Abstract
Objective
Absence of significant epicardial coronary artery disease (CAD) in patients with acute onset of chest pain and elevation of myocardial necrosis markers is occasionally observed. The aim of this study was to analyse the clinical characteristics and outcome of such patients with advanced age.
Methods
We retrospectively analysed 4,311 patients with acute onset of chest pain plus necrosis marker elevation. Two hundred and seventy two patients without CAD on angiogram (6.3%) were identified. Out of them, 50 (1.2%) patients ≥ 75 years (Group I) were compared with (1) 222 acute coronary syndrome (ACS) patients without CAD on angiogram < 75 years (Group II), and (2) 610 consecutive patients ≥ 75 years with Non-ST-elevation Myocardial Infarction (NSTEMI) undergoing percutaneous coronary intervention (Group III).
Results
Group I compared to Group III patients made up for more females (64.0% vs. 49.2%; P < 0.0001), and had more severe anginal symptoms on presentation [Canadian Cardiovascular Society (CCS) class I/II, 26.0% vs. 49.8%; P = 0.02]. Group I patients also had lower troponin levels (0.62 ± 0.8 ng/mL vs. 27 ± 74 ng/mL; P < 0.02), lower leukocyte count (9.4 ± 3.13 × 109 vs. 12 ± 5.1 × 109; P = 0.001) and better preserved left ventricular function (56.7% ± 14.3% vs. 45% ± 11%; P < 0.0001). Event-free survival (cardiac death, myocardial infarction, recurrent angina, and re-hospitalisation) was more frequent in Group I and II patients compared to Group III patients (64.9%, 66.7%, and 41.6%, respectively; P < 0.0001).
Conclusions
ACS in patients ≥ 75 years without CAD is very infrequent, associated with a (1) similar outcome compared to ACS patients < 75 years without CAD, and (2) significant better outcome compared to NSTEMI patients ≥ 75 years.
Keywords: Acute coronary syndrome, Angina, Biological markers, Coronary stenosis, Myocarditis, Syndrome
1. Introduction
In patients presenting with acute coronary syndrome (ACS), an elevated level of cardiac troponins (suggestive of myocardial necrosis) is a well-known risk factor for fatal events.[1],[2]
The characterisation and prognosis of patients presenting with evidence of myocardial necrosis and detection of elevated of cardiac biomarkers (preferably troponin) above the 99th percentile of the upper reference limit—but with insignificant coronary artery disease defined—remain uncertain. Independent predictors of insignificant coronary artery disease (CAD) included, among others, younger age.[3] The importance of this problem in patients with advanced age appears to be underestimated in clinical practice.
The purpose of our analysis was to compare demographic, clinical findings, and prognosis in ACS patients with insignificant CAD ≥ 75 years of age with two groups of patients: (1) ACS patients with insignificant CAD but younger than 75 years, and (2) to ACS patients ≥ 75 years of age with significant coronary artery stenosis undergoing percutaneous coronary intervention [true non-ST-elevation myocardial infarction (NSTEMI)].
2. Patients and methods
Using the database of the Zentralklinik Bad Berka, we retrospectively analyzed the records of all consecutive patients who had been admitted from May 2002 to April 2011 with acute (< 12 h) onset of chest pain and elevation of troponin I, creatine kinase, or both. All patients underwent cardiac catheterization within 24 h of hospital admission. Patients without significant coronary stenosis (coronary artery diameter stenosis ≤ 50%) constitute the study population and were assigned to Group I and II depending on their age (≥ 75 years vs. < 75 years). Exclusion criteria were: (1) evidence of bundle-branch block or pacemaker rhythm; (2) renal failure, defined as decrease of the glomerular filtration rate below 30 mL/min or as a new or permanent requirement for hemodialysis, which could interfere with measurement of troponin I; (3) sepsis or other infectious disease (clinical signs: fever > 38°C or C-reactive protein > 100 mg/L); and (4) pulmonary embolism (diagnosed by pathological findings at computed tomographic scans of the lungs in patients with elevated D-dimer levels). The thresholds used to define positive tests were > 0.1 ng/mL for Troponin I and > 0.3 ng/mL for D-dimers.
2.1. Electrocardiographic analysis
All electrocardiograms were analyzed retrospectively by an independent observer (Memisevic N), who recorded ST-segment depression, Q waves, and T-wave inversion.
2.2. Cardiac catheterization
All angiograms recorded after intracoronary application of nitrates were analyzed retrospectively by an independent operator (von Korn H). Intraluminal thrombus was defined as an intraluminal filling defect separate from the adjacent vascular wall, an ulcer was defined as a breakdown of the plaque surface, and vasospasm was defined as a stenosis that could be reversed by the application of nitrates.
Takotsubo-like left ventricular cardiomyopathy was defined as hypokinesis or akinesis from the mid-portion to the apex of the left ventricle together with hyperkinesis in the base, whenever this extended over a territory supplied by more than one coronary artery.[4]
2.3. Follow-up and end-points
Follow-up data was obtained by reviewing the patient's hospital charts, conducting standardized telephone interviews, contacting the patient's physician if necessary, or conducting periodic outpatient visits (Farah A and Tukhiashvili K). Anginal status was noted, and adverse events were identified as myocardial infarction or as those requiring re-intervention or re-admission to the hospital. The cause of death was sub-classified as cardiac or non-cardiac.
2.4. Statistics
For continuous variables and percentages, median values (interquartile ranges) were reported and comparisons between them were made using the t-test. For categorical variables, mean ± SD were reported and comparisons between them were made using the chi-square test or Fisher's exact test. The log-rank test (Mantel-Cox) statistic was used to compare event rates. Clinical outcomes (death, congestive heart failure, recurrent angina, re-hospitalisation, and repeat revascularisation) are presented with the Kaplan-Meier method. A two-sided probability value of P < 0.05 was considered to be statistically significant.
3. Results
Between May 2002 and April 2011, 4311 patients were admitted at our institution with recent onset of chest pain and a serum elevation of troponin I and/or creatine kinase. Of those, 4039 (93.7%) patients were excluded due to STEMI (1249 patients, 30.9%), and NSTEMI (2499 patients, 61.9%) diagnosis. An additional 291 (7.2%) patients have been excluded because troponin elevation was related to non-cardiac disease or other excluding factors were present (Table 1). During the study period, 272 (6.3%) patients with ACS did not show a critical stenosis of any coronary artery. Out of them, 50 (1.16%) were ≥ 75 years of age (Group I). The control groups were established by analysing (1) patients with ACS without critical narrowing of a coronary artery and being younger than 75 years (Group II; n = 222); and (2) all patients with NSTEMI ≥ 75 years (Group III; n = 610), and Table 2 provides relevant clinical information.
Table 1. Diagnoses in patients with acute coronary syndrome but without significant coronary artery stenoses (n = 563).
| No detectable cause (Group I + II patients) | 272 | (48.3%) |
| Myocarditis/inflammatoric cardiomyopathy | 78 | (13.9%) |
| Pulmonary diseases | 41 | (7.3%) |
| Pulmonary embolism | 23 | (4.1%) |
| Chronic obstructive pulmonary disease + right heart failure | 7 | (1.2%) |
| Spontaneous pneumothorax | 2 | (0.4%) |
| Tension pneumothorax with atrio-ventricular-block grade 3 | 2 | (0.4%) |
| Pneumonia with pericarditis | 2 | (0.4%) |
| Acute respiratory distress syndrome | 2 | (0.4%) |
| Porto-pulmonary hypertension | 2 | (0.4%) |
| Non-small cell lung cancer | 1 | (0.2%) |
| Hypertension related | 39 | (6.9%) |
| Tako-Tsubo-syndrome | 39 | (6.9%) |
| Rhythm disturbances | 35 | (6.2%) |
| Atrio-ventricular-block grade 3 | 10 | (1.8%) |
| Atrial fibrillation | 7 | (1.2%) |
| Coronary embolic events | 5 | (0.9%) |
| Tachymyopathy | 2 | (0.4%) |
| Ventricular tachycardia | 4 | (0.8%) |
| Sinu-atrial-block | 3 | (0.5%) |
| Atrio-ventricular nodal re-entry tachycardia | 2 | (0.4%) |
| Frequent premature ventricular complexes | 1 | (0.2%) |
| Implantable defibrillator discharge | 1 | (0.2%) |
| Pericarditis | 9 | (1.6%) |
| Worsened heart failure in known dilated cardiomyopathy | 9 | (1.6%) |
| Aortic stenosis | 8 | (1.4%) |
| Endocarditis | 6 | (1.1%) |
| Sepsis | 5 | (0.9%) |
| Hypovolemia | 4 | (0.8%) |
| Ischemic stroke/transistoric ischemic cerebral event | 4 | (0.8%) |
| Lab error | 2 | (0.4%) |
| Ruptured coronary plaque with spontaneous lysis | 2 | (0.4%) |
| Borelliosis | 1 | (0.2%) |
| Coronary spasm | 1 | (0.2%) |
| Hypertrophic obstructive cardiomyopathy | 1 | (0.2%) |
| Hyperthyroidism | 1 | (0.2%) |
| Amyloidosis | 1 | (0.2%) |
| Percutaneous coronary intervention 10 days before | 1 | (0.2%) |
| Cholecystitis | 1 | (0.2%) |
| Pancreatitis | 1 | (0.2%) |
| Aortic aneurysm | 1 | (0.2%) |
| Hypoglycemia | 1 | (0.2%) |
Data are presented as n (%).
Table 2. Baseline characteristics.
|
Group I TNI+, no CAD ≥ 75 years (n = 50) |
Group II TNI+, no CAD < 75 years (n = 222) |
Group III TNI+, CAD+ ≥ 75 years (n = 610) |
P-Value Group I vs. Group II |
P-Value Group I vs. Group III |
|
| Age, yrs | 79.34 ± 3.6 | 57.89 ± 12.3 | 80 ± 3.5 | < 0.0001 | 0.6079 |
| Gender (male percent) | 18/50 (36%) | 117/219 (53.4%) | 309/610 (50.8%) | 0.0288 | 0.0493 |
| History of AMI | 1/49 (2.1%) | 6/215 (2.8%) | 90/610 (14.8%) | 1.000 | 0.0405 |
| Lysis | 0/50 (0%) | 1/219 (0.5%) | 9/610 (1.6%) | 1.000 | 0.9245 |
| Resuscitation | 2/50 (4%) | 9/217 (4.2%) | 21/610 (3.2%) | 1.000 | 1.000 |
| CCS | |||||
| Grade I and II | 13/50 (26%) | 81/217 (37.2%) | 299/610 (49.2%) | 0.1425 | 0.0184 |
| Grade III and IV | 22/50 (44%) | 95/217 (43.8%) | 310/610 (50.8%) | 1.000 | 0.5674 |
| Diabetes | 19/50 (38%) | 57/217 (26.3%) | 252/605 (41.7%) | 0.1176 | 0.8452 |
| Hypertension | 43/50 (86%) | 161/217 (74.2%) | 470/602 (78.3%) | 0.0959 | 0.2777 |
| Hypercholesterolemia | 25/50 (50%) | 94/217 (43.3%) | 171/600 (28.3%) | 0.4318 | 0.0297 |
| Active smoking | 7/50 (14%) | 55/216 (25.5%) | 182/604 (30%) | 0.0962 | 0.0147 |
| Atrial fibrillation | 18/40 (45%) | 25/171 (14.6%) | 140/610 (23%) | 0.0001 | 0.0284 |
AMI: acute myocardial infarction; CAD: coronary artery disease; CCS: Canadian Cardiovascular Society; TNI+: troponin I positive.
Significant differences between older and younger ACS-patients without coronary stenoses at baseline (Group I vs. II) were only found in terms of age, sex, and prevalence of atrial fibrillation. Comparing Group I patients to patients ≥ 75 years with NSTEMI (Group III), the latter group had more frequently a history of previous acute myocardial infarction, presented more frequently with Canadian Cardiovascular Society (CCS) Grade I and II class anginal symptoms and atrial fibrillation, but had less frequently hypercholesterolemia.
3.1. Laboratory values, electrocardiograph analysis, and ejection fraction
The mean values of troponin and leukocytes were significantly higher in Group III patients compared to Group I patients. Abnormal electrocardiograph (ECG) patterns were also significantly more frequent in Group III patients, and finally left ventricular ejection fraction was significantly lower in Group III patients (Table 3).
Table 3. Ejection fraction, laboratory values, and ECG analysis.
|
Group I TNI+, no CAD ≥ 75 years (n = 50) |
Group II TNI+, no CAD < 75 years (n = 222) |
Group III TNI+, CAD+ ≥ 75 years (n = 610) |
P-Value Group I vs. Group II |
P-Value Group I vs. Group III |
|
| LVEF, % | 56.74 ± 14.13 | 59.17 ± 12.22 | 45 ± 11 | 0.22 | < 0.0001 |
| Laboratory values | |||||
| Creatinine, µmol/L | 105.90 ± 53.71 | 90.38 ± 56.81 | 116 ± 78 | 0.08 | 0.4289 |
| C-reactive protein, mg/L | 21.43 ± 31.73 | 18.35 ± 42.49 | 19 ± 36 | 0.63 | 0.6983 |
| White cell count, × 109 | 9.38 ± 3.13 | 9.28 ± 4.37 | 12 ± 5.12 | 0.87 | 0.0011 |
| Creatin kinase, mmol/L | 3.99 ± 6.47 | 3.13 ± 3.63 | 12 ± 18 | 0.22 | 0.2689 |
| Troponin I, ng/mL | 0.62 ± 0.80 | 1.86 ± 6.88 | 27 ± 74 | 0.23 | 0.0200 |
| Hemoglobin, mmol/L | 8.22 ± 1 | 8.59 ± 1 | 8.2 ± 1 | 0.019 | 0.7360 |
| Hematocrit | 0.40 ± 0.05 | 0.41 ± 0.05 | 0.45 ± 0.09 | 0.07 | 0.3448 |
| ECG | |||||
| ST-depression | 12/46 (26.1%) | 36/211 (17.1%) | 310/610 (50.8%) | 0.21 | 0.0107 |
| T-wave alterations | 16/46 (34.8%) | 68/213 (31.9%) | 440/609 (72.1%) | 0.73 | 0.0002 |
| Q wave | 2/46 (4.4%) | 7/213 (3.3%) | 338/610 (55.7%) | 0.9 | < 0.0001 |
| S1Q3-pattern | 0/46 (0%) | 3/170 (1.8%) | 10/598 (1.7%) | 1.00 | 1.0000 |
| AV-Block | 3/46 (6.5%) | 6/169 (3.6%) | 49/601 (8.2%) | 0.41 | 1.0000 |
| BBB | 11/46 (23.9%) | 41/209 (19.6%) | 160/610 (26.2%) | 0.55 | 0.8256 |
AV: atrio-ventricular; BBB: bundle branch block; CAD: coronary artery disease; ECG: electrocardiograph; LVEF: left ventricular ejection fraction; TNI+: troponin I positive.
3.2. Follow-up data
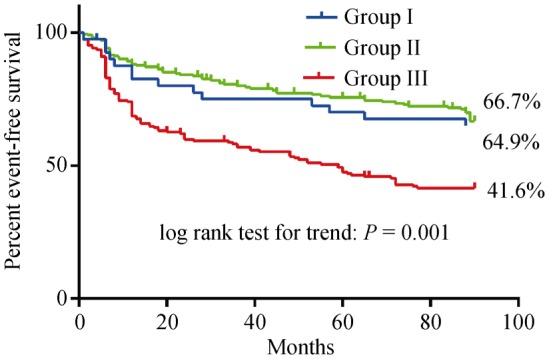
Clinical follow-up was available for 41/50 Group I patients (82%), 163/222 (73.4%) in Group II patients, and in 431/610 (70.5%) Group III patients, respectively. The mean follow-up duration was 22.3 ± 22.9 months. As demonstrated in Table 4 and Figure 1, the rate of adverse events was significantly lower in Group I patients compared with Group III patients (35.1% vs. 58.4%; P = 0.001).
Table 4. Follow-up data.
|
Group I TNI+, no CAD ≥ 75 years (n = 50) |
Group II TNI+, no CAD < 75 years (n = 222) |
Group III TNI+, CAD+ ≥ 75 years (n = 610) |
P-Value Group I vs. Group II |
P-Value Group I vs. Group III |
|
| Number of patients in follow-up | 41/50 (82%) | 163/222 (73.4%) | 431/610 (70.5%) | 0.2777 | 0.1032 |
| Follow-up duration, months | 26.2 ± 20.4 | 27.3 ± 21.2 | 17.5 ± 19.8 | 0.4832 | < 0.01 |
| Cardiac death | 1/41 (2.4%) | 5/163 (3.1%) | 49/431 (11.6%) | 1.0000 | 0.1064 |
| Myocardial infarction | 1/41 (2.4%) | 1/163 (0.6%) | 30/431 (7%) | 0.3624 | 0.5031 |
| Recurrent angina | 8/41 (19.5%) | 6/163 (3.7%) | 101/431 (23.3%) | 0.0017 | 0.6993 |
| Readmission to hospital | 7/41 (17.1%) | 21/163 (12.9%) | 211/430 (48.8%) | 0.4566 | < 0.0001 |
| CHF (NYHA II-IV) | 7/41 (17.1%) | 15/163 (9.2%) | 92/430 (20.9%) | 0.1617 | 0.6882 |
| Event-free survival | 64.9% | 66.7% | 41.6% | Log rank test for trend 0.001 | |
CAD: coronary artery disease; CHF: congestive heart failure; NYHA: New York Heart Association; pts: patients; TNI+: troponin I positive.
Figure 1. Event-free survival of Group I, II and Group III patients during follow-up.
4. Discussion
In the current study, 1.2% of patients ≥ 75 years with acute onset of chest pain and elevated markers of myocardial necrosis did not show significant (≥ 50%) coronary stenosis at angiography (Group I). Their prognosis is better compared to NSTEMI patients of comparable age undergoing percutaneous coronary intervention (Group III), but not different to ACS patients without CAD younger than 75 years (Group II).
4.1. Incidence
Elevated troponin values may be encountered in 1%–3% of a healthy reference population.[5] A troponin increase reflects acute or chronic myocardial damage but is not exclusive to ACS, and this can lead to difficulties in the interpretation of the result. The term false-positive has been used to describe the situation in which acute onset of chest pain is associated with an elevated troponin level, but no significant coronary disease is found at coronary angiography. In this setting, several differential diagnoses have to be considered where troponin elevation may be related to underlying cardiac but non-coronary pathology or extracardiac disease, such as severe renal dysfunction.[5]–[8] However, in 272/563 (48%) patients (6% of all screened 4,311 patients) elevated troponin levels could not be explained despite thorough clinical examination. Some of the cases might be related to heterophilic antibodies,[5] or several analytical issues including non-specific binding, effect of matrix selection, and lot-to-lot variation.[5],[9] The incidence of patients presenting with ACS and troponin elevation but without significant CAD varies in the literature. Older publications report 11% to 19%,[10],[11] whereas more recent publications report normal coronary arteries in ACS and troponin positive patients in 6% to 9%,[2],[12] which is well comparable to our results. However, data specifically reporting on patients older than 75 years are not available and the widespread use of high-sensitive troponin might change the incidence of ACS plus elevated troponin but without CAD in the future.[13]–[15]
4.2. Clinical presentation and possible mechanisms of troponin release in absence of significant CAD
Group I patients were more likely to be women, and presented with more severe anginal symptoms compared to patients undergoing angioplasty due to significant coronary obstruction. They were less likely to have a history of myocardial infarction, and the incidence of ST-segment or T-wave alterations was significantly lower. There has been much discussion in published reports about coronary microvascular dysfunction and its prevalence in elderly patients without significant obstructive CAD.[15]–[18] Similarly, coronary erosions on mild coronary plaques have been described to occur more often in patients with advanced age.[19] This phenomenon may represent a potential explanation for troponin release, increased C-reactive protein levels, and higher rate of future events without significant coronary stenosis. Several studies have suggested that troponins may be released from cardiac myocytes in situation other than myocyte necrosis. For example: (1) normal cell turnover might lead to increase in troponin and approximately 50% of cells are exchanged during life.[20] (2) Cellular release of proteolytic troponin degradation products: proteolysis can create small fragments being able to pass the cellular membrane with normal membrane integrity.[21] Induction of a very short (< 15 min) and mild ischemia has been shown to cause development of troponin I degradation products.[22] (3) Increased cellular wall permeability: another potential cause of troponin release is increase permeability of the cell membrane without cell necrosis and may occur due to myocardial stretch. A rat model increasing pre-load has been shown to be associated with release of troponin I, independent of ischemia.[23],[24] (4) Formation and release of membraneous blebs: active secretion of vesicles (blebs) has been hypothesized to be a mechanism to enable troponin to be released from cardiac cells. Cultured cardiac myocytes have been shown to develop blebs during anoxia and to release cytosolic enzymes without undergoing necrosis.[25] (5) There are also likely to be unknown causes of troponin elevations. It is not known as to why sepsis causes the release of troponin although heat shock proteins and tumor necrosis factor have been implicated.[26] ver Elst, et al.[27] did not find evidence of irreversible myocyte necrosis in autopsy cases of septic shock were there was a positive premortem Troponin. Increased troponin levels in patients with renal failure are not solely related to decreased renal excretion.[26]
Although several clinical, electrocardiographic and laboratory parameters differed significantly between the two groups, it seems to be impossible to identify a patient subgroup in which clinical presentation obviated the need for coronary angiography. Attempts were made to reliably predict the probability of insignificant CAD before angiography. Roe, et al.[3] proposed a simple nomogram based on 15 clinical and electrocardiographic parameters. The sum of the 15 parameters represents the probability that a given patient has insignificant CAD. However, this model was never prospectively evaluated.
4.3. Clinical outcome
In our study, we were able to demonstrate that the prognosis is worse in NSTEMI patients compared Group I or Group II patients. However, the event-rate of the latter groups of patients was 33.3% (Group II) and 35.1% (Group I) over a period of 86 months, respectively. This translates into an annual event-rate of roughly 4.5% in these groups, which is higher than the reported 2.4% per year cardiovascular events in a healthy population of comparable age.[28] Taking this into account, troponin-positive ACS without relevant coronary artery stenosis does not seem to be a benign condition and may warrant a more aggressive medical therapy. Whether a treatment similar to acute coronary syndrome with relevant coronary artery stenoses (e.g., dual platelet inhibition for 12 months and statin medication) can significantly reduce adverse events during follow-up and whether patients ≥ 75 years have to be treated specifically, needs to be investigated in further studies.
4.4. Limitations
This study was a single centre analysis, which was non-randomized and retrospective. Troponin data was available at baseline only. The investigator determined coronary artery stenosis is subjective and angiographic CAD is not an accurate assessment of underlying atherosclerosis, especially if including up to 50% stenosis. Finally, a bias due to loss of patients during follow-up may have played an important role.
4.5. Conclusion
Approximately 1.2% of all patients ≥= 75 years admitted for acute onset of chest pain and elevated markers of myocardial necrosis do not show significant (≥ 50%) coronary stenosis at angiography. The use of several different clinical variables did not help to differentiate patients with and without significant coronary stenosis. The composite outcome (cardiac death, re-infarction and re-hospitalization) of patients undergoing angioplasty due to coronary artery disease is worse than that of patients without significant coronary stenosis. Nonetheless, this latter group is associated with significant morbidity/mortality, and the cardiac event free survival is lower than expected for a population of comparable age.
Acknowledgments
The author takes responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation. There was no grant support and none of the authors has any potential conflicts of interest, including related consultancies, shareholdings and funding grants.
References
- 1.Heidenreich PA, Alloggiamento T, Melsop K, et al. The prognostic value of troponin in patients with non-ST elevation acute coronary syndromes: a meta-analysis. J Am Coll Cardiol. 2001;38:478–485. doi: 10.1016/s0735-1097(01)01388-2. [DOI] [PubMed] [Google Scholar]
- 2.von Korn H, Graefe V, Ohlow MA, et al. Acute coronary syndrome without significant stenosis on angiography: characterization and prognosis. Tex Heart Inst J. 2008;35:406–412. [PMC free article] [PubMed] [Google Scholar]
- 3.Roe M, Harrington R, Prosper D, et al. Clinical and therapeutic profile of patients presenting with acute coronary syndromes who do not have significant coronary artery disease. Circulation. 2000;102:1101–1106. doi: 10.1161/01.cir.102.10.1101. [DOI] [PubMed] [Google Scholar]
- 4.Kurisu S, Sato H, Kawagoe T, et al. Tako-tsubo-like left ventricular dysfunction with ST-segment elevation: a novel cardiac syndrome mimicking acute myocardial infarction. Am Heart J. 2002;143:448–455. doi: 10.1067/mhj.2002.120403. [DOI] [PubMed] [Google Scholar]
- 5.Agewall S, Giannitsis E, Jernberg T, et al. Troponin elevation in coronary vs. non-coronary disease. Eur Heart J. 2011;32:404–411. doi: 10.1093/eurheartj/ehq456. [DOI] [PubMed] [Google Scholar]
- 6.Hamm CW, Giannitsis E, Katus HA. Cardiac troponin elevations in patients without acute coronary syndrome. Circulation. 2002;106:2871–2872. doi: 10.1161/01.cir.0000044342.50593.63. [DOI] [PubMed] [Google Scholar]
- 7.Ohlow MA, Geller JC, Richter S, et al. Incidence and predictors of ventricular arrhythmias after ST-segment elevation myocardial infarction. Am J Emerg Med. 2011;30:580–586. doi: 10.1016/j.ajem.2011.02.029. [DOI] [PubMed] [Google Scholar]
- 8.Ohlow MA, Beierlein A, Müller S, et al. Stable tachycardia with wide QRS-complex in pre-hospital emergency medicine. Dtsch Med Wschr. 2005;130:2694–2698. doi: 10.1055/s-2005-922056. [DOI] [PubMed] [Google Scholar]
- 9.Panteghini M. Assay-related issues in the measurement of cardiac troponins. Clin Chim Acta. 2009;402:88–93. doi: 10.1016/j.cca.2008.12.037. [DOI] [PubMed] [Google Scholar]
- 10.Diver DJ, Bier JD, Ferreira PE, et al. Clinical and arteriographic characterization of patients with unstable angina without critical coronary arterial narrowing (from the TIMI-IIIA Trial) Am J Cardiol. 1994;74:531–537. doi: 10.1016/0002-9149(94)90739-0. [DOI] [PubMed] [Google Scholar]
- 11.William AE, Freeman MR, Chisholm RJ, et al. Angiographic morphology in unstable angina pectoris. Am J Cardiol. 1988;62:1024–1027. doi: 10.1016/0002-9149(88)90541-3. [DOI] [PubMed] [Google Scholar]
- 12.Dokainish H, Pillai M, Murphy SA, et al. Prognostic implications of elevated troponin in patients with suspected acute coronary syndrome but no critical epicardial coronary disease: a TACTICS-TIMI-18 substudy. J Am Coll Cardiol. 2005;45:19–24. doi: 10.1016/j.jacc.2004.09.056. [DOI] [PubMed] [Google Scholar]
- 13.Giannitsis E, Becker M, Kurz K, et al. High-sensitivity cardiac troponin T for early prediction of evolving non-ST-segment elevation myocardial infarction in patients with suspected acute coronary syndrome and negative troponin results on admission. Clin Chem. 2010;56:642–650. doi: 10.1373/clinchem.2009.134460. [DOI] [PubMed] [Google Scholar]
- 14.Wu AHB, Lu QA, Todd J, et al. Short- and long-term biological variation in cardiac troponin I measured with a high-sensitivity assay: implications for clinical practice. Clin Chem. 2009;55:52–58. doi: 10.1373/clinchem.2008.107391. [DOI] [PubMed] [Google Scholar]
- 15.Ohlow MA. Is elective coronary angiography overused in patients with suspected coronary artery disease? Future Cardiol. 2010;6:455–457. doi: 10.2217/fca.10.26. [DOI] [PubMed] [Google Scholar]
- 16.Sheifer S, Canos M, Weinfurt K, et al. Sex difference in coronary size assessed by intravascular ultrasound. Am Heart J. 2000;139:649–653. doi: 10.1016/s0002-8703(00)90043-7. [DOI] [PubMed] [Google Scholar]
- 17.Vaccarino V, Krumholz H, Berkman L, et al. Sex differences in mortality after myocardial infarction: is there evidence for increased risk in women. Circulation. 1995;91:1861–1871. doi: 10.1161/01.cir.91.6.1861. [DOI] [PubMed] [Google Scholar]
- 18.Marroquin O, Holubkow R, Edmundovicz L, et al. Heterogeneity of microvascular dysfunction in women with chest pain not attributable to coronary artery disease: implications for clinical practice. Am Heart J. 2003;145:628–635. doi: 10.1067/mhj.2003.95. [DOI] [PubMed] [Google Scholar]
- 19.Farb A, Burke A, Tang A, et al. Coronary plaque erosion without rupture into a lipid core: a frequent cause of coronary thrombosis in sudden cardiac death. Circulation. 1996;93:1354–1363. doi: 10.1161/01.cir.93.7.1354. [DOI] [PubMed] [Google Scholar]
- 20.Bergmann O, Bhardwaj RD, Bernard S, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao WD, Atar D, Liu Y, et al. Role of troponin I proteolysis in the pathogenesis of stunned myocardium. Circ Res. 1997;80:393–399. [PubMed] [Google Scholar]
- 22.McDonough J, Arrell D, Van Eyk J. Troponin I degradation and covalent complex formation accompanies myocardial ischemia/reperfusion injury. Circ Res. 1999;84:9–20. doi: 10.1161/01.res.84.1.9. [DOI] [PubMed] [Google Scholar]
- 23.Feng J, Schaus B, Fallavollita J, et al. Preload induces troponin I degradation independently of myocardial ischemia. Circulation. 2001;103:2035–2037. doi: 10.1161/01.cir.103.16.2035. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y, Serfass RC, Mackey-Bojack SM, et al. Cardiac troponin T alterations in myocardium and serum of rats after stressful, prolonged intense exercise. J Appl Physiol. 2000;88:1749–1755. doi: 10.1152/jappl.2000.88.5.1749. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz P, Piper H, Spahr R, et al. Ultrastructure of cultured adult myocardial cells during anoxia and reoxygenation. Am J Pathol. 1984;115:349–361. [PMC free article] [PubMed] [Google Scholar]
- 26.Babuin L, Jaffe A. Troponin: The biomarker of choice for the detection of cardiac injury. CMAJ. 2006;173:1191–1202. doi: 10.1503/cmaj.050141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.ver Elst KM, Spapen HD, Nguyen DN, et al. Cardiac troponins I and T are biological markers of left ventricular dysfunction in septic shock. Clin Chem. 2000;46:650–657. [PubMed] [Google Scholar]
- 28.Hayden M, Pignone M, Phillips C, et al. Aspirin for the primary prevention of cardiovascular events: a summary of the evidence for the U.S. preventive service task force. Ann Intern Med. 2002;136:161–172. doi: 10.7326/0003-4819-136-2-200201150-00016. [DOI] [PubMed] [Google Scholar]


