. 2003;116B(1):1–7. doi: 10.1002/ajmg.b.10027. [DOI] [PubMed] [Google Scholar]
- Ventura J, Nuechterlein KH, Subotnik KL, Gutkind D, Gilbert EA. Symptom dimensions in recent-onset schizophrenia and mania: a principal components analysis of the 24-item Brief Psychiatric Rating Scale. Psychiatry Res. 2000;97(2–3):129–135. doi: 10.1016/s0165-1781(00)00228-6. [DOI] [PubMed] [Google Scholar]
- Walther S, Federspiel A, Horn H, Bianchi P, Wiest R, Wirth M, Strik W, Muller TJ. Encoding deficit during face processing within the right fusiform face area in schizophrenia. Psychiatry Res. 2009;172(3):184–191. doi: 10.1016/j.pscychresns.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Wang L, Laviolette P, O'Keefe K, Putcha D, Bakkour A, Van Dijk KR, Pihlajamaki M, Dickerson BC, Sperling RA. Intrinsic connectivity between the hippocampus and posteromedial cortex predicts memory performance in cognitively intact older individuals. Neuroimage. 2010;51(2):910–917. doi: 10.1016/j.neuroimage.2010.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss AP, Heckers S. Neuroimaging of declarative memory in schizophrenia. Scand J Psychol. 2001;42(3):239–250. doi: 10.1111/1467-9450.00234. [DOI] [PubMed] [Google Scholar]
- Whittaker JF, Deakin JF, Tomenson B. Face processing in schizophrenia: defining the deficit. Psychol Med. 2001;31(3):499–507. doi: 10.1017/s0033291701003701. [DOI] [PubMed] [Google Scholar]
- Whyte MC, McIntosh AM, Johnstone EC, Lawrie SM. Declarative memory in unaffected adult relatives of patients with schizophrenia: a systematic review and meta-analysis. Schizophrenia Research. 2005;78(1):13–26. doi: 10.1016/j.schres.2005.05.018. [DOI] [PubMed] [Google Scholar]
- Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. American Journal of Psychiatry. 2000;157(1):16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- Yang Y, Nuechterlein KH, Phillips O, Hamilton LS, Subotnik KL, Asarnow RF, Toga AW, Narr KL. The contributions of disease and genetic factors towards regional cortical thinning in schizophrenia: the UCLA family study. Schizophr Res. 2010;123(2–3):116–125. doi: 10.1016/j.schres.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Nuechterlein KH, Phillips OR, Gutman B, Kurth F, Dinov I, Thompson PM, Asarnow RF, Toga AW, Narr KL. Disease and genetic contributions toward local tissue volume disturbances in schizophrenia: a tensor-based morphometry study. Hum Brain Mapp. 2012;33(9):2081–2091. doi: 10.1002/hbm.21349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeineh MM, Engel SA, Thompson PM, Bookheimer SY. Dynamics of the hippocampus during encoding and retrieval of face-name pairs. Science. 2003;299(5606):577–580. doi: 10.1126/science.1077775. [DOI] [PubMed] [Google Scholar]