Abstract
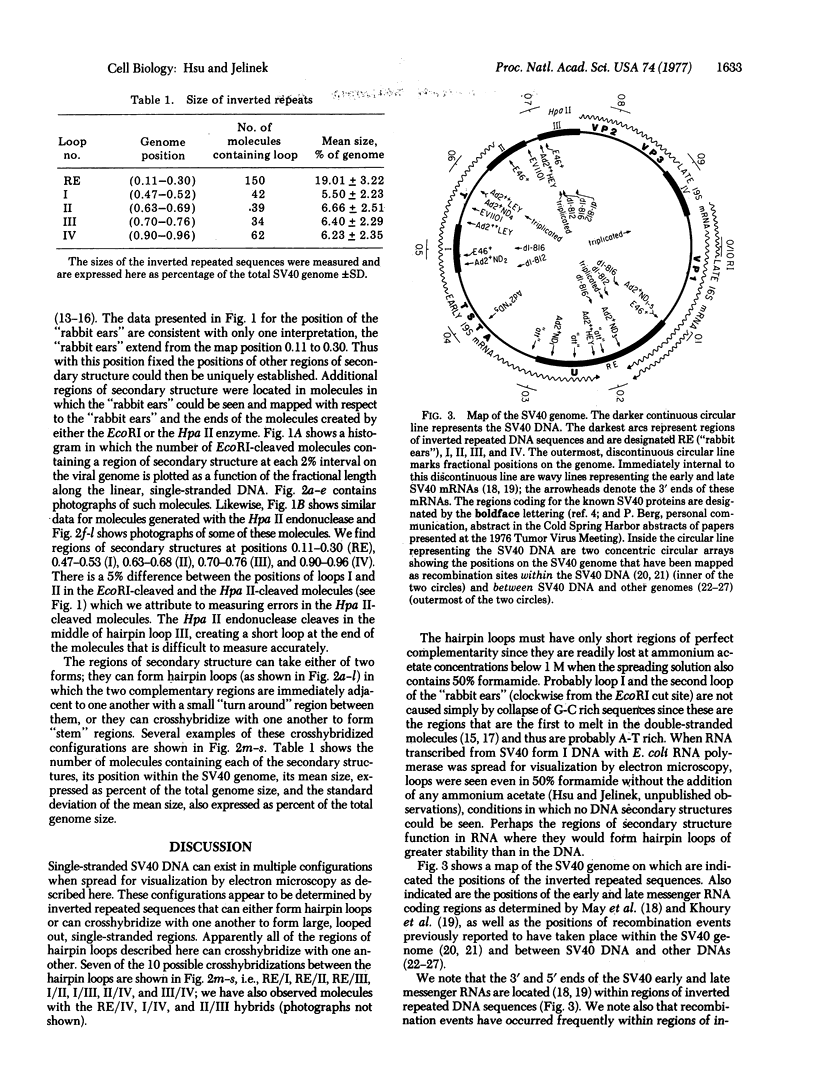
Single-stranded, linear DNA of simian virus 40 (SV40) created by denaturing the endonuclease EcoRI- or Hpa II-generated, linear, double-stranded products from form I DNA of SV40 was analyzed for regions of inverted repeated sequences by visualization with the electron microscope. Six hairpin loops were found at positions 0.11-0.30 (two loops forming a "rabbit ears" structure), 0.47-0.52, 0.63-0.68, 0.70-0.76, and 0.90-0.96. The nucleotide sequences within all of these inverted repeats may be related since the looped regions can crosshybridize with one another and, thus, the SV40 genome may contain regions of interspersed repeated and unique sequences. The map positions of the 3' and 5' ends of the early and late messenger RNAs, as determined by others, lie within regions of inverted repeated sequences. Previously recorded recombination events that occurred either within the SV40 genome or between SV40 DNA and other genomes have apparently occurred frequently at positions of inverted repeated sequences within the SV40 DNA.
Full text
PDF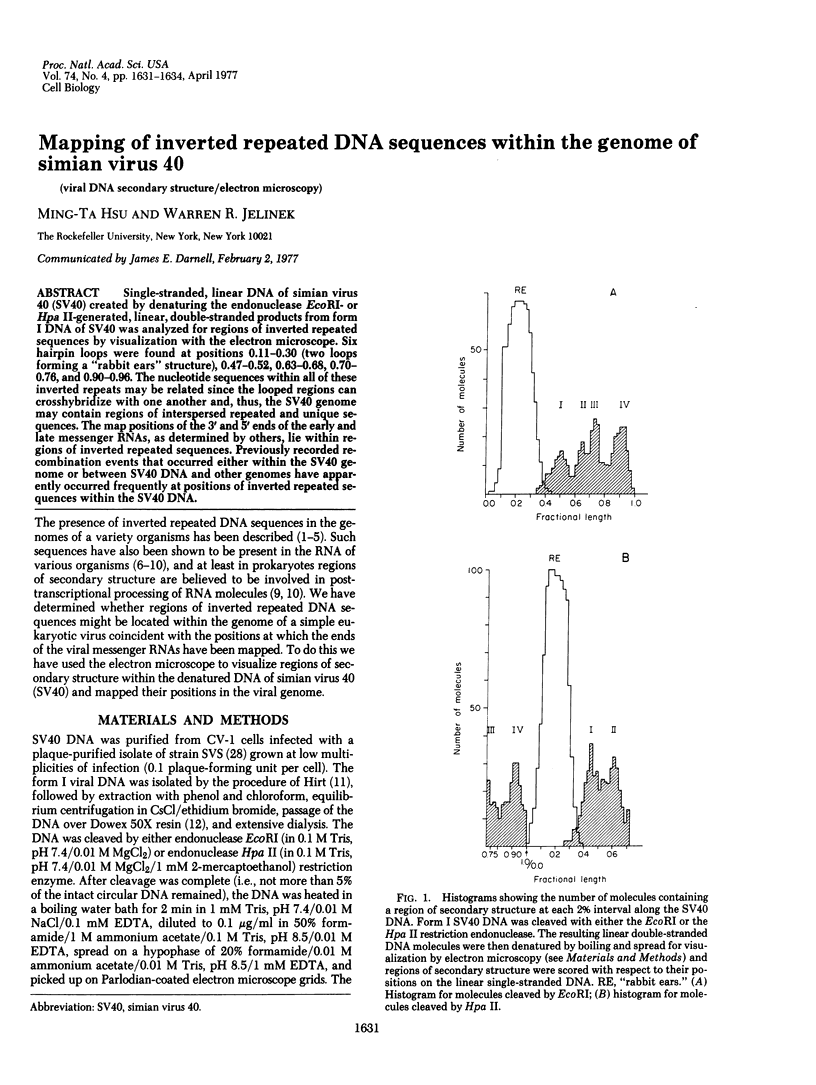


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beard P., Morrow J. F., Berg P. Cleavage of circular, superhelical simian virus 40 DNA to a linear duplex by S1 nuclease. J Virol. 1973 Dec;12(6):1303–1313. doi: 10.1128/jvi.12.6.1303-1313.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockman W. W., Lee T. N., Nathans D. Characterization of cloned evolutionary variants of simian virus 40. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):119–127. doi: 10.1101/sqb.1974.039.01.017. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Boyer H. W., Tischer E. G., Goodman H. M. Electron microscopic mapping of the attachment sites on SV40 DNA during lytic infection. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):109–117. doi: 10.1101/sqb.1974.039.01.016. [DOI] [PubMed] [Google Scholar]
- Danna K. J., Sack G. H., Jr, Nathans D. Studies of simian virus 40 DNA. VII. A cleavage map of the SV40 genome. J Mol Biol. 1973 Aug 5;78(2):363–376. doi: 10.1016/0022-2836(73)90122-8. [DOI] [PubMed] [Google Scholar]
- Davidson E. H., Hough B. R., Amenson C. S., Britten R. J. General interspersion of repetitive with non-repetitive sequence elements in the DNA of Xenopus. J Mol Biol. 1973 Jun 15;77(1):1–23. doi: 10.1016/0022-2836(73)90359-8. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. T7 early RNAs and Escherichia coli ribosomal RNAs are cut from large precursor RNAs in vivo by ribonuclease 3. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3296–3300. doi: 10.1073/pnas.70.12.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Jelinek W., Darnell J. E. Double-stranded regions in heterogeneous nuclear RNA from Hela cells. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2537–2541. doi: 10.1073/pnas.69.9.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek W., Molloy G., Fernandez-Munoz R., Salditt M., Darnell J. E. Secondary structure in heterogeneous nuclear RNA: involvement of regions from repeated DNA sites. J Mol Biol. 1974 Jan 25;82(3):361–370. doi: 10.1016/0022-2836(74)90597-x. [DOI] [PubMed] [Google Scholar]
- Kelly T. J., Jr, Lewis A. M., Jr, Levine A. S., Siegel S. Structural studies on two adenovirus 2-SV40 hybrids containing the entire SV40 genome. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):409–417. doi: 10.1101/sqb.1974.039.01.053. [DOI] [PubMed] [Google Scholar]
- Kelly T. J., Jr, Lewis A. M., Jr, Levine A. S., Siegel S. Structure of two adenovirus-simian virus 40 hybrids which contain the entire SV40 genome. J Mol Biol. 1974 Oct 15;89(1):113–126. doi: 10.1016/0022-2836(74)90165-x. [DOI] [PubMed] [Google Scholar]
- Kelly T. J. Structure of the DNA of the adenovirus 7-simian virus 40 hybrid, e46, by electron microscopy. J Virol. 1975 May;15(5):1267–1272. doi: 10.1128/jvi.15.5.1267-1272.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury G., Fareed G. C., Berry K., Martin M. A., Lee T. N., Nathans D. Characterization of a rearrangement in viral DNA: mapping of the circular simian virus 40-like DNA containing a triplication of a specific one-third of the viral genome. J Mol Biol. 1974 Aug 5;87(2):289–301. doi: 10.1016/0022-2836(74)90150-8. [DOI] [PubMed] [Google Scholar]
- Khoury G., Martin M. A., Lee T. N., Danna K. J., Nathans D. A map of simian virus 40 transcription sites expressed in productively infected cells. J Mol Biol. 1973 Aug 5;78(2):377–389. doi: 10.1016/0022-2836(73)90123-x. [DOI] [PubMed] [Google Scholar]
- Lebowitz P., Kelly T. J., Jr, Nathans D., Lee T. N., Lewis A. M., Jr A colinear map relating the simian virus 40 (SV40) DNA segments of six adenovirus-SV40 hybrids to the DNA fragments produced by restriction endonuclease cleavage of SV40 DNA. Proc Natl Acad Sci U S A. 1974 Feb;71(2):441–445. doi: 10.1073/pnas.71.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May E., Maizel J. V., Salzman N. P. Mapping of transcription sites of simian virus 40-specific late 16S and 19S mRNA by electron microscopy. Proc Natl Acad Sci U S A. 1977 Feb;74(2):496–500. doi: 10.1073/pnas.74.2.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz J. E., Carbon J., Herzberg M., Davis R. W., Berg P. Isolation and characterization of individual clones of simian virus 40 mutants containing deletions duplications and insertions in their DNA. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):69–84. doi: 10.1101/sqb.1974.039.01.012. [DOI] [PubMed] [Google Scholar]
- Morrow J. F., Berg P. Cleavage of Simian virus 40 DNA at a unique site by a bacterial restriction enzyme. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3365–3369. doi: 10.1073/pnas.69.11.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder C., Delius H. Specificity of the break produced by restricting endonuclease R 1 in Simian virus 40 DNA, as revealed by partial denaturation mapping. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3215–3219. doi: 10.1073/pnas.69.11.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Kramer R. A., Steitz J. A. T7 early messenger RNAs are the direct products of ribonuclease III cleavage. J Mol Biol. 1974 Nov 15;89(4):777–782. doi: 10.1016/0022-2836(74)90052-7. [DOI] [PubMed] [Google Scholar]
- Ryskov A. P., Saunders G. F., Farashyan V. R., Georgiev G. P. Double-helical regions in nuclear precursor of mRNA (pre-mRNA). Biochim Biophys Acta. 1973 Jun 8;312(1):152–164. doi: 10.1016/0005-2787(73)90060-9. [DOI] [PubMed] [Google Scholar]
- Schmid C. W., Deininger P. L. Sequence organization of the human genome. Cell. 1975 Nov;6(3):345–358. doi: 10.1016/0092-8674(75)90184-1. [DOI] [PubMed] [Google Scholar]
- Schmid C. W., Manning J. E., Davidson N. Inverted repeat sequences in the Drosophila genome. Cell. 1975 Jun;5(2):159–172. doi: 10.1016/0092-8674(75)90024-0. [DOI] [PubMed] [Google Scholar]
- Takemoto K. K., Kirschstein R. L., Habel K. Mutants of simian virus 40 differing in plaque size, oncogenicity, and heat sensitivity. J Bacteriol. 1966 Oct;92(4):990–994. doi: 10.1128/jb.92.4.990-994.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. A., Thomas C. A., Jr Palindromes in chromosomes. J Mol Biol. 1974 Mar 25;84(1):115–138. doi: 10.1016/0022-2836(74)90216-2. [DOI] [PubMed] [Google Scholar]




