Abstract
Frailty is a state of late life decline and vulnerability, typified by physical weakness and decreased physiologic reserve. The epidemiology and pathophysiology of frailty share features with those of cardiovascular disease. Gait speed can be used as a measure of frailty and is a powerful predictor of mortality. Advancing age is a potent risk factor for cardiovascular disease and has been associated with an increased risk of adverse outcomes. Older adults comprise approximately half of cardiac surgery patients, and account for nearly 80% of the major complications and deaths following surgery. The ability of traditional risk models to predict mortality and major morbidity in older patients being considered for cardiac surgery may improve if frailty, as measured by gait speed, is included in their assessment. It is possible that in the future frailty assessment may assist in choosing among therapies (e.g., surgical vs. percutaneous aortic valve replacement for patients with aortic stenosis).
Keywords: Cardiac surgery, Frailty, Gait speed, Risk scores, Risk stratification
Proverbs
“When a person gets old, their legs get old first”—Chinese Proverb
“Slow and steady wins the race”—Aesop's “The Tortoise and the Hare”
1. Demographics/Epidemiology
The United States Population is aging. According to current projections, people 65 years of age and older will represent over 20% of the population by 2060 (an increase from about 14% currently). It is anticipated that those 85 years of age and older will more than triple by that time.[1] Life expectancy has also been steadily increasing, and is estimated to nearly reach 80 years by 2015.[2]
Age is a potent risk factor for virtually all forms of cardiovascular disease, especially coronary artery disease, hypertension, heart failure and stroke, underlying a prevalence that rises dramatically with age. The prevalence of these disorders rises from 12.8% in men and 10.1% in women in people 20–39 years old to 83% in men and 8.1% in women in those 80 years old and older.[3]
2. Age related physiologic changes to the cardiovascular system
There are multiple age related changes that occur in the cardiovascular system. These do not inevitably lead to disease states, but do reduce the reserve that older persons have. Table 1 lists several changes as well as diseases states the changes may predispose older adults to (i.e., reduced cardiovascular reserve).
Table 1. Age-associated changes and cardiovascular disease in the cardiovascular system.[114].
| Age associated changes | Organ | Cardiovascular disease |
| Increased intimal thickness | Vasculature | Systolic hypertension |
| Arterial stiffening | Coronary artery stenosis | |
| Increased pulse pressure | Peripheral artery stenosis | |
| Increased pulse wave velocity | Carotid artery stenosis | |
| Early central wave reflections | ||
| Decreased endothelium-mediated vasodilatation | ||
| Increased left atrial size | Atria | Atrial fibrillation |
| Atrial premature complexes | ||
| Decreased maximal heart rate | Sinus node | Sinus node dysfunction, sick sinus syndrome |
| Decreased heart rate variability | ||
| Increased conduction time | Atrioventricular node | Second, third-degree block |
| Sclerosis, calcification | Valves | Stenosis, regurgitation |
| Increased left ventricular wall tension | Ventricles | Left ventricular hypertrophy |
| Prolonged myocardial contraction | ||
| Prolonged early diastolic filling rate | Heart failure (with or without systolic dysfunction) | |
| Decreased maximal cardiac output | ||
| Right bundle branch block | ||
| Ventricular premature complexes | Ventricular tachycardia, fibrillation | |
Notably, age related changes occur throughout the body, and in virtually all organ systems.
3. Frailty
3.1. What is frailty?
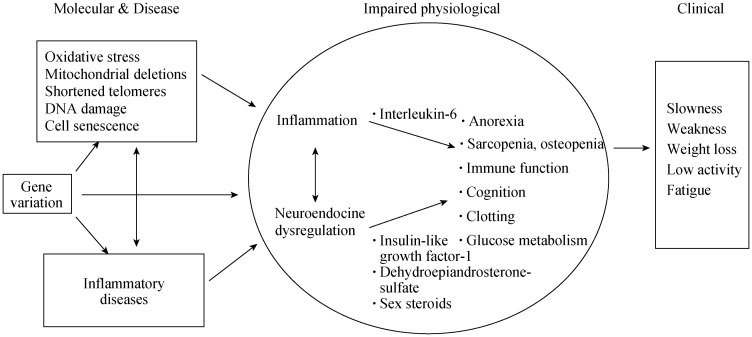
Frailty has been defined as a state of late life decline and vulnerability, typified by physical weakness and decreased physiologic reserve.[4] Multiple domains are affected, including neuromuscular, metabolic, immune, mobility, strength, endurance, nutrition and cognitive, resulting in increased vulnerability.[5]–[8] While there is more work to be done, there is a growing understanding of the underlying pathophysiology of frailty. Abnormalities including endocrine and immune dysfunction feature prominently in current theories. Chronic low-grade systemic inflammation and abnormalities in the endocrine system such as low testosterone and insulin resistance have been noted.[9]–[12] A common final pathway of sarcopenia, a decline in skeletal muscle resulting from excessive breakdown with insufficient muscle building, has also been suggested.[10],[13] It has been noted that some abnormalities are similar to those found in some cancers and in rheumatoid arthritis (Figure 1).[14] The presence of frailty has been associated with a number of adverse outcomes including an increased risk of falls, institutionalization, disability, health care resource use as well as mortality.[15]–[18]
Figure 1. Pathophysiology of frailty.
Proposed pathway for frailty from genetics to environment to clinical phenotype. Reproduced with permission, John Wiley and Sons.[4]
3.2. Frailty, comorbidity, disability
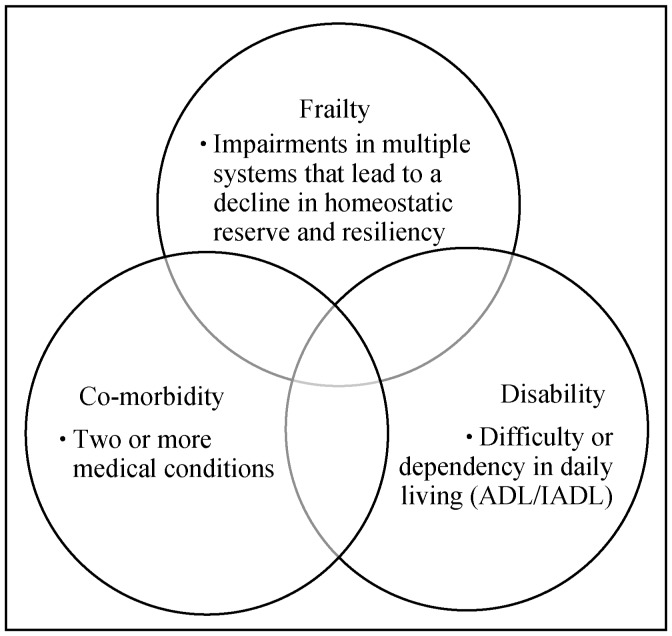
Although there is some degree of overlap, frailty is not synonymous with old age, disability or co-morbidity. Frailty usually precedes disability which results from longstanding frailty and medical co-morbidities. Disability [defined as difficulty performing activities of daily living (ADLs) and instrumental activities of daily living (IADLs)] may result from a specific event that befalls a frail person (e.g., pneumonia resulting in dyspnea and O2 dependence) or something that affects a previously non-frail person (e.g., previously healthy skier falls, suffering a broken leg which leads to disability). The International Academy on Nutrition and Frailty Task Force endorses separating the three domains.[19] (Figure 2).
Figure 2. Frailty, co-morbidity and disability.
Distinct domains with some overlap. ADL: activities of daily living (basic self-care tasks); IADL: instrumental ADL (household management tasks). Reproduced with permission, Elsevier.[115]
3.3. Frailty—indices/measures
More than 20 indices or measures of frailty have been developed.[20] The Cardiovascular Health Study (CHS) index (also called the Fried Index) is a widely used tool that requires a subject have at least three of the following to be considered frail: weight loss ≥ 5% of their body weight in the last year, exhaustion (effort required for activities), weakness (as measured by grip strength) slow walking speed (> 6–7 s per 15 ft), decreased physical activity (kilocalories/week: M < 383, F < 270). Subjects meeting one or two criteria are considered “pre-frail,” and those with none are considered “robust.” [5]
The Rockwood frailty index utilizes 70 variables, where higher scores mean a subject is more frail. The included items range from medical conditions to functional decline.[21] The Study of Osteoporotic Fractures Index includes an assessment of weight loss of 5% in last year, an inability to rise from a chair 5 times without using ones arms, and a “no” answer to the question “Do you feel full of energy?”[22] There are many other measures/scales/tools.
3.4. Frailty—epidemiology
The prevalence and incidence of frailty depends on the population studied and on the measure used to define frailty. The prevalence in 15 studies (44,894) was 9.9%, and in eight studies, when psycho-social factors were included in the assessment, the prevalence was 13.6%.[23] Using the CHS criteria, in the CHS study population (5317 men and women ≥ 65 years), frailty was present in 7%, 44% were pre-frail and, 46% were robust.[5] In the Women's Health and Aging study, 11.3% were frail.[24]
In the Women's Health Initiative Observational Study of 40,000 women who were aged 65–70 years at study entry, utilizing the CHS criteria, 16.3% were classified as frail, 28.3% were pre-frail and incident frailty at three years was 14.8%. Those who became frail were: older, less-educated, smokers and hormone replacement therapy users.[25] In a study of 6000 US men ≥ 65 years of age, 4% were frail as defined by modified CHS criteria (skeletal mass was substituted for weight loss). Forty percent were defined as pre-frail and 56% robust.[26] Frailty was more common in African Americans and Asians followed by Caucasians and Hispanics. Frail persons were older, more often single, and less-educated. 1.6% met frailty criteria in the 65–69 year age group and 11.1% in those > 80 years. With regard to incident frailty, at an average of 4.6 years of follow-up, 54.4% who were robust at baseline remained so, 25.3% became pre-frail and 1.6% became frail.
3.5. Frailty and outcomes
Frailty has been associated with an increased risk for mortality and morbidity. Utilizing the CHS criteria, in a longitudinal study of 754 independently living and non-disabled subjects ≥ 70 years of age who were followed for 6 years, frailty was associated with disability, long-term skilled nursing facility residence, and death.[27] In surgical patients, it independently predicted post-operation complications, hospital length of stay, and discharges to skilled nursing or assisted living facilities.[28] It has been shown that frail patients are less likely to mount adequate immune response to flu vaccine,[29] to have worse outcomes after renal transplantation,[28] and to be at greater risk for hip fractures, disability, and hospitalization, even after adjustment for co-morbidities.[25]
3.6. Frailty—interventions
It is believed that one of the most effective ways to improve the status of frail patients is exercise. Exercise has been shown to increase mobility and balance, decrease falls, improve ADL performance and bone mineral density.[30]–[32] Resistance training, only twice per week has revealed benefits.[33] Walking as little as a mile per week reduced functional limitations in one study.[34] Other interventions that have been proposed include hormone replacement (e.g., testosterone), and dietary supplements (e.g., vitamin D) as well as more comprehensive outpatient programs.[35],[36]
Outpatient programs that have the potential to benefit frail elderly have focused on utilizing comprehensive geriatric assessment (CGA). CGA utilizes an interdisciplinary team of medical providers, nurses, social workers, pharmacists and physical and occupational therapists with the goal to improve physical and psychosocial functioning, reduce disability, institutionalization, mortality and to increase quality of life.[37] Program for all-inclusive care of the elderly (PACE) programs are a medicare program which takes over patient's primary care and provides the components of CGA outlined above.[38] They have been shown to be effective and cost-favorable.[39]
In addition to the illness that necessitates inpatient treatment, hospitalization itself presents challenges to frail elderly, which includes a change in environment, new medications, and immobility. Frail hospitalized patients are at 7 times the risk of developing disability within one month of discharge versus non-frail patients (35% vs. 7%).[40] Interventions include acute care of the elderly (ACE) units which provide a more home-like environment, patient-centered care focusing on preventing disability and comprehensive discharge planning. In a randomized control trial of 1531 patients, ACE unit admission decreased ADL decline and skilled nursing facility placement at discharge and at one year, without increasing length of stay or cost.[41]
As older adults progress from robust to increasingly frail to end-stage frailty, possible interventions range from exercise, to comprehensive geriatric assessment to ACE units and PACE programs, and finally to hospice and end of life care.[42]
3.7. Frailty and cardiovascular disease
There are multiple commonalities in frailty and cardiovascular disease, and chronic low-level inflammation may represent a common pathophysiologic basis for both conditions.[11],[43]–[45] Proposed instigators of the inflammation common to patients with frailty and those with cardiovascular disease are lifelong antigen exposure, angiotensin 1R activation, obesity, insulin resistance, and imbalance in redox.[45],[46] Markers of inflammation such as activation of neutrophils and monocytes, elevations in high sensitivity C-reactive protein (CRP), interleukin (IL-6) and thrombotic markers, including factor VIII and D-dimers are found in frail patients and in those with cardiovascular disease.[9],[47]–[52]
Inflammation appears to contribute to the two conditions via distinct pathways. In cardiovascular disease, inflammation can lead to oxidation of lipoproteins and atherosclerotic plaque activation.[53] In frail patients it is thought that inflammation can cause a catabolic neurohormonal state, taking amio acids from muscle, impairing stress response and repair.[54]–[57] Insulin resistance is common in patients with both conditions. In addition to its relationship to chronic inflammation, insulin resistance can lead to impaired muscle protein breakdown, limiting available amino acids for repair. Vitamin D deficiency appears to affect both as well and other commonalities include anemia, elevated leukocytes and high fibrinogen.[44],[58],[59] Cardiovascular disease and frailty share many common features and suggest a common biological pathway and also appear to facilitate each other, leading to adverse outcomes (cardiac and noncardiac) which can result in disability, institutionalization and mortality.[60]
3.8. Frailty and cardiovascular disease—epidemiology
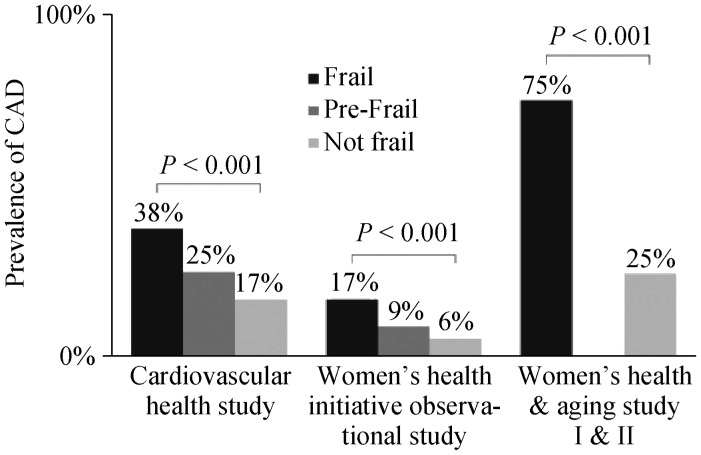
Several studies have revealed a cross-sectional association between frailty and prevalent cardiovascular disease in the community. These have included populations from 450 to over 4700 subjects (Cardiovascular Health Study,[61] Zutphen Elderly Men's Study,[7] Women's Health and Aging Studies,[59] Beaver Dam Eye Study[62]), with odds ratios (OR) ranging from 1.41–4.1, (Table 2, Figure 3). In the Women's Health Initiative Observational Study of 40,657 subjects who were ≥ 65 years old and either had a diagnosis of cardiovascular disease or its risk factors were at higher risk of developing incident frailty.[25] Those with a diagnosis of coronary heart disease or stroke had the highest risk (OR: 1.47–1.71). In the French 3 City Study & Health ABC Study, frail older adults were at higher risk of developing incident cardiovascular disease events such as heart failure and mortality.[63]–[65] It has been estimated that the prevalence of frailty prevalence in the cardiovascular disease population ranges from 25%–60%.[58],[66],[67]
Table 2. Studies revealing an association between cardiovascular disease and frailty.[67].
| Study | Variable |
| Zutphen Elderly Men's Study (1999)[7] | Prevalent frailty in elders with CVD |
| OR 4.1 (95%CI: 1.8–9.3) | |
| CHS (2001)[61] | OR 2.79 (95%CI: 2.12–3.67) |
| Beaver Dam Eye Study (2005)[62] | OR 2.67 (95%CI: 1.33–5.41) |
| WHI-OS (2005)[25] | OR 3.36 (95%CI: 3.09–3.66) |
| WHAS I and II (2005)[59] | OR 2.72 (95%CI: 1.72–4.30 |
| Incident frailty in elderly with CVD | |
| WHI-OS (2005)[25] | OR 1.47 (95%CI: 1.25–1.73) |
| Incident CVD in frail elders | |
| HABC Study (2006)[64] | HR 1.61 (95%CI: 1.05–2.45) |
| Mortality in frail elders with severe CVD | |
| Cacciatore et al. (2005)[116] | HR 1.62 (95%CI: 1.08–2.45) |
| Purser et al (2006)[66] | OR 4.0 (95%CI: 1.1–13.8) |
CVD: cardiovascular disease.
Figure 3. Cardiovascular disease and frailty.
Prevalence of cardiovascular disease stratified by frailty status in major studies. Depicted studies used the Fried criteria (3 of 5 = frail; 1 or 2 of 5 = pre-frail). CAD: coronary artery disease. Reproduced with permission, Elsevier.[67]
3.9. Gait speed—is that the ticket?
Indcies of frailty can be cumbersome, and as described above, scales with as many as 70 items have been used. These are unlikely to be used in clinical practice. Most have been modifications of or are similar to the Cardiovascular Health Study (CHS) criteria and have in common elements of slowness, weakness, and inactivity. Gait speed, like other measures of frailty, has been associated with elevated levels of CRP, IL-6 and tumor necrosis factor-α.[48] Many have found that slow gait speed (SGS) is most predictive of frailty and adverse outcomes, outperforming other more complex measures.[60],[66],[68] Gait speed has good inter-rater and retest reliability.[69] In fact, one professional organization has recommended its use as the measure of frailty in a task force statement.[19] The procedure for measuring gait speed is described in Table 3.
Table 3. Protocol for the 5-m gait speed test.
| *In an unobstructed area, position the patient with his/her feet behind, but just touching the 0 m start line |
| Instruct the subject to “walk at your comfortable pace” until a few steps past the 5 m mark (he/she should not start to slow down before reaching that mark) |
| Begin each trial on the word “Go” |
| Start the timer with the first footfall after the 0 m line |
| Stop the timer with the first footfall after the 5 m line |
| Allow sufficient time for recuperation between trials |
| Repeat three times and record the average |
| #Frailty is defined as an average time taken to walk the 5-meter course ≥ 6 s. |
*Walking aids such as canes and walkers may be used if needed; #Depending on the source, time cutoffs for frailty have varied between 5.0–7.7 s (0.65–1 m/s).
3.10. Gait speed and survival
The relationship between gait speed and survival was analyzed in a landmark meta-analysis of 9 studies (1986–2000) involving 34,485 community dwelling adults 65 years of age or older followed for from 6 to 21 years.[70] The subjects mean age was 73.5 years, 59.6% were women, and 79.8% were Caucasian. Their mean gait speed was 0.92 m/s. In all studies, gait speed was associated with survival (pooled HR per 0.1 m/s: 0.88, 95%CI: 0.87–0.90, P < 0.001). Survival increased across the range of gait speed, with significant increments per 0.1 m/s. At age 75, predicted 10 year survival across the range of gait speeds ranged from 19%–87% in men and 35%–91% in women.
Predicted survival based on age, sex, and gait speed was as accurate as that based on age, sex, use of mobility aids, and self-reported function, or age, sex, chronic conditions, smoking history, blood pressure, body mass index, and hospitalization.
Gait speed has also been associated with cardiovascular mortality specifically. For example, in the French 3 City Study, slow gait speed was associated with a threefold increase in cardiovascular mortality in 5 years (OR: 3.00, 95%CI: 1.65–5.57, n = 3208) but no difference in death from cancer or other causes.[63] Frailty in heart failure patients predicts mortality, as well as emergency department visits (92% increase) and hospitalizations (30%–65% increase).[71],[72]
3.11. Frailty in cardiology
Frailty is marked by impaired resistance to stressors, and cardiovascular events such as acute coronary syndromes, arrhythmias, and cardiac surgery (among others) are significant threats to maintaining homeostasis. For example, in a study of 309 elderly patients admitted to a cardiology service with severe coronary artery disease, slow gait speed as a surrogate for frailty was the strongest predictor of mortality at 6 months (OR: 3.8, 95%CI: 1.1–13.1).[66] In patients who had a recent ST segment elevation myocardial infarction (STEMI), adding reduced gait speed as a measure of frailty was independently associated with increased cardiovascular events (non-fatal myocardial infarction, ischemic stroke).[73] Frailty can help predict adverse events post PCI in stable coronary disease.[74] Frailty also increases the risk of incident heart failure,[65] and frail heart failure patients have increased mortality, hospitalizations and decreased quality of life.[75] Frailty has also been associated with subclinical cardiovascular disease such as prehypertension, left ventricular hypertrophy, and elevated carotid intima-media thickness.[61],[76]
3.12. Cardiac surgery in elderly
Older adults comprise approximately half of cardiac surgery patients, but suffer nearly 80% of the major complications and deaths following surgery.[77] Post-operative complications increase cost, reduce quality of life and affect long-term mortality. However, many elderly do well with cardiac surgery, benefit from enhanced survival and quality of life. Predicting which patients will do well and which may be at elevated risk for complications is difficult. Our current methods of mortality and morbidity estimation in cardiac surgery patients may not be ideal.
3.13. Frailty and pre-existing cardiac surgery risk scores
As noted, several risk models have been developed to predict operative risk and help guide patient selection as they are evaluated for cardiac surgery. Examples of risk scores include the ambler risk model, the Age, Creatinine, Ejection Fraction (ACEF) score,[78] and the Parsonnet score.[79] Perhaps the most commonly used scores are versions of the Society of Thoracic Surgeons (STS) score[77] and EuroSCORE.[80] These scores/models generally utilize demographic information, chronic and acute medical conditions, as well as information about the intended procedure. These models have been used extensively, but have their limitations. The predictions are derived from a population of patients at one point in time and then applied to patients who undergo the procedures subsequently, when surgical, anesthetic, and perioperative medical practice has changed, and thus need to be continually updated. Applying the models to a particular patient can be challenging. For example, certain combinations of procedures may not be addressed by certain models, and characteristics of the population that the measures were initially validated/developed on may not apply to the patient of interest. Several important factors are not addressed by current risk scores, including severe calcific atherosclerotic disease of the aorta, mitral annular calcification, pulmonary hypertension, nutritional status and frailty.
The STS score was based on 774,881 surgical procedures that took place between 2002 and 2006, and the registry includes data from nearly 90% of the cardiac surgery providers in the US. It provides an estimate of risk of mortality and major morbidity (stroke, renal failure, prolonged ventilation, deep sternal wound infection, and all cause re-operation). It addresses, isolated coronary artery bypass grafting (CABG), valve or combined CABG and valve surgeries.[77],[81],[82]
The EuroSCORE was developed using data from 1995 for estimating risk of in-hospital mortality.[80] It has been supplanted by the 2011 EuroSCORE II model based on data from 22,381 patients in 43 countries who had cardiac surgery in the summer of 2010.[83]
The ability of any of these scoring systems to accurately predict outcomes varies significantly. In particular, the original EuroSCORE has been noted to overestimate operative risk in robust elderly and underestimate it in frail ones with similar co-morbidities.[84]–[87] In other evaluations, the STS score has both over and under-estimated early post-operative mortality, and their predictive ability for long-term mortality has varied as well.[88],[89]
Although these scoring systems provide a place to start, as noted, they do not take important factors into account. Specifically, adverse events are not simply a result of manifest co-morbid diagnoses, but may come from an accumulation of subclinical impairments which may manifest as frailty. In addition, most scores were designed to predict mortality, not morbidity, and perform less well in this domain.[90],[91] Importantly, the so-called “eye test” for frailty often fails. It has been shown that chronologic age and low body mass index drive the “eye test,” whereas frail patients often actually have a high body mass index and excess central adiposity.[11],[92] Frailty, rigorously defined, has been proposed as a factor that can aid in predicting outcomes, and possibly aid in selecting treatments.
3.14. Frailty and cardiac surgery
Several studies have shown that frailty can predict mortality and morbidity after cardiac surgery. Using various frailty definitions and populations from 208–3826 patients, frailty was shown to predict: mortality (in-hospital and mid-term), prolonged institutional care, and the combined endpoint of death, myocardial infarction (MI), or stroke.[93]–[95] A study using the CHS scale and impairments in IADL and other factors in the Heart Center Leipzig cohort showed that frailty was associated with higher post-operative mortality.[95] Impairments in mobility and ADLs predicted post-operative mortality in the Maritime Heart Center Cardiac Surgery Registry.[94]
The frailty ABC's Study was a multicenter prospective cohort of 131 patients, ≥ 70 years of age who underwent CABG and/or valve replacement/repair via standard sternotomy (2008–2009).[96] A gait speed cutoff of 0.833 m/s (≥ 6 s needed to walk 5 m) was used to define slow gait speed based on analysis of receiver operator curves. Surgeons were blinded as to gait speed. The primary endpoint was mortality or major morbidity utilizing STS all cause death and major morbidity (stroke, renal failure, prolonged ventilation, deep sternal wound infection, and all cause re-operation).
Subjects in the study had a mean age of 75.8 ± 4.4 years, with 23% being ≥ 80 years. Thirty four percent were women and 46% had slow gait speed. Those with slow gait speed were more likely to be female (43% vs. 25%, P = 0.03), shorter (1.65 m vs. 1.69 m, P = 0.01), diabetic (50% vs. 28%, P = 0.01), and have more with one disability in IADL (48% vs. 18%, P < 0.0001).
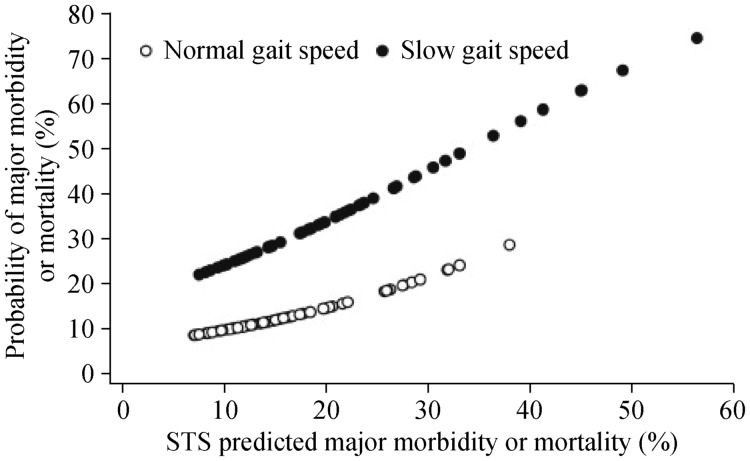
In a model, slow gait speed and Society of Thoracic Surgeons (STS) score both independently predicted the primary endpoint (OR: 3.05, 95%CI: 1.23–7.54), although model performance improved when gait speed was added. For a given STS predicted mortality or major morbidity (listed above), the predicted risk based on the model was 2–3 times greater in patients with slow gait speed vs. normal. (Figure 4). Notably, there was no correlation between gait speed and STS score (Figure 5). The dual risk factors of slow gait speed (≥ 6 s to walk 5 m) and high STS score (≥ 15% predicted mortality or major morbidity) identified patients at the highest risk. Among those with dual risk factors, 43% experienced major morbidity or mortality compared with only 5.9% of those without either risk factor. Subjects with high STS risk but normal gait speed and those with low STS risk but slow gait speed had intermediate probability of major morbidity or mortality (18.9% and 21.7%, respectively).[96]
Figure 4. Predicted probability of mortality or major morbidity according to Gait Speed and the Society of Thoracic Surgeons (STS) risk score.
Slow gait speed (solid circles) conferred a 2- to 3-fold increase risk for any given level of STS predicted mortality or major morbidity compared with normal gait speed (open circles). The adjusted odds ratio for mortality or major morbidity was 3.05 (95%CI: 1.23–7.54). Reproduced with permission, Elsevier.[96]
Figure 5. Lack of correlation between Gait Speed and the Society of Thoracic Surgeons (STS) risk Score, reflecting distinct domains.
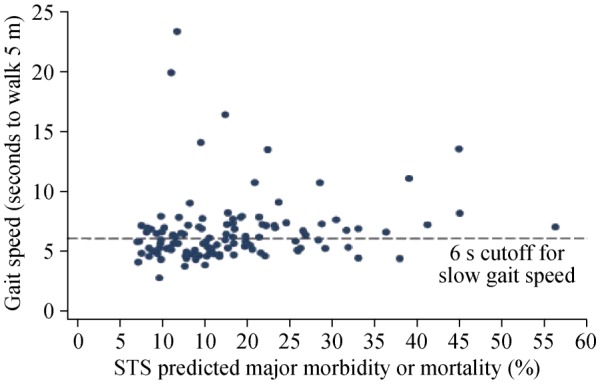
Lack of correlation between gait speed and STS score (R = 0.14, P = 0.13), showing that gait speed represents a distinct domain. In addition, there was no correlation between gait speed and age or left ventricular ejection fraction. Reproduced with permission, Elsevier.[96]
Thus, this study showed that 5 m gait speed incrementally predicted mortality and major morbidity, finding that slow gait speed was associated with a 2–3 fold increase in risk. Model performance improved when gait speed was added. In addition, slow gait speed also predicted discharge to a health care facility. An intriguing finding was that women and aortic valve replacement patients with slow gait speed may have been at particularly high risk.
3.15. Frailty and cardiac surgery study (FCSS) follow-up study
While the FCSS added an assessment of frailty to traditional cardiac surgery risk scores which focus on co-morbid conditions, the researchers hypothesized that the addition of disability to these other domains might further enhance patients risk stratification for morbidity and mortality. Thus after the recruitment of an additional 21 more subjects, an analysis that included an evaluation of four commonly used frailty scales,[97] three commonly used disability scales and five cardiac surgery risk scores was performed. These are shown in Table 4.
Table 4. Frailty, disability and cardiac surgery risk scores used in the FCSS follow-up study.[97].
| Frailty scales |
| 5-m gait speed |
| CHS frailty scale[5] |
| Expanded CHS frailty scale[27],[117] |
| MacArthur study of successful aging sub-dimensions[118] |
| Disability scales |
| Nagi scale [119] |
| Katz activities of daily living [120] |
| Older americans research and services instrumental activities of daily living [121] |
| Cardiac surgery risk scores |
| Revised Parsonnet score [79] |
| Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) [77] |
| Society of Thoracic Surgeons Predicted Risk of Mortality or Major Morbidity (STS-PROMM) [77] |
| Logistic EuroSCORE [80] |
| Age-Creatinine-Ejection Fraction (ACEF) score [78] |
Inclusion, exclusion criteria and study sites were unchanged, and basic subject characteristics were quite similar. With regard to the measures added in this study, the proportion of patients with any ADL disability was 5%, 32% for any IADL, and 76% for any Nagi disability.
In this analysis, gait speed was better than the other frailty scales (it was the only statistically significant measure) at predicting postoperative mortality or major morbidity, which is in keeping with prior studies.[27],[66],[68] Slow gait speed was associated with an increased OR of mortality or major morbidity of 2.63, 95%CI: 1.17–5.90), and had better predictive capacity compared to the other scales (AUC = 0.64, AIC = 154.1).
The Nagi scale outperformed the ADL and IADL scales, likely because patients who are unable to dress themselves or independently use a telephone are usually not referred for surgery. The Nagi scale contains impairments that are more prevalent in this population, such as difficulty climbing a flight of stairs. Only Nagi disability showed a statistically significant increase in risk (28% per impairment) (OR: 1.28, 95%CI: 1.06–1.54), and “fair” predictive ability (AUC = 0.65, AIC = 1.61.2), suggesting it may be a more clinically useful tool in this setting.
Of the five cardiac surgery risk scores, all were significant predictors of mortality or major morbidity, except the ACEF score. There was a trend suggesting that the Parsonnet (AUC = 0.72, AIC = 155.3) and the Society of Thoracic Surgeonse Predicted Risk of Mortality or Major Morbidity (STS-PROMM) score (AUC = 0.68, AIC = 166.5) scores had the best predictive ability. Of note, STS-PROMM was specifically designed to predict both mortality and major morbidity, while the others were designed to predict mortality, and these do not predict morbidity very well.[98],[99]
When combinations of scales were evaluated, a multivariable model combining one scale from each domain: 5 meter gait speed, Parsonnet and Nagi gave the greatest AUC (0.76 vs. 0.72 with Parsonet alone), suggesting this might be the optimal strategy for predicting the primary outcome. For a given level of risk adjusted for STS-PROMM and Parsonnet, 5 m gait speed ≥ 6 s and ≥ 3 Nagi impairments were incremental predictors of mortality or major morbidity.
Sensitivity analyses suggested that model performance was stable when sociodemographic and operative covariates were added, and interestingly, there was an apparent interaction between frailty and disability such that frailty (by gait speed) was most predictive when disability was not present (OR: 1.39 vs. 3.13, without disability), supporting the concept that frailty's effect is lessened when patients have progressed to disability on the proposed continuum.
In summary, both frailty and disability were complementary and additive to existing risk scores in predicting mortality or morbidity in elderly patients undergoing cardiac surgery. Specifically, a 5 m gait speed of at least six seconds (two times increased risk) and three or more Nagi scale impairments were each predictive of an increased risk of in hospital morbidity and mortality over that predicted by standard surgical risk scores.
3.16. Frailty and transcatheter aortic valve implantation (TAVI)
Frailty is common in patients with symptomatic severe aortic stenosis and TAVI was developed as a possible intervention for patients who were not candidates for surgical aortic valve replacement (AVR) due to concerns about their ability to tolerate and/or recover from the procedure due to comorbidities, and general health status. Frailty by the CHS (Fried) Scale was found in one-third of TAVI patients, in one study,[100] and was the strongest predictor of death, MI, stroke or heart failure at nine months. One single center study in TAVI patients showed that frail patients had higher one year mortality at one year and a trend towards more complications (including major bleeding, vascular complications and length of stay),[101] and another study showed increased functional decline at six months and major cardiac and cerebral events at one year.[102],[103]
3.17. Frailty and decision-making
Adding a frailty measure, in particular, gait speed, to traditional risk assessment tools and disability (e.g., Nagi) may provide better estimate of risk to patients prior to cardiac surgery or other procedures. It may help steer patients toward TAVI and other less invasive techniques (an approach that has yet to be proven) or identify patients who are at low risk for traditional surgical AVR.[104] It has already been shown that frail patients receive less aggressive care than non-frail ones, including less often being referred for intensive care unit admission, CABG, cardiac catheterization and are less often prescribed angiotensin converting enzyme inhibitors and beta-blockers.[66],[75],[105] Frailty assessment may also identify patients who might benefit from pre-, peri-, post-operation interventions.[104] Such interventions might include comprehensive geriatric assessment, intensive monitoring, early mobilization, and planned discharges to rehabilitation facilities. As noted, gait speed as a measure of frailty has many advantages over more complex evaluative tools, in that it appears to be simple, fast, reproducible, and inexpensive.
4. Frailty—the future
Future research in the area of frailty should include further delineating the underlying molecular pathophysiology of frailty and its relationship to cardiovascular disease.[106]–[108] With regard to measurement, understanding which measures are best used in which populations and clinical situations and validating cut-offs for diagnosis of frailty including slow gait speed (recognizing that frailty is a continuous condition), and integrating frailty and gait speed into existing models of risk and testing interventions.[104] In addition, understanding the relationship between frailty, disability and co-morbidity and their relevance in different conditions and populations is of importance. There are already ongoing studies evaluating frailty assessment measures in AVR, other cardiac surgery patients, and in patients with acute MI (CoreValve,[109] Frailty-AVR,[110] Partner II,[111] Silver AMI,[112],[113]).
5. Frailty—summary
Frailty is a common syndrome of late life vulnerability based on subclinical impairments in multiple organ systems. It shares both epidemiologic and pathophysiologic features with cardiovascular disease, principally inflammation. Frailty is associated with adverse outcomes. While there are multiple methods to measure or characterize it, gait speed may be the best measure of frailty. Gait speed is a powerful predictor of mortality, and when added to standard risk scores can help predict outcomes in cardiac surgery patients. Thus, frailty as assessed by gait speed may help direct patient evaluation and direct cardiovascular disease therapy, and may itself be a target for therapy. It seems that the Chinese proverb noted at the beginning of this review may be more accurate than Aesop's.
Acknowledgments
The author would like to thank Carla Griswold and her staff for her assistance with manuscript preparation.
References
- 1.Bureau USC. U.S. Census bureau projections show a slower growing, older, more diverse nation a half century from now, 2012. http://www.census.gov/newsroom/releases/archives/population/cb12-243.html (accessed on September 20, 2014)
- 2.Life expectancy, final 2007 data. http://www.cdc.gov/nchs/fastats/lifexpec.htm. Accessed November 5, 2014.
- 3.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2014 update: A report from the American heart association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walston J, Hadley EC, Ferrucci L, et al. Research agenda for frailty in older adults: Toward a better understanding of physiology and etiology: Summary from the american geriatrics society/national institute on aging research conference on frailty in older adults. J Am Geriatr Soc. 2006;54:991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 5.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 6.Ferrucci L, Guralnik JM, Studenski S, et al. Designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons: A consensus report. J Am Geriatr Soc. 2004;52:625–634. doi: 10.1111/j.1532-5415.2004.52174.x. [DOI] [PubMed] [Google Scholar]
- 7.Chin A, Paw MJ, Dekker JM, et al. How to select a frail elderly population? A comparison of three working definitions. J Clin Epidemiol. 1999;52:1015–1021. doi: 10.1016/s0895-4356(99)00077-3. [DOI] [PubMed] [Google Scholar]
- 8.Cigolle CT, Ofstedal MB, Tian Z, et al. Comparing models of frailty: The health and retirement study. J Am Geriatr Soc. 2009;57:830–839. doi: 10.1111/j.1532-5415.2009.02225.x. [DOI] [PubMed] [Google Scholar]
- 9.Walston J, McBurnie MA, Newman A, et al. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: Results from the cardiovascular health study. Arch Intern Med. 2002;162:2333–2341. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- 10.Cesari M, Leeuwenburgh C, Lauretani F, et al. Frailty syndrome and skeletal muscle: Results from the invecchiare in chianti study. Am J Clin Nutr. 2006;83:1142–1148. doi: 10.1093/ajcn/83.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barzilay JI, Blaum C, Moore T, et al. Insulin resistance and inflammation as precursors of frailty: The cardiovascular health study. Arch Intern Med. 2007;167:635–641. doi: 10.1001/archinte.167.7.635. [DOI] [PubMed] [Google Scholar]
- 12.Travison TG, Nguyen AH, Naganathan V, et al. Changes in reproductive hormone concentrations predict the prevalence and progression of the frailty syndrome in older men: The concord health and ageing in men project. J Clin Endocrinol Metab. 2011;96:2464–2474. doi: 10.1210/jc.2011-0143. [DOI] [PubMed] [Google Scholar]
- 13.Boirie Y. Physiopathological mechanism of sarcopenia. J Nutr Health Aging. 2009;13:717–723. doi: 10.1007/s12603-009-0203-x. [DOI] [PubMed] [Google Scholar]
- 14.Candore G, Caruso C, Jirillo E, et al. Low grade inflammation as a common pathogenetic denominator in age-related diseases: Novel drug targets for anti-ageing strategies and successful ageing achievement. Curr Pharm Des. 2010;16:584–596. doi: 10.2174/138161210790883868. [DOI] [PubMed] [Google Scholar]
- 15.Clegg A, Young J, Iliffe S, et al. Frailty in elderly people. Lancet. 2013;381:752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rochat S, Cumming RG, Blyth F, et al. Frailty and use of health and community services by community-dwelling older men: The concord health and ageing in men project. Age ageing. 2010;39:228–233. doi: 10.1093/ageing/afp257. [DOI] [PubMed] [Google Scholar]
- 17.Ensrud KE, Ewing SK, Taylor BC, et al. Frailty and risk of falls, fracture, and mortality in older women: The study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2007;62:744–751. doi: 10.1093/gerona/62.7.744. [DOI] [PubMed] [Google Scholar]
- 18.Shamliyan T, Talley KM, Ramakrishnan R, et al. Association of frailty with survival: A systematic literature review. Ageing Res Rev. 2013;12:719–736. doi: 10.1016/j.arr.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Abellan van Kan G, Rolland Y, Bergman H, et al. The I.A.N.A task force on frailty assessment of older people in clinical practice. J Nutr Health Aging. 2008;12:29–37. doi: 10.1007/BF02982161. [DOI] [PubMed] [Google Scholar]
- 20.de Vries NM, Staal JB, van Ravensberg CD, et al. Outcome instruments to measure frailty: A systematic review. Ageing Res Rev. 2011;10:104–114. doi: 10.1016/j.arr.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci. 2007;62:738–743. doi: 10.1093/gerona/62.7.738. [DOI] [PubMed] [Google Scholar]
- 22.Ensrud KE, Ewing SK, Taylor BC, et al. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med. 2008;168:382–389. doi: 10.1001/archinternmed.2007.113. [DOI] [PubMed] [Google Scholar]
- 23.Collard RM, Boter H, Schoevers RA, et al. Prevalence of frailty in community-dwelling older persons: A systematic review. J Am Geriatr Soc. 2012;60:1487–1492. doi: 10.1111/j.1532-5415.2012.04054.x. [DOI] [PubMed] [Google Scholar]
- 24.Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: Characterization in the women's health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61:262–266. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 25.Woods NF, LaCroix AZ, Gray SL, et al. Frailty: Emergence and consequences in women aged 65 and older in the women's health initiative observational study. J Am Geriatr Soc. 2005;53:1321–1330. doi: 10.1111/j.1532-5415.2005.53405.x. [DOI] [PubMed] [Google Scholar]
- 26.Cawthon PM, Marshall LM, Michael Y, et al. Frailty in older men: Prevalence, progression, and relationship with mortality. J Am Geriatr Soc. 2007;55:1216–1223. doi: 10.1111/j.1532-5415.2007.01259.x. [DOI] [PubMed] [Google Scholar]
- 27.Rothman MD, Leo-Summers L, Gill TM. Prognostic significance of potential frailty criteria. J Am Geriatr Soc. 2008;56:2211–2116. doi: 10.1111/j.1532-5415.2008.02008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210:901–908. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 29.Yao X, Hamilton RG, Weng NP, et al. Frailty is associated with impairment of vaccine-induced antibody response and increase in post-vaccination influenza infection in community-dwelling older adults. Vaccine. 2011;29:5015–5021. doi: 10.1016/j.vaccine.2011.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keysor JJ. Does late-life physical activity or exercise prevent or minimize disablement? A critical review of the scientific evidence. Am J Prev Med. 2003;25:129–136. doi: 10.1016/s0749-3797(03)00176-4. [DOI] [PubMed] [Google Scholar]
- 31.Province MA, Hadley EC, Hornbrook MC, et al. The effects of exercise on falls in elderly patients. A preplanned meta-analysis of the ficsit trials. Frailty and injuries: Cooperative studies of intervention techniques. JAMA. 1995;273:1341–1347. [PubMed] [Google Scholar]
- 32.Chou CH, Hwang CL, Wu YT. Effect of exercise on physical function, daily living activities, and quality of life in the frail older adults: A meta-analysis. Arch Phys Med Rehabil. 2012;93:237–244. doi: 10.1016/j.apmr.2011.08.042. [DOI] [PubMed] [Google Scholar]
- 33.Hunter GR, McCarthy JP, Bamman MM. Effects of resistance training on older adults. Sports Med. 2004;34:329–348. doi: 10.2165/00007256-200434050-00005. [DOI] [PubMed] [Google Scholar]
- 34.Miller ME, Rejeski WJ, Reboussin BA, et al. Physical activity, functional limitations, and disability in older adults. J Am Geriatr Soc. 2000;48:1264–1272. doi: 10.1111/j.1532-5415.2000.tb02600.x. [DOI] [PubMed] [Google Scholar]
- 35.Tenover JS. Androgen replacement therapy to reverse and/or prevent age-associated sarcopenia in men. Baillieres Clin Endocrinol Metab. 1998;12:419–425. doi: 10.1016/s0950-351x(98)80153-5. [DOI] [PubMed] [Google Scholar]
- 36.Ensrud KE, Blackwell TL, Cauley JA, et al. Circulating 25-hydroxyvitamin d levels and frailty in older men: The osteoporotic fractures in men study. J Am Geriatr Soc. 2011;59:101–106. doi: 10.1111/j.1532-5415.2010.03201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Devons CA. Comprehensive geriatric assessment: Making the most of the aging years. Curr Opin Clin Nutr Metab Care. 2002;5:19–24. doi: 10.1097/00075197-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Eng C, Pedulla J, Eleazer GP, et al. Program of all-inclusive care for the elderly (pace): An innovative model of integrated geriatric care and financing. J Am Geriatr Soc. 1997;45:223–232. doi: 10.1111/j.1532-5415.1997.tb04513.x. [DOI] [PubMed] [Google Scholar]
- 39.Mukamel DB, Peterson DR, Temkin-Greener H, et al. Program characteristics and enrollees' outcomes in the program of all-inclusive care for the elderly (pace) Milbank Q. 2007;85:499–531. doi: 10.1111/j.1468-0009.2007.00497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gill TM, Allore HG, Gahbauer EA, et al. Change in disability after hospitalization or restricted activity in older persons. JAMA. 2010;304:1919–1928. doi: 10.1001/jama.2010.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Counsell SR, Holder CM, Liebenauer LL, et al. Effects of a multicomponent intervention on functional outcomes and process of care in hospitalized older patients: A randomized controlled trial of acute care for elders (ACE) in a community hospital. J Am Geriatr Soc. 2000;48:1572–1581. doi: 10.1111/j.1532-5415.2000.tb03866.x. [DOI] [PubMed] [Google Scholar]
- 42.Walston JD, Fried LP. Frailty and its implications for care. In: Morrison RS, Meire DE, editors. Geriatric palliative care. New York, USA: Oxford University Press; 2003. p. 93. [Google Scholar]
- 43.Hubbard RE, Woodhouse KW. Frailty, inflammation and the elderly. Biogerontology. 2010;11:635–641. doi: 10.1007/s10522-010-9292-5. [DOI] [PubMed] [Google Scholar]
- 44.Leng SX, Xue QL, Tian J, et al. Inflammation and frailty in older women. J Am Geriatr Soc. 2007;55:864–871. doi: 10.1111/j.1532-5415.2007.01186.x. [DOI] [PubMed] [Google Scholar]
- 45.De Martinis M, Franceschi C, Monti D, et al. Inflammation markers predicting frailty and mortality in the elderly. Exp Mol Pathol. 2006;80:219–227. doi: 10.1016/j.yexmp.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 46.Bossoni S, Mazziotti G, Gazzaruso C, et al. Relationship between instrumental activities of daily living and blood glucose control in elderly subjects with type 2 diabetes. Age ageing. 2008;37:222–225. doi: 10.1093/ageing/afm158. [DOI] [PubMed] [Google Scholar]
- 47.Visser M, Pahor M, Taaffe DR, et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: The health abc study. J Gerontol A Biol Sci Med Sci. 2002;57:M326–M332. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- 48.Cesari M, Penninx BW, Pahor M, et al. Inflammatory markers and physical performance in older persons: The inchianti study. J Gerontol A Biol Sci Med Sci. 2004;59:242–248. doi: 10.1093/gerona/59.3.m242. [DOI] [PubMed] [Google Scholar]
- 49.Leng SX, Xue QL, Tian J, et al. Associations of neutrophil and monocyte counts with frailty in community-dwelling disabled older women: Results from the women's health and aging studies ? Exp Gerontol. 2009;44:511–516. doi: 10.1016/j.exger.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 50.Di Napoli M, Papa F. Villa Pini Stroke Data Bank Investigators. Inflammation, hemostatic markers, and antithrombotic agents in relation to long-term risk of new cardiovascular events in first-ever ischemic stroke patients. Stroke. 2002;33:1763–1771. doi: 10.1161/01.str.0000019124.54361.08. [DOI] [PubMed] [Google Scholar]
- 51.Mora S, Rifai N, Buring JE, et al. Additive value of immunoassay-measured fibrinogen and high-sensitivity C-reactive protein levels for predicting incident cardiovascular events. Circulation. 2006;114:381–387. doi: 10.1161/CIRCULATIONAHA.106.634089. [DOI] [PubMed] [Google Scholar]
- 52.Tzoulaki I, Murray GD, Lee AJ, et al. Relative value of inflammatory, hemostatic, and rheological factors for incident myocardial infarction and stroke: The edinburgh artery study. Circulation. 2007;115:2119–2127. doi: 10.1161/CIRCULATIONAHA.106.635029. [DOI] [PubMed] [Google Scholar]
- 53.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 54.Chevalier S, Gougeon R, Nayar K, et al. Frailty amplifies the effects of aging on protein metabolism: Role of protein intake. Am J Clin Nutr. 2003;78:422–429. doi: 10.1093/ajcn/78.3.422. [DOI] [PubMed] [Google Scholar]
- 55.Wolfe RR. Optimal nutrition, exercise, and hormonal therapy promote muscle anabolism in the elderly. J Am Coll Surg. 2006;202:176–180. doi: 10.1016/j.jamcollsurg.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 56.Morais JA, Ross R, Gougeon R, et al. Distribution of protein turnover changes with age in humans as assessed by whole-body magnetic resonance image analysis to quantify tissue volumes. J Nutr. 2000;130:784–791. doi: 10.1093/jn/130.4.784. [DOI] [PubMed] [Google Scholar]
- 57.Wu G. Amino acids: Metabolism, functions, and nutrition. Amino Acids. 2009;37:1–17. doi: 10.1007/s00726-009-0269-0. [DOI] [PubMed] [Google Scholar]
- 58.Boxer RS, Dauser DA, Walsh SJ, et al. The association between vitamin D and inflammation with the 6-minute walk and frailty in patients with heart failure. J Am Geriatr Soc. 2008;56:454–461. doi: 10.1111/j.1532-5415.2007.01601.x. [DOI] [PubMed] [Google Scholar]
- 59.Chaves PH, Semba RD, Leng SX, et al. Impact of anemia and cardiovascular disease on frailty status of community-dwelling older women: The women's health and aging studies I and II. J Gerontol A Biol Sci Med Sci. 2005;60:729–735. doi: 10.1093/gerona/60.6.729. [DOI] [PubMed] [Google Scholar]
- 60.Afilalo J. Frailty in patients with cardiovascular disease: Why, when, and how to measure. Curr Cardiovasc Risk Rep. 2011;5:467–472. doi: 10.1007/s12170-011-0186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Newman AB, Gottdiener JS, McBurnie MA, et al. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. 2001;56:M158–M166. doi: 10.1093/gerona/56.3.m158. [DOI] [PubMed] [Google Scholar]
- 62.Klein BE, Klein R, Knudtson MD, et al. Frailty, morbidity and survival. Arch Gerontol Geriatr. 2005;41:141–149. doi: 10.1016/j.archger.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 63.Dumurgier J, Elbaz A, Ducimetière P, et al. Slow walking speed and cardiovascular death in well functioning older adults: Prospective cohort study. BMJ. 2009;339:B4460. doi: 10.1136/bmj.b4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295:2018–2026. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 65.Khan H, Kalogeropoulos AP, Georgiopoulou VV, et al. Frailty and risk for heart failure in older adults: The health, aging, and body composition study. Am Heart J. 2013;166:887–894. doi: 10.1016/j.ahj.2013.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Purser JL, Kuchibhatla MN, Fillenbaum GG, et al. Identifying frailty in hospitalized older adults with significant coronary artery disease. J Am Geriatr Soc. 2006;54:1674–1681. doi: 10.1111/j.1532-5415.2006.00914.x. [DOI] [PubMed] [Google Scholar]
- 67.Afilalo J, Karunananthan S, Eisenberg MJ, et al. Role of frailty in patients with cardiovascular disease. Am J Cardiol. 2009;103:1616–1621. doi: 10.1016/j.amjcard.2009.01.375. [DOI] [PubMed] [Google Scholar]
- 68.Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314–322. doi: 10.1046/j.1532-5415.2003.51104.x. [DOI] [PubMed] [Google Scholar]
- 69.Munoz-Mendoza CL, Cabanero-Martinez MJ, Millan-Calenti JC, et al. Reliability of 4-m and 6-m walking speed tests in elderly people with cognitive impairment. Arch Gerontol Geriatr. 2011;52:e67–e70. doi: 10.1016/j.archger.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 70.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McNallan SM, Singh M, Chamberlain AM, et al. Frailty and healthcare utilization among patients with heart failure in the community. JACC Heart Fail. 2013;1:135–141. doi: 10.1016/j.jchf.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chaudhry SI, McAvay G, Chen S, et al. Risk factors for hospital admission among older persons with newly diagnosed heart failure: Findings from the cardiovascular health study. J Am Coll Cardiol. 2013;61:635–642. doi: 10.1016/j.jacc.2012.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Matsuzawa Y, Konishi M, Akiyama E, et al. Association between gait speed as a measure of frailty and risk of cardiovascular events after myocardial infarction. J Am Coll Cardiol. 2013;61:1964–1972. doi: 10.1016/j.jacc.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 74.Singh M, Rihal CS, Lennon RJ, et al. Influence of frailty and health status on outcomes in patients with coronary disease undergoing percutaneous revascularization. Circ Cardiovasc Qual Outcomes. 2011;4:496–502. doi: 10.1161/CIRCOUTCOMES.111.961375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lupon J, Gonzalez B, Santaeugenia S, et al. Prognostic implication of frailty and depressive symptoms in an outpatient population with heart failure. Rev Esp Cardiol. 2008;61:835–842. [PubMed] [Google Scholar]
- 76.Elbaz A, Ripert M, Tavernier B, et al. Common carotid artery intima-media thickness, carotid plaques, and walking speed. Stroke. 2005;36:2198–2202. doi: 10.1161/01.STR.0000181752.16915.5c. [DOI] [PubMed] [Google Scholar]
- 77.Shahian DM, O'Brien SM, Filardo G, et al. The society of thoracic surgeons 2008 cardiac surgery risk models: Part 1--coronary artery bypass grafting surgery. Ann Thorac Surg. 2009;88:S2–S22. doi: 10.1016/j.athoracsur.2009.05.053. [DOI] [PubMed] [Google Scholar]
- 78.Ranucci M, Castelvecchio S, Menicanti L, et al. Risk of assessing mortality risk in elective cardiac operations: Age, creatinine, ejection fraction, and the law of parsimony. Circulation. 2009;119:3053–3061. doi: 10.1161/CIRCULATIONAHA.108.842393. [DOI] [PubMed] [Google Scholar]
- 79.Bernstein AD, Parsonnet V. Bedside estimation of risk as an aid for decision-making in cardiac surgery. Ann Thorac Surg. 2000;69:823–828. doi: 10.1016/s0003-4975(99)01424-1. [DOI] [PubMed] [Google Scholar]
- 80.Nashef SA, Roques F, Michel P, et al. European system for cardiac operative risk evaluation (EuroSCORE) Eur J Cardiothorac Surg. 1999;16:9–13. doi: 10.1016/s1010-7940(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 81.Shahian DM, O'Brien SM, Filardo G, et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: Part 3--valve plus coronary artery bypass grafting surgery. Ann Thorac Surg. 2009;88:S43–S62. doi: 10.1016/j.athoracsur.2009.05.055. [DOI] [PubMed] [Google Scholar]
- 82.O'Brien SM, Shahian DM, Filardo G, et al. The society of thoracic surgeons 2008 cardiac surgery risk models: Part 2--isolated valve surgery. Ann Thorac Surg. 2009;88:S23–S42. doi: 10.1016/j.athoracsur.2009.05.056. [DOI] [PubMed] [Google Scholar]
- 83.Nashef SA, Roques F, Sharples LD, et al. EuroSCORE II. Eur J Cardiothorac Surg. 2012;41:734–744. doi: 10.1093/ejcts/ezs043. [DOI] [PubMed] [Google Scholar]
- 84.Nilsson J, Algotsson L, Hoglund P, et al. Comparison of 19 pre-operative risk stratification models in open-heart surgery. Eur Heart J. 2006;27:867–874. doi: 10.1093/eurheartj/ehi720. [DOI] [PubMed] [Google Scholar]
- 85.Dupuis JY. Predicting outcomes in cardiac surgery: Risk stratification matters? Curr Opin Cardiol. 2008;23:560–567. doi: 10.1097/HCO.0b013e32831217ed. [DOI] [PubMed] [Google Scholar]
- 86.Collart F, Feier H, Kerbaul F, et al. Valvular surgery in octogenarians: Operative risks factors, evaluation of euroscore and long term results. Eur J Cardiothorac Surg. 2005;27:276–280. doi: 10.1016/j.ejcts.2004.10.041. [DOI] [PubMed] [Google Scholar]
- 87.Chikwe J, Adams DH. Frailty: The missing element in predicting operative mortality. Semin Thorac Cardiovasc Surg. 2010;22:109–110. doi: 10.1053/j.semtcvs.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 88.Dewey TM, Brown D, Ryan WH, et al. Reliability of risk algorithms in predicting early and late operative outcomes in high-risk patients undergoing aortic valve replacement. J Thorac Cardiovasc Surg. 2008;135:180–187. doi: 10.1016/j.jtcvs.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 89.Ben-Dor I, Pichard AD, Gonzalez MA, et al. Correlates and causes of death in patients with severe symptomatic aortic stenosis who are not eligible to participate in a clinical trial of transcatheter aortic valve implantation. Circulation. 2010;122:S37–S42. doi: 10.1161/CIRCULATIONAHA.109.926873. [DOI] [PubMed] [Google Scholar]
- 90.Leontyev S, Walther T, Borger MA, et al. Aortic valve replacement in octogenarians: Utility of risk stratification with euroscore. Ann Thorac Surg. 2009;87:1440–1445. doi: 10.1016/j.athoracsur.2009.01.057. [DOI] [PubMed] [Google Scholar]
- 91.Speziale G, Nasso G, Barattoni MC, et al. Operative and middle-term results of cardiac surgery in nonagenarians: A bridge toward routine practice. Circulation. 2010;121:208–213. doi: 10.1161/CIRCULATIONAHA.108.807065. [DOI] [PubMed] [Google Scholar]
- 92.Gallagher D, Ruts E, Visser M, et al. Weight stability masks sarcopenia in elderly men and women. Am J Physiol Endocrinol Metab. 2000;279:E366–E375. doi: 10.1152/ajpendo.2000.279.2.E366. [DOI] [PubMed] [Google Scholar]
- 93.de Arenaza DP, Pepper J, Lees B, et al. Preoperative 6-minute walk test adds prognostic information to euroscore in patients undergoing aortic valve replacement. Heart. 2010;96:113–117. doi: 10.1136/hrt.2008.161174. [DOI] [PubMed] [Google Scholar]
- 94.Lee DH, Buth KJ, Martin BJ, et al. Frail patients are at increased risk for mortality and prolonged institutional care after cardiac surgery. Circulation. 2010;121:973–978. doi: 10.1161/CIRCULATIONAHA.108.841437. [DOI] [PubMed] [Google Scholar]
- 95.Sündermann S, Dademasch A, Praetorius J, et al. Comprehensive assessment of frailty for elderly high-risk patients undergoing cardiac surgery. Eur J Cardiothorac Surg. 2011;39:33–37. doi: 10.1016/j.ejcts.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 96.Afilalo J, Eisenberg MJ, Morin JF, et al. Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J Am Coll Cardiol. 2010;56:1668–1676. doi: 10.1016/j.jacc.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 97.Afilalo J, Mottillo S, Eisenberg MJ, et al. Addition of frailty and disability to cardiac surgery risk scores identifies elderly patients at high risk of mortality or major morbidity. Circ Cardiovasc Qual Outcomes. 2012;5:222–228. doi: 10.1161/CIRCOUTCOMES.111.963157. [DOI] [PubMed] [Google Scholar]
- 98.Kurki TS, Jarvinen O, Kataja MJ, et al. Performance of three preoperative risk indices; cabdeal, euroscore and cleveland models in a prospective coronary bypass database. Eur J Cardiothorac Surg. 2002;21:406–410. doi: 10.1016/s1010-7940(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 99.Xu J, Ge Y, Hu S, et al. A simple predictive model of prolonged intensive care unit stay after surgery for acquired heart valve disease. J Heart Valve Dis. 2007;16:109–115. [PubMed] [Google Scholar]
- 100.Ewe SH, Ajmone Marsan N, Pepi M, et al. Impact of left ventricular systolic function on clinical and echocardiographic outcomes following transcatheter aortic valve implantation for severe aortic stenosis. Am Heart J. 2010;160:1113–1120. doi: 10.1016/j.ahj.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 101.Green P, Woglom AE, Genereux P, et al. The impact of frailty status on survival after transcatheter aortic valve replacement in older adults with severe aortic stenosis: A single-center experience. JACC Cardiovasc Interv. 2012;5:974–981. doi: 10.1016/j.jcin.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schoenenberger AW, Stortecky S, Neumann S, et al. Predictors of functional decline in elderly patients undergoing transcatheter aortic valve implantation (TAVI) Eur Heart J. 2013;34:684–692. doi: 10.1093/eurheartj/ehs304. [DOI] [PubMed] [Google Scholar]
- 103.Stortecky S, Schoenenberger AW, Moser A, et al. Evaluation of multidimensional geriatric assessment as a predictor of mortality and cardiovascular events after transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2012;5:489–496. doi: 10.1016/j.jcin.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 104.Afilalo J, Alexander KP, Mack MJ, et al. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol. 2014;63:747–762. doi: 10.1016/j.jacc.2013.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ekerstad N, Swahn E, Janzon M, et al. Frailty is independently associated with short-term outcomes for elderly patients with non-ST-segment elevation myocardial infarction. Circulation. 2011;124:2397–2404. doi: 10.1161/CIRCULATIONAHA.111.025452. [DOI] [PubMed] [Google Scholar]
- 106.Rathore SS, Weinfurt KP, Foody JM, et al. Performance of the thrombolysis in myocardial infarction (TIMI) ST-elevation myocardial infarction risk score in a national cohort of elderly patients. Am Heart J. 2005;150:402–410. doi: 10.1016/j.ahj.2005.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Koller MT, Steyerberg EW, Wolbers M, et al. Validity of the framingham point scores in the elderly: Results from the rotterdam study. Am Heart J. 2007;154:87–93. doi: 10.1016/j.ahj.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 108.Rathore SS, Weinfurt KP, Gross CP, et al. Validity of a simple ST-elevation acute myocardial infarction risk index: Are randomized trial prognostic estimates generalizable to elderly patients? Circulation. 2003;107:811–816. doi: 10.1161/01.cir.0000049743.45748.02. [DOI] [PubMed] [Google Scholar]
- 109.Safety and efficacy study of the Medtronic CoreValve® System in the treatment of symptomatic severe aortic stenosis with significant comorbidities in very high risk subjects who need aortic valve replacement. http://clinicaltrials.gov/show/NCT01675440. Accessed November 5, 2014.
- 110.Frailty Assessment Before Cardiac Surgery & Transcatheter Interventions. http://clinicaltrials.gov/show/NCT01845207. Accessed November 5, 2014.
- 111.The PARTNER II Trial: Placement of AoRTic TraNscathetER Valves. http://clinicaltrials.gov/show/NCT01314313. Accessed November 5, 2014.
- 112.SILVER-AMI: Outcomes in older persons with heart attacks. http://clinicaltrials.gov/show/NCT01755052. Accessed November 5, 2014.
- 113.Society of thoracic surgeons adult cardiac surgery database data collection form version 2.8. http://www.sts.org/sites/default/files/documents/STSAdultCVDataCollectionFormV2_8.pdf. Accessed November 5, 2014.
- 114.Schwartz JB, Zipes DP. Cardiovascular disease in the elderly. In: Bonow RO, Mann DL, Zipes DP, Libby P, editors. Braunwald's heart disease: A textbook of cardiovascular medicine. Philadelphia, USA: Saunders/Elsevier, Inc.; 2012. [Google Scholar]
- 115.Afilalo J, Mottillo S, Alexander KP, et al. Identification of vulnerable elderly before cardiac surgery: A comparison of different survey scales. J Am Coll Cardiol. 2011;57:E1395–E1395. [Google Scholar]
- 116.Cacciatore F, Abete P, Mazzella F, et al. Frailty predicts long-term mortality in elderly subjects with chronic heart failure. Eur J Clin Invest. 2005;35:723–730. doi: 10.1111/j.1365-2362.2005.01572.x. [DOI] [PubMed] [Google Scholar]
- 117.Retornaz F, Monette J, Batist G, et al. Usefulness of frailty markers in the assessment of the health and functional status of older cancer patients referred for chemotherapy: A pilot study. J Gerontol A Biol Sci Med Sci. 2008;63:518–522. doi: 10.1093/gerona/63.5.518. [DOI] [PubMed] [Google Scholar]
- 118.Sarkisian CA, Gruenewald TL, John Boscardin W, et al. Preliminary evidence for subdimensions of geriatric frailty: The macarthur study of successful aging. J Am Geriatr Soc. 2008;56:2292–2297. doi: 10.1111/j.1532-5415.2008.02041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nagi SZ. An epidemiology of disability among adults in the united states. Milbank Mem Fund Q Health Soc. 1976;54:439–467. [PubMed] [Google Scholar]
- 120.Katz NM, Chase GA. Risks of cardiac operations for elderly patients: Reduction of the age factor. Ann Thorac Surg. 1997;63:1309–1314. doi: 10.1016/s0003-4975(97)00240-3. [DOI] [PubMed] [Google Scholar]
- 121.Fillenbaum GG, Smyer MA. The development, validity, and reliability of the oars multidimensional functional assessment questionnaire. J Gerontol. 1981;36:428–434. doi: 10.1093/geronj/36.4.428. [DOI] [PubMed] [Google Scholar]