Abstract
Objective
To investigate the role of oxidative stress, inflammation, hypercoagulability and neuroendocrine activation in the transition of hypertensive heart disease to heart failure with preserved ejection fraction (HFPEF).
Methods
We performed echocardiography for 112 patients (≥ 60 years old) with normal EF (18 controls and 94 with hypertension), and determined protein carbonylation (PC), and tetrahydrobiopterin (BH4), C-reactive protein (CRP), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), fibrinogen, plasminogen activator inhibitor type-I (PAI-I), von Willebrand factor, chromogranin A (cGA) and B-type natriuretic peptide (BNP) levels from their blood samples.
Results
We found that 40% (38/94) of the patients with hypertension (HT) had no diastolic dysfunction (HTDD−), and 60% (56/94) had diastolic dysfunction (HTDD+). Compared to the controls, both patient groups had increased PC and BH4, TNF-α, PAI-I and BNP levels, while the HTDD+ group had elevated cGA and CRP levels. Decreased atrial and longitudinal left ventricular (LV) systolic and diastolic myocardial deformation (strain and strain rate) was demonstrated in both patient groups versus the control. Patients whose LV diastolic function deteriorated during the follow-up had elevated PC and IL-6 level compared to their own baseline values, and to the respective values of patients whose LV diastolic function remained unchanged. Oxidative stress, inflammation, BNP and PAI-I levels inversely correlated with LV systolic, diastolic and atrial function.
Conclusions
In patients with HT and normal EF, the most common HFPEF precursor condition, oxidative stress and inflammation may be responsible for LV systolic, diastolic and atrial dysfunction, which are important determinants of the transition of HT to HFPEF.
Keywords: Hypertension, Heart failure, Inflammation, Oxidative stress
1. Introduction
Hypertension is the main underlying cause of heart failure with preserved ejection fraction (HFPEF). This condition partially or entirely accounts for 78% to 88% of HFPEF cases.[1]–[3] The most common diseases causing HFPEF (hypertension, diabetes mellitus, obesity and chronic renal disease) are associated with a proinflammatory, prothrombotic state and increased oxidative stress.[2] Proinflammatory cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) are raised in HFPEF,[4] and predict future HFPEF development.[5] This proinflammatory state promotes the activation, adhesion and subendothelial migration of circulating leukocytes in two ways: by increasing the expression of the vascular cell adhesion molecule (VCAM) and E-selectin, and by increasing the activity of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, which leads to increased reactive oxygen species production in the coronary microvasculature endothelial cells.[2],[6]–[8] The presence of nitrosative/oxidative stress was recently demonstrated by using nitrotyrosine and dihydroethidium staining in cardiomyocytes of HFPEF patients. Nitrotyrosine staining reflects low nitric oxide (NO) bioavailability due to peroxynitrite formation from NO by superoxide anion (O2−.).[6],[9]
Peripheral endothelial dysfunction was recently identified as an independent predictor of the outcome of HFPEF, thus suggesting a causal role of endothelial dysfunction in the pathogenesis of HFPEF.[10] In cardiomyocytes adjacent to dysfunctional endothelium, low NO bioavailability because of high nitrosative/oxidative stress decreases cyclic guanosine monophosphate (cGMP) production without altering soluble guanylate cyclase activity. Low cGMP level accounts for low protein kinase G (PKG) activity in HFPEF myocardium, which results in hypertrophy and increased stiffness of the myocardium.[2],[9],[11] Both low cGMP content and low PKG activity were recently demonstrated in myocardial homogenates of HFPEF patients.[9]
Besides peripheral endothelial dysfunction, another important concept in the pathogenesis of HFPEF is oxidative stress due to myocardial nitric oxide synthase (NOS) uncoupling. This myocardial NOS uncoupling is caused mainly by oxidative depletion of the NOS cofactor tetrahydrobiopterin (BH4).[12] In hypertonic animal HFPEF models, treatment with BH4 reversed left ventricular (LV) hypertrophy, fibrosis and diastolic dysfunction. However, this effect could not be observed when the animals were treated with an antihypertensive agent (hydralazine) or non-specific antioxidants that do not affect NOS uncoupling (Tempol and tetrahydroneopterin). We can thus conclude that the above LV alterations (LV hypertrophy, fibrosis and diastolic dysfunction) were specifically due to oxidative stress resulting from myocardial NOS uncoupling and were not the results of hypertension or oxidative stress in general.[13],[14]
We hypothesized that oxidative stress and inflammation have an important (potentially primary) role in the pathogenesis of LV diastolic and subtle systolic dysfunction in patients with hypertension and normal ejection fraction (EF), which is the most common precursor state of HFPEF. To this end, we investigated the presence of oxidative stress, a proinflammatory and prothrombotic state and endothelial dysfunction in patients with hypertension and normal EF.
2. Methods
The study was conducted from December 2007 to July 2012 at the 3rd Department of Medicine, Semmelweis University, Budapest. We prospectively enrolled a total of 112 patients ≥ 60 years old with normal LVEF (> 50%). Ninety-four participants were patients with uncomplicated hypertension and eighteen participants were age-matched normotensive, healthy controls. The average follow-up period was 23.3 ± 12.5 months, depending on the time of enrollment. Forty-four of the patients were followed for three years, and each patient was followed for a minimum of one year. Annual follow-up examinations were set up, with each patient undergoing a physical examination, an electrocardiograph (ECG), a detailed echocardiography, a carotid ultrasound and a chest X-ray. Biochemical measurements were also performed. We used the same tests during the follow-up period and the baseline examinations.
Note that measurements of BH4, total biopterin and B-type natriuretic peptide (BNP) were added to the study protocol only later. As a result, biopterin and BNP levels were measured in all patients at least once, BNP was measured twice in about 75% of the patients and three times in case of a few patients, while biopterin levels were measured twice only in a few patients.
Seventeen patients did not finish the follow-up. Eight of them did not want to continue with the study, and nine patients satisfied one of the exclusion criteria (HFPEF developed in two of them). The study complied with the Declaration of Helsinki and was approved by the Institutional Committee on Human Research. An informed consent was obtained from all patients. Hypertension was defined by a systolic blood pressure > 140 mmHg and/or a diastolic blood pressure > 90 mmHg, or by antihypertensive pharmacotherapy. Blood pressure was measured three times according to the standard procedure, i.e., in a sitting position following a 10-min rest. Then the values were averaged.
Exclusion criteria included diabetes mellitus, more than a mild degree valvular or congenital heart disease, the presence of electrical pacemakers or implantable cardiac defibrillators, prior cardiovascular surgery, an established history of coronary heart disease, prior or ongoing atrial tachyarrhythmias, prior or manifest heart failure, any malignant or immunological disease, anticoagulant or antioxidant treatment, or conditions associated with acute inflammation or stress.
2.1. Biochemical measurements
All measurements were carried out from plasma or serum samples.
2.1.1. Oxidative stress parameters
Protein carbonylation (PC) assays were based on the photometric method of Levine, et al.[15]
Plasma BH4 levels were measured using high performance liquid chromatography (HPLC) based on the method of Fukushima and Nixon[16] and modified by Fekkes and Voskuilen-Kooijman.[17] Briefly, blood was collected into 4 mL Vacutainer tubes containing 7.2 mg K-EDTA and 0.1% (w/v) dithiothreitol (DTT) and centrifuged at 2650 r/min for 20 min after standing at room temperature in the dark for 2 to 3 h. Plasma was separated and frozen immediately at −80 °C until analysis. The amount of BH4 was determined by the differential oxidation method of Fukushima and Nixon.[16] The BH4 level was calculated from the difference between total biopterin [BH4 plus 7,8-dihydrobiopterin (BH2) plus biopterin] level and the alkaline-stable oxidized biopterin level (BH2 plus biopterin). Total biopterin level was determined by acid iodine oxidation of BH2 and BH4 to biopterin and the alkaline-stable oxidized biopterin level was determined by using alkaline iodine, which oxidizes only BH2 to biopterin.
2.1.2. Inflammatory parameters
C-reactive protein (hs-CRP) concentrations were measured in serum samples with the ultrasensitive particle-enhanced immunoturbidimetric assay in Cobas Integra 800 analyzer (Roche, Basel, Switzerland). Interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) levels were measured by high-sensitivity sandwich ELISA Kits (R&D Systems, Minneapolis, MN, USA), according to the instructions of the manufacturer.
2.1.3. Coagulation parameters
The von Willebrand factor antigen (VWF: Ag) level was measured by an in-house sandwich ELISA assay using rabbit polyclonal anti-human VWF antibodies (Dako, Glostrup, Denmark). Commercial reference plasma (Diagnostica Stago, Taverny, France) was used as calibration in each experiment. In addition, assay control samples (both pooled normal and commercially available plasma) were used in each experiment.
Human tissue plasminogen activator inhibitor type I (PAI-I) concentrations were measured in citrated plasma samples with a commercially available ELISA kit according to manufacturer's instructions (Hyphen BioMed, Neuville– Sur-Oise, France).
Plasma fibrinogen was measured on an ACL TOP coagulation analyzer (Instrumentation Laboratory, Milano, Italy) using the prothrombin-derived method, according to the manufacturer's instructions.
2.1.4. Neuroendocrine parameters
The chromogranin A (cGA) levels were determined by the commercially available chromogranin A assay (Cisbio Bioassays, IBA, Gif-sur-Yvette, France) according to manufacturer's instructions.
The concentration of the BNP levels were measured by immune fluorescence method using 250 µL EDTA-plasma (Triage BNP test, Biosite Diagnostics Incorporated, San Diego, CA, USA) as described in detail earlier.[18],[19]
2.2. Pulse wave velocity (PWV) measurement
PWV was measured using the validated PulsePen tonometer (DiaTecne, Milan, Italy) with the patient in supine position as described earlier.[20],[21] Briefly, two sequences of measurements were performed in each subject, and their mean was used for statistical analysis. The PulsePen device measures the time difference between the R wave of the ECG and the ‘foot’ of the pulse pressure wave, which was obtained sequentially above the carotid and the femoral arteries using a handheld tonometer, to calculate pulse transit time between these two sites. The average signal of at least 10 heart cycles was used in the measurements at both sites. Surface tape measurements were performed between the carotid site and the jugular notch, and between the jugular notch and the femoral site. The difference of these two distances was considered to be the pulse wave travel distance and this data was used to calculate PWV using PulsePen software.
2.3. Carotid ultrasonography
Echo-Doppler examination was performed using a Philips iE33 system (Philips Ultrasound, Bothell, WA, USA). The common, bulb, internal and external carotids of both left and right sides were studied in longitudinal and transverse planes with anterior, lateral and posterior approaches. The carotid ultrasonography finding was scored as follows: score 0: absence of plaque and intima media thickness (IMT) < 1 mm; score 1: increased (≥ 1 mm) IMT; score 2: non-stenotic plaque (with or without increased IMT); and score 3: stenotic (≥ 50% stenosis) plaque.
2.4. Standard echocardiography
Echocardiographic imaging was carried out using a Philips iE33 system (Philips Ultrasound, Bothell, WA, USA). Cardiac dimensions and wall thicknesses were measured from two-dimensionally guided M-mode tracings according to the recommendations of the American Society of Echocardiography.[22] LV mass was computed by the Devereux-modified cube formula.[23] Left atrial volume was calculated using the biplane area-length method. The biplane Simpson method was applied to calculate LV end-diastolic and end-systolic volumes, stroke volume and LVEF. LV diastolic function was assessed using the combination of transmitral Doppler flow, pulmonary venous flow, isovolumic relaxation time (IVRT) and myocardial tissue Doppler septal early diastolic filling velocity (E'). LV diastolic function was graded according to Nishimura and Tajik:[24] Grade 1= impaired relaxation with normal filling pressure; Grade 1a = impaired relaxation pattern with increased filling pressure; Grade 2 = pseudonormalized pattern; and Grade 3 = restrictive pattern. Transmitral flow was acquired from the apical four-chamber view with the sample volume placed at the level of the tips of mitral leaflets. E/A ratio, E deceleration time, A wave duration and IVRT were determined. Pulmonary venous flow was acquired from the same apical four-chamber view by placing the sample volume within the right upper pulmonary vein. Peak systolic forward flow, diastolic forward flow, atrial reversal flow duration and peak velocity were measured from this trace.
2.5. Color tissue Doppler imaging
Real time color Doppler myocardial imaging data were obtained in the apical four-chamber, two-chamber and five-chamber views. Mitral annular velocities [peak systolic velocity, peak E' and peak late diastolic (A') velocities] were recorded from the lateral, septal, inferior, anterior, posterior, anteroseptal LV walls. The width of the image sector and the depth of the imaging were adjusted to achieve a frame rate more than 180 frames/s. Pulse repetition frequency was set at the lowest possible level without aliasing. An insonation angle not exceeding 20° was maintained to ensure an appropriate alignment of the Doppler beam with the myocardial segment that was examined. Color-coded tissue Doppler images were also acquired over three consecutive cardiac cycles for each of the six LV walls and four atrial walls (in the apical four-chamber and two-chamber views from the mid lateral, inferior and anterior left atrial and mid lateral right atrial walls). We used narrow sector images (≤ 20°) with frame rates of 200–300 frames/s and the data were analyzed offline using the QLAB 6.0 advanced ultrasound quantification software (Koninklijke, Philips Electronics N.V. 2007). During the analysis, we measured the longitudinal LV peak systolic strain (S), end-systolic S, peak systolic, early diastolic and late diastolic strain rates (SR) in the basal and mid myocardial segments of the six LV walls in the apical views. The peak atrial contraction period, atrial reservoir period and atrial conduit period Ss and SRs were measured in the apical four- and two-chamber views from the mid lateral, inferior and anterior left atrial and the mid lateral right atrial myocardium.
2.6. Statistical analysis
All continuous data are expressed as mean ± SD unless otherwise stated. Categorical variables are expressed as proportions. Comparisons between two groups were performed with two-tailed t test or two-tailed t test with Welch's correction if variances were not equal according to the F test. Comparisons among groups used one-way analysis of variance (ANOVA) followed by the Tukey's multiple comparisons test for between-groups comparisons. The Kruskal–Wallis one-way analysis of variance by ranks was performed if Bartlett's test indicated heterogeneity of variances and comparisons between groups used the two-tailed t test with Welch's correction. Time series were compared using two-way ANOVA for repeated measures followed by the Bonferroni post-hoc test. Linear correlation coefficients were determined by calculating Pearson correlation. All tests were conducted at the two-sided 5% significance level. Statistical analysis was performed using GraphPad Prism5 (GraphPad Software Inc., San Diego, CA, USA).
3. Results
3.1. Patient characteristics
The control group and both patient groups (HTDD− and HTDD+) had similar gender distribution, height, weight, body surface area, diastolic blood pressure, heart rate, estimated glomerular filtration rate values and hemoglobin concentration. No significant difference was found between patient groups in terms of medication (Table 1). There was no difference in age between the control group and the whole hypertensive patient group (66.1 ± 4.4 vs. 69.4 ± 7.7 years, the data of the whole hypertensive patient group is not shown in Table 1) and between the control and the HTDD− group.
Table 1. Patient characteristics.
| Controls (n = 18) | HTDD− (n = 38) | HTDD+ (n = 56) | |
| Age, yrs | 66.1 ± 4.4 | 66.1 ± 5.6 | 71.6 ± 8.1*, ## |
| Sex, F/M | 12/6 | 29/9 | 33/23 |
| Duration of HT, yrs | 0 | 11.5 ± 11.6 | 14.4 ± 12.2 |
| Height, cm | 168.7 ± 8.4 | 164.3 ± 7.5 | 164.8 ± 8.6 |
| Weight, kg | 70.1 ± 13.2 | 74.3 ± 18 | 80.2 ± 25 |
| BMI, kg/m2 | 24.6 ± 3.7 | 27.6 ± 5.8* | 28 ± 4**, # |
| BSA, m2 | 1.8 ± 0.2 | 1.8 ± 0.2 | 1.8 ± 0.3 |
| Serum creatinine, µmol/L | 71.6 ±14.8 | 70.3 ± 14.8 | 82.8 ± 25.2# |
| eGFR, mL/min | 82.6 ± 19.9 | 88.4 ± 26.6 | 75.4 ± 27.1 |
| SBP, mmHg | 129.5 ± 16.6 | 146.5 ± 16.2** | 148.9 ± 17.9** |
| DBP, mmHg | 83.8 ± 9.1 | 85.9 ± 10.9 | 88.9 ± 10.8 |
| Heart rate, beats/min | 71.1 ± 8.3 | 74.9 ± 9.3 | 72.2 ± 8.0 |
| Hemoglobin concentration, g/L | 140.9 ± 12.6 | 137.2 ± 13.1 | 138.9 ± 14.8 |
| Medications (number of patients) | |||
| BB | 1 | 22 | 30 |
| ACEI | 0 | 19 | 35 |
| ARB | 0 | 9 | 13 |
| CCB | 0 | 15 | 27 |
| Diuretics | 0 | 22 | 35 |
| Aldosterone antagonists | 0 | 0 | 0 |
| Platelet inhibitors | 0 | 14 | 24 |
| Statin | 3 | 13 | 29 |
| PPI | 2 | 10 | 8 |
*P < 0.05, **P < 0.01 vs. control; #P < 0.05, ##P < 0.01 vs. HTDD− groups. ACEI: angiotensin convertase enzyme inhibitor; ARB: angiotensin receptor blocker; BB: beta-adrenergic receptor blocker; BMI: body mass index; BSA: body surface area; CCB: calcium channel antagonist; DBP: diastolic blood pressure; eGFR: estimated glomerular filtration rate; HT: hypertension; SBP: systolic blood pressure; PPI: proton pump inhibitor.
However, patients in the HTDD+ group were slightly older than those in the control (P < 0.05) and HTDD− (P < 0.01) groups. The body mass index (BMI) was higher in both patient groups than in the control group (P < 0.05 for HTDD− and P < 0.01 for HTDD+ groups). Serum creatinine levels were higher in the HTDD+ group than in the control group (P < 0.05). Compared to the control group, systolic blood pressure was similarly elevated in both patient groups (P < 0.01 for HTDD− and P < 0.001 for HTDD+ groups) (Table 1).
3.2. LV diastolic dysfunction
At the baseline examination, 40% (38/94) of the patients with hypertension and normal EF had no LV diastolic dysfunction (HTDD− group), and 60% (56/94) of the patients had mild, Grade 1 (54 patients) or Grade 1a (2 patients) LV diastolic dysfunction (HTDD+ group).
3.3. LV systolic and atrial function
Echocardiography results are summarized in Table 2. No significant between-groups differences were found either in traditional LV systolic function indices [2D-guided M-mode EF measurement using the LVIDd2−LVIDs2/LVIDd2 × 100 formula (LVIDd: diastolic left ventricular internal dimension; LVIDs: systolic left ventricular internal dimension), EF Simpson, stroke volume (SV), LV outflow tract time velocity integral (LVOT-TVI), mitral annulus M-mode excursion] or in LV systolic function parameters based on myocardial velocity measurements by tissue Doppler imaging (TDI) such as mitral annulus peak systolic velocity.
Table 2. Echocardiographic measurements.
| Parameter | Controls (n = 18) | HTDD− (n = 38) | HTDD+ (n = 56) |
| 2D guided M-mode EF, % | 65.89 ± 7.99 | 66.21 ± 5.96 | 68.66 ± 8.17 |
| EF Simpson, % | 67.61 ± 5.6 | 66.45 ± 4.8 | 64.53 ± 7.03 |
| Stroke volume, mL | 71.4 ± 2 | 68.8 ± 17.9 | 63.7 ± 18.8 |
| ESV, mL | 34.8 ± 12.4 | 34.7 ± 12.0 | 36.9 ± 14.6 |
| EDV, mL | 106.2 ± 30.2 | 103.6 ± 28.2 | 101.7 ± 27.3 |
| LV outflow tract time velocity integral, cm | 23.75 ± 3.98 | 23.55 ± 2.91 | 23.55 ± 4.88 |
| Mitral annulus M-mode excursion, mm | 15.3 ± 2.3 | 15.05 ± 2.26 | 15 ± 2.11 |
| Mitral annulus peak systolic velocity, cm/s | 8.44 ± 1.04 | 7.96 ± 1.06 | 8.12 ± 1.4 |
| LVM, g | 166.2 ± 38 | 196.9 ± 77.6 | 226.4 ± 66.2**,# |
| LVM/BSA, g/m2 | 91.6 ± 14.9 | 106.56 ± 35.9* | 122.7 ± 31**,# |
| LVM/BMI, g × m2/kg | 6.779 ± 1.218 | 7.255 ± 2.608 | 8.253 ± 2.287** |
| LAV, mL | 59.3 ± 18.2 | 63.5 ± 21.2 | 65 ± 21 |
| LAV/BSA, mL/m2 | 32.7 ± 8.5 | 33.7 ± 9.5 | 35.5 ± 10.4 |
| LAV/BMI, mL × m2/kg | 2.45 ± 0.785 | 2.32 ± 0.686 | 2.41 ± 0.829 |
| The mean of the longitudinal LV peak strain, % | −16.5 ± 3.0 | −14.7 ± 2.7* | −14.5 ± 2.0* |
| The mean of the longitudinal LV peak systolic SR, 1/s | −0.9 ± 0.14 | −0.76 ± 0.16** | −0.81 ± 0.12**, # |
| The mean of the longitudinal LV peak early diastolic wave SR, 1/s | 1.12 ± 0.22 | 0.97 ± 0.2*,## | 0.84 ± 0.16**, |
| Mean atrial contraction period SR, 1/s | −2.89 ± 0.65 | −2.4 ± 0.59* | −2.45 ± 0.54* |
| Mean atrial reservoir period SR, 1/s | 1.95 ± 0.39 | 1.53 ± 0.42** | 1.55 ± 0.35** |
*P < 0.05, **P < 0.01 vs. the control group; #P < 0.05, ##P < 0.01 vs. the HTDD− group. BMI: body mass index; BSA: body surface area; EDV: left ventricular end-diastolic volume; EF: ejection fraction; ESV: left ventricular end-systolic volume; LAV: left atrial volume; LV: left ventricle; LVM: left ventricular mass; SR: strain rate.
However, the more sensitive myocardial deformation parameters (strain and strain rate) revealed LV systolic dysfunction in our patients with hypertension and normal EF. Compared to the control group, we found reduced absolute values for the following parameters in both patient groups: the mean peak longitudinal LV systolic S (P < 0.05 for both patient groups), systolic (P < 0.001 for both patient groups) and early diastolic SRs (P < 0.05 for HTDD− and P < 0.001 for HTDD+ groups). In the HTDD+ group, the absolute value of the mean peak longitudinal LV systolic SR was increased (P < 0.05) and the longitudinal LV early diastolic SR was reduced (P < 0.01) compared to the HTDD− group.
The absolute values of the mean peak atrial contraction period (P < 0.05 for both patient groups) and the atrial reservoir period (P < 0.001 for both patient groups) SRs were decreased in both patient groups compared to the control.
3.4. Left atrial volume (LAV) and LV mass
No differences were found between groups in terms of LAV, LAV indexed to body surface area (LAV/BSA) or LAV indexed to BMI (LAV/BMI). LV mass (LVM) increased in the HTDD+ group compared to the control (P < 0.001) and HTDD− groups (P < 0.05), while LVM was similar in the HTDD− and the control groups. LVM/BSA increased (P < 0.05 for HTDD−, P < 0.001 for HTDD+ groups) in both patient groups versus the control, and in the HTDD+ group versus the HTDD− group (P < 0.05). LVM/BMI increased (P < 0.01) in the HTDD+ group versus the control and in a borderline significant manner (P = 0.063) in the HTDD+ group versus the HTDD− group (see Table 2) [these LAV and LVM results will be published in another article written from this multipurpose study: (J Hypertens accepted for publication, 2014)].
3.5. Biochemical measurements
Table 3 shows the biochemical measurement results. There was no significant difference in any of the investigated biochemical parameters between the HTDD+ and HTDD− groups.
Table 3. Biochemical results.
| Parameter | Control (n = 18) | HTDD− (n = 38) | HTDD+ (n = 56) |
| PC, µmol/g | 0.191 ± 0.131 | 0.306 ± 0.214* | 0.29 ± 0.246* |
| Plasma BH4, nmol/mL | 0.917 ± 0.351 | 1.98 ± 2.1** | 2.04 ± 1.99** |
| Total biopterin, nmol/mL | 2.01 ± 0.86 | 3.05 ± 2.8 | 2.98 ± 2.46* |
| CRP, mg/L | 1.97 ± 1.67 | 2.91 ± 2.63 | 4.17 ± 4.21** |
| IL-6, pg/mL | 1.88 ± 1.04 | 2.4 ± 2.91 | 3.0 ± 4.49 |
| TNF-α, pg/mL | 0.854 ± 0.778 | 1.75 ± 2.19* | 2.26 ± 4.62* |
| FIB, g/L | 4.03 ± 0.61 | 4.46 ± 0.93 | 4.56 ± 0.95 |
| PAI-I, ng/mL | 11.62 ± 5.44 | 21.11 ± 11.21** | 21.23 ± 16.16** |
| VWF, Ag% | 168.03 ± 57.59 | 148.27 ± 39.15 | 156.58 ± 48.93 |
| cGA, ng/mL | 75.85 ± 17.3 | 136.04 ± 162.71 | 148.16 ± 166.34** |
| BNP, pg/mL | 15.89 ± 9.24 | 29.8 ± 32.3* | 43.49 ± 69.41** |
*P < 0.05, **P < 0.01 vs. the control group. BH4: tetrahydrobiopterin; BNP: B-type natriuretic peptide; cGA: chromogranin A; CRP: C-reactive protein; FIB: fibrinogen; IL-6: interleukin-6; PAI-I: plasminogen activator inhibitor type I; PC: protein carbonylation; TNF-α: tumor necrosis factor-α; VWF: von Willebrand factor.
3.5.1. Oxidative stress parameters
Protein carbonylation (PC) (P < 0.05 for both) and plasma BH4 levels (P < 0.01 for both) increased in both patient groups. Plasma total biopterin level (BH4 + BH2 + biopterin) increased in the HTDD+ group (P < 0.05).
3.5.2. Inflammatory parameters
Only a non-significant increase in IL-6 levels could be detected in the patient groups. While TNF-α levels were increased in both patient groups (P < 0.05 for both), CRP level increased (P < 0.01) only in the HTDD+ group.
3.5.3. Coagulation parameters
There was no significant difference in fibrinogen and VWF: Ag levels. PAI-I levels increased (P < 0.001) in both patient groups.
3.5.4. Neuroendocrine activation parameters
BNP level increased in both patient groups (P < 0.05 for the HTDD− group, P < 0.01 for the HTDD+ group). cGA level increased only in the HTDD+ group (P < 0.01) and showed a non-significant elevation in the HTDD− group.
3.6. The relation of LV diastolic function to the biochemical parameters during follow-up
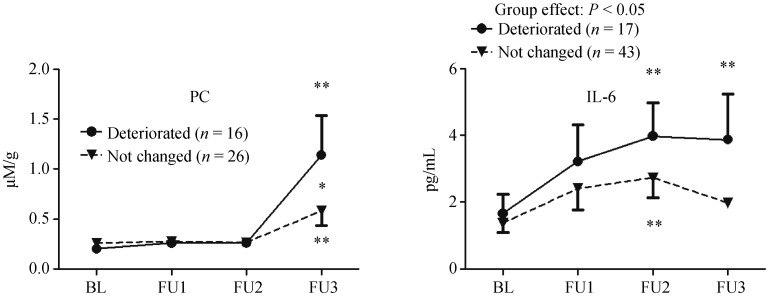
During follow-up, we found no change in LV diastolic function in case of 89 patients. However, in case of 19 patients LV diastolic function deteriorated, and in case of four patients LV diastolic function improved with at least one grade. As the number of patients with improved LV diastolic function was limited, the data was not sufficient for statistical analysis. In case of patients whose LV diastolic function deteriorated during the follow-up, we found increased PC and IL-6 levels compared to the patients' own baseline values (P < 0.01 for both PC and IL-6) and to the respective values of those patients whose LV diastolic function remained unchanged (P < 0.05 for IL-6; and at the time of the 3rd follow-up examination, P < 0.05 for PC) (Figure 1). During the follow-up, there was no difference in any other biochemical parameters between the two groups of patients whose LV diastolic function deteriorated and remained unchanged.
Figure 1. Comparison of protein carbonylation and IL-6 levels during follow-up in patients whose LV diastolic function remained unchanged or deteriorated.
*P < 0.05, **P < 0.01. The table includes the results of only those patients who had at least three measurements of the investigated parameters during the follow-up. BL: baseline; IL-6: interleukin-6; FU: follow-up; LV: left ventricular; PC: protein carbonylation.
3.7. PWV and carotid score
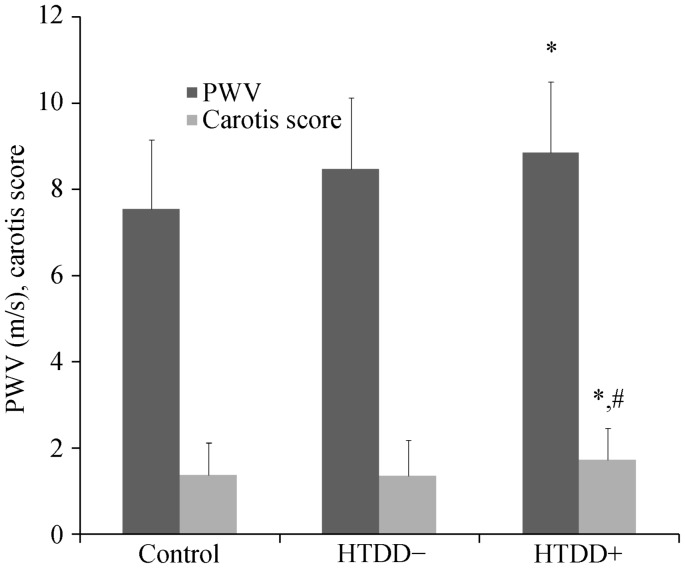
The PWV increased in the HTDD+ group compared to the control group (P < 0.05), indicating the presence of atherosclerosis in the great arteries (Figure 2). The PWV of both patient groups combined was also greater than that of the control group (P < 0.05). The carotid score was greater in the HTDD+ group versus both the control and the HTDD− groups (P < 0.05 for both) (Figure 2).
Figure 2. Pulse wave velocity and carotid score results.
*P < 0.05 versus the control group, #P < 0.05 versus the HTDD− group. HTDD−: hypertensive patients without left ventricular diastolic dysfunction; HTDD+: hypertensive patients with left ventricular diastolic dysfunction; PWV: pulse wave velocity.
3.8. Relevant correlations among the investigated parameters
Table 4 highlights only the most important and relevant correlations.
Table 4. The most relevant correlations.
| Correlated parameters | P | r |
| BH4 with mean longitudinal LV peak S | 0.02 | 0.251 |
| With mean longitudinal LV peak systolic SR | 0.002 | 0.329 |
| With mean longitudinal LV peak early diastolic wave SR | 0.008 | −0.285 |
| PAI-I with mean longitudinal LV peak S | 0.014 | 0.232 |
| With mean longitudinal LV peak early diastolic wave SR | 0.009 | −0.246 |
| IL-6 with mean longitudinal LV peak S | 0.008 | 0.249 |
| With mean longitudinal LV peak early diastolic wave SR | 0.009 | −0.285 |
| TNF-α with mean longitudinal LV peak S | 0.025 | 0.213 |
| cGA with (P wave-LAcontr int.) − (P wave-RAcontr int.) | 0.026 | 0.22 |
| With carotid score | 0.018 | 0.254 |
| BNP with mean atrial contraction period peak SR | 0.004 | 0.274 |
| With mean LA reservoir period peak S | 0.016 | −0.23 |
| With mean LA reservoir period peak SR | 0.004 | −0.272 |
| With mean mitral annulus M-mode peak excursion | 0.036 | −0.21 |
| With mean mitral annulus peak systolic velocity | < 0.001 | −0.352 |
| With mean E' | 0.001 | −0.301 |
| With mean E/E' | 0.022 | 0.218 |
| With LVM/BSA | 0.028 | 0.257 |
| With EF Simpson/LVM | 0.03 | −0.207 |
| With EF Simpson/LVM/BSA | 0.013 | −0.243 |
| With LAV | 0.001 | 0.309 |
| With LAV/BSA | < 0.001 | 0.35 |
| PWV with BNP | 0.035 | 0.239 |
| With LVM | 0.041 | 0.23 |
| With EF Simpson | 0.009 | −0.294 |
| With ESV | 0.01 | 0.293 |
| With mean longitudinal LV peak S | < 0.001 | 0.535 |
| With mean longitudinal LV peak systolic SR | < 0.001 | 0.51 |
| With mean longitudinal LV peak early diastolic wave SR | < 0.001 | −0.496 |
| With mean atrial reservoir period peak S | 0.012 | −0.282 |
| With mean atrial reservoir period peak SR | < 0.001 | −0.389 |
BH4: tetrahydrobiopterin; BNP: B-type natriuretic peptide; BSA: body surface area; cGA: chromogranin A; contr: contraction; E: transmitral flow peak early diastolic filling velocity; E': mitral annular peak early diastolic filling velocity; EF: ejection fraction; ESV: left ventricular end-systolic volume; IL-6: interleukin-6; Int.: interval; LA: left atrium; LAV: left atrial volume; LV: left ventricle; LVM: left ventricular mass; PAI-I: plasminogen activator inhibitor type I; S: strain; SR: strain rate; RA: right atrium; TNF-α: tumor necrosis factor-α.
BH4 and PAI-I levels showed an inverse correlation with the absolute values of longitudinal LV systolic and early diastolic myocardial deformation parameters.
The TNF-α and IL-6 levels also showed an inverse correlation with the absolute values of longitudinal LV systolic myocardial deformation parameters. Similarly, IL-6 level correlated inversely with the absolute values of longitudinal LV early diastolic myocardial deformation (SR).
cGA levels showed a positive correlation with the atrial conduction time estimated from the [(P wave-LA contraction) − (P wave-right atrial contraction)] interval and the carotid score. Plasma BNP level showed a negative correlation with the absolute values of atrial myocardial deformation parameters (Table 3) and with some more traditional parameters of LV systolic and diastolic function, such as mitral annulus M-mode excursion, mitral annulus peak systolic velocity, mean E'. Plasma BNP level also showed a negative correlation with some recently proposed parameters of LV systolic dysfunction such as EF Simpson/LVM and EF Simpson/LVM/BSA.[25] Plasma BNP level showed a positive correlation with LAV, LAV/BSA, LVM/BSA and mean E/E'.
The PWV showed a strong inverse correlation with the absolute values of LV systolic and LV early diastolic myocardial deformation parameters and with EF Simpson. The PWV showed a negative correlation with the mean atrial reservoir period peak S and SR and a positive correlation with ESV, LVM and BNP.
4. Discussion
4.1. LV dysfunction and transition to HFPEF are due to oxidative stress and inflammation in hypertension
The increased PC and plasma BH4 levels in both patient groups versus the control group indicates the presence of oxidative stress in patients with hypertension and normal EF. The markedly increased PAI-I levels in both patient groups compared to the control group are consistent with the presence of increased oxidative stress, as it has been proved that PAI-I levels increase in parallel with oxidative stress.[26],[27] There is some evidence that increased plasma BH4 levels are associated with decreased tissue BH4 levels leading to nitric oxide NOS uncoupling, and as a corollary, increased oxidative stress.[28],[29] The increased TNF-α levels in both patient groups (HTDD+ and HTDD−) and the increased CRP level in the HTDD+ group compared with the control group verifies the presence of inflammation in our patients.
Hypertension is the most common underlying cause and precursor condition of HFPEF. The transition of hypertensive heart disease to HFPEF is characterized by progressive LV hypertrophy and the deterioration of LV diastolic and atrial functions.[30] Our results demonstrated that in patients whose LV diastolic function deteriorated during follow-up, PC and IL-6 levels increased compared to their own baseline values and to those patients' values whose LV diastolic function remained unchanged during follow-up. LVM increased in line with the progressive LV diastolic dysfunction. Increased plasma BH4 levels have been shown to be associated with decreased endothelial and possibly with decreased tissue BH4 levels.[28],[29] Thus, it is likely that the increased plasma BH4 levels in our patients can be associated with decreased NOS cofactor myocardial BH4 levels. This leads to myocardial NOS uncoupling and causes myocardial oxidative stress. In hypertensive mouse HFPEF models,[13],[14],[31] it was elegantly shown that it is myocardial oxidative stress due to myocardial NOS uncoupling that is responsible for HFPEF, not oxidative stress in general or hypertension in itself. Thus, our results demonstrate that inflammation, oxidative stress and decreased tissue BH4 levels–particularly myocardial oxidative stress due to myocardial NOS uncoupling–promote unfavorable changes that might accelerate the transition of hypertensive heart disease to HFPEF.
Plasma BH4, PAI-I, TNF-α and IL-6 levels showed an inverse correlation with the absolute values of longitudinal LV systolic and, with the exception of TNF-α, with the longitudinal early diastolic myocardial deformation parameters as well. This further reinforces the hypothesis that increased oxidative stress and inflammation may not only play a major role in the deterioration of LV diastolic function, but may have a potential pathogenetic role in LV systolic dysfunction as well.
4.2. Increased atherosclerosis in line with progressive LV diastolic dysfunction
The increased PWV and the increased carotid score in the HTDD+ group versus the control and HTDD− groups indicate the presence of advanced atherosclerosis in the great arteries in line with the deterioration of LV diastolic function. Based on the evidence for a pathogenetic role of oxidative stress and inflammation in atherosclerosis,[32],[33] it can be assumed that increased oxidative stress and inflammation contribute to the transition of hypertensive heart disease to HFPEF and LV systolic and diastolic dysfunction in patients with hypertension, and at the same time result in increased atherosclerosis of great arteries.
4.3. Possible causes of atrial dysfunction in patients with hypertension and normal EF
Our findings that cGA levels increased in the HTDD+ group versus the controls, showed a positive correlation with the atrial conduction time, showed an inverse correlation with the absolute values of atrial myocardial deformation parameters and a positive correlation with LA volume and LA volume/BSA, indicate that neuroendocrine activation occurring as a sequela of LV systolic and diastolic dysfunction may significantly contribute to the development of atrial dysfunction in hypertensive patients. Atrial dysfunction might be also an important determinant of the transition of hypertensive heart disease to HFPEF.[30] BNP levels correlate inversely with some more traditional markers of LV systolic and diastolic dysfunction, and it suggests that neuroendocrine activation contributes to the deterioration of LV systolic and diastolic function in these patients.
4.4. Clinical implications
We believe that our results have significant clinical applications. To the best of our knowledge, currently there is no evidence-based treatment for HFPEF. In line with the results of other authors,[13],[14],[28],[31] our findings also support the observation that administrating BH4 to inhibit myocardial NOS uncoupling might prevent the underlying conditions from developing into HFPEF, or might be able to reverse manifestations of HFPEF.
4.5. Limitations
An important limitation of our study is that we were not in the position to perform biochemical measurements from myocardial samples for ethical reasons. Therefore, we could not measure myocardial BH4 levels and thus could not provide direct evidence for myocardial NOS uncoupling in our patients. Moreover, while at the start of the study none of our patients had HFPEF, during the follow-up only two patients developed this condition, which is a further limitation.
Thus we could only verify that in case of patients with hypertension and normal EF (the most common precursor of HFPE) oxidative stress and inflammation can be linked to the deterioration of LV diastolic function, atrial dysfunction and progression of LV hypertrophy. All of these are important determinants of the transition of hypertensive heart disease to HFPEF.
As it was difficult to find ≥ 60-year-old healthy controls in a hospital environment who met all the selection criteria, the number of control patients was also relatively small.
4.6. Conclusions
Our results suggest that increased oxidative stress (particularly myocardial oxidative stress due to myocardial NOS uncoupling) and inflammation are not just epiphenomena in patients with hypertension and normal EF, but it is more likely that they play a pathogenetic role in subtle LV systolic dysfunction and deterioration of LV diastolic function and contribute to progressive atherosclerosis in the great arteries. Subtle LV systolic dysfunction and the deterioration of LV diastolic function are associated with progressive LV hypertrophy, neuroendocrine activation and atrial dysfunction, and all these factors are considered to be important determinants of the transition of hypertensive heart disease to HFPEF.
Acknowledgments
Vereckei A received the K 67971 grant from the Hungarian National Scientific Research Fund (OTKA). The authors declare no conflict of interest.
References
- 1.Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis and treatment. Eur Hear J. 2011;32:670–679. doi: 10.1093/eurheartj/ehq426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 3.van Heerebeck L, Franssen CPM, Hamdani N, et al. Molecular and cellular basis for diastolic dysfunction. Curr Heart Fail Rep. 2012;9:293–302. doi: 10.1007/s11897-012-0109-5. [DOI] [PubMed] [Google Scholar]
- 4.Kalogeropoulos A, Georgiopoulou V, Psaty BM, et al. Inflammatory markers and incident heart failure risk in older adults. The health ABC (Health, Aging and Body Composition) Study. J Am Coll Cardiol. 2010;55:2129–2137. doi: 10.1016/j.jacc.2009.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collier P, Watson CJ, Voon V, et al. Can emerging biomarkers of myocardial remodelling identify asymptomatic hypertensive patients at risk for diastolic dysfunction and diastolic heart failure? Eur J Heart Fail. 2011;13:1087–1095. doi: 10.1093/eurjhf/hfr079. [DOI] [PubMed] [Google Scholar]
- 6.Westermann D, Lindner D, Kasner M, et al. Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ Heart Fail. 2011;4:44–52. doi: 10.1161/CIRCHEARTFAILURE.109.931451. [DOI] [PubMed] [Google Scholar]
- 7.van Heerebeck L, Hamdani N, Handoko ML, et al. Diastolic stiffness of the failing diabetic heart: importance of fibrosis, advanced glycation end products and myocyte resting tension. Circulation. 2008;117:43–51. doi: 10.1161/CIRCULATIONAHA.107.728550. [DOI] [PubMed] [Google Scholar]
- 8.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 9.van Heerebeck L, Hamdani N, Falcao-Pires I, et al. Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation. 2012;126:830–839. doi: 10.1161/CIRCULATIONAHA.111.076075. [DOI] [PubMed] [Google Scholar]
- 10.Akiyama E, Sugiyama S, Matsuzawa Y, et al. Incremental prognostic significance of peripheral endothelial dysfunction in patients with heart failure and normal left ventricular ejection fraction. J Am Coll Cardiol. 2012;60:1778–1786. doi: 10.1016/j.jacc.2012.07.036. [DOI] [PubMed] [Google Scholar]
- 11.Schulz E, Jansen T, Wenzel P, et al. Nitric oxide, tetrahydrobiopterin, oxidative stress and endothelial dysfunction in hypertension. Antioxid Redox Signal. 2008;10:1115–1126. doi: 10.1089/ars.2007.1989. [DOI] [PubMed] [Google Scholar]
- 12.Cunnington C, Channon KM. Tetrahydrobiopterin: pleiotropic roles in cardiovascular pathophysiology. Heart. 2010;96:1872–177. doi: 10.1136/hrt.2009.180430. [DOI] [PubMed] [Google Scholar]
- 13.Silberman GA, Fan T-HM, Liu H, et al. Uncoupled cardiac nitric oxide synthase mediates diastolic dysfunction. Circulation. 2010;121:519–528. doi: 10.1161/CIRCULATIONAHA.109.883777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moens AL, Takimoto E, Tocchetti CG, et al. Reversal of cardiac hypertrophy and fibrosis from pressure overload by tetrahydrobiopterin. Efficacy of recoupling nitric oxide synthase as a therapeutic strategy. Circulation. 2008;117:2626–2636. [Google Scholar]
- 15.Levine RL, Williams JA, Stadtman ER, et al. Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol. 1994;233:346–573. doi: 10.1016/s0076-6879(94)33040-9. [DOI] [PubMed] [Google Scholar]
- 16.Fukushima T, Nixon JC. Analysis of reduced forms of biopterin in biological tissues and fluids. Anal Biochem. 1980;102:176–188. doi: 10.1016/0003-2697(80)90336-x. [DOI] [PubMed] [Google Scholar]
- 17.Fekkes D, Voskuilen-Kooijman A. Quantitation of total biopterin and tetrahydrobiopterin in plasma. Clin Biochem. 2007;40:411–413. doi: 10.1016/j.clinbiochem.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Szabo G, Molvarec A, Stenczer B, et al. Natriuretic peptide precursor B gene (TTTC)(n) microsatellite polymorphism in pre-eclampsia. Clinica Chimica Acta. 2011;412:1371–1375. doi: 10.1016/j.cca.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 19.Szabo G, Molvarec A, Nagy B, et al. Increased B-type natriuretic peptide levels in early-onset versus late-onset preeclampsia. Clin Chem Lab Med. 2014;52:281–288. doi: 10.1515/cclm-2013-0307. [DOI] [PubMed] [Google Scholar]
- 20.Salvi P, Lio G, Labat C, et al. Validation of a new non-invasive tonometer for determining arterial pressure wave and pulse wave velocity: the PulsePen device. J Hypertens. 2004;22:285–293. doi: 10.1097/00004872-200412000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Othmane TEH, Nemcsik J, Fekete BC, et al. Arterial stiffness in hemodialysis: Which parameter to measure to predict cardiovascular mortality? Kidney Blood Press Res. 2009;32:250–257. doi: 10.1159/000238822. [DOI] [PubMed] [Google Scholar]
- 22.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1988;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 24.Nishimura R, Tajik AJ. Evaluation of diastolic filling of left ventricle in health and disease: Doppler echocardiography is the clinician's rosetta stone. J Am Coll Cardiol. 1997;30:8–18. doi: 10.1016/s0735-1097(97)00144-7. [DOI] [PubMed] [Google Scholar]
- 25.MacIver DH. Current controversies in heart failure with a preserved ejection fraction. Future Cardiol. 2010;6:97–111. doi: 10.2217/fca.09.56. [DOI] [PubMed] [Google Scholar]
- 26.Nakayama N, Nakamura T, Okada H, et al. Modulators of induction of plasminogen activator inhibitor type-1 in HepG2 cells by transforming growth factor. Coron Artery Dis. 2011;22:468–478. doi: 10.1097/MCA.0b013e32834a3817. [DOI] [PubMed] [Google Scholar]
- 27.Rui-Ming L. Oxidative stress, plasminogen activator inhibitor 1, and lung fibrosis. Antioxid Redox Signal. 2008;10:303–319. doi: 10.1089/ars.2007.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antoniades C, Shirodaria C, Crabtree M, et al. Altered plasma versus vascular biopterins in human atherosclerosis reveal relationships between endothelial nitric oxide synthase coupling, endothelial function and inflammation. Circulation. 2007;116:2851–2859. doi: 10.1161/CIRCULATIONAHA.107.704155. [DOI] [PubMed] [Google Scholar]
- 29.Katusic ZS, d'Uscio L, Nath KA. Vascular protection by tetrahydrobiopterin: Progress and therapeutic prospects. Trends Pharmacol Sci. 2009;30:48–54. doi: 10.1016/j.tips.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melenovsky V, Borlaug BA, Rosen B, et al. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban Baltimore community. J Am Coll Cardiol. 2007;49:198–207. doi: 10.1016/j.jacc.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 31.Takimoto E, Champion HC, Li M, et al. Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J Clin Invest. 2005;115:1221–1231. doi: 10.1172/JCI21968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peluso I, Morabito G, Urban L, et al. Oxidative stress in atherosclerosis development: the central role of LDL and oxidative burst. Endocr Metab Immune Disord Drug Targets. 2012;12:351–360. doi: 10.2174/187153012803832602. [DOI] [PubMed] [Google Scholar]
- 33.Violi F, Pignatelli P, Pignata C, et al. Reduced atherosclerotic burden in subjects with genetically determined low oxidative stress. Arterioscler Thromb Vasc Biol. 2013;33:406–412. doi: 10.1161/ATVBAHA.112.300438. [DOI] [PubMed] [Google Scholar]