Abstract
Bacterial pathogens secrete protein toxins and effectors that hijack metabolites to covalently modify key host proteins and interfere with their function during infection. Adenosine metabolites, such as nicotinamide adenine dinucleotide (NAD) and adenosine triphosphate (ATP), have in particular been co-opted by these secreted virulence factors to reprogram host pathways. While some host targets for secreted virulence factors have been identified, other toxin and effector substrates have been elusive, which require new methods for their characterization. In this review, we focus on chemical reporters based on NAD and ATP that should facilitate the discovery and characterization of adenosine diphosphate (ADP)-ribosylation and adenylylation/AMPylation in bacterial pathogenesis and cell biology.
Introduction
Bacterial pathogens have evolved a variety of complex mechanisms to modulate host pathways for infection [1]. Amongst these diverse virulence mechanisms, altering posttranslational modifications (PTMs) of host proteins has emerged as a common strategy by which bacterial pathogens rewire cellular pathways [2,3]. Bacteria can manipulate PTMs by activating host factors or secreting their own enzymes to add or remove metabolites from key proteins [2,3]. To bypass the physical barrier of host membranes, bacteria have acquired pore-forming factors to translocate toxins or specialize secretion systems to inject protein effectors into host cells (Figure 1A). These bacterial toxins and effectors often encode enzymes responsible for PTMs such as proteolysis, phosphorylation, glycosylation, lipidation, ubiquitylation, acetylation, methylation, ADP-ribosylation, AMPylation and others [2-5], which can activate or inhibit the function of the target proteins. The characterization of these pathogen-encoded proteins has revealed unpredicted enzymatic activities, often achieved by unique protein sequence and architecture that have begun to reveal important host targets and mechanisms of pathogenesis. Nonetheless, many protein targets of bacterial toxins and effectors in specific cell-types are still unknown. The analysis of PTMs during bacterial infection can be particularly challenging as endogenous protein modifications within host cells may mask less abundant targets of bacterial toxins or effectors.
Figure 1.
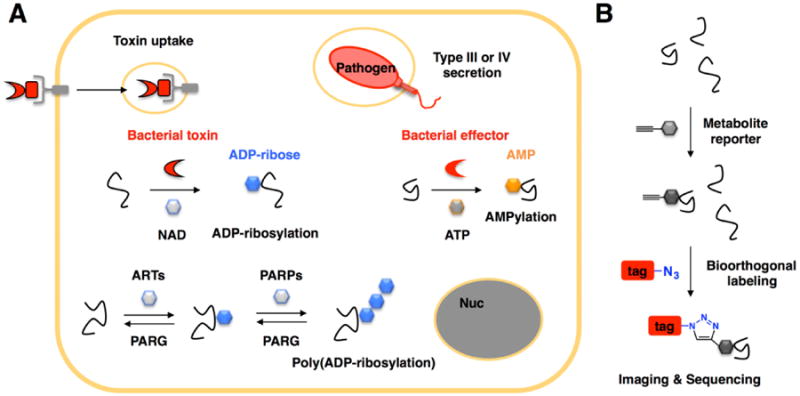
Protein ADP-ribosylation and AMPylation in cells and during bacterial infection. A) Proteins can be ADP-ribosylated by endogenous enzymes or secreted bacterial toxins/effectors that use the cofactor NAD. Protein AMPylation by secreted bacterial toxins/effectors. B) Chemical (metabolite) reporter labeling strategy for bioorthogonal detection of post-translational modifications. Proteins tagged with an alkyne can then be reacted with azide-modified reagents for imaging or affinity enrichment and sequencing.
To facilitate the analysis of PTMs, specific chemical reporters, metabolite analogs bearing uniquely reactive functionality such as an alkyne or azide, have been developed to improve the detection and discovery of various PTMs using bioorthogonal ligation methods (Figure 1B) [6]. In this review, we will summarize adenosine-based chemical reporters of ADP-ribosylation and AMPylation and highlight chemical biology strategies that may be use to characterize bacterial toxins and effectors targets as well as endogenously regulated PTMs.
ADP-Ribosylation
First reported as a histone modification in nuclear extracts [7••], ADP-ribosylation of proteins is now known to play key roles in a variety of cellular pathways in eukaryotes (Figure 2A) [8]. ADP-ribosylation is catalyzed by ADP-ribosyltransferases (ARTs, 17 in humans) that use the cofactor NAD to covalently modify different amino acid side chains (Figure 2A). Mono-ADP-ribosylated proteins can then be elaborated by poly-ADP-ribose polymerases (PARPs) to form poly-ADP-ribosylated proteins (Figure 1B). ADP-ribosylation is reversible and can be removed by poly-ADP-ribose glycohydrolases (PARGs, 3 active isoforms in humans) (Figure 2A). Recently, proteomic studies have suggested over a hundred mammalian proteins are ADP-ribosylated [9-11]. These results collectively suggest that this dynamic and complex PTM is involved in a many cellular functions and is regulated by a family of enzymes.
Figure 2.
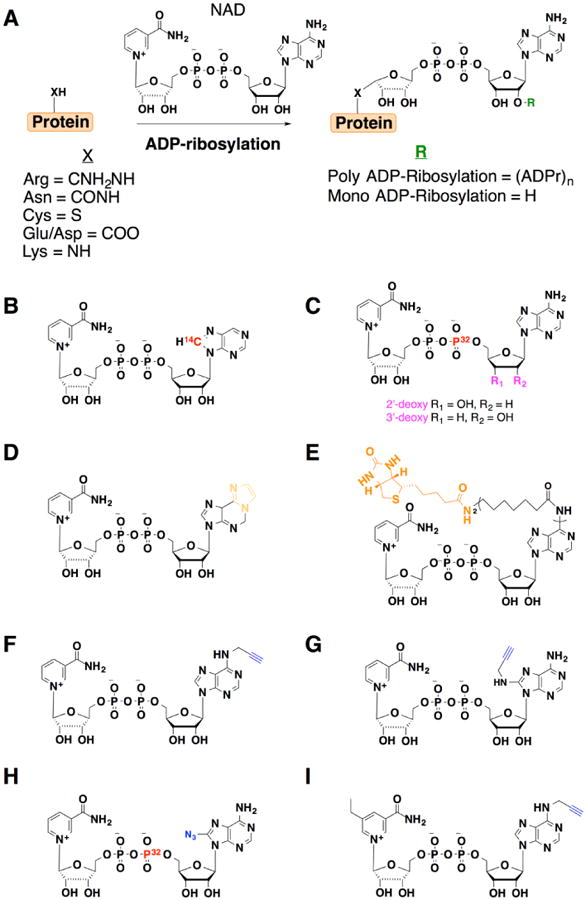
NAD analogs used to study ADP-ribosylation. Groups key to detection or enrichment are highlighted in color. A) Summary of mono- and poly-ADP-ribosylation. B) 14C-NAD. C) 2′- and 3′-deoxy-NAD. D) N4-ethenoadenine dinucleotide (ENAD). E) N6-biotinylated NAD. F) 6-propargyl-NAD. G) 8-propargyl-NAD. H) 8-azido-NAD. I) 5-ethyl-nicotinamide-N6-propargyl-NAD.
In the context of bacterial pathogenesis, diphtheria toxin catalyzed ADP-ribosylation of mammalian elongation factor-2 (EF-2) was reported shortly after the discovery of histone ADP-ribosylation [12••,13••], which revealed how this secreted toxin could utilize NAD to inhibit host protein synthesis. Following these studies, several other secreted bacterial proteins have also been shown to exhibit ADP-ribosyltransferase activity against different host protein targets [4,5]. For example, ExoS from Pseudomonas aeruginosa can ADP-ribosylate host proteins, such as H-Ras and other small GTPases (Rab5) to inhibit cell signaling and membrane trafficking [14,15]. Alternatively, Photorhabdus luminescens TccC3 [16•] and Clostridium botulinum C3 [17] toxins can ADP-ribosylate actin and small GTPase RhoA, respectively, to remodel the host cytoskeleton and cell signaling. These examples demonstrate that ADP-ribosylation can function as a potent inhibitory or activating modification. Interestingly, bacteriophage T4 can also co-opt NAD to ADP-ribosylate and inhibit RNA polymerase in bacteria [18•,19•], demonstrating ADP-ribosylation is a conserved mechanism for pathogen modulation of host proteins in animals, plants and bacteria. While some host targets of some bacterial ARTs have been characterized [4,5], the precise substrates of other toxins or effectors during bacterial infection of specific cell types are still unknown. Both experimental and in silico methods have identified several of these new bacterial ARTs, such as typhoid toxin in Salmonella typhi [20], certhrax in Bacillus cereus and chelt in Vibrio cholera. [21] Characterizing the host targets of these bacterial ARTs will be crucial for understanding the virulence mechanisms of these pathogens.
The discovery of endogenous and pathogen-induced ADP-ribosylation in mammalian cells highlights the significance and challenge of investigating this PTM and their associated enzymes. For example, ADP-ribosylation can occur on several amino acid residues and is chemically labile, which hinders the prediction and biochemical mapping of modification sites [4,5,8]. In addition, ADP-ribosylation can occur in polymeric form that can vary in length and linkage. Poly-ADP-ribosylation can also mask less abundant mono-ADP-ribosylated proteins in cell lysates. During infection of host cells, discriminating between endogenous and pathogen-induced ADP-ribosylation can further complicate the characterization of secreted toxin or bacterial effector targets. These technical challenges demand new methods and reagents to characterized mono- and poly-ADP-ribosylated proteins and their associated enzymes in vitro and in cells.
A variety of NAD analogs have been developed to study ADP-ribosylation. ADP-ribosylation has historically been analyzed with radioactivity using 14C-adenosyl NAD labeling [7••] (Figure 2B). To specifically monitor mono-ADP-ribosylation, 2′-deoxy and 3′-deoxy NAD with 32P-α-phosphate were developed for radioactive detection and prevention of ADP chain elongation (Figure 2C) [22•,23•,24]. These NAD analogs were utilized by ARTs and revealed a variety of proteins such as histones [25] and EF-2 [26] were mono-ADP-ribosylated on specific amino acids, which also helped demonstrate that a single modification was sufficient to alter protein function. Non-radioactive analogs of NAD have also been developed. For example, the fluorescence properties of nicotinamide N4-ethenoadenine dinucleotide (ENAD) were used to monitor activity of diphtheria toxin (Figure 2D) [27]. Antibodies to ethenoadenosine have also been generated and used for immunostaining of poly-ADP-ribose in cells [28]. An NAD analog with biotin at the N6 position of the adenosine ring has also facilitated the detection of ADP-ribosylated proteins and characterization of some PARPs with streptavidin reagents (Figure 2E) [29•]. While biotinylated NAD has been useful and is commercially available, it may not be utilized by some enzymes and is not compatible with live cell labeling experiments.
To circumvent the limitations of radioactive and larger NAD analogs, less sterically demanding alkyne analogs of NAD were developed to enable sensitive bioorthogonal detection and discovery of ADP-ribosylated proteins. NAD analogs modified with alkynyl groups at the 6 or 8 position of adenosine ring were shown to be substrates for PARPs, such as PARP-1 and tankyrase-1 (Figure 2F,G) [30•]. After enzymatic incorporation onto proteins in cell lysates, 6-and 8-alkyne modified ADP-ribosylated proteins could be detected by in-gel fluorescence after bioorthogonal labeling with azide-functionalized fluorophores [30•]. Moreover, the alkyne-modified proteins could be reacted with azide-biotin reagents and affinity purified with streptavidin beads for proteomic analysis [30•]. Using this in vitro chemical proteomics labeling strategy, a total of 79 candidate ADP-ribosylated proteins were identified as potential PARP-1 substrates, including histones, heterogeneous nuclear ribonucleoproteins and nucleophosmin [30•]. It should be noted that an azide-modified NAD analog initially generated for photocrosslinking studies (Figure 2H) [31,32] could also be employed as a chemical reporter of ADP-ribosylation [6]. While NAD reporters can be used to profile the substrates of recombinant or overexpressed enzymes in cell lysates, these chemical reporters may also be utilized by endogenously expressed enzymes and complicate studies of individual enzyme targets.
To address potential overlapping substrate specificity of ARTs or PARPs, the “bump-hole” strategy developed for ATP and kinases [33] has been has been applied to ADP-ribosylation [34•]. To utilize the “bump-hole” strategy, an orthogonal substrate-enzyme pair was developed by mutation of the target enzyme to bind a chemical derivative of the substrate. By creating the unique enzyme-substrate pair, the activity and targets of an individual enzyme can be investigated. By initially profiling nicotinamide-based inhibitors, a 5-ethyl-nicotinamide derivative of NAD was found to complement engineered PARPs. Further modification of this NAD analog with an N6-propargyl group afforded a “bumped” NAD chemical reporter that could be used for bioorthogonal detection and chemical proteomics (Figure 2I) [34•]. Using this orthogonal NAD reporter and engineered PARPs, in vitro profiling of PARP1 and PARP2 targets in HEK293T nuclear extracts revealed overlapping and distinct protein substrates of these enzymes [34•]. These studies should motivate the further the development of orthogonal NAD reporter-ART/PARP pairs.
AMPylation
Protein adenylylation or AMPylation, the covalent modification of proteins with AMP on side chain hydroxyl groups (Ser, Thr or Tyr) through a phosphodiester bond, was first described on Escherichia coli glutamine synthetase (GS) adenylyl transferase (Figure 3A) [35••]. Subsequent biochemical studies of VopS revealed that this secreted effector from Vibrio parahaemolyticus could catalyze AMPylation of small GTPases such as RhoA, Cdc42, and Rac to interfere with downstream effectors and modulate the host cytoskeleton [36••]. Based on these studies, proteins containing either the filamentation-induced by cAMP (fic) domain or the adenylyl transferase (ATase) domain have been identified as candidate adenylyltransferases or AMPylators from bacteria to humans [37••,38,39••,40]. AMPylation also appears to be reversible in the context of bacteria infections, as the secreted effector SidD from Legionella pneumophila can deAMPylate Rab1 to regulate exocytosis [41••,42••]. These studies demonstrate protein AMPylation is a dynamic PTM that may regulate a broad range of cellular functions.
Figure 3.
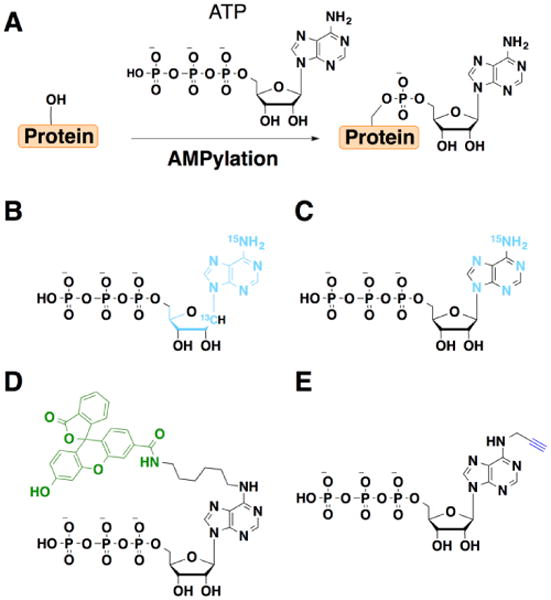
ATP analogs used to study AMPylation. Groups key to detection or enrichment are highlighted in color. A) Protein adenylylation or AMPylation. B) 15N513C10-ATP C) 15N513C0-ATP. D) Fluorescein-ATP (Fl-ATP). E) N6-pATP.
To facilitate the analysis of AMPylated proteins, chemically functionalized ATP analogs have been developed. While AMPylation was originally discovered using radiolabeled nucleotides [35••] and mass spectrometry analysis of purified substrates [36••,43], neither approach is ideal for unbiased discovery of novel AMPylated proteins. Antibodies to AMPylated peptides have been generated, but these reagents have been largely restricted to modified Thr residues [44]. To address these limitations, several ATP analogs have been developed to characterize AMPylated substrates. For mass spectrometry-based detection, 13C- and 15N-ATP analogs have been developed that enable direct identification of AMPylated substrates (Figure 3B,C) [45•]. This stable-isotope tagging strategy was used to identify vimentin as an AMPylation target of the Bep2, the secreted Fic-domain containing protein effector from Bartonella rochalimae [45•]. Fluorescein-ATP (Fl-ATP) has also been used to monitor AMPylation (Figure 3D) [46•]. For example, Fl-ATP can be utilized by VopS to label Cdc42 in vitro and used to screen for candidate AMPylation inhibitors [47•].
An alkyne analog of ATP, N6-propargyl ATP (N6-pATP) has been developed as a bioorthogonal chemical reporter for AMPylation (Figure 3E) [48•]. N6-pATP is substrate for ATase and Fic-domain containing AMPylators and can be used for fluorescent detection or affinity enrichment of labeled substrates following bioorthogonal ligation with complementary azide-modified fluorophores or affinity tags [48•]. For example, N6-pATP can be used with recombinant AMPylators to identify potential substrates in whole cell lysates [48•]. To complement profiling of cell lysates, N6-pATP and recombinant AMPylators can also be used with nucleic acid-programmable protein arrays (NAPPA) to identify candidate substrates in vitro [49•,50•]. These studies have revealed new candidate GTPase and non-GTPase substrates for the secreted bacterial effectors VopS and IbpA [49•]. Application of NAPPA to protein-protein interactions and AMPylation profiling for the type IV secretion effector SidM also identified several new substrates such as Rab8 and Rab10, which may be important for regulating the intracellular trafficking of Legionella pneumophila [50•]. These studies highlight the utility of ATP analogs as chemical reporters for protein AMPylation that should facilitate further in vitro profiling studies of complex cell lysates or protein arrays.
Conclusion and Future Directions
The chemical reporters developed for ADP-ribosylation and AMPylation have provided new methods for charactering these interesting PTMs in cell biology and bacterial pathogenesis. While these reagents have facilitated in vitro studies, new methods are still needed to characterize these protein modifications in live cells and during bacterial infection. The study of ADP-ribosylation and AMPylation face several common challenges. The key challenge for these and other PTMs remains mapping sites of modification on diverse amino acid side chains. In this regard, the adenosine-based chemical reporters may facilitate the enrichment of the tagged-peptides for unbiased characterization of modification sites by mass spectrometry. For both protein modifications, differentiating toxin/effector targets from endogenous host regulated PTMs remain a major challenge. To address these issues, the use of protein arrays with chemical reporters should continue to reveal new candidate targets in vitro and application of engineered enzymes with bumped chemical reporters should facilitate studies with cell lysates. Additionally, the chemical reporters that have been developed for both of these protein modifications have been restricted to in vitro studies. The development of enzyme-specific chemical reporters that function in living cells are therefore desperately needed for exploring these complex protein modification in bacterial pathogenesis as well as host cell biology.
Highlights.
Pathogens use ADP-ribosylation and AMPylation to manipulate host proteins.
Adenosine-based chemical reporters provide new tools to characterize modified proteins.
Acknowledgments
NPW is a Leukemia and Lymphoma Society postdoctoral fellow. HCH acknowledges support from NIH-NIGMS R01 GM087544 grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bhavsar AP, Guttman JA, Finlay BB. Manipulation of host-cell pathways by bacterial pathogens. Nature. 2007;449:827–834. doi: 10.1038/nature06247. [DOI] [PubMed] [Google Scholar]
- 2.Salomon D, Orth K. What pathogens have taught us about posttranslational modifications. Cell Host Microbe. 2013;14:269–279. doi: 10.1016/j.chom.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cui J, Shao F. Biochemistry and cell signaling taught by bacterial effectors. Trends Biochem Sci. 2011;36:532–540. doi: 10.1016/j.tibs.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Aktories K. Bacterial protein toxins that modify host regulatory GTPases. Nat Rev Micro. 2011;9:487–498. doi: 10.1038/nrmicro2592. [DOI] [PubMed] [Google Scholar]
- 5.Deng Q, Barbieri JT. Molecular mechanisms of the cytotoxicity of ADP-ribosylating toxins. Annu Rev Microbiol. 2008;62:271–288. doi: 10.1146/annurev.micro.62.081307.162848. [DOI] [PubMed] [Google Scholar]
- 6.Grammel M, Hang HC. Chemical reporters for biological discovery. Nat Chem Biol. 2013;9:475–484. doi: 10.1038/nchembio.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7••.Nishizuka Y, Ueda K, Honjo T, Hayaishi O. Enzymic adenosine diphosphate ribosylation of histone and poly adenosine diphosphate ribose synthesis in rat liver nuclei. J Biol Chem. 1968;243:3765–3767. The initial report and characterization of histone poly(ADP-ribosylation) in rat liver nuclear extracts. [PubMed] [Google Scholar]
- 8.Gibson BA, Kraus WL. New insights into the molecular and cellular functions of poly(ADP-ribose) and PARPs. Nat Rev Mol Cell Biol. 2012;13:411–424. doi: 10.1038/nrm3376. [DOI] [PubMed] [Google Scholar]
- 9.Jungmichel S, Rosenthal F, Altmeyer M, Lukas J, Hottiger Michael O, Nielsen Michael L. Proteome-wide Identification of Poly(ADP-Ribosyl)ation Targets in Different Genotoxic Stress Responses. Molecular Cell. 2013;52:272–285. doi: 10.1016/j.molcel.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 10.Gagne JP, Isabelle M, Lo KS, Bourassa S, Hendzel MJ, Dawson VL, Dawson TM, Poirier GG. Proteome-wide identification of poly(ADP-ribose) binding proteins and poly(ADP-ribose)-associated protein complexes. Nucleic Acids Research. 2008;36:6959–6976. doi: 10.1093/nar/gkn771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Wang J, Ding M, Yu Y. Site-specific characterization of the Asp- and Glu- ADP-ribosylated proteome. Nat Meth. 2013;10:981–984. doi: 10.1038/nmeth.2603. [DOI] [PubMed] [Google Scholar]
- 12••.Honjo T, Nishizuka Y, Hayaishi O. Diphtheria toxin-dependent adenosine diphosphate ribosylation of aminoacyl transferase II and inhibition of protein synthesis. J Biol Chem. 1968;243:3553–3555. The study demonstrated the ADP-ribosylation of EF-2 by Diptheria toxin, which halts protein synthesis, during infection. [PubMed] [Google Scholar]
- 13.Collier RJ, Cole HA. Diphtheria toxin subunit active in vitro. Science. 1969;164:1179–1181. doi: 10.1126/science.164.3884.1179. The report described a subunit of diptheria toxin that ADP-ribosylates aminoacyl-transferase II in vitro. [DOI] [PubMed] [Google Scholar]
- 14.Coburn J, Wyatt RT, Iglewski BH, Gill DM. Several GTP-binding proteins, including p21c-H-ras, are preferred substrates of Pseudomonas aeruginosa exoenzyme S. J Biol Chem. 1989;264:9004–9008. [PubMed] [Google Scholar]
- 15.Simon NC, Barbieri JT. Exoenzyme S ADP-ribosylates Rab5 effector sites to uncouple intracellular trafficking. Infect Immun. 2014;82:21–28. doi: 10.1128/IAI.01059-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16•.Lang AE, Schmidt G, Schlosser A, Hey TD, Larrinua IM, Sheets JJ, Mannherz HG, Aktories K. Photorhabdus luminescens Toxins ADP-Ribosylate Actin and RhoA to Force Actin Clustering. Science. 2010;327:1139–1142. doi: 10.1126/science.1184557. This report described the use of ADP-ribosyaltion by the plant pathogen Photorhabdus luminescens of actin and RhoA to disrupt the cellular cytoskeleton. [DOI] [PubMed] [Google Scholar]
- 17.Aktories K, Braun U, Rösener S, Just I, Hall A. The rho gene product expressed in E. Coli is a substrate of botulinum ADP-ribosyltransferase C3. Biochemical and Biophysical Research Communications. 1989;158:209–213. doi: 10.1016/s0006-291x(89)80199-8. [DOI] [PubMed] [Google Scholar]
- 18•.Tiemann B, Depping R, Gineikiene E, Kaliniene L, Nivinskas R, Ruger W. ModA and ModB, two ADP-ribosyltransferases encoded by bacteriophage T4: catalytic properties and mutation analysis. J Bacteriol. 2004;186:7262–7272. doi: 10.1128/JB.186.21.7262-7272.2004. A study that performed mutational analysis of bacteriophage enzymes ModA and ModB to define key residues for their ADP-ribosylation activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19•.Rohrer H, Zillig W, Mailhammer R. ADP-ribosylation of DNA-dependent RNA polymerase of Escherichia coli by an NAD+: protein ADP-ribosyltransferase from bacteriophage T4. Eur J Biochem. 1975;60:227–238. doi: 10.1111/j.1432-1033.1975.tb20995.x. This study detailed ADP-ribosylation of a bacterial RNA polymerase by bacteriophage ModA and ModB, which disrupts transcription. [DOI] [PubMed] [Google Scholar]
- 20.Song J, Gao X, Galan JE. Structure and function of the Salmonella Typhi chimaeric A2B5 typhoid toxin. Nature. 2013;499:350–354. doi: 10.1038/nature12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fieldhouse RJ, Turgeon Z, White D, Merrill AR. Cholera- and Anthrax-Like Toxins Are among Several New ADP-Ribosyltransferases. PLoS Comput Biol. 2010;6:e1001029. doi: 10.1371/journal.pcbi.1001029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lichtenwalner DM, Suhadolnik RJ. Adenosine diphosphate ribosylation of histone and nonhistone chromosomal proteins with oxidized nicotinamide adenine dinucleotide and 2′-deoxynicotinamide adenine dinucleotide using nuclei isolated from rat liver and HeLa cells. Biochemistry. 1979;18:3749–3755. doi: 10.1021/bi00584a016. This study utilized 2′-deoxyNAD to label PARP1 ADP-ribosylated proteins in nuclear extracts. [DOI] [PubMed] [Google Scholar]
- 23•.Alvarez-Gonzalez R. 3′-Deoxy-NAD+ as a substrate for poly(ADP-ribose)polymerase and the reaction mechanism of poly(ADP-ribose) elongation. Journal of Biological Chemistry. 1988;263:17690–17696. This report developed and tested 3′-deoxyNAD to label ADP-ribosylated PARP1 and histone H1 in vitro. [PubMed] [Google Scholar]
- 24.Mendoza-Alvarez H, Alvarez-Gonzalez R. Biochemical Characterization of Mono(ADP-ribosyl)ated Poly(ADP-ribose) Polymerase†. Biochemistry. 1999;38:3948–3953. doi: 10.1021/bi982148p. [DOI] [PubMed] [Google Scholar]
- 25.Alvarez-Gonzalez R, Moss J, Niedergang C, Althaus FR. Selective probing of ADP-ribosylation reactions with oxidized 2′-deoxynicotinamide adenine dinucleotide. Biochemistry. 1988;27:5378–5383. doi: 10.1021/bi00414a063. [DOI] [PubMed] [Google Scholar]
- 26.Martinez M, Russ Price S, Moss J, Alvarez-Gonzalez R. Mono(ADP-ribosyl)ation of poly(ADP-ribose)polymerase by cholera toxin. Biochemical and Biophysical Research Communications. 1991;181:1412–1418. doi: 10.1016/0006-291x(91)92096-3. [DOI] [PubMed] [Google Scholar]
- 27.Giovane A, Balestrieri C, Quagliuolo L, Servillo L. 1-N6-Etheno-ADP-ribosylation of elongation factor-2 by diphtheria toxin. FEBS Letters. 1985;191:191–194. doi: 10.1016/0014-5793(85)80006-5. [DOI] [PubMed] [Google Scholar]
- 28.Davis RE, Mysore V, Browning JC, Hsieh JC, Lu QAT, Katsikis PD. In Situ Staining for Poly(ADP-Ribose) Polymerase Activity Using an NAD Analogue. Journal of Histochemistry & Cytochemistry. 1998;46:1279–1289. doi: 10.1177/002215549804601108. [DOI] [PubMed] [Google Scholar]
- 29•.Zhang J, Snyder SH. Purification of a nitric oxide-stimulated ADP-ribosylated protein using biotinylated .beta.-nicotinamide adenine dinucleotide. Biochemistry. 1993;32:2228–2233. doi: 10.1021/bi00060a014. The study utilized biotinylated NAD to purify ADP-ribosylated mammalian proteins after oxidative DNA damage and diptheria toxin ADP-ribosylated EF-2. [DOI] [PubMed] [Google Scholar]
- 30•.Jiang H, Kim JH, Frizzell KM, Kraus WL, Lin H. Clickable NAD analogues for labeling substrate proteins of poly(ADP-ribose) polymerases. J Am Chem Soc. 2010;132:9363–9372. doi: 10.1021/ja101588r. This study developed two clickable NAD analogs for labeling ADP-ribosylated targets of PARP1 and tankyrase 1 in cellular extracts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaillancourt RR, Dhanasekaran N, Johnson GL, Ruoho AE. 2-Azido-[32P]NAD+, a photoactivatable probe for G-protein structure: evidence for holotransducin oligomers in which the ADP-ribosylated carboxyl terminus of alpha interacts with both alpha and gamma subunits. Proceedings of the National Academy of Sciences. 1990;87:3645–3649. doi: 10.1073/pnas.87.10.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim H, Jacobson MK, Rolli V, Ménissier-de Murcia J, Reinbolt J, Simonin F, Ruf A, Schulz G, de Murcia G. Photoaffinity labelling of human poly(ADP-ribose) polymerase catalytic domain. Biochemical Journal. 1997;322:469–475. doi: 10.1042/bj3220469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allen JJ, Li M, Brinkworth CS, Paulson JL, Wang D, Hubner A, Chou WH, Davis RJ, Burlingame AL, Messing RO, et al. A semisynthetic epitope for kinase substrates. Nat Methods. 2007;4:511–516. doi: 10.1038/nmeth1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34•.Carter-O'Connell I, Jin H, Morgan RK, David LL, Cohen MS. Engineering the Substrate Specificity of ADP-Ribosyltransferases for Identifying Direct Protein Targets. Journal of the American Chemical Society. 2014;136:5201–5204. doi: 10.1021/ja412897a. This study utilized the bump-hole strategy to develop orthognal NAD-ART pairs to study protein targets for PARP1 and PARP2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35••.Brown MS, Segal A, Stadtman ER. Modulation of Glutamine Synthetase Adenylylation and Deadenylylation Is Mediated by Metabolic Transformation of the PII-Regulatory Protein. Proceedings of the National Academy of Sciences. 1971;68:2949–2953. doi: 10.1073/pnas.68.12.2949. The initial report of protein adenylylation on glutamine synthetase in E. coli by the PII regulatory protein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yarbrough ML, Li Y, Kinch LN, Grishin NV, Ball HL, Orth K. AMPylation of Rho GTPases by Vibrio VopS Disrupts Effector Binding and Downstream Signaling. Science. 2009;323:269–272. doi: 10.1126/science.1166382. The initial report of protein adenylylation or AMPylation of Cdc42, Rho, and Rac by VopS, which disrupted cytoskeletal orgainization during infection. [DOI] [PubMed] [Google Scholar]
- 37.Worby CA, Mattoo S, Kruger RP, Corbeil LB, Koller A, Mendez JC, Zekarias B, Lazar C, Dixon JE. The Fic Domain: Regulation of Cell Signaling by Adenylylation. Molecular Cell. 2009;34:93–103. doi: 10.1016/j.molcel.2009.03.008. The study investigated the Histophilus somni effector, IbpA. The AMPylator contained a fic domain, which was identified as key to AMPylation activity in bacterial and human proteins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woolery AR, Luong P, Broberg CA, Orth K. AMPylation: Something old is new again. Frontiers in Microbiology. 2010;1 doi: 10.3389/fmicb.2010.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39••.Engel P, Goepfert A, Stanger FV, Harms A, Schmidt A, Schirmer T, Dehio C. Adenylylation control by intra- or intermolecular active-site obstruction in Fic proteins. Nature. 2012;482:107–110. doi: 10.1038/nature10729. This report detailed the regulation of Fic domain proteins by conserved mechanism of ATP-binding-site obstruction that involves an inhibitory α-helix. [DOI] [PubMed] [Google Scholar]
- 40.Rahman M, Ham H, Liu X, Sugiura Y, Orth K, Kramer H. Visual neurotransmission in Drosophila requires expression of Fic in glial capitate projections. Nat Neurosci. 2012;15:871–875. doi: 10.1038/nn.3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41••.Tan Y, Luo ZQ. Legionella pneumophila SidD is a deAMPylase that modifies Rab1. Nature. 2011;475:506–509. doi: 10.1038/nature10307. This report investigted the deAMPylation activity of SidD on Rab1, which is necessary for bacterial exocytosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42••.Neunuebel MR, Chen Y, Gaspar AH, Backlund PS, Yergey A, Machner MP. De-AMPylation of the Small GTPase Rab1 by the Pathogen Legionella pneumophila. Science. 2011;333:453–456. doi: 10.1126/science.1207193. The study explored both Rab1 AMPylation and deAMPylation during legionella infection by the effectors SidM and SidD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Y, Al-Eryani R, Yarbrough M, Orth K, Ball H. Characterization of AMPylation on Threonine, Serine, and Tyrosine Using Mass Spectrometry. Journal of The American Society for Mass Spectrometry. 2011;22:752–761. doi: 10.1007/s13361-011-0084-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hao YH, Chuang T, Ball HL, Luong P, Li Y, Flores-Saaib RD, Orth K. Characterization of a rabbit polyclonal antibody against threonine-AMPylation. Journal of Biotechnology. 2011;151:251–254. doi: 10.1016/j.jbiotec.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45•.Pieles K, Glatter T, Harms A, Schmidt A, Dehio C. An experimental strategy for the identification of AMPylation targets from complex protein samples. PROTEOMICS. 2014;14:1048–1052. doi: 10.1002/pmic.201300470. This study coupled mass spectrometry with heavy isotope ATP analogs to identify AMPylated substrates including vimentin, which was validated by biochemical assays. [DOI] [PubMed] [Google Scholar]
- 46•.Lewallen DM, Steckler CJ, Knuckley B, Chalmers MJ, Thompson PR. Probing adenylation: using a fluorescently labelled ATP probe to directly label and immunoprecipitate VopS substrates. Molecular BioSystems. 2012;8:1701–1706. doi: 10.1039/c2mb25053e. This study utilized fluorescent ATP to directly label VopS substrates like Cdc42 both in vitro and in MCF7 cell extracts. [DOI] [PubMed] [Google Scholar]
- 47•.Lewallen DM, Sreelatha A, Dharmarajan V, Madoux F, Chase P, Griffin PR, Orth K, Hodder P, Thompson PR. Inhibiting AMPylation: A Novel Screen To Identify the First Small Molecule Inhibitors of Protein AMPylation. ACS Chemical Biology. 2013;9:433–442. doi: 10.1021/cb4006886. This study utilized fluorescent ATP to screen for VopS AMPylation small molecule inhibitors with selectivity against the human AMPylator, HYPE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48•.Grammel M, Luong P, Orth K, Hang HC. A Chemical Reporter for Protein AMPylation. Journal of the American Chemical Society. 2011;133:17103–17105. doi: 10.1021/ja205137d. This report developed a clickable ATP analog used by both Fic and ATase domain AMPylators. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49•.Yu Xiaobo, W AR, Luong Phi, Hao Yi Heng, Grammel Markus, Westcott Nathan, Park Jin, Wang Jie, Bian Xiaofang, Demirkan Gokhan, Hang Howard C, Orth Kim, LaBaer Joshua. Click chemistry-based detection of global pathogen-host AMPylation on self-assembled human protein microarrays. 2014 doi: 10.1074/mcp.M114.041103. Molecular & Cellular Proteomics. in press. This report used a clickable ATP analog to study pathogen AMPylation on host proteome microarrays to discover new substrates of VopS and IbpaFic2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50•.Yu Xiaobo, D KB, Baker Kristi, Neunuebel M Ramona, Graves Morgan, Westcott Nathan, Hang Howard, LaBaer Joshua, Qiu Ji, Machner Matthias P. Host-Pathogen Interaction Profiling Using Self-Assembling Human Protein Arrays. mBio. 2014 doi: 10.1021/pr5013015. This study used a clickable ATP analog to investigate protein-protein interactions and AMPylation targets of LidA and SidM in a mammalian proteome microarray. [DOI] [PMC free article] [PubMed] [Google Scholar]


