Abstract
Heart failure (HF), a complex clinical syndrome due to structural or functional disorder of the heart, is a major global health issue, with a prevalence of over 5.8 million in the USA alone, and over 23 million worldwide. As a leading cause of hospitalizations among patients aged 65 years or older, HF is a major consumer of healthcare resources, creating a substantial strain on the healthcare system. This paper discusses the epidemiology of HF, financial impact, and multifaceted predicaments in end-stage HF care. A search was conducted on the U.S. National Library of Medicine website (www.pubmed.gov) using keywords such as end-stage heart failure, palliative care, ethical dilemmas. Despite the poor prognosis of HF (worse than that for many cancers), many HF patients, caregivers, and clinicians are unaware of the poor prognosis. In addition, the unpredictable clinical trajectory of HF complicates the planning of end-of-life care, such as palliative care and hospice, leading to underutilization of such resources. In conclusion, ethical dilemmas in end-stage HF are numerous, embroiling not only the patient, but also the caregiver, healthcare team, and society.
Keywords: Ethical dilemmas, Heart failure, Palliative care
1. Introduction
Heart failure (HF) is defined as a complex clinical syndrome resulting from structural or functional disorders of the heart.[1] The current HF classification scheme emphasizes both the development and progression of the disease by identification of four stages.[2] Stage A includes those patients at risk for HF but without structural heart disease or HF symptoms (e.g., patients with diabetes mellitus, hypertension, or coronary artery disease); stage B comprises those patients who are asymptomatic but have structural heart disease (e.g., left ventricular hypertrophy or impaired left ventricular function); stage C consists of those patients with structural heart disease with current or prior heart failure symptoms (this stage accounts for the majority of HF patients); and stage D, which encompasses patients with refractory HF requiring advanced specialized treatments such as mechanical circulatory support, fluid removal procedures such as ultrafiltration, continuous inotropic infusions, heart transplant, experimental surgery or drugs, and end-of-life care or hospice.[2]
Although stage D HF patients account for 5% of the HF population,[3] and may benefit from palliative care consultation, the importance of palliative care was only acknowledged and incorporated into clinical guidelines in 2005.
2. Epidemiology
The elderly population (65 years or older) are the primary victims of HF, and comprise 80% of patients hospitalized with HF.[1] The incidence of HF has steadily increased with the aging population, and as of 2012, 2.4% of the US population was reported to have HF.[4] It is projected that HF prevalence will increase by 23%, from 2.42% to 2.97% in 2030, affecting more than 8 million Americans.[4] Of these 8 million Americans, 2 million will be over the age of 80 years, accounting for more than 26% of all HF patients.[4]
Although HF is a progressive, incurable condition with an unpredictable clinical trajectory,[5],[6] many patients have an inaccurate perception of HF as a benign condition compared to cancer,[7] with limited understanding of their poor prognosis.[8],[9] In fact, in 2004, deaths attributed to heart failure numbered 284, 365, exceeding the combined death toll for lung cancer, breast cancer, prostate cancer, and HIV/AIDS.[10] This prevailing ignorance of patients and caregivers about their impending death is compounded by the reluctance of healthcare providers to acknowledge the terminal nature of HF,[9] with subsequent failure to assist patients and their caregivers in end-of-life planning.
3. Prevalence
HF is a major global health issue, with a prevalence of over 5.8 million in the USA alone, and over 23 million worldwide.[11] Listed on the death certificates in 1 out of 9 Americans,[12] HF is a leading cause of hospitalizations among patients aged 65 years or older.[13] As such, HF is a major consumer of healthcare resources, creating a substantial strain on the healthcare system.
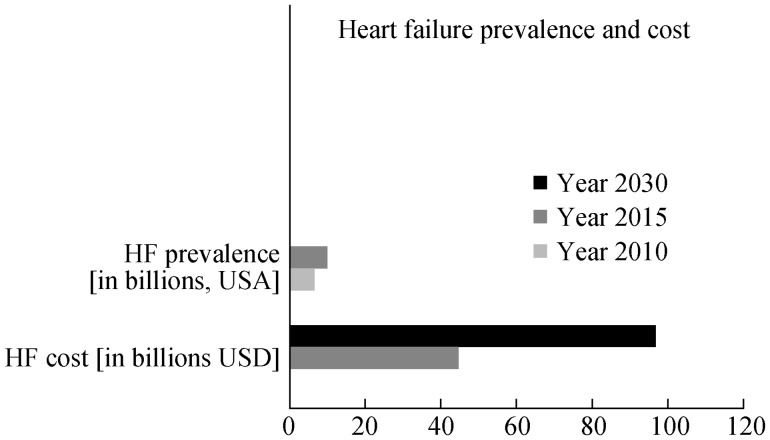
Based on the National Health & Nutrition Examination Survey (NHANES) data from 2005–2008, in the United States alone, approximately 5.7 million people ages 20 years or older were afflicted with HF, with a projected increase in HF prevalence to 6.6 million among US adults ages 18 years or older in the year 2010.[14] By the year 2030, it is predicted that an additional 3 million people will develop HF (see Figure 1).[14] Based on data from the Framingham Heart study, the lifetime risk of developing HF at age 40 years is 1 in 5, regardless of gender.[14] After age 65 years, HF incidence is estimated at 10 per 1000 population.[14]
Figure 1. Heart failure (HF) prevalence in 2010 was estimated at 6.6 million, with projected increase to 9.6 million in year 2030.
Concurrently, the cost of HF has skyrocketed from an estimated 44.6 billion dollars in 2015 to a projected 97 billion dollars in year 2030.
In 2008, HF was a contributing cause in more than 280,000 deaths (approximately 1 in 9).[14] HF is the primary cause of mortality in more than 55,000 Americans each year,[15] with an estimated 50% of HF patients dying within 5 years of the diagnosis.[14]
4. Financial burden
In addition to the lives lost, HF is a costly disease, costing the USA more than $34.4 billion dollars each year in health care services, medications, and lost productivity.[16] The total cost of HF in the USA is projected to grow from 44.6 billion dollars in 2015, to 97 billion dollars by the year 2030 (see Figure 1).[14]
Although not viewed in the same fearful context as cancer, heart failure is a progressive and incurable condition with an unpredictable clinical trajectory. That is, some cancers are curable and the clinical development is somewhat predictable in terminal cancers, thereby facilitating end-of-life planning. In contrast, the clinical course of HF is unpredictable, rendering it difficult to plan end-of-life care. In addition, many patients, their caregivers, and clinicians fail to recognize the terminal, progressive nature of HF. In Canada, although cancer accounts for 25% of deaths, 90% of cancer patients receive palliative or end-of-life care compared to patients with cardiovascular disease, which accounts for over one third of deaths, but utilize a disproportionately smaller proportion of palliative care resources.[6] Similarly, in the US, while almost 50% of patients enrolled in hospice have cancer as a primary diagnosis, only 12.2% have a primary diagnosis of cardiac disease, reflecting underutilization of hospice among HF patients.[10]
Kirkpatrick, et al.[17] reported that the prognosis of the HF patient is poor, worse than that for many cancers, yet many HF patients, caregivers, and clinicians are unaware of the poor prognosis. Acute HF is the leading medical cause of hospitalization among people aged 65 years or older in the United States, European countries, Australia, and New Zealand, with in-hospital mortality rates ranging from 2% to 20%.[18] Of those who survive to hospital discharge, 55% of hospitalized HF patients are readmitted within 6 months.[19] Furthermore, 30-day readmission rates are high, with HF being the most frequent diagnosis for hospital readmission.[20]
An analysis of 2007–2009 Medicare fee-for-service claims revealed that the median time to 30-day readmission was 12 days for those patients initially hospitalized for HF.[21] Despite a reduction in risk-adjusted 1-year mortality (decreased from 31.7% in 1999 to 29.6% in 2008; P < 0.001), 1-year mortality remained high (about 30%) for medicare beneficiaries 1998–2008, hospitalized with HF.[22] Thus, the initial hospitalization for HF should alert providers to initiate end-of-life care planning, if not already started. These HF hospitalizations and frequent readmissions with early mortality should be a harbinger to patients, caregivers, and providers about the need for end-of-life care planning. It is reported that 38% of patients will die within the first year of diagnosis of heart failure, while another 60% will die within 5 years.[5] There is a 30% mortality among heart failure patients within the year following the first hospitalization for heart failure, but mortality may be as high as 60% among those patients with multiple comorbidities.[6]
The lifetime prevalence of heart failure for all adults is 15%–20%, and increases to over 20% prevalence among octogenarians.[23] In addition, with advances in heart failure technologies (including pharmacologic agents, device therapies, mechanical assist devices, etc), and advances in coronary revascularization (drug-eluting stents and complex percutaneous coronary interventions, etc), more patients are surviving acute myocardial infarctions and living longer, but going on to develop heart failure. The primary mode of death among HF patients is variable, according to NYHA functional class. Patients with NYHA class II symptoms are at a proportionally higher risk of sudden cardiac death while those with NYHA class IV symptoms have a one-year mortality as high as 75% with a significantly higher risk of dying of progressive heart failure characterized by worsening shortness of breath, orthopnea, hypotension, and decreasing level of consciousness.[24]
Since 50% of patients died suddenly from arrhythmias and acute ischemic events,[25] implantable cardiac defibrillators (ICDs) are recommended for primary prevention of sudden cardiac death. Although many patients preferred a “do not resuscitate” (DNR) status as death became more imminent, their perceived quality of life did not diminish significantly, with 29% to 58% of patients reporting good to excellent quality of life in all intervals before death.[26] In contrast, progressive pump failure is a more common mode of death in advanced heart failure,[25],[27] with these patients experiencing disabling symptoms such as fatigue, shortness of breath, and limited mobility, leading to restricted socialization, complicated medication regimens, and poor quality of life, as well as exerting a significant toll on the caregivers, as well as on the healthcare resources.
Compared with HF patients with reduced ejection fraction (HFREF), cardiovascular deaths, sudden death, and HF deaths are lower in patients with HF with preserved ejection fraction (HFPEF).[28] Nonetheless, mortality remains high in HFPEF patients, with non-cardiovascular deaths responsible for the majority of deaths in HFPEF.[28],[29] In addition, HFPEF is actually more common than HFREF, with an escalating incidence and prevalence in those aged 70 years or older.[30] Thus, all HF patients would benefit from early referral to palliative care but lack of awareness and understanding by both patients/caregivers and clinicians limit utilization of this much-needed resource in HF management.
5. Palliative care, hospice and end-of-life planning
Despite being used interchangeably by many healthcare providers, palliative care, hospice, and end-of-life planning are not synonymous terms.
Palliative care, as defined by the World Health Organization (WHO), is an approach that improves the quality of life of patients, who are faced with life-threatening illnesses. This approach focuses not only on symptom alleviation, but is holistic in its approach by addressing physical, psychosocial and spiritual needs of patients. Such model is applicable early in the course of a disease and can be applied in conjunction with life-prolonging therapies.[31] Palliative care is an active process with a multidisciplinary, holistic approach which strives to address physical, emotional, psychological and spiritual aspects of care while offering a support system to both patients and their caregivers.[32]
Hospice, in contrast to palliative care, is a passive process, and focuses on symptom management in patients with a prognosis of six months or less.[32] Thus, while hospice care is based on patient prognosis, palliative care is based on patient and family/caregiver needs, independent of the prognosis which may be greater than six months. Furthermore, palliative care patients may continue life-prolonging therapies, but hospice patients must agree to forego curative or life-prolonging medical treatment.[10]
The goal of palliative care includes symptom alleviation and commonly is associated with patients with terminal cancer.[33] In contrast, end-of-life care encompasses not only symptom management, but also includes hospice care, advanced directives and advanced care planning.[34] Consequently, end-of-life care is now “mainstream” and an integral part of the process of heart failure care.[35] End-of-life care refers to a process incorporating advance directives (i.e., living wills, healthcare power of attorney) as well as preparation for end of life, including a discussion of death. End of life planning is not defined by a specific timeframe and address numerous transitions: physical, emotional, spiritual, financial, as well as respect for choice (patient or proxy) at the end of life.[36]
6. End-of-life care dilemmas in HF
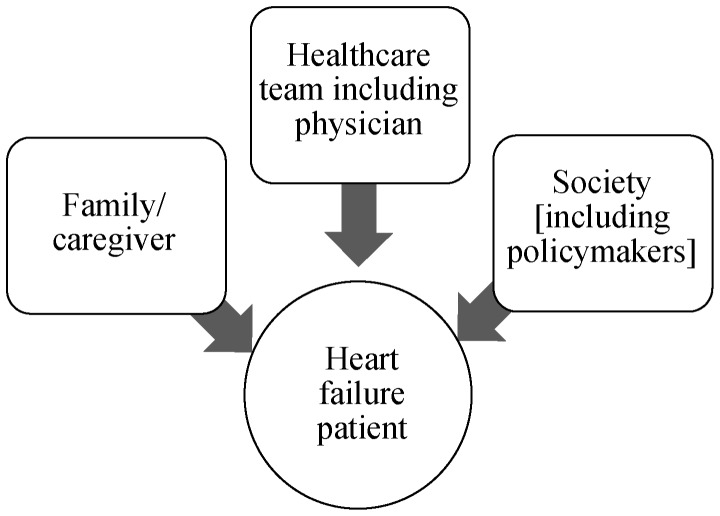
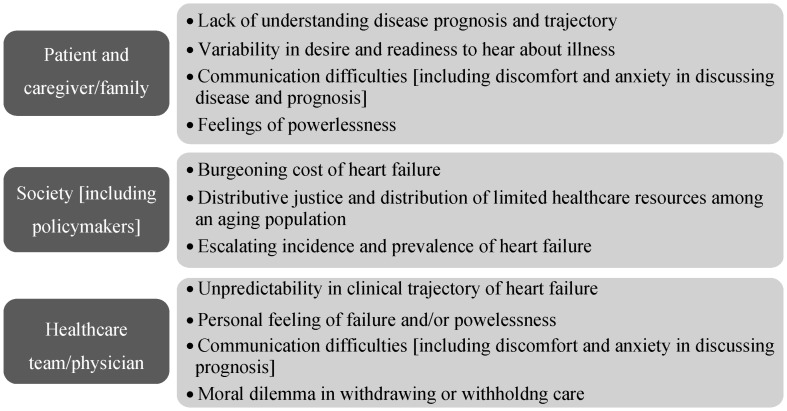
End-of-life care dilemmas are numerous and vary according to the stakeholder (Figures 2 & 3). Patients, their caregivers, clinicians, and policymakers/society each have their own agenda and perspective on end-of-life care in HF, and these perspectives are often incongruent among the stakeholders. For example, the end stage HF patient may wish to pursue aggressive therapies to prolong life, while the clinician, in order to avoid conflict, or to avoid taking away hope, may agree to provide such therapies. At the other end, policymakers and society may view such therapies as an unnecessary financial burden to society, with cost-effectiveness of such therapies and unequal allocation of finite healthcare resources as significant considerations. Ethicists and democratic proponents may have their own perspective and view such medical therapies as distributive justice, examples of which include public programs that provide social security or medical care to all elderly and retired persons.[37]
Figure 2. Stakeholders in end-of-life care dilemmas in heart failure, with the heart failure patient being the central stakeholder.
Figure 3. Dilemmas of various stakeholders in the care of the heart failure patient.
One of the most prominent dilemmas at end of life in HF is represented by poor or ineffective communication (for various reasons) between patients/caregivers and their clinical providers. This may be related to discomfort for both parties in addressing the terminal, progressive nature of the disease, as well as discussing end-of-life care planning. Other issues include lack of adequate training and education in the discussion of end-of-life, as well as discerning the timing of when to broach the subject of end-of-life planning.[5] The deactivation of ICDs and left ventricular assist devices (LVADs) poses another challenging quandary in care of the end stage HF patient.
Other dilemmas involve the ethical and medico-legal issues associated with deciding which patients are appropriate candidates for which therapies, provision of maximal possible care or withholding such therapies.[38] There is clinician or therapeutic inertia [failure of the clinician to titrate therapy when the treatment goals are unmet;[39] resource limitations, including limited organ availability for heart transplant, patient compliance (such as continued smoking or substance abuse), and the lack of adequate psycho-social support for the patient].[38] Another essential, though often overlooked aspect is the financial burden placed on patients and on society when dealing with a chronic condition such as organ transplantation,[38] device therapies (such as ICDs, cardiac resynchronization therapy devices-with pacemakers only/CRT-P, and cardiac resynchronization therapy devices-with defibrillators/CRT-D) and mechanical circulatory assist systems (such as LVADs as destination therapy or bridge to transplant), all of which requires continuous medical care, diagnostic assessment, and long-term care with regular interval follow-up visits.
Although the financial burden is an uncomfortable topic when discussing clinical care of patients with advanced heart failure, the cost effectiveness of technologies in heart failure is a necessary topic of discussion and consideration in many countries with a public healthcare system, as well as for countries such as the United States, with both private insurance systems and public healthcare systems, including Medicare and Medicaid.
Between 2005 and 2009, the incidence of orthotopic heart transplantation increased only marginally, whereas the annual left ventricular assist device implantation rates almost tripled. This rapid escalation in LVAD implantation rates was accompanied by a significant increase in healthcare expenditures, as the cumulative left ventricular assist device cost increased 232% within 5 years (from $143 million to $479 million). The impact of such inflation in healthcare expenses was shouldered to a great extent by taxpayers since Medicare and Medicaid were the primary payers for nearly one half of all patients (orthotopic heart transplantation, 45%; left ventricular assist device, 51%) by 2009.[40] Thus, cost-effectiveness, distributive justice, and allocation of finite resources are important factors when utilization of these technologies is considered.[41]
Based on the physician clinical model, these providers may have a treatment imperative which fosters pursuing more aggressive therapy, even when clinically inappropriate in end-stage heart failure. Clinicians often feel a sense of failure, and both clinicians and patients may fail to accept the limitations of medicine and the available treatments. The uncertain and unpredictable disease trajectory of heart failure is another obstacle in planning end-of-life care for patients in their caregivers.
7. Dilemmas from the patient and caregiver perspective
In an interview study of 64 caregivers at 6 months after the patient's death, there were common themes regarding end of life planning issues: (1) lack of availability of treatment options for certain patients, (2) changes in preferences at the very end of illness, (3) variability in patient and caregiver desire for and readiness to hear information about the patient's illness, and (4) difficulties with patient–caregiver communication.[42] Patients and their caregivers may fail to raise end-of-life issues for various reasons including: lack of understanding their condition; unpredictability of the clinical trajectory of HF; discomfort and anxiety in raising end-of-life discussions; and a feeling of powerlessness while viewing clinicians as unapproachable or reluctant to give out information.[5]
In a study of patient preferences regarding end-of-life treatment in advanced HF, two distinct groups of patients were identified: one group preferred life-prolonging treatments, while the other group favoring strategies that improved quality of life despite reduced survival time. As treatment preferences were independent of functional or symptom status, this may present an important opportunity for clinicians to discuss such treatment preferences early in the course of illness and help facilitate end-of-life planning.[43]
Patients with HF often have a poor understanding of their condition and prognosis. A review of the literature identified three common themes or areas of need: education to enhance professional communication skills, communication to improve patient understanding, and communication skills to facilitate advance care planning.[44]
Patients and families are frequently unaware about the imminence of death in end-stage HF during the last few days or hours preceding their demise.[9] Bereaved family members of HF patients who succumbed to non-sudden cardiac deaths reported that they received minimal communication about what to expect, and only 1/3 reported being aware of a poor prognosis, while only 8% of patients and 44% of family members were informed about the limited remaining time, with 36% of these patients ending up dying alone.
Pain and other symptoms such as dyspnea and edema, as well as progressive and profound fatigue, may also hinder the patient's ability to engage in end of life care discussions. HF patients report a high prevalence of pain (63%–80%), anxiety (49%) and breathlessness (60%–88%), with rates similar to those for advanced cancers and AIDS.[7]
In a qualitative and open unstructured interview of advanced HF patients, four main themes emerged: “Living in the Shadow of Fear; Running on Empty; Living a Restricted life; and Battling the System”, with patients describing their experience as a “fearful and tired sort of living”, characterized by growing dependence and powerlessness.[45]
Deactivation of ICDs, CRT-Ds, and LVADs may also create a conflict for patients and their caregivers. In 2010, the Heart Rhythm Society (HRS) published a consensus statement in collaboration with other professional societies, including the American Academy of Hospice and Palliative Medicine. The document, reporting that only few physicians discuss device deactivation with their heart failure patients, attempts to educate providers about paramount issues, such as legal, ethical and religious principles, withdrawal of life-sustaining therapies (including device deactivation in patients who chose this option) and importance of proactive communication with the patients nearing end of life in order to minimize fear, anxiety and suffering. In addition, such document provides the caregivers with a clinical guide to help managing the request for device deactivation.[46]
In a study of internal medicine physicians and internal medicine subspecialists at Beth Israel Deaconess Medical Center, an academic tertiary care center in Boston, Massachusetts, physicians (surveyed via an anonymous email link survey) were consistently less comfortable discussing cessation of pacemakers and ICDs compared to other life-sustaining therapies (P < 0.005), such as withdrawal or removal of mechanical ventilation, dialysis and feeding tubes.[47]
Patients with LVADs, despite improved survival and potential for improved quality of life due to symptomatic improvement, may experience not only complications, such as recurrent infections, but also reduced quality of life in terms of frequency of follow-up and monitoring. Open, honest communication regarding the impact of LVAD on daily life, as well as prognosis of HF and end-of-life planning are imperative prior to implantation.[48]
8. Clinician/healthcare provider dilemma
Ineffective communication or lack of communication skills are recognized hindrances in raising end-of-life discussions.[44] In addition, physicians are faced with a moral and ethical dilemma of withdrawal of life-sustaining therapies in a model in which they are more accustomed to rescue and prolong life. Thus, some may incorrectly perceive device deactivation as physician-assisted suicide or euthanasia. However, the HRS consensus statement provides clarification about the differences between device deactivation and extreme measures.[46]
Although clinical guidelines now exist for recommendation for end-of-life considerations in HF,[1] they do not address the timing of when to refer end-stage HF patients for non-hospice palliative care or hospice care.[10] Since HF is characterized by unpredictable decompensations and improvements, the clinical trajectory makes timing of referrals difficult to determine, and may also lead patients and caregivers to have unrealistic expectations that, having survived and recovered from an episode of HF exacerbation, they see no reason why they should not recover again from future episodes. Thus, prognostication in HF is difficult due to the unpredictable course of the disease, which makes it even more important to discuss palliative care and end-of-life care early in the disease process.[49] Furthermore, there is a lack of research regarding the best models to implement for palliative care and end-of-life care provision in HF patients.[7]
9. Society/policymaker dilemma
Despite the documented benefits (i.e., improved survival and symptomatic improvement) of advanced technologies, such as ventricular assist devices and cardiac resynchronization therapy for treatment of HF, the costliness of such therapies is of significant concern to policymakers. Cost-effectiveness and distributive justice will be important factors in the policy decision-making for implementation of such technologies, as many countries cannot afford the financial burden imposed by these technologies.[41]
Current medicare hospice benefit limits include, for example, a 6-month prognostication to death as a prerequisite to enrollment, a forced choice between skilled care and hospice care for Medicare patients entering nursing homes from hospitals, and limitations on the availability of therapies.[36]
Prolongation of the final stages of HF exerts a toll on patients, caregivers, medical system and society. It is reported that more than 25% of Medicare spending occurs in the last year of life. Furthermore, the costs of care during the last 6 months for a HF patient has steadily increased with a projection estimating that, by the year 2030, each US adult will pay on average $244 per year to care for the 10 million patients with HF.[4]
10. Practical considerations
Healthcare providers who care for patients with end-stage HF need to examine their own attitudes and beliefs about terminal illness and death in order to effectively advocate for patients in the final stages of heart failure. In particular, physicians, who are at the helm in regards to continuation of care and resuscitation decisions, exert a significant impact on patients' end of life care, even in the presence of advance directives. In a physician survey study examining the impact of external factors and perceived patients' preferences on physicians' decisions to honor or forgo patients' advance directives, the majority of physicians (59%) reported that they would resuscitate a patient in ventricular fibrillation despite the known existence of an advance directive requesting no resuscitation.[50]
Likewise, a recent 2013 survey of physicians at academic medical centers exploring their personal resuscitation preferences and attitudes towards advance directives found that while the vast majority of physicians (88.3%) chose the do-not-resuscitate status for themselves if diagnosed with a terminal illness, many physicians continue to aggressively pursue futile treatments for their terminally ill patients.[51] Besides awareness of one's own biases, the clinician should meet with the individual patient to discuss the patient's wishes and set realistic expectations at the initial meeting or diagnosis, and revisit such wishes and goals of care at regular intervals, especially when there is a change in the patient's clinical status. This personalized acknowledgement of the patient's right to self-determination is important to ensure that the patient's advance directives will be upheld.
Furthermore, a recent task force identified that early discussion between patients and their clinician was not only cost-effective (with reduced utilization of futile, non-beneficial medical care at end of life), but more importantly, with better quality of life and positive family outcomes.[52]
Early involvement of the multidisciplinary team is essential to develop trust between the patient and their team members, as well as facilitate timely interventions to assist patients and their caregivers. A thorough assessment of the patient with end-stage HF should incorporate evaluation of (a) the patient's psychosocial support (e.g., who drives the patient to his/her appointments? Does the patient live alone? Who helps to prepare meals and assist the patient with activities of daily living and personal hygiene?); (b) financial resources (medical insurance; medication prescription coverage; hospice coverage; co-payments); (c) the patient's emotional state and well-being; and (d) spiritual and religious needs. Holistic care is critical, and requires coordination of the entire multidisciplinary team. Consultations with the social worker (for assistance with living wills, advance directives, exploring available resources) and with palliative care team if available (to help identify the patient's expectations, needs, pain and symptom management) are indispensable in the end of life care for the HF patient. Furthermore, these team members may ease the personal discomfort that many physicians feel when faced with the situation of “giving up the fight”. Death is inevitable for all, but helping patients and family members have closure at the terminal stages of the disease is treating not only the body, but the soul, and allows patients to “go gentle into that good night”.
11. Conclusions
Prevention of HF appears to be the most logical approach to curtail costs of HF care. Control of risk factors, such as hypertension, may help reduce HF development. Lifetime risk for CHF doubled for subjects with blood pressure ≥ 160/100 mmHg vs. < 140/90 mmHg. In those patients without an antecedent myocardial infarction (MI), at age 40 years, the lifetime risk for CHF was 11.4% (95%CI: 9.6%–13.2%) for men and 15.4% (95%CI: 13.5%–17.3%) for women. Thus, the lifetime risk for developing HF (in patients without MI) is one in nine for men and one in six for women, thereby highlighting the fact that CHF is largely attributable to hypertension.[23] Risk factor modification and prevention of HF require understanding the risk factors for the two types of HF: ischemic heart disease is the predominant risk factor for HFREF, while hypertension, atrial fibrillation and diabetes are the main risk factors for HFPEF.[53]
Through adherence to clinical guidelines (including appropriate criteria use), optimization of medical therapy, empowerment and accountability of patients/caregivers in healthcare management, as well as reduction of clinician inertia, the incidence of HF may decrease, with subsequent reduction in healthcare costs. Patient involvement and accountability, including weight loss, smoking cessation, and lifestyle modification, will be essential for a successful outcome. In the meantime, HF prevalence continues to grow and patients, their caregivers, and clinicians must become more educated and proactive in navigation of end-of-life care, in order to minimize the patient's fears and suffering, as death becomes imminent. Life is a continuum, with death being the end of the journey. Yet, few are comfortable in accepting the inevitable. In the end, the physician's moral and ethical imperative is primum non nocere (“first, do not harm”), which extends to the terminal patient and may entail advocating for his death.
References
- 1.Hunt SA, Abraham WT, Chin MH, et al. American College of Cardiology; American Heart Association Task Force on Practice Guidelines; American College of Chest Physicians; International Society for Heart and Lung Transplantation; Heart Rhythm Society ACC/AHA 2005 Guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (writing committee to update the 2001 guidelines for the evaluation and management of heart failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 2.Jessup M, Abraham WT, Casey DE, et al. 2009 focused update: ACCF/AHA Guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 3.Costanzo MR, Mills RM, Wynne J. Characteristics of “Stage D” heart failure: insights from the acute decompensated heart failure national registry longitudinal module (ADHERE LM) Am Heart J. 2008;155:339–347. doi: 10.1016/j.ahj.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 4.Heidenreich PA, Albert NM, Allen LA, et al. American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barclay S, Momen N, Case-Upton S, et al. End-of-life care conversations with heart failure patients: a systematic literature review and narrative synthesis. Br J Gen Pract. 2011;61:e49–e62. doi: 10.3399/bjgp11X549018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howlett J, Morin L, Fortin M, et al. End-of-life planning in heart failure: it should be the end of the beginning. Can J Cardiol. 2010;26:135–141. doi: 10.1016/s0828-282x(10)70351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selman L, Harding R, Beynon T, et al. Improving end-of-life care for patients with chronic heart failure: “Let's hope it'll get better, when I know in my heart of hearts it won't”. Heart. 2007;93:963–967. doi: 10.1136/hrt.2006.106518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willems DL, Hak A, Visser F, et al. Thoughts of patients with advanced heart failure on dying. Palliat Med. 2004;18:564–572. doi: 10.1191/0269216304pm919oa. [DOI] [PubMed] [Google Scholar]
- 9.Wotton K, Borbasi S, Redden M. When all else has failed: Nurses' perception of factors influencing palliative care for patients with end-stage heart failure. J Cardiovasc Nurs. 2005;20:18–25. doi: 10.1097/00005082-200501000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Adler ED, Goldfinger JZ, Kalman J, et al. Palliative care in the treatment of advanced heart failure. Circulation. 2009;120:2597–2606. doi: 10.1161/CIRCULATIONAHA.109.869123. [DOI] [PubMed] [Google Scholar]
- 11.Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8:30–41. doi: 10.1038/nrcardio.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Go AS, Mozaffarian D, Roger VL, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roger VL, Weston SA, Redfield MM, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 14.Roger VL, Go AS, Lloyd-Jones DM, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miniño AM, Murphy SL, Xu J, et al. Deaths: final data for 2008. Natl Vital Stat Rep. 2011;59:1–126. [PubMed] [Google Scholar]
- 16.Heidenreich PA, Trogdon JG, Khavjou OA, et al. American Heart Association Advocacy Coordinating Committee; Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Arteriosclerosis; Thrombosis and Vascular Biology; Council on Cardiopulmonary; Critical Care; Perioperative and Resuscitation; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease;Council on Cardiovascular Surgery and Anesthesia, and Interdisciplinary Council on Quality of Care and Outcomes Research Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 17.Kirkpatrick JN, Guger CJ, Arnsdorf MF, et al. Advance directives in the cardiac care unit. Am Heart J. 2007;154:477–481. doi: 10.1016/j.ahj.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 18.Fonarow GC. Epidemiology and risk stratification in acute heart failure. Am Heart J. 2008;155:200–207. doi: 10.1016/j.ahj.2006.10.043. [DOI] [PubMed] [Google Scholar]
- 19.Giamouzis G, Kalogeropoulos A, Georgiopoulou V, et al. Hospitalization epidemic in patients with heart failure: risk factors, risk prediction, knowledge gaps, and future directions. J Card Fail. 2011;17:54–75. doi: 10.1016/j.cardfail.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 20.Fida N, Piña IL. Trends in heart failure hospitalizations. Curr Heart Fail Rep. 2012;9:346–353. doi: 10.1007/s11897-012-0117-5. [DOI] [PubMed] [Google Scholar]
- 21.Dharmarajan K, Hsieh AF, Lin Z, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309:355–363. doi: 10.1001/jama.2012.216476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen J, Normand SL, Wang Y, et al. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998–2008. JAMA. 2011;306:1669–1678. doi: 10.1001/jama.2011.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lloyd-Jones DM, Larson MG, Leip EP, et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068–3072. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 24.Arnold JM, Liu P, Demers C, et al. Canadian Cardiovascular Society Canadian Cardiovascular Society consensus conference recommendations on heart failure 2006: diagnosis and management. Can J Cardiol. 2006;22:23–45. doi: 10.1016/s0828-282x(06)70237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orn S, Dickstein K. How do heart failure patients die? Eur Heart J. 2002;4(Supplement D):D59–D65. [Google Scholar]
- 26.Levenson JW, McCarthy EP, Lynn J, et al. The last six months of life for patients with congestive heart failure. J Am Geriatr Soc. 2000;48(5 Suppl):S101–S109. doi: 10.1111/j.1532-5415.2000.tb03119.x. [DOI] [PubMed] [Google Scholar]
- 27.Cleland JG, Chattopadhyay S, Khand A, et al. Prevalence and incidence of arrhythmias and sudden death in heart failure. Heart Fail Rev. 2002;7:229–242. doi: 10.1023/a:1020024122726. [DOI] [PubMed] [Google Scholar]
- 28.Chan MM, Lam CS. How do patients with heart failure with preserved ejection fraction die? Eur J Heart Fail. 2013;15:604–613. doi: 10.1093/eurjhf/hft062. [DOI] [PubMed] [Google Scholar]
- 29.Lam CS, Donal E, Kraigher-Krainer E, et al. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:18–28. doi: 10.1093/eurjhf/hfq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaila K, Haykowsky MJ, Thompson RB, et al. Heart failure with preserved ejection fraction in the elderly: scope of the problem. Heart Fail Rev. 2012;17(4–5):555–562. doi: 10.1007/s10741-011-9273-z. [DOI] [PubMed] [Google Scholar]
- 31.World Health Organization [WHO] definition of palliative care. http://www.who.int/cancer/palliative/definition/en/; accessed online 5/10/13 (accessed September 24, 2014)
- 32.Hospice care definition. http://aahfn2012conference.com/pdf/B12_Donaho.pdf (accessed September 24, 2014)
- 33.McClung JA. End-of-life care in the treatment of heart failure in the elderly. Clin Geriatr Med. 2007;23:235–248. doi: 10.1016/j.cger.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 34.Goodlin SJ. End-of-life care in heart failure. Curr Cardiol Rep. 2009;11:184–191. doi: 10.1007/s11886-009-0027-7. [DOI] [PubMed] [Google Scholar]
- 35.Lumb J. Practical issues across the lifetime of heart failure. Expert Rev Cardiovasc Ther. 2011;9:151–153. doi: 10.1586/erc.10.193. [DOI] [PubMed] [Google Scholar]
- 36.End-of-life care definition. http://consensus.nih.gov/2004/2004EndOfLifeCareSOS024html.htm (accessed September 24, 2014)
- 37.Distributive justice definition. http://www.annenbergclassroom.org/glossaries.aspx?term=distributive-justice&AspxAutoDetectCookieSupport=1 (accessed September 24, 2014)
- 38.Schwarz ER, Philip KJ, Simsir SA, et al. Maximal care considerations when treating patients with end-stage heart failure: ethical and procedural quandaries in management of the very sick. J Relig Health. 2011;50:872–879. doi: 10.1007/s10943-010-9326-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okonofua EC, Simpson KN, Jesri A, et al. Therapeutic inertia is an impediment to achieving the Healthy People 2010 blood pressure control goals. Hypertension. 2006;47:345–351. doi: 10.1161/01.HYP.0000200702.76436.4b. [DOI] [PubMed] [Google Scholar]
- 40.Mulloy DP, Bhamidipati CM, Stone ML, et al. Orthotopic heart transplant versus left ventricular assist device: a national comparison of cost and survival. J Thorac Cardiovasc Surg. 2013;145:566–573. doi: 10.1016/j.jtcvs.2012.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kini V, Kirkpatrick JN. Ethical challenges in advanced heart failure. Curr Opin Support Palliat Care. 2013;7:21–28. doi: 10.1097/SPC.0b013e32835c4915. [DOI] [PubMed] [Google Scholar]
- 42.Fried TR, O'Leary JR. Using the experiences of bereaved caregivers to inform patient- and caregiver-centered advance care planning. J Gen Intern Med. 2008;23:1602–1607. doi: 10.1007/s11606-008-0748-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.MacIver J, Rao V, Delgado DH, et al. Choices: a study of preferences for end-of-life treatments in patients with advanced heart failure. J Heart Lung Transplant. 2008;27:1002–1007. doi: 10.1016/j.healun.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 44.Barnes S, Gardiner C, Gott M, et al. Enhancing patient-professional communication about end-of-life issues in life-limiting conditions: a critical review of the literature. J Pain Symptom Manage. 2012;44:866–879. doi: 10.1016/j.jpainsymman.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 45.Ryan M, Farrelly M. Living with an unfixable heart: a qualitative study exploring the experience of living with advanced heart failure. Eur J Cardiovasc Nurs. 2009;8:223–231. doi: 10.1016/j.ejcnurse.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 46.Lampert R, Hayes DL, Annas GJ, et al. American College of Cardiology; American Geriatrics Society; American Academy of Hospice and Palliative Medicine, American Heart Association; European Heart Rhythm Association; Hospice and Palliative Nurses Association HRS expert consensus statement on the management of cardiovascular implantable electronic devices (CIEDs) in patients nearing end of life or requesting withdrawal of therapy. Heart Rhythm. 7:1008–1026. doi: 10.1016/j.hrthm.2010.04.033. [DOI] [PubMed] [Google Scholar]
- 47.Kramer DB, Kesselheim AS, Brock DW, et al. Ethical and legal views of physicians regarding deactivation of cardiac implantable electrical devices: a quantitative assessment. Heart Rhythm. 2010;7:1537–1542. doi: 10.1016/j.hrthm.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ben Gal T, Jaarsma T. Self-care and communication issues at the end of life of recipients of a left-ventricular assist device as destination therapy. Curr Opin Support Palliat Care. 2013;7:29–35. doi: 10.1097/SPC.0b013e32835d2d50. [DOI] [PubMed] [Google Scholar]
- 49.McClung JA. End-of-life care in the treatment of advanced heart failure in the elderly. Cardiol Rev. 2013;21:9–15. doi: 10.1097/CRD.0b013e31826d23ea. [DOI] [PubMed] [Google Scholar]
- 50.Burkle CM, Mueller PS, Swetz KM, et al. Physician perspectives and compliance with patient advance directives: the role external factors play on physician decision making. BMC Med Ethics. 2012;13:31. doi: 10.1186/1472-6939-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Periyakoil VS, Neri E, Fong A, et al. Do unto others: doctors' personal end-of-life resuscitation preferences and their attitudes toward advance directives. PLoS One. 2014;9:e98246. doi: 10.1371/journal.pone.0098246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bernacki RE, Block SD, for the American College of Physicians High Value Care Task Force Communication about serious illness care goals: a review and synthesis of best practices. JAMA Intern Med. doi: 10.1001/jamainternmed.2014.5271. Published Online First: Oct 20 2014. [DOI] [PubMed] [Google Scholar]
- 53.Brouwers FP, Hillege HL, van Gilst WH, et al. Comparing new onset heart failure with reduced ejection fraction and new onset heart failure with preserved ejection fraction: an epidemiologic perspective. Curr Heart Fail Rep. 2012;9:363–368. doi: 10.1007/s11897-012-0115-7. [DOI] [PubMed] [Google Scholar]