Abstract
Objective
To determine the extent to which differences in generic quality of life (QOL) between transcatheter aortic valve implantation (TAVI) and surgical aortic valve replacement (AVR) patients explained by EuroSCORE and heart-team operability assessment.
Methods
A total of 146 high-risk patients with EuroSCORE > 6 and aged ≥ 75 years underwent TAVI (n = 80) or aortic valve replacement (n = 66) between February 2010 and July 2013. A total of 75 patients also completed preoperative and six month SF-12 QOL measures. Analyses examined incident major morbidity, compared six month QOL between groups adjusted for EuroSCORE and operability, and quantified rates of clinically significant QOL improvement and deterioration.
Results
The AVR group required longer ventilation (> 24 h) (TAVI 5.0% vs. AVR 20.6%, P = 0.004) and more units of red blood cells [TAVI 0 (0–1) vs. AVR 2 (0–3), P = 0.01]. New renal failure was higher in TAVI (TAVI 5.0% vs. AVR 0%, P = 0.06). TAVI patients reported significantly lower vitality (P = 0.01) by comparison to AVR patients, however these findings were no longer significant after adjustment for operability. In both procedures, clinically significant QOL improvement was common [range 25.0% (general health) – 62.9% (physical role)] whereas deterioration in QOL occurred less frequently [range 9.3% (physical role) – 33.3% (mental health)].
Conclusions
Clinically significant improvement and deterioration in QOL was evident at six months in high risk elderly aortic valve replacement patients. Overall QOL did not differ between TAVI and AVR once operability was taken into consideration.
Keywords: Aortic valve replacement, Cardiac surgery, EuroSCORE, Transcatheter aortic valve implantation, Quality of life
1. Introduction
Non-inferiority of transcatheter aortic valve implantation (TAVI) by comparison to surgical aortic valve replacement (AVR) is demonstrated in high-risk patients in terms of survival and major morbidity.[1]–[10] A recent meta-analysis of 17 separate TAVI vs. AVR studies corroborated this in all-cause mortality analyses.[11] Previous studies also support consistent improvements in functional New York Heart Association (NYHA) Class,[12]–[18] and heart failure specific quality of life (QOL) in patients undergoing TAVI procedures.[13],[17],[18] However, findings with generic QOL measures, which are those reflecting day-to-day functioning,[19] are less conclusive.
With respect to previous QOL studies, neither the transfemoral nor transapical intervention group in the PARTNER study showed significant improvements on the EuroQOL at one year.[13] By contrast, Bekeredjian, et al.[16] showed significant improvement at six months in all generic Short Form-36 (SF-36) QOL domains with the greatest gains evident in physical functioning. Krane, et al.[14] also reported improvement in the majority of SF-36 QOL domains, however, no significant improvement was found for three others, whereas emotional-role functioning decreased. These findings raise the possibility that TAVI may not provide uniform improvements in aspects of daily functioning. In general, the lack of clarity over findings with respect to generic QOL is conspicuous and does not parallel what has been reported for heart failure specific QOL measures.
This prospective study extends beyond previous reports in two ways, firstly by comparing major morbidity outcomes and QOL outcomes among an Australian cohort of TAVI and AVR patients. By comparison to international experiences, there is little data from Australia where transcatheter aortic valves are only approved for use in patients deemed either inoperable or high-risk for AVR by a heart team. A second unique aspect of the current study is that we sought to determine the extent to which there is clinically significant change in generic QOL 6-months after TAVI by comparison to AVR taking into consideration operability, which has not been reported previously.
2. Methods
2.1. Patients
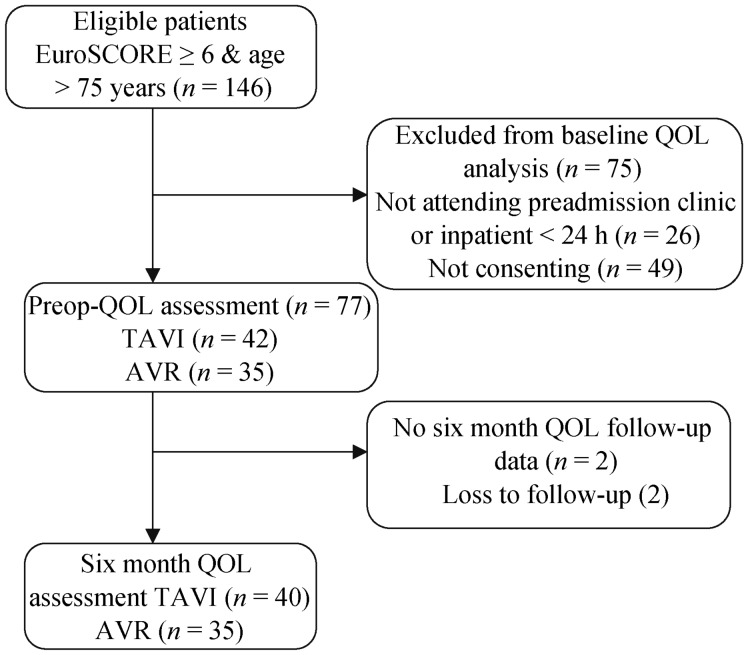
Consecutive patients undergoing TAVI or isolated AVR at Flinders Medical Centre, South Australia, Australia, from February 2010 to July 2013 were eligible for the study. To make comparable TAVI and AVR groups exclusion criteria was EuroSCORE < 6 and age < 75 years. A flow chart of participants through the study is shown in Figure 1. From 146 eligible patients, 26 were not approached for consent because of not attending pre-admission clinic or being an urgent procedure. Total 77 completed baseline QOL assessment and 75 completed six-month follow-up assessment. Medical data was collected prospectively according to standardized definitions,[20] and ethics approval was granted (Approval Number 148.13).
Figure 1. Flow chart of patients through the study.
AVR: aortic valve replacement; QOL: quality of life; TAVI: transcatheter aortic valve implantation.
2.2. TAVI patient selection
The process for TAVI is described elsewhere.[21],[22] Briefly, transcatheter aortic valves are not commercially available in Australia and are implanted in patients deemed either inoperable or high-risk for AVR by a heart team (comprising at least one cardiac surgeon and at least one interventional cardiologist) or as part of a clinical trial. Patients with severe aortic stenosis referred for intervention were assessed by the heart team, taking into consideration age, comorbidities, risk scores and frailty. Patients were classified as being suitable for either open AVR or TAVI or as inoperable and only suitable for the TAVI procedure. A clinical decision was made whether the individual proceeded to AVR or TAVI.
2.3. TAVI technique
All transcatheter valve procedures were performed by an experienced interventional cardiologist and cardiac surgeon in the catheterization laboratory using combined fluoroscopy and transesophegeal echocardiography (TEE) guidance. All patients had a general anesthetic and femoral vascular access. Procedures in which successful deployment was achieved utilized either the Edwards Sapien® (Edwards Lifesciences, Irvine CA, n = 31) or Edwards Sapien XT® (Edwards Lifesciences, Irvine CA, n = 45) prosthesis deployed either transfemoral (n = 70), transapical (n = 5), or transaortic (n = 1) approach utilizing rapid ventricular pacing. In the cohort, a failed procedure was defined as a procedure in which a valve was not successfully deployed or abandoned prior to valve deployment for technical and procedural limitations (n = 4).
2.4. AVR technique
All AVR procedures were performed by one of four experienced cardiothoracic surgeons. The technique was similar between surgeons. Access was via standard median sternotomy. Cardiopulmonary bypass was established using aortic and right atrial cannulation with arrest achieved by high dose tepid blood cardioplegia delivered in an antegrade fashion. Four different tissue valve prostheses were used; two porcine (Medtronic Mosaic®, Medtronic Inc. Minneapolis MN; St Jude Medical Epic®, St Jude Medical Inc. Minneapolis MN), two pericardial (St Jude Medical Trifecta®, St Jude Medical Inc. Minneapolis MN; Edwards Perimount Magna®, Edwards Lifesciences, Irvine CA). No patient received a mechanical prosthesis. Intra-operative TEE was used as needed.
2.5. Morbidity endpoints
Postoperative morbidity and mortality were defined consistent with the Society of Thoracic Surgeons database [23] and included: (a) mortality during the index hospitalisation or within 30 days of surgery regardless of location; (b) neurological injury (permanent stroke, cerebrovascular accident or central neurological deficit persisting for longer than 72 h); (c) renal failure (new requirement for renal dialysis or increase in serum creatinine to more than 2.0 mg/dL and double the most recent preoperative creatinine level); (d) prolonged ventilation (> 24 h postoperatively); (e) reoperation procedure or intervention for any reason during the index admission; or (f) postoperative myocardial infarction (MI) (two or more of cardiac enzyme level elevation, presence of new wall motion abnormality on echocardiography, or presence of new Q waves on ECG). In addition, we assessed the units of red blood cells transfused.
2.6. QOL and depression symptoms
Assessments were performed in the week prior to procedure and at six-month follow-up using the QOL Short Form-12 (SF-12) that covers eight general QOL domains with good validity in cardiac populations.[19], [24] Depression symptoms were measured at 30-days and 6-months with the Patient health questionnaire (PHQ-9) as recommended elsewhere.[25] PHQ-9 scores ≥ 10 have favorable psychometric validity to identify depression in cardiac patients including cardiac surgery patients.[26],[27]
2.7. Statistical analysis
Statistical analyses were performed with SPSS® 20.0 (SPSS Inc., Chicago, IL). In all analyses, P ≤ 0.05 was considered significant and no adjustment was made for multiple comparisons.[28] Values are expressed as mean ± SD or median [interquartile range (IQR), 25% to 75%]. Descriptive comparisons were made with the General Linear Model, chi-square statistic with Fishers exact test, or Mann-Whitney test. Individual QOL domains were analyzed with analysis of covariance (ANCOVA). Three models were run with incremental adjustment for preoperative QOL domain (Model 1), preoperative QOL domain and EuroSCORE (Model 2), and preoperative QOL domain, EuroSCORE and operability (Model 3). Ancillary analysis of clinically significant deterioration was based on the a priori moderate effect size (effect size change – 0.50) [29] and calculated as; ΔQOLTime2 − QOLTime1 / SD QOLTime1. With respect to depression symptoms, analysis examined rates of clinically significant depressive symptoms between groups (i.e., PHQ ≥ 5 and ≥ 10) with the chi-square statistic.
3. Results
3.1. Descriptive comparisons
Descriptive comparisons between groups are shown in Table 1. TAVI patients were older, spent less time in hospital post-procedure, and were characterized by a lower proportion of operable patients, patients with previous valvuloplasty, lung disease, higher chronic kidney disease (CKD) staging and higher NYHA class. Patients lost follow-up were characterized by a higher proportion of peripheral vascular disease (26.1% vs. 9.1%, P = 0.006) and larger BSA (1.94 ± 0.24 vs. 1.92 ± 0.27) but were otherwise similar.
Table 1. Descriptive comparisons by procedure group.
| Total n = 146 | AVR n = 66 | TAVI n = 80 | P | |
| Age, yrs | 83.31 ± 4.62 | 81.14 ± 3.53 | 85.10 ± 4.67 | < 0.001 |
| Male | 76 (52.1) | 29 (43.9) | 47 (58.8) | 0.08 |
| Operability | 107 (73.3) | 66 (100.0) | 41 (51.2) | < 0.001 |
| Logistic EuroSCORE, median IQR | 12.0 (8–18) | 10.5 (7–15.5) | 13 (9–19) | 0.08 |
| Body surface area | 1.93 ± 0.25 | 1.95 ± 0.21 | 1.91 ± 0.28 | 0.32 |
| CKD | 21 (14.4) | 6 (9.1) | 15 (18.8) | 0.10 |
| Stage 1 | 15 (10.3) | 11 (16.7) | 4 (5) | |
| Stage 2 | 60 (41.1) | 30 (45.5) | 30 (37.5) | |
| Stage 3 | 60 (41.1) | 22 (33.3) | 38 (47.5) | |
| Stage 4 | 8 (5.5) | 3 (4.5) | 5 (6.2) | |
| Stage 5 | 3 (2.1) | 0 | 3 (3.8) | |
| Atrial fibrillation | 43 (29.5) | 17 (25.8) | 26 (29.5) | 0.37 |
| Diabetes | 46 (31.5) | 22 (33.3) | 24 (30.0) | 0.67 |
| Redo | 44 (30.1) | 4 (6.1) | 40 (50.0) | < 0.001 |
| Previous valvuloplasty | 22 (15.1) | 3 (4.5) | 19 (23.8) | < 0.001 |
| Peripheral vascular disease | 25 (17.1) | 7 (10.6) | 18 (22.5) | 0.06 |
| Lung disease | 56 (8.4) | 19 (28.8) | 37 (46.2) | 0.03 |
| Cerebrovascular disease | (22.6) | 14 (21.2) | 19 (2.8) | 0.72 |
| Hypertension | 122 (83.6) | 54 (81.8) | 68 (85.0) | 0.61 |
| Hypercholesterolemia | 116 (79.5) | 49 (74.2) | 67 (83.8) | 0.16 |
| NYHA Class | 2.57 ± 0.86 | 2.41 ± 0.94 | 2.70 ± 0.77 | 0.04 |
| LVEF % > 60 | 93 (6.7) | 43 (65.2) | 50 (62.5) | 0.82 |
| 45– 60 | 30 (20.5) | 13 (19.7) | 17 (21.2) | |
| 30–45 | 16 (11.0) | 6 (9.1) | 10 (12.5) | |
| < 30 | 7 (4.8) | 4 (6.1) | (3.8) | |
| Hospital stay (days), median IQR | 7 (5–9) | 8 (6–10.5) | 6 (4–9) | 0.04 |
Data presented as mean ± SD or n (%) unless otherwise stated. Groups inclusive of aborted procedures. AVR: aortic valve replacement; CKD: chronic kidney disease; IQR: interquartile range; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association; TAVI: transcatheter aortic valve implantation.
3.2. Short-term morbidity and mortality
In general, TAVI patients did not experience disproportionate incident morbidity or mortality (Table 2). The renal failure/dialysis endpoint tended to be higher among the TAVI group (TAVI 5.0% vs. AVR 0%, P = 0.06). Prolonged ventilation was higher among the AVR group (TAVI 11.9% vs. AVR 20.6%, P = .004) and AVR group also required more transfused units of red blood cells [TAVI 0 (0–1) vs. AVR 2 (0–3), P = 0.01]. Otherwise TAVI patients experienced comparable rates of incident MI and requirement for reoperation.
Table 2. In-hospital morbidity by procedure group.
| Incident morbidity* | Total n = 146 | AVR n = 66 | TAVI n = 80 | P |
| Mortality < 30 days | 6 (4.1) | 3 (4.5) | 3 (3.8) | 1.0 |
| Stroke, CVA | 5 (3.4) | 2 (3.0) | 3 (3.8) | 1.0 |
| New renal failure/dialysis | 4 (2.7) | - | 4 (5.0) | 0.06 |
| Ventilation > 24 h | 17 (11.9) | 13 (20.6) | 4 (5.0) | 0.004 |
| Reoperation | 3 (2.1) | 2 (3.0) | 1 (1.3) | 0.71 |
| Myocardial infarction | 5 (3.4) | 3 (4.5) | 2 (2.5) | 0.66 |
| Combined endpoint (binary) | 36 (24.7) | 20 (30.3) | 16 (20.0) | 0.15 |
| RBC transfused, median IQR | 2 (0–2) | 2 (0–3) | 0 (0–1) | 0.01 |
| Aborted procedure# | 4 (2.7) | - | 4 (5.0)# | 0.13 |
Data presented as mean ± SD or n (%) unless otherwise stated. *Society of Thoracic Surgeons definitions were used; #Aborted procedures. 1: bleeding and haematoma following heparinzation; 2: unable to deploy catheter system due to sheath kinking; 3: balloon valvuloplasty performed, valve not deployed; 4: left coronary artery occluded with balloon valvuloplasty, valve not deployed. AVR: aortic valve replacement; CVA: cerebrovascular accident; IQR: interquartile range; RBC: red blood cells; TAVI: transcatheter aortic valve implantation.
3.3. QOL and depression symptoms
Analysis of mean NYHA class showed significant improvement in symptoms for both TAVI and surgical AVR patients following surgery (P < 0.001), however there were no differences between groups at six months (Table 3). Sensitivity and unadjusted analysis according to categorical NYHA class corroborated that there were no differences between groups at six-months (P = 0.75). Comparison of generic QOL showed that TAVI patients were found to report, on average, comparable QOL at six month follow-up in most SF-12 domains (all P > 0.20). Only vitality was significantly higher after AVR after adjustment for EuroSCORE (P = 0.01) though the finding was no longer significant after adjustment for operability (P = 0.10). Analyses showed moderate effect size partial η2 = 0.13.
Table 3. Quality of life at six months by procedure group§.
| QOL domain | AVR n = 35 |
TAVI n = 40 |
Model 1* P |
Model 2# P |
Model 3† P |
| NHYA class | |||||
| Preoperative | 2.49 ± 1.01 | 2.55 ± 0.90 | |||
| Six-months | 1.60 ± 1.04 | 1.67 ± 1.05 | 0.79 | 0.82 | 0.86 |
| Physical function | |||||
| Preoperative | 27.86 ± 2.52 | 15.00 ± 25.19 | |||
| Six-months | 40.00 ± 37.96 | 28.25 ± 29.56 | 0.56 | 0.63 | 0.91 |
| Vitality | |||||
| Preoperative | 32.86 ± 24.8 | 34.76 ± 26.97 | |||
| Six-months | 47.14 ± 26.96 | 30.98 ± 25.64 | 0.01 | 0.01 | 0.26 |
| Physical role | |||||
| Preoperative | 33.21 ± 28.28 | 32.50 ± 29.66 | |||
| Six-months | 57.93 ± 34.70 | 48.06 ± 29.41 | 0.37 | 0.41 | 0.41 |
| Bodily pain | |||||
| Preoperative | 72.86 ± 25.85 | 63.13 ± 31.21 | |||
| Six-months | 83.14 ± 26.65 | 73.70 ± 33.88 | 0.24 | 0.91 | 0.51 |
| General health | |||||
| Preoperative | 55.00 ± 26.98 | 46.38 ± 26.14 | |||
| Six-months | 55.57 ± 27.51 | 47.89 ± 23.53 | 0.60 | 0.68 | 0.61 |
| Mental health | |||||
| Preoperative | 44.28 ± 11.48 | 45.00 ± 9.72 | |||
| Six-months | 42.50 ± 11.42 | 45.48 ± 14.84 | 0.40 | 0.50 | 0.75 |
| Emotional role | |||||
| Preoperative | 54.29 ± 34.02 | 59.38 ± 33.22 | |||
| Six-months | 78.21 ± 26.1 | 73.80 ± 28.42 | 0.45 | 0.42 | 0.76 |
| Social function | |||||
| Preoperative | 58.57 ± 33.73 | 56.25 ± 35.25 | |||
| Six-months | 77.14 ± 28.03 | 75.03 ± 33.01 | 0.81 | 0.70 | 0.25 |
Data presented as mean ± SD. *Model 1 adjusted for baseline SF-12 score; #Model 2 adjusted for baseline score + EuroSCORE; †Model 3 adjusted for adjusted for baseline score + EuroSCORE + operability; §ANCOVA analysis on square root transformations; backtransformed scores shown. AVR: aortic valve replacement; NYHA: New York Heart Association; QOL: quality of life; SF-12: short form-12; TAVI: transcatheter aortic valve implantation.
As shown in Table 4, clinically significant improvement was common in all QOL domains with nearly 50% of patients improving in bodily pain, social functioning and emotional role. By contrast, one in every six patients experienced deterioration in vitality, mental health, bodily pain and social functioning (range: 17.3%–33.3%). Unadjusted analysis for trend suggested that there were no differences between TAVI and AVR patients (all P > 0.05). There was no significant difference between groups in mild to moderate depressive symptoms at either 30-days and 6 months (P > 0.20).
Table 4. Deterioration and improvement in six-month QOL by procedure group.
| AVR, n = 35 |
TAVI, n = 40 |
P | |||
| Decline | Improvement | Decline | Improvement | ||
| General health | 9 (25.7) | 12 (34.3) | 9 (22.5) | 10 (25.0) | 0.27 |
| Physical function | 6 (17.1) | 15 (42.9) | 5 (12.5) | 16 (40.0) | 0.63 |
| Vitality | 5 (14.3) | 16 (45.7) | 16 (40.0) | 13 (32.5) | 0.97 |
| Physical role | 5 (14.3) | 22 (62.9) | 4 (10.0) | 18 (45.0) | 0.06 |
| Mental health | 16 (45.7) | 9 (25.7) | 10 (25.0) | 12 (30.0) | 0.52 |
| Bodily pain | 6 (17.1) | 18 (51.4) | 8 (20.0) | 19 (47.5) | 0.81 |
| Social function | 6 (17.1) | 19 (54.3) | 7 (17.5) | 18 (45.0) | 0.38 |
| Emotional role | 6 (17.1) | 19 (54.3) | 5 (12.5) | 18 (45.0) | 0.28 |
Data presented as n (%). AVR: aortic valve replacement; QOL: quality of life; TAVI: transcatheter aortic valve implantation; Clinically significant change effect sizes ≥ 0.50 and ≤ −0.50.
4. Discussion
The present study has shown that incident morbidity for MI, reoperation and combined morbidity endpoint was generally not significantly different in TAVI by comparison to AVR, corroborating international literature.[1]–[9] Comorbidity characteristics of TAVI patients by definition contribute to their high-risk or inoperable status, and TAVI patients were found to experience significantly higher incident renal failure supporting general short term findings,[10] yet perhaps diverging from documented improvements in estimated glomerular filtration rate after TAVI.[30] By contrast, AVR patients experienced a significantly higher proportion of prolonged ventilation and required more transfused red blood cells. It was evident here that operability attenuated differences in six-month QOL between groups. It was further evident that clinically significant improvement and deterioration in QOL was common in both groups. As our finding was based on a relatively small sample, further validation in larger samples will help answer the extent to which TAVI and AVR is associated with clinically significant improvement and deterioration in QOL.
This study adds to the international literature comparing TAVI and AVR by reporting Australian results where TAVI is approved only for inoperable or high-risk patients. The morbidity findings can be compared alongside international reports,[31] and others concerning Australian TAVI patients,[32],[33] and generally higher risk aortic stenosis patients. Here we found few differences in major morbidity outcomes delineated by the Society for Thoracic Surgeons.[23] This study also extends the extant literature which to date has been inconclusive with regards to TAVI impact on generic QOL predominantly because of the relative infancy of TAVI procedures. By contrast to survival, morbidity and heart failure, previous studies are less consistent regarding group differences in generic QOL.[34],[35] Previous TAVI studies have reported improvements in functional NYHA Class from as early as 30 days and sustained in 75% of patients at 2-year follow-up.[12]–[18] In terms of heart failure specific QOL, improvement in Minnesota Living With Heart Failure Questionnaire and Kansas City Cardiomyopathy Questionnaire was reported from 30 days to 1 year follow-up.[13],[17],[18] Here, it was evident that once adjustment was made for operability status there was no difference between TAVI and AVR patients with regards to average QOL at six months.
It was further evident that inspection of mean QOL alone may obscure clinically significant QOL improvement and deterioration. This approach to improvement and decline in QOL parallels what has been reported for neurocognitive deficits.[36] Though it was evident that there were no between group differences in QOL change in this sample, it was nevertheless noteworthy that one in every six patients experienced deterioration in general health, vitality, mental health, bodily pain and social functioning (range: 17.3%–33.3%). Yet, nearly 50% of all patients showed clinically significant improvements in bodily pain, social functioning and emotional role, thus contrasting to Krane et al's German TAVI cohort.[14] The latter finding is important to contextualize in terms of the inoperable status of Australian TAVI patients and the direct comparison to AVR patients.
The strengths of this study included selection of older high-risk patients in a contemporary dataset and use of advanced statistical analyses to determine the effects of operability, and also clinically significant change in QOL. The practical implications of this study include informing clinical decision making and rehabilitation practices for elderly aortic stenosis patients.[24] Similarly, the failure to make QOL gains,[14],[18],[35] may provide valuable information to aid patients' decision making, considering that QOL is salient to patients.[19]
These findings are presented with several limitations that may temper the generalizability including small sample size and recruitment from a single surgical centre. Moreover, the study is based on early experience with first and second generation TAVI devices performed under general anaesthetic, whereas some centres insert second generation devices under local anaesthetic without transthoracic echocardiography guidance. The surgical centre also provides service from a broad geographical region inclusive of remote patients yet accessibility to postoperative services was not included in these analyses which may affect long term outcomes.[37],[38] The analysis of incident morbidity was constrained by few endpoints as reflected in the width of the confidence intervals. It is possible that selection bias at six month follow-up may overestimate QOL benefits of either TAVI or AVR. Also we opted not to utilize SF-12 QOL summary scores as they may not be sensitive to discrete effects for individual and heterogeneous QOL domains. Finally, the methodology to determine clinically significant change in QOL needs to be considered among other statistical methods as there is no universal agreement as to the best approach.
In conclusion, we observed clinically significant improvement and deterioration in six month QOL among high risk elderly aortic valve replacement patients. However overall QOL, and morbidity did not differ between TAVI and AVR once operability was taken into consideration.
Acknowledgments
Tully PG was supported by the National Health and Medical Research Council of Australia (Clinical Overseas Fellowship # 1053578). The funding body had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript. The authors acknowledge the support of the participating clinicians Hugh Cullen, and David Lance, and research staff Nicole Canning and Bronwyn Pesudovos.
References
- 1.Tamburino C, Capodanno D, Ramondo A, et al. Incidence and predictors of early and late mortality after transcatheter aortic valve implantation in 663 patients with severe aortic stenosis. Circulation. 2011;123:299–308. doi: 10.1161/CIRCULATIONAHA.110.946533. [DOI] [PubMed] [Google Scholar]
- 2.Ye J, Cheung A, Lichtenstein SV, et al. Transapical transcatheter aortic valve implantation: follow-up to 3 years. J Thorac Cardiovasc Surg. 2010;139:1107–1113, 13 e1. doi: 10.1016/j.jtcvs.2009.10.056. [DOI] [PubMed] [Google Scholar]
- 3.Webb JG, Altwegg L, Boone RH, et al. Transcatheter aortic valve implantation: impact on clinical and valve-related outcomes. Circulation. 2009;119:3009–3016. doi: 10.1161/CIRCULATIONAHA.108.837807. [DOI] [PubMed] [Google Scholar]
- 4.Thielmann M, Wendt D, Eggebrecht H, et al. Transcatheter aortic valve implantation in patients with very high risk for conventional aortic valve replacement. Ann Thorac Surg. 2009;88:1468–1474. doi: 10.1016/j.athoracsur.2009.07.033. [DOI] [PubMed] [Google Scholar]
- 5.Ruiz CE, Laborde JC, Condado JF, et al. First percutaneous transcatheter aortic valve-in-valve implant with three year follow-up. Catheter Cardiovasc Interv. 2008;72:143–148. doi: 10.1002/ccd.21597. [DOI] [PubMed] [Google Scholar]
- 6.Bosmans JM, Kefer J, De Bruyne B, et al. Procedural, 30-day and one year outcome following CoreValve or Edwards transcatheter aortic valve implantation: results of the Belgian national registry. Interact Cardiovasc Thorac Surg. 2011;12:762–767. doi: 10.1510/icvts.2010.253773. [DOI] [PubMed] [Google Scholar]
- 7.Piazza N, van Gameren M, Juni P, et al. A comparison of patient characteristics and 30-day mortality outcomes after transcatheter aortic valve implantation and surgical aortic valve replacement for the treatment of aortic stenosis: a two-centre study. EuroIntervention. 2009;5:580–588. doi: 10.4244/eijv5i5a94. [DOI] [PubMed] [Google Scholar]
- 8.Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 9.Tissot CM, Attias D, Himbert D, et al. Reappraisal of percutaneous aortic balloon valvuloplasty as a preliminary treatment strategy in the transcatheter aortic valve implantation era. EuroIntervention. 2011;7:49–56. doi: 10.4244/EIJV7I1A11. [DOI] [PubMed] [Google Scholar]
- 10.Alsara O, Alsarah A, Laird-Fick H. Advanced age and the clinical outcomes of transcatheter aortic valve implantation. J Geriatr Cardiol. 2014;11:163–170. doi: 10.3969/j.issn.1671-5411.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takagi H, Niwa M, Mizuno Y, et al. A meta-analysis of transcatheter aortic valve implantation versus surgical aortic valve replacement. Ann Thorac Surg. 2013;96:513–519. doi: 10.1016/j.athoracsur.2013.04.049. [DOI] [PubMed] [Google Scholar]
- 12.Buellesfeld L, Gerckens U, Schuler G, et al. Two year follow-up of patients undergoing transcatheter aortic valve implantation using a self-expanding valve prosthesis. J Am Coll Cardiol. 2011;57:1650–1657. doi: 10.1016/j.jacc.2010.11.044. [DOI] [PubMed] [Google Scholar]
- 13.Lefevre T, Kappetein AP, Wolner E, et al. One year follow-up of the multi-centre European PARTNER transcatheter heart valve study. Eur Heart J. 2011;32:148–157. doi: 10.1093/eurheartj/ehq427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krane M, Deutsch MA, Bleiziffer S, et al. Quality of life among patients undergoing transcatheter aortic valve implantation. Am Heart J. 2010;160:451–457. doi: 10.1016/j.ahj.2010.05.038. [DOI] [PubMed] [Google Scholar]
- 15.Ussia GP, Mule M, Barbanti M, et al. Quality of life assessment after percutaneous aortic valve implantation. Eur Heart J. 2009;30:1790–1796. doi: 10.1093/eurheartj/ehp171. [DOI] [PubMed] [Google Scholar]
- 16.Bekeredjian R, Krumsdorf U, Chorianopoulos E, et al. Usefulness of percutaneous aortic valve implantation to improve quality of life in patients > 80 years of age. Am J Cardiol. 2010;106:1777–1781. doi: 10.1016/j.amjcard.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 17.Gotzmann M, Hehen T, Germing A, et al. Short-term effects of transcatheter aortic valve implantation on neurohormonal activation, quality of life and 6-minute walk test in severe and symptomatic aortic stenosis. Heart. 2010;96:1102–1106. doi: 10.1136/hrt.2009.180661. [DOI] [PubMed] [Google Scholar]
- 18.Goncalves A, Marcos-Alberca P, Almeria C, et al. Quality of life improvement at midterm follow-up after transcatheter aortic valve implantation. Int J Cardiol. 2013;162:117–122. doi: 10.1016/j.ijcard.2011.05.050. [DOI] [PubMed] [Google Scholar]
- 19.Tully PJ. Quality-of-life measures for cardiac surgery practice and research: a review and primer. J Extra Corpor Technol. 2013;45:8–15. [PMC free article] [PubMed] [Google Scholar]
- 20.ASCTS cardiac surgery database project: data definitions., T.A.S.o.C.a.T. Surgeons, Editor 2008: Melbourne, Victoria, Australia
- 21.Lee L, Prakash R, Joseph M, et al. Transcatheter aortic valve implantation (TAVI) for severe aortic stenosis, experience from the flinders medical centre. Heart Lung Circ. 2011;20:S152–S153. [Google Scholar]
- 22.Crouch G, Sinhal A, Baker R, et al. Trans-apical aortic valve replacement: the flinders experience. Heart Lung Circ. 2011;20:795. [Google Scholar]
- 23.O'Brien SM, Shahian DM, Filardo G, et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: Part 2--isolated valve surgery. Ann Thorac Surg. 2009;88:S23–S42. doi: 10.1016/j.athoracsur.2009.05.056. [DOI] [PubMed] [Google Scholar]
- 24.Székely A, Nussmeier NA, Miao Y, et al. A multinational study of the influence of health-related quality of life on in-hospital outcome after coronary artery bypass graft surgery. Am Heart J. 2011;161:1179–1185.e2. doi: 10.1016/j.ahj.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 25.Tully PJ, Baker RA. Depression, anxiety, and cardiac morbidity outcomes after coronary artery bypass surgery: a contemporary and practical review. J Geriatr Cardiol. 2012;9:197–208. doi: 10.3724/SP.J.1263.2011.12221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunkel A, Kendel F, Lehmkuhl E, et al. Predictors of preoperative depressive risk in patients undergoing coronary artery bypass graft surgery. Clin Res Cardiol. 2009;98:643–650. doi: 10.1007/s00392-009-0050-0. [DOI] [PubMed] [Google Scholar]
- 27.Tully PJ. Psychological depression and cardiac surgery: a comprehensive review. J Extra Corpor Technol. 2012;44:224–232. [PMC free article] [PubMed] [Google Scholar]
- 28.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- 29.Crosby RD, Kolotkin RL, Williams GR. Defining clinically meaningful change in health-related quality of life. J Clin Epidemiol. 2003;56:395–407. doi: 10.1016/s0895-4356(03)00044-1. [DOI] [PubMed] [Google Scholar]
- 30.Keleş T, Ayhan H, Durmaz T, et al. Improvement in renal functions with transcatheter aortic valve implantation. J Geriatr Cardiol. 2013;10:317–322. doi: 10.3969/j.issn.1671-5411.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 32.Clarke A, Wiemers P, Poon KKC, et al. Early experience of transaortic TAVI—The future of surgical TAVI? Heart Lung Circ. 2013;22:265–269. doi: 10.1016/j.hlc.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 33.Ho E, Mathur MN, Brady PW, et al. Surgical aortic valve replacement in very elderly patients aged 80 years and over: evaluation of early clinical outcomes. Heart Lung Circ. 2014;23:242–248. doi: 10.1016/j.hlc.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Kala P, Tretina M, Poloczek M, et al. Quality of life after transcatheter aortic valve implantation and surgical replacement in high-risk elderly patients. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2013;157:75–80. doi: 10.5507/bp.2012.062. [DOI] [PubMed] [Google Scholar]
- 35.Amonn K, Stortecky S, Brinks H, et al. Quality of life in high-risk patients: comparison of transcatheter aortic valve implantation with surgical aortic valve replacement. Eur J Cardiothorac Surg. 2013;43:34–42. doi: 10.1093/ejcts/ezs173. [DOI] [PubMed] [Google Scholar]
- 36.Kneebone A, Andrew M, Baker R, et al. Neuropsychologic changes after coronary artery bypass grafting: use of reliable change indices. Ann Thorac Surg. 1998;65:1320–1325. doi: 10.1016/s0003-4975(98)00158-1. [DOI] [PubMed] [Google Scholar]
- 37.Clark RA, Coffee N, Turner D, et al. Appliction of geopgraphic modeling techniques to quantify spatial access to health services before and after an cute crdiac event: the Cardiac Accessibility and Remoteness Index of Australia (ARIA) project. Circulation. 2012;125:2006–2014. doi: 10.1161/CIRCULATIONAHA.111.083394. [DOI] [PubMed] [Google Scholar]
- 38.Prabhu A, Tully PJ, Bennetts JS, et al. The morbidity and mortality outcomes of indigenous Australian peoples after isolated coronary artery bypass graft surgery: the influence of geographic remoteness. Heart Lung Circ. 2013;22:599–605. doi: 10.1016/j.hlc.2013.01.003. [DOI] [PubMed] [Google Scholar]