Abstract
Objectives
Endovascular surgery has revolutionized the treatment of aortic aneurysms; however these improvements have come at the cost of increased radiation and contrast exposure, particularly for more complex procedures. Three dimensional (3D) fusion computed tomographic (CT) imaging is a new technology that may facilitate these repairs. The purpose of this analysis was to determine the impact of utilizing intraoperative 3D fusion CT on performance of fenestrated endovascular aortic repair.
Methods
A review of our institutional database was performed to identify patients undergoing fenestrated/branched endovascular aortic repair (FEVAR). Subjects treated using 3D fusion CT were compared to patients treated in the immediate 12-month period prior to implementation of this technology when procedures were performed in a standard hybrid operating room without CT fusion capabilities. Primary endpoints included patient radiation exposure (air kerma area product: milliGray; mGy*cm2), fluoroscopy time (minutes; min), contrast usage (mL) and procedure time (min). Patients were grouped by number of aortic graft fenestrations revascularized with a stentgraft and operative outcomes were compared.
Results
A total of 72 patients (N = 41 before vs. N = 31 after 3D fusion CT implementation) underwent FEVAR from September 2012 through March 2014. For 2-vessel fenestrated endografts, there was a significant decrease in radiation exposure (3400±1900 vs. 1380±520 mGy*cm2; P=.001), fluoroscopy time (63±29 vs. 41±11min; P=.02), and contrast usage (69±16 vs. 26±8 mL; P=.0002) with intraoperative 3D fusion CT. Similarly, for combined 3 and 4-vessel FEVAR, significantly decreased radiation exposure (5400±2225 vs. 2700±1400 mGy*cm2; P<.0001), fluoroscopy time (89±36 vs 6±21min; P=.02), contrast usage (90±25 vs. 39±17mL; P<.0001), as well as procedure time (330±100 vs. 230±50min; P=.002) was noted. Estimated blood loss was significantly less (P<.0001) and length of stay had a trend (P=.07) toward being lower for all patients in the 3D CT group.
Conclusions
These results demonstrate that use of intraoperative 3D fusion CT imaging during FEVAR can significantly decrease radiation exposure and procedure time, as well as contrast usage, which may also decrease the overall physiologic impact of the repair.
Introduction
Fenestrated endovascular aortic repair (FEVAR) is becoming increasingly common, and is now commercially available in the United States with recent Food and Drug Administration (FDA) approval of a customized fenestrated device. With this technological advancement, there is an expected decrease in the physiologic insult to the patient compared to open repair1–3, however this comes with increased radiation and contrast exposure risk during the procedures4. Flouroscopy is an important radiation source in contemporary practice, and an increasing focus on the effects of cumulative radiation exposure to patients and providers is present in the literature4–14. Notably, a recent report has demonstrated that FEVAR is one of the most radiation intensive procedures that vascular specialists perform4.
The FEVAR radiation dose is related not only to the fluoroscopy time, but also the density of the abdomen/pelvis, and the frequent obliquity that is necessary to visualize target vessels adequately13. Additionally, these procedures often require large amounts of iodinated contrast, which may, in part, be responsible for the known decrement in renal function that can occur after FEVAR15,16. Importantly, advanced imaging techniques have been shown to decrease operative times and radiation exposure, as well as mitigate the need for contrast usage during routine EVAR17,18.
Our institution has recently upgraded to a fixed imaging unit capable of 3D fusion CT, and we sought to evaluate how this technology has affected the conduct of FEVAR, and whether this has afforded any demonstrable benefit with regard to radiation exposure and contrast usage.
Methods
The Institutional Review Board (FWA00005790) at the University of Florida approved this study protocol (#201300781). A waiver of informed consent was granted because all collected data pre-existed in medical records and no study related interventions or subject contact occurred. Therefore, the rights and welfare of these subjects was not adversely affected.
Patient selection
A review of our institutional endovascular aortic database was performed for patients who have undergone FEVAR before and after inauguration of a hybrid operating room capable of 3D fusion CT imaging. To mitigate the effect of learning curve over time, we compared patients treated using 3D fusion CT imaging to those patients treated in the immediate 12 months prior to the availability of the new hybrid unit. Specifically, the dates of collection included patients receiving FEVAR in the 3D CT capable room beginning in September 2013 versus those treated in the 365 days before. FEVAR began being offered by a single surgeon (AWB) at our institution in January 2010 and the initial 68 cases [69% (N = 47) were 3 or 4-vessel fenestration procedures] spanning a 2.5 year period were intentionally excluded in an attempt to minimize the impact of the operative team learning curve on the results of this study.
Additionally, no significant operative team personnel or device implantation technique changes occurred during the study interval. The method of target visceral vessel catheterization has been described previously and did not change significantly over the study period19. Patients were grouped by number of fenestrations/branches revascularized (i.e. excluding fenestrations or scallops that were not supported with a stent). Subjects undergoing a 2-vessel FEVAR were compared separately from 3 and 4-vessel FEVAR patients, and three and four vessel patients were grouped to increase numbers for comparison. Demographics, comorbidities, intraoperative characteristics (including adjuncts as defined per Society for Vascular Surgery standards20,21) and postoperative outcomes were abstracted from our prospective database and/or the electronic medical record, as needed. Comorbidities were defined per reporting guidelines1.
Clinical practice
All patients were recovered in a dedicated cardiovascular intensive care unit and patients were mobilized and given a normal diet on postoperative day 1 if there were no clinical concerns (e.g. neurologic, cardiopulmonary, gastrointestinal, hematologic, or renal system derangements). Thereafter, patients were transferred to the floor (unless on the spinal drain protocol) and all indwelling lines and catheters were removed if clinical recovery continued to be uneventful. Once patients tolerated a regular diet and received evaluation by physical therapy, they were discharged from the hospital. A restrictive transfusion protocol exists at our institution and patients are generally transfused only for a hemoglobin < 7g/dL unless there is evidence of hypovolemic anemia or cardiac ischemia. Need for spinal drainage was determined by the operating surgeon. Spinal drain protocols did not change during the study interval and have been previously published22.
Equipment and procedural details
Patients undergoing FEVAR prior to 3D capable OR (“no CT”) availability were treated in a hybrid operating room utilizing a fixed imaging Toshiba Infinix™ Vc-i ceiling mounted (Toshiba, Minato, Tokyo, Japan) single plane system.
Fluoroscopy was generally performed at a low dose of 7.5 frames per second (fps), and digital subtraction angiography (DSA) was performed at 3fps, unless an increased frame rate was necessary for improved imaging, in which case 6fps was utilized. This system was routinely used in conjunction with intravascular ultrasound (IVUS) using a Volcano catheter (Volcano Corporation, San Diego, CA). Prior to device delivery, IVUS was used to determine branch vessel locations. After device delivery, a flush catheter was used to perform digital subtraction angiography (DSA) in the anterior-posterior (AP) and lateral projections to mark the branch vessel locations. Next, the device was deployed while triangulating the radiopaque fenestration/branch markers against the DSA roadmap imaging. DSA imaging was performed intermittently to facilitate vessel catheterization and confirm successful access to the respective target vessels. DSA runs were also routinely utilized to perform individual completion runs for the branch vessels, as well as a completion aortogram. Contrast was minimized by diluting it to 1/3 strength (Visipaque™ 320; GE Healthcare, United Kingdom) for hand-injection imaging, and 1/2 strength for completion imaging.
Use of the 3D CT capable hybrid operating room began in September 2013, which employs a Siemens Artis Zeego® system (Siemens, Munich, Germany). Of note, the radiology technicians for our dedicated vascular operating team underwent advanced imaging training through Siemens before our first procedure, which allowed a rather seamless introduction of this technology into our practice. This unit was also used at a low dose of 7.5fps, and DSA was performed at 4fps. Prior to the procedure, the operating surgeon used the Siemens Leonardo workstation (Siemens AG, Forchheim, Germany) to process the preoperative CT arteriogram (CTA) by placing digital marks around the orifice of each branch vessel on the CTA (Figure 1). These marks were saved, and an intraoperative non-contrasted abdominal CT was then performed before prepping and draping, a process that takes no more than two or three minutes, which adds little to the overall operating room time.
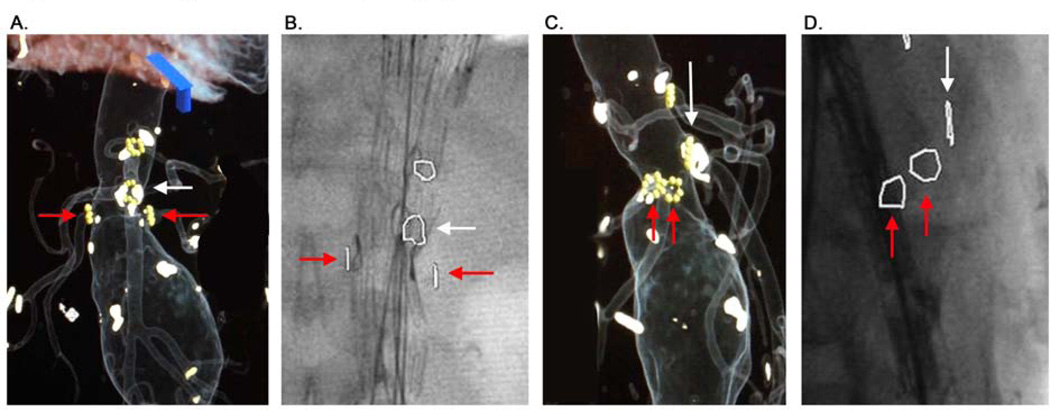
Figure 1. 3D Overlay Fusion and Overlay Imaging.
This image demonstrates the method of overlay mark production and utilization. Panel A demonstrates the marks placed around the origins of the SMA (white arrow) and renal arteries (red arrows). Panel B demonstrates intraoperative overlay of these marks on live fluoroscopy. The radiopaque markers on the graft can be seen to closely approximate these overlay marks during deployment. Panel C and D demonstrate the same patient in a lateral projection. Note that a perfectly orthogonal image of the vessel origin can be obtained by aligning the origin marker such that it appears as a line on the overlay, and this can be aligned before initiating fluoroscopy, minimizing radiation during adjustment of the c-arm.
The Artis Zeego® system (http://usa.healthcare.siemens.com/angio/artis-zee/artis-zee) acquired (DynaCT) images by rotating around the patient 200 degrees and obtaining an array of equally spaced two-dimensional x-ray projection images. For performance of our overlay imaging with the preoperative CTA, the system initially acquired a non-contrasted CT centered at the visceral segment of the aorta (133 frames over 5 seconds). The images were sent to the Leonardo workstation to create the native mask (bony anatomy and vascular structures) DynaCT. DynaCT image data sets were then reconstructed as a 3D image on the Syngo X workplace. Next, this intraoperative non-contrasted CT was fused to the preoperative CTA using bony landmarks. After prepping, draping and obtaining arterial access, stiff Lunderquist wires (Cook Medical, Bloomington, IN) were placed and IVUS was used to confirm the preoperatively placed marks, which are seen as an overlay image on live fluoroscopy (Figure 1). Next, the vessel origin overlay image was adjusted to correspond to IVUS with the stiff wires in place before device delivery. The device was then inserted and deployed using the radiopaque graft markers and the overlay marks displayed on live fluoroscopy. Notably, contrast was not routinely given or required until after each branch vessel was revascularized using a stentgraft.
The 3D overlay image was used selectively to confirm the wire path as the target vessel of interest, decreasing the need for DSA during fenestration/branch catheterization. DSA was typically reserved for a completion aortogram and/or during difficult vessel catheterizations. In patients requiring iliac limbs, IVUS was used to determine hypogastric location, and contrast injections were typically not used for iliac limb deployment. Similar to FEVAR performed in the other hybrid operating room, contrast was diluted to 1/3 strength for all hand-injection imaging, and 1/2 strength for completion digital subtraction aortography. Notably, the power injector was not routinely used until completion aortogram, and to prevent settling of contrast, the ½ strength mixed contrast was not loaded until the time of the aortogram.
Radiation dose calculation
Radiation exposure is reported as air kerma area product (KAP), recorded in milligray [mGy*cm2]), along with total fluoroscopy time (minutes), both of which are routinely collected in our hybrid operating rooms for each procedure. Kerma is an acronym for “kinetic energy released per unit mass,” and provides an international standard for estimating radiation dose. Notably, our Toshiba Infinix™ VC-i system and the Siemens Artis Zeego® system have similar methods of KAP determination. This method is guided by FDA standards, with the reference location for determination of KAP at15cm from the isocenter toward the X-ray source along the beam axis.
Endpoints and statistics
Primary endpoints included radiation dose (KAP), fluoroscopy time, contrast use, and procedure time (defined as time from skin incision/puncture to bandage application). Secondary endpoints that were analyzed included total estimated blood loss, length of stay, complications and 30-day mortality. Complications were defined and tabulated based on reporting standards for endovascular aneurysm repair21. Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration(CKD-EPI) formula23.
Differences in renal function were calculated using the non-parametric Kruskal-Wallis and exact Wilcoxon scores tests. Continuous variables were analyzed using a Student t test or Wilcoxon Mann-Whitney test, and categorical variables were compared with a chi-square or Fisher’s exact test, when indicated. All analysis was completed using the R statistical software package (Vienna, Austria; V.2.15.0). A P-value < .05 was considered significant.
Results
Between September 2013 and March 2014, 32 patients underwent FEVAR, and 31 of these were performed using 3D fusion CT by a single surgeon. One FEVAR was performed in the older hybrid operating room due to the emergent nature of the procedure and room availability. In the 12 months prior, 40 patients underwent FEVAR in the non-CT capable hybrid OR, and these were combined with the single patient described previously, making the total “no CT” cohort 41 patients. Demographics, comorbidities and aneurysm characteristics of all patients are displayed in Table I. Notably, the mean BMI of the two groups is one of the only patient characteristics that was different, and was significantly lower in the 3D CT group (no CT, 30±6 vs. 3D CT, 26±4; P=.01). Table II demonstrates demographics and patient characteristics stratified by the number of vessels revascularized.
Table I.
Demographics and comorbidities for all patients
| Feature, No.(%) | No CT (N = 41) | 3D CT (N = 31) | P-valuea |
|---|---|---|---|
| Age (mean±SD) | 71±7 | 72±11 | .6 |
| Gender (male) | 32 (78%) | 21 (67%) | .5 |
| Body mass index | 30±6 | 26±4 | .01 |
| Comorbidity | |||
| Hypertension | 39 (95%) | 25 (81%) | .06 |
| Dyslipidemia | 23 (56%) | 16 (51%) | .8 |
| Chronic pulmonary disease | 20 (48%) | 11 (35%) | .3 |
| Smoking | 18 (43%) | 16 (51%) | .6 |
| Coronary artery disease | 18 (44%) | 12 (39%) | .8 |
| Diabetes mellitus | 12 (29%) | 8 (25%) | .8 |
| Cerebrovascular disease | 10 (14%) | 6 (15%) | 1 |
| Chronic renal insufficiencyϮ | 8 (19%) | 7 (22%) | .8 |
| Congestive heart failure | 6 (14%) | 3 (9%) | .7 |
| Arrhythmia | 5 (12%) | 3 (9%) | 1 |
| End stage renal disease | 0 | 2(7%) | .2 |
P-value determined with t-tests, Chi-square or Fischer’s exact tests when indicated.
Table II.
Patient demographics and comorbidities by number of revascularized vessels
| Feature, No.(%) | No CT | 3D CT | P-valuea |
|---|---|---|---|
| 2-vessel FEVAR | N = 8 | N = 12 | |
| Hypertension | 7 | 10 | 1 |
| Dyslipidemia | 4 | 8 | .6 |
| Chronic pulmonary disease | 3 | 5 | 1 |
| Smoking | 1 | 1 | .6 |
| Coronary artery disease | 5 | 5 | .6 |
| Diabetes mellitus | 3 | 4 | 1 |
| Cerebrovascular disease | 1 | 1 | 1 |
| Chronic renal insufficiencyϮ | 1 | 2 | 1 |
| Congestive heart failure | 1 | 2 | 1 |
| Arrhythmia | 1 | 1 | 1 |
| End stage renal disease | 0 | 2 | .5 |
| 3 or 4-vessel FEVAR | N = 33 | N = 19 | |
| Hypertension | 32 | 15 | .05 |
| Dyslipidemia | 19 | 8 | .4 |
| Chronic pulmonary disease | 17 | 6 | .3 |
| Smoking | 17 | 12 | .5 |
| Coronary artery disease | 13 | 7 | 1 |
| Diabetes mellitus | 9 | 4 | .7 |
| Cerebrovascular disease | 5 | 3 | 1 |
| Chronic renal insufficiencyϮ | 7 | 5 | .7 |
| Congestive heart failure | 5 | 1 | .4 |
| Arrhythmia | 4 | 2 | 1 |
| End stage renal disease | 0 | 0 | NA |
P-value determined with t-tests, Chi-square or Fischer’s exact test when indicated.
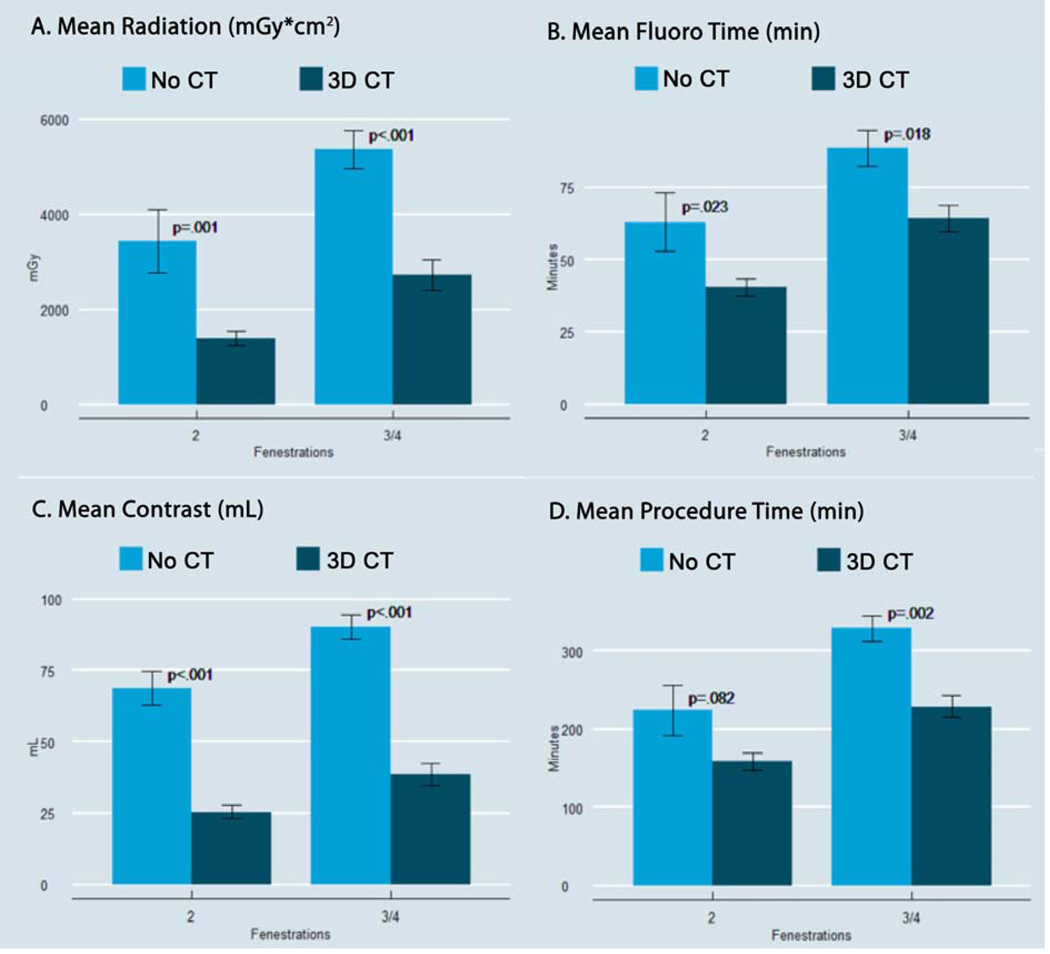
Radiation exposure (no CT, 5000±280 vs. 3D CT, 2200±1300mGy*cm2; P <.0001) and fluoroscopy time (no CT, 84±36 vs. 3D CT, 55±21 minutes; P=.0004) were both significantly lower in the 3D CT patients (Figure 2A/B). Additionally, overall contrast utilization was lower for the 3D CT patients (no CT, 86±25 vs. 3D CT, 34±15 mL; P<.0001). When divided into 2 vessel and 3/4 vessel fenestration groups, the difference remained significant [2V: no CT (N = 8), 69±16 vs. 3D CT (N = 12), 26±8; P=.0002; 3/4V: no CT (N = 33), 90±25 vs. 3D CT (N = 19) 39±17; P<.0001) (Figure 2C). Notably, the procedure times were less in the 3D CT groups, with a much larger impact in the 3/4 vessel patients (Figure 2D), and a significantly lower estimated blood loss (EBL) was found in the 3D CT group (no CT-390 ± 380 vs. 200 ± 180mL; P<.0001).
Figure 2. Radiation, Fluroscopy, Contrast and Procedure Time.
This figure demonstrates the reduction in radiation exposure (panel A), fluoroscopy time (panel B), contrast usage (panel C), and overall procedural time (panel D) with use of 3D CT fusion technology in FEVAR.
Outcomes
Several features of the perioperative details that may have impacted postoperative outcomes are tabulated in Table III. Notably, no difference existed in aneurysm size, extent, urgency or adjunct utilization. The 30-day mortality between the no CT and 3D CT groups was 5% (N = 2) and 3% (N =1; P = 1), respectively. A trend toward a decreased length of stay for the 3D CT patients (P = .07) was also observed. Although no significant differences in the rate of postoperative complications was noted between the No CT and 3D CT groups, no spinal cord ischemia events were observed in the 3D CT group (P = .06).
Table III.
Operative details and postoperative outcomes of all fenestrated EVAR patients
| Feature, No. (%) | No CT (N = 41) | 3D CT (N = 31) | P-valuea |
|---|---|---|---|
| Aneurysm diameter (mean, mm±SD) | 67±13 | 65±14 | .3 |
| Suprarenal/TAAA extent | 26 (65%) | 21 (72%) | 0.7 |
| Elective | 30 (73%) | 26 (84%) | |
| Urgent/emergent indication | 11 (27%) | 5 (16%) | .5 |
| Intraoperative adjunct | 9 (22%) | 5 (16%) | .8 |
| EBL (mL±SD) | 390±380 | 200±180 | <.0001 |
| 30-day mortality | 2 (5%) | 1 (3%) | 1 |
| Length of stay (median, days, IQR) | 5 [4, 11] | 5 [3, 8] | .07 |
| Complication | |||
| Cardiac | 5 (12%) | 3 (10%) | 1 |
| Pulmonary | 5 (12%) | 1 (3%) | .2 |
| Renal | 6 (15%) | 1 (3%) | .2 |
| Bleeding | 3 (7%) | 1 (3%) | .6 |
| Gastrointestinal | 3 (7%) | 1 (3%) | .6 |
| Stroke | 1 (2%) | 0 | 1 |
| Spinal cord ischemia | 5 (12%) | 0 | .06 |
P-value determined with t-tests, Chi-square or Fischer’s exact test when indicated.
Finally, the rates of endoleak and description of the reinterventions are catalogued in Table IV any reintervention: No CT (N=5) 12% vs. 3D CT (N = 4) 13%; P = 1)]. Fenestration patency all vessels patent in each group: No CT (N = 34) vs. 3D CT (N = 27); P = 1) during follow-up did not differ between the two groups. Additionally, no significant differences were seen with respect to eGFR change during follow-up (delta eGFR from preop to most recent follow-up: No CT [median, interquartile range] 0 [−5, 0] vs. 3D CT 0 [−4, 1.5]; P = .6).
Table IV.
Comparison of endoleak rates and description of reintervention after fenestrated EVAR with and without intraoperative 3D fusion CT
| Endoleak Typea | No CT (N = 32) No. (%) |
3D CT (N =25) No. (%) |
P-valueb |
|---|---|---|---|
| No endoleak | 27 (84%) | 19 (76%) | |
| Type 1a/b | 0 | 0 | |
| Type 2 | 3 (9%) | 4 (16%) | |
| Type 3 | 2 (6%) | 1 (4%) | |
| Type 4 | 0 | 0 | |
| Indeterminate | 0 | 1 (4%) | .4 |
| Description of Reinterventions | |||
No intraoperative CT patients (N = 5)
| |||
Intraoperative 3D CT patients (N = 4)
| |||
Data based on patients with available contrasted postoperative CT scans;
P-value determined with Fischer’s exact test.
Discussion
This study demonstrates that utilization of intraoperative 3D fusion CT significantly reduces radiation exposure, contrast utilization and overall procedural time in FEVAR. Although this study is not powered or designed to detect the effects of this technology on perioperative outcomes, important metrics including blood loss and length of stay appeared to be favorably impacted. These findings highlight the potential physiologic benefits of implementing innovative imaging techniques in advanced endovascular procedures. Additionally, while we were not able to reliably calculate the radiation dosage to the provider who performed these procedures, one may reasonably assume that the substantial reduction in radiation exposure to the patient also translated to decreased exposure to all healthcare providers participating in these repairs.
There are a number of factors that led to a reduction in radiation exposure and fluoroscopy time in this study. Most importantly, the intraoperative CT and vessel origin overlay facilitate graft deployment and catheterization of the branch vessels, thereby decreasing fluoroscopy time. Additional elements that may have contributed to the observed decrease in radiation dose/fluoroscopy over time include subtle technical aspects regarding the vessel marking method.
Figure 1 demonstrates the markings placed on the vessel origins, which are traced around the orifice immediately at the origin of the branch vessel on the aortic wall, producing a circle on the live fluoroscopy overlay. This circle facilitates precise fluoroscopy usage by allowing the provider to position the c-arm such that a perpendicular view of the vessel will be seen immediately upon initiation of live fluoroscopy. This decreases the overall fluoroscopy time, and thus decreases the radiation exposure.
Further, although lateral fluoroscopy is typically required for successful celiac/SMA catheterization and stenting, the c-arm can be positioned such that the desired field of view and vessel orientation are ensured before fluoroscopy is initiated. Also, the fenestrated device markers can be aligned with the vessel origin in the anterior-posterior c-arm position, or in slight obliquity, rather than the full lateral position. Eliminating the need for deep oblique and lateral fluoroscopy in this manner significantly reduces the amount of radiation required24. Indeed, reducing the need for prolonged obliquity of the c-arm during FEVAR is tantamount to reduction the overall radiation exposure to the patient and operating team alike, and it is our opinion that this advantage of 3D fusion CT should be exploited as much as possible. Lastly, and also very importantly, the need for DSA imaging is greatly decreased with this new technology, and is reserved primarily for completion imaging. DSA is used for difficult vessel catheterization at times, but the frequency is diminished substantially with improved imaging and 3D overlay on the live fluoroscopic image, despite the addition of the radiation from the intraoperative CT itself. We should note that the operator cannot rely entirely on the intraoperative 3D overlay for device to alterations in branch vessel location with the stiff delivery systems in place. Frequently, once the stiff wires/delivery system is in the aorta, the relationships of the vessels are altered, and the aorta itself may move substantially. Thus, the operator must be proficient with IVUS, and comfortable with manipulating the overlay to match the IVUS imaging before device deployment. This concept has recently been corroborated by Maurel et al.25 in their study demonstrating vessel movement upon introduction of the stiff deployment system during these procedures. Our routine is to perform IVUS with two stiff Lunderquist wires in place, and to adjust the overlay to match our IVUS as closely as possible before device delivery.
With increasing awareness of the possible detrimental health effects of radiation exposure to both patients and healthcare providers, investigators and the developers of imaging products have sought to decrease the radiation required for these procedures7,13. Some have suggested that maximum doses of radiation should be used as benchmarks for hospital quality, and have shown that education and protocol changes can have a drastic impact on radiation exposure to both patient and healthcare providers26,27.
To that end, providing advanced imaging technologies to supplement increasing use of fluoroscopy-based procedures is important, and all of the advanced imaging companies have newer systems with their own respective proprietary technology to reduce radiation exposure. However, the importance of adhering to ALARA (as low as [is] reasonably achievable) principles is equally, if not more important. This includes such things as decreasing the time on the fluoroscopy pedal, reducing frame rates, increasing the distance between the provider and the radiation source (especially important during DSA imaging), diligent use of collimation, avoidance of obliquity unless necessary, and the proper use of protective shielding during the procedure. These techniques can also dramatically reduce radiation exposure to patient and healthcare providers, perhaps as much as any other intervention13,28.
Additionally, FEVAR has a known association with renal dysfunction, and has been reported to have an associated >30% decrement in renal function in 25–33% of patients after the procedure15,16. This is likely a multifactorial phenomenon, related not only to the patient’s underlying medical comorbidities, but also to the performance of the operation (atheroemboli and contrast nephropathy), alterations in branch vessel flow due to configuration changes induced by the stents, as well as the postoperative contrast-based imaging that is often necessary. All of these factors can be mitigated, and we feel that the initial insult related to performance of the procedure is an excellent starting point for implementation of renal protection strategies. Here we demonstrate that the contrast dose can be reduced by as much as 60% using advanced imaging techniques and conservative dye utilization. Of note, we have had a longstanding policy of minimal contrast usage with FEVAR, and in addition to the imaging improvements, we typically use 1/3 strength Visipaque™ 320 (GE Healthcare, United Kingdom) for hand injection images (both DSA and live fluoroscopy), and 1/2 strength for completion aortography. The ability to use these decreased concentrations is certainly facilitated by high quality imaging in both of our hybrid operating rooms.
The limitations of this analysis include the fact that this is a single surgeon, single institution experience, which inherently introduces bias into the analysis, and may not be applicable to all practices. Additionally, the impact of learning curve on the analysis cannot be fully determined, and there were certainly ongoing improvements in technical efficiency during the course of the study period. Despite this issue, there were no significant differences in contrast, radiation and dye exposure in the initial 68 patients excluded from the analysis compared to the ‘no CT’ control group (data not shown). The lack of staff exposure data is an unfortunate weakness, but inconsistencies in radiation badge usage were such that we felt that a valid analysis would not be possible.
Further, due to the variability in the complexity of these procedures with respect to access issues, adjunctive procedures and the variability in visceral anatomy/occlusive disease, we cannot account for the effect of procedural difficulty and the impact on radiation exposure/procedure time. We felt that an analysis based on revascularized vessels would allow the fairest analysis in terms of difficulty level, and our data suggest that there were no significant differences in the group before and after initiation of 3D CT usage with regard to aneurysm extent or adjunct usage. Finally, the BMI of our 3D CT patients was on average lower than the no CT group, which would certainly bias the total radiation exposure to be lower in the 3D CT group; however, the fluoroscopy time corroborates the 3D CT radiation dose data, mitigating this weakness.
Conclusion
FEVAR is an evolving technology that offers reduced physiologic impact of complex aortic repair compared to standard open repair; however the elevated procedural complexity leads to increased radiation and dye exposure risk. These results demonstrate that use of intraoperative 3D CT fusion imaging during FEVAR decreases radiation exposure, procedure time, and contrast usage. This technology has promising potential to improve the safety and overall outcomes of these procedures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 27th Annual Meeting of the Florida Vascular Society, Friday, May 2nd, 2014, Palm Beach, FL
References
- 1.Greenberg R, Eagleton M, Mastracci T. Branched endografts for thoracoabdominal aneurysms. J Thorac Cardiovasc Surg. 2010;140:S171–S178. doi: 10.1016/j.jtcvs.2010.07.061. [DOI] [PubMed] [Google Scholar]
- 2.Haulon S, Greenberg RK. Part two: Treatment of type iv thoracoabdominal aneurysms--fenestrated stent-graft repair is now the best option. Eur J Vasc Endovasc Surg. 2011;42:4–8. doi: 10.1016/j.ejvs.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Kristmundsson T, Sonesson B, Dias N, Tornqvist P, Malina M, Resch T. Outcomes of fenestrated endovascular repair of juxtarenal aortic aneurysm. J Vasc Surg. 2014;59:115–120. doi: 10.1016/j.jvs.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Kirkwood ML, Arbique GM, Guild JB, Timaran C, Chung J, Anderson JA, et al. Surgeon education decreases radiation dose in complex endovascular procedures and improves patient safety. J Vasc Surg. 2013;58:715–721. doi: 10.1016/j.jvs.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Berrington de Gonzalez A, Mahesh M, Kim KP, Bhargavan M, Lewis R, Mettler F, et al. Projected cancer risks from computed tomographic scans performed in the united states in 2007. Arch Intern Med. 2009;169:2071–2077. doi: 10.1001/archinternmed.2009.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J, Einstein AJ, Fazel R, Krumholz HM, Wang Y, Ross JS, et al. Cumulative exposure to ionizing radiation from diagnostic and therapeutic cardiac imaging procedures: A population-based analysis. J Am Coll Cardiol. 2010;56:702–711. doi: 10.1016/j.jacc.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Einstein AJ, Henzlova MJ, Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. Jama. 2007;298:317–323. doi: 10.1001/jama.298.3.317. [DOI] [PubMed] [Google Scholar]
- 8.Fazel R, Krumholz HM, Wang Y, Ross JS, Chen J, Ting HH, et al. Exposure to low-dose ionizing radiation from medical imaging procedures. N Engl J Med. 2009;361:849–857. doi: 10.1056/NEJMoa0901249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howells P, Eaton R, Patel AS, Taylor P, Modarai B. Risk of radiation exposure during endovascular aortic repair. Eur J Vasc Endovasc Surg. 2012;43:393–397. doi: 10.1016/j.ejvs.2011.12.031. [DOI] [PubMed] [Google Scholar]
- 10.Kaul P, Medvedev S, Hohmann SF, Douglas PS, Peterson ED, Patel MR. Ionizing radiation exposure to patients admitted with acute myocardial infarction in the united states. Circulation. 2010;122:2160–2169. doi: 10.1161/CIRCULATIONAHA.110.973339. [DOI] [PubMed] [Google Scholar]
- 11.Maurel B, Sobocinski J, Perini P, Guillou M, Midulla M, Azzaoui R, et al. Evaluation of radiation during evar performed on a mobile c-arm. Eur J Vasc Endovasc Surg. 2012;43:16–21. doi: 10.1016/j.ejvs.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 12.Miller DL, Hilohi CM, Spelic DC. Patient radiation doses in interventional cardiology in the u.S.: Advisory data sets and possible initial values for u.S. Reference levels. Med Phys. 2012;39:6276–6286. doi: 10.1118/1.4754300. [DOI] [PubMed] [Google Scholar]
- 13.Mohapatra A, Greenberg RK, Mastracci TM, Eagleton MJ, Thornsberry B. Radiation exposure to operating room personnel and patients during endovascular procedures. J Vasc Surg. 2013;58:702–709. doi: 10.1016/j.jvs.2013.02.032. [DOI] [PubMed] [Google Scholar]
- 14.Noor M, Shekhdar J, Banner NR. Radiation exposure after heart transplantation: Trends and significance. J Heart Lung Transplant. 2011;30:309–314. doi: 10.1016/j.healun.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Haddad F, Greenberg RK, Walker E, Nally J, O'Neill S, Kolin G, et al. Fenestrated endovascular grafting: The renal side of the story. J Vasc Surg. 2005;41:181–190. doi: 10.1016/j.jvs.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 16.Verhoeven EL, Vourliotakis G, Bos WT, Tielliu IF, Zeebregts CJ, Prins TR, et al. Fenestrated stent grafting for short-necked and juxtarenal abdominal aortic aneurysm: An 8-year single-centre experience. Eur J Vasc Endovasc Surg. 2010;39:529–536. doi: 10.1016/j.ejvs.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Varu VN, Greenberg JI, Lee JT. Improved efficiency and safety for evar with utilization of a hybrid room. Eur J Vasc Endovasc Surg. 2013;46:675–679. doi: 10.1016/j.ejvs.2013.09.023. [DOI] [PubMed] [Google Scholar]
- 18.von Segesser LK, Marty B, Ruchat P, Bogen M, Gallino A. Routine use of intravascular ultrasound for endovascular aneurysm repair: Angiography is not necessary. Eur J Vasc Endovasc Surg. 2002;23:537–542. doi: 10.1053/ejvs.2002.1657. [DOI] [PubMed] [Google Scholar]
- 19.Scali ST, Waterman A, Feezor RJ, Martin TD, Hess PJ, Jr, Huber TS, et al. Treatment of acute visceral aortic pathology with fenestrated/branched endovascular repair in high-surgical-risk patients. J Vasc Surg. 2013;58:56–65. e51. doi: 10.1016/j.jvs.2012.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaikof EL, Blankensteijn JD, Harris PL, White GH, Zarins CK, Bernhard VM, et al. Reporting standards for endovascular aortic aneurysm repair. J Vasc Surg. 2002;35:1048–1060. doi: 10.1067/mva.2002.123763. [DOI] [PubMed] [Google Scholar]
- 21.Chaikof EL, Fillinger MF, Matsumura JS, Rutherford RB, White GH, Blankensteijn JD, et al. Identifying and grading factors that modify the outcome of endovascular aortic aneurysm repair. J Vasc Surg. 2002;35:1061–1066. doi: 10.1067/mva.2002.123991. [DOI] [PubMed] [Google Scholar]
- 22.DeSart K, Scali ST, Feezor RJ, Hong M, Hess PJ, Jr, Beaver TM, et al. Fate of patients with spinal cord ischemia complicating thoracic endovascular aortic repair. J Vasc Surg. 2013;58:635–642. e632. doi: 10.1016/j.jvs.2013.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller DL, Balter S, Cole PE, Lu HT, Berenstein A, Albert R, et al. Radiation doses in interventional radiology procedures: The rad-ir study: Part ii: Skin dose. J Vasc Interv Radiol. 2003;14:977–990. doi: 10.1097/01.rvi.0000084601.43811.cb. [DOI] [PubMed] [Google Scholar]
- 25.Maurel B, Hertault A, Sobocinski J, Le Roux M, Martin Gonzalez T, Azzaoui R, et al. Techniques to reduce radiation and contrast volume during evar. J Cardiovasc Surg (Torino) 2014;55:123–131. [PubMed] [Google Scholar]
- 26.Duncan JR, Street M, Strother M, Picus D. Optimizing radiation use during fluoroscopic procedures: A quality and safety improvement project. J Am Coll Radiol. 2013;10:847–853. doi: 10.1016/j.jacr.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 27.Duran A, Hian SK, Miller DL, Le Heron J, Padovani R, Vano E. A summary of recommendations for occupational radiation protection in interventional cardiology. Catheter Cardiovasc Interv. 2013;81:562–567. doi: 10.1002/ccd.24520. [DOI] [PubMed] [Google Scholar]
- 28.Wagner LK, Archer BR, Cohen AM. Management of patient skin dose in fluoroscopically guided interventional procedures. J Vasc Interv Radiol. 2000;11:25–33. doi: 10.1016/s1051-0443(07)61274-3. [DOI] [PubMed] [Google Scholar]