Abstract
Matrix Metalloproteinase 1 (MMP1, collagenase-1) expression is implicated in a number of diseased states including emphysema and malignant tumors. The cigarette-smoke induced expression of this interstitial collegenase has been studied extensively and its inhibition proposed as a novel therapeutic treatment for tobacco related diseases such as chronic obstructive pulmonary disease (COPD) and lung cancer. However, a limitation in MMP1 research is the inability to take advantage of natural in vivo studies as most research has been performed in vitro or via animal models expressing human forms of the gene due to the lack of a rodent ortholog of MMP1. The present study examines the function of two possible mouse orthologs of human MMP1 known as Mmp 1a and Mmp 1b. Using genomic sequence analysis and expression analysis of these enzymes, the data demonstrate that neither MMP 1a nor MMP 1b behave in the same manner as human MMP1 in the presence of cigarette smoke. These findings establish that the two commonly proposed orthologs of MMP1, MMP 1a and MMP 1b, provide substantial limitations for use in examining MMP1 induced lung disease in mouse models of cigarette smoke emphysema.
Keywords: gene expression regulation, animal model, mice, proteinase
1. Introduction
More then five million deaths occur each year from tobacco related diseases and increasing trends project this number to reach eight million by 2030 (Mathers and Loncar, 2006, World Health Organization, 2008). Two diseases that smoking particularly accentuates are chronic pulmonary obstructive disease (COPD) and lung cancer. Compared to non-smokers, smokers are 13 times as likely to die from COPD as well as 23 times (male) and 13 times (female) as likely to develop lung cancer (U.S. Department of Health and Human Services, 2004). Induction of matrix metalloproteinase by cigarette smoke is a known detrimental effect of cigarettes that can lead to COPD and contribute to lung cancer growth.
The induction of protease expression is observed in the airway epithelial cells in the smoke exposed lung (Cohen, 1973, Imai et al. , 2001). The ultimate destruction of the lung in COPD is likely the end result of an imbalance in proteases and inhibitors (Churg and Wright, 2005). One very important protease in this proposed model is the interstitial collagenase known as Matrix Metalloproteinase 1 (MMP1, collagenase-1). MMP1 is part of a family of secreted zinc metalloproteinases responsible for degrading collagens of the extracellular matrix. These enzymes are important in tissue remodeling during development and are expressed at low levels during normal physiological conditions (Greenlee et al. , 2007). However, increased expression of MMPs, and specifically MMP1, occurs during injury that accompanies many pathological conditions or tissue repair such as wound healing. Increased MMP1 expression has been associated with emphysema, multiple types of malignant tumors, rheumatoid arthritis, as well as cardiovascular and fibrotic diseases (Pardo and Selman, 2005). In addition, patients with emphysema demonstrate higher levels of MMP1 mRNA as well as MMP1 protein levels and enzymatic activity than control patients without emphysema (Imai, Dalal, 2001). In another study, MMP1 was shown to increase tumor progression and invasion as well as being associated with a poor prognosis in esophageal, breast, colorectal, and ovarian cancers (Boire et al., 2005, Bostrom et al. , 2011, Kanamori et al. , 1999, Murray et al. , 1996, Tao et al. , 2012).
These high levels of MMP1 associated with different diseased states are mediated by multiple environmental factors. Cigarette smoke has clearly been a key culprit in increasing MMP1 activity resulting in the aforementioned lung diseases such as emphysema and cancer (Joos et al. , 2002, Selman et al. , 1996). However, what is important to understand is the pathway by which cigarette smoke actually induces MMP1 expression. Delineation of this pathway will allow the development of therapeutic methods inhibiting MMP1 induction without impeding other important matrix metalloproteinases in the process (Fingleton, 2003). The mitogen activated protein kinase pathway (MAPK) is one important pathway in the cigarette smoke induced up regulation of not only MMP1, but also other molecules important to COPD pathology such as mucin and cytokines (Mercer and D'Armiento, 2006, Mercer et al. , 2004).
An important aspect of the MMP1 pathway research is identifying a viable animal model in which MMP1 expression in vivo can be manipulated. Current research is limited since it is predominantly performed in vitro and does not fully take advantage of the effects and possible therapeutic uses for emphysema, cancer or other pathological issues heightened by MMP1 activity since rodent animals lack MMP1 (Elkington et al. , 2011). Recently, a duplication of the MMP1 gene was found in mice coding for two separate genes labeled Mmp 1a (Mcol-A) and Mmp 1b (Mcol-B). These two genes are 82% identical, while Mmp 1a is 58% identical to the human MMP1 gene (Balbin et al. , 2001). Mmp 1a is thought to be a more likely ortholog to MMP1, since Mmp 1b exhibits no collagenolytic activity (Balbin, Fueyo, 2001). More promising similarities were identified with the overexpression of Mmp 1a in mouse models. There is evidence linking the overexpression of Mmp 1a to tumor growth and angiogenesis (Foley et al. , 2013). Additionally, Mmp 1a deficiency in knockout mice can suppress tumor growth suggesting a role in cancer similar to MMP1 (Fanjul-Fernandez et al. , 2013). Even more interesting, the co-implantation of wild type mmp1a fibroblasts to the lung cancer cells in this same study completely restored tumor growth (Foley, Fanjul-Fernandez, 2013). Due to findings such as these, it has been proposed that mouse Mmp 1a and Mmp 1b are viable orthologs for human MMP1 research. However, studies have not yet examined if these orthologs are similarly regulated under smoke exposure conditions. The present work examined the effects of cigarette smoke on Mmp 1a and Mmp 1b expression as a means to compare the findings to the known effect of cigarette smoke on the activity of the human MMP1 promoter. In addition, sequence analysis was utilized to compare the consensus between the human MMP1 genome with the orthologs Mmp 1a and Mmp 1b to specifically analyze the differences in the important distal 1kb promoter region required for cigarette smoke induction of human MMP1 which could account for functional differences between the proteases (Mercer et al. , 2009).
2. Materials and Methods
2.1 Genomic sequence analysis
CLC Main Workbench software (CLC bio·EMEA, Aarhus, Denmark) was used to compare human MMP1, mouse Mmp 1a and Mmp 1b and rabbit MMP1 genomic sequences. Specifically, the one kb distal portion of the gene’s sequences was given special consideration as this is the cigarette smoke responsive region (Mercer, Wallace, 2009).
2.2 Cells and cigarette smoke extract treatment
Lewis lung carcinoma cells (LLC, mouse lung carcinoma cells) and L cells (mouse fibroblasts) were grown in DMEM (Life Technologies Corp., Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Life Technologies Corp.). MH-S cells (mouse alveolar macrophages) were grown in RPMI 1640 medium (Mediatech Inc., Manassas, VA, USA) supplemented with 10% FBS (Life Technologies Corp.). All cells were grown at 37°C in a humidified incubator with 5% CO2. LLC (CRL-1642), L cells (CRL-2648) and MH-S (CRL-2019) were obtained from American Tissue Culture Collection (Manassas, VA, USA). Cigarette smoke extract (CSE) was prepared using constant suction to draw the smoke of a filtered 3R4F reference cigarette (University of Kentucky, Lexington, KY, USA) through 25 ml of Dulbecco’s PBS (Life Technologies Corp.). The pH of the CSE exposed PBS was adjusted to 7.4, filtered, and added to cell growth media at final concentration of 0.5%, 2.0%, and 5.0% (v/v), immediately. For gene expression analysis, cells were treated for 12, 24, and 72 hours with CSE in 10% FBS or serum starved condition. For protein expression analysis, cells were treated for 24 and 72 hours with CSE and no serum. The media supplemented with PBS was used as a vehicle control. Cells treated with LPS (Sigma-Aldrich Co. LLC., St. Louis, MO, USA) were cultured for 24 hours at the final concentration of 500 ng/ml.
2.3 Mouse cigarette smoking models
Inbred mouse lines C57BL/6J and AKR were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). All of the experiments were conducted according to Institutional Animal Care and Use Committee-approved protocol. Eight week old adult C57BL/6J mice were exposed to cigarette smoke (80-100 mg/m3 total particular matter) via a full body smoke exposure chamber (Teague Enterprises, Woodland, CA, USA) for 4 hours per day 5 days per week for 2 weeks as per standard laboratory protocol (Geraghty et al. , 2011). Another group of adult AKR mice were exposed to smoke via the same method for 2 weeks and a longer duration of 3 months. Both sets of mice were given food and water ad libitum and sacrificed 12 hours after smoke exposure to harvest samples. Alveolar macrophages were obtained from AKR mice using the lavage method of extraction with two separate washes using 1 ml of PBS each time. Lung tissue for RNA and protein isolation was snap-froze with liquid nitrogen. Samples for histological analysis were fixed with 10% formalin (Thermo Fisher Scientific Inc., Waltham, MA, USA).
2.4 Quantitative RT-PCR
RNA was isolated from cell culture experiments, the AKR alveolar macrophages, and lung tissues of the C57BL/6J and AKR mice using RNAeasy Mini Kit (QIAGEN Inc., Valencia, CA, USA). cDNA synthesis was achieved using High Capacity cDNA Reverse Transcription Kit (Life Technologies Corp.). Quantitative reverse transcription polymerase chain reaction (qRT-PCR) was used to quantify the gene expression of mouse Mmp 1a and Mmp 1b using TaqMan Gene Expression Assays (catalog# 4331182 and 4331182 respectively, Life Technologies Corp.). The primers/probe set for mouse glyceraldehyde-3-phosphate dehydrogenase (GAPD) also from Life Technologies Corp. (catalog# 4352339E) was used as an endogenous control. Forty cycles of PCR were run using Applied Biosystems 7300 Real-Time PCR System (Life Technologies Corp.) and the data was analyzed with the supplied software (7300 System SDS Software with SDS Relative Quantification Study Plug-In, version 1.4.0.25, Life Technologies Corp.).
2.5 Western Blotting
For protein samples from culture cells, serum-free culture media was collected 24 hours post-CSE treatment to analyze changes in MMP1a/b secretion. One ml of culture media from each condition was applied to the analysis by trichloroacetic acid (Sigma-Aldrich Co. LLC.) protein concentration. From the mouse lung and placenta tissue, samples were lysed with RIPA buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS) supplemented with proteinase inhibitor cocktail (cOmplete Mini, Roche Diagnostics Corp, Indianapolis, IN, USA). Samples were separated using 10% SDS-polyacrylamide gel and transferred to a nitrocellulose membrane (Thermo Fisher Scientific Inc.). Anti-(human and mice) MMP1 rabbit polyclonal antibodies (orb11056, Biorbyt LLC, San Francisco, CA, USA) was used at 1:1,000 dilutions, detected using chemiluminescent technique (Amercham ECL Western Blotting Detection Reagent, GE Healthcare Bio-Sciences Corp., Piscataway, NJ, USA) and captured the image by ImageQuant LAS 4010 (GE Healthcare Bio-Sciences Corp.).
2.6 Immunohistochemistry
The fixed tissue was embedded in paraffin. The section was cut 5μm thick and mounted on charged slide glasses (Thermo Fisher Scientific Inc.). The slides were deparaffinized and treated with pH 9.0 10 mM Tris-HCl buffer under 121°C for 20 min as antigen retrieval procedure. Then, the sections were pre-incubated with 5.0% horse serum at room temperature for 1 hr and incubated with the rabbit polyclonal antibodies against mouse MMP1 (orb11056, Biorbyt LLC) at 1,000 times dilution 4°C over night. After the wash, the sections were incubated with ImmPRESS Anti-Rabbit Ig (peroxidase) polymer (Vector Laboratories Inc., Burlingame, CA, USA) and the color developed with ImmPACT DAB peroxidase substrate (Vector Laboratories Inc.). The slides were counter-stained with hematoxylin solution (Sigma-Aldrich Corp LLC.) and cover-slipped. The image was taken with Nikon Eclipse E400 microscope (Nikon Instruments Inc., Melville, NY, USA) and QIClick digital camera (QImaging, Surrey, BC, Canada).
3. Results
3.1 Synteny between human and mouse chromosomes show MMP 1a as a possible ortholog to human MMP1
Fig. 1A exhibits the synteny between human and mouse chromosomes. Human MMP1 is located on chromosome 11. Fig. 1A demonstrates that chromosome 11 coincides with a partial distribution to mouse chromosome 9 including the top portion of the chromosome where mouse Mmp 1a is located. As indicated by the arrow, the direction of the chromosome fragment is reversed between human and mouse. Fig. 1B shows the human MMP1 to be downstream of MMP3 as well as upstream of MMP10. In comparison, Fig. 1B also illustrates mouse Mmp 1a to be downstream from Mmp3 as well as upstream of Mmp10. By observing chromosome mapping, it is shown that mouse Mmp 1a is a possible ortholog of human MMP1.
Fig. 1.
A) Synteny between human and mouse chromosomes illustrating where MMP 1a would be if true ortholog. B) Chromosome mapping of MMP1 in various spices.
3.2 Neither Mmp 1a nor Mmp 1b was upregulated by cigarette smoke in vitro
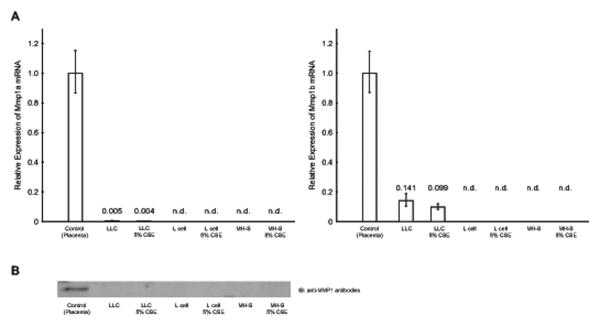
LLC, L cell and MH-S cells were treated with CSE or LPS and analyzed for the expression of Mmp 1a and Mmp 1b by qRT-PCR. Treatment with 5% CSE did not alter the expression level of Mmp 1a or Mmp 1b (p = 0.6130 and 0.2403, respectively; Fig. 2A). The cells treated with 0.5% and 2% CSE and with LPS also exhibited little expression of both Mmp 1a and Mmp 1b with no significant difference compared to control cells (data not shown). There were no expressions of Mmp 1a and Mmp 1b in L cells and MH-S cells (Fig. 2A). The cells treated with 0.5% and 2% CSE and with LPS also exhibited no expression of Mmp 1a and Mmp 1b (data not shown). Serum starved culture conditions did not affect the results (data not shown). This data was confirmed at the protein level by western blotting with an anti-Mmp1 antibodies (Fig. 2B). This experimental condition with 5% CSE was used for the treatment of human lung cells and shown to up regulate MMP1 expression as described in our previous reports (Geraghty, Dabo, 2011, Moon et al. , 2014).
Fig. 2.
A) qRT-PCR. The expression level of Mmp 1a (left) and Mmp 1b (right) after 24 hr exposure to CSE was analyzed by qRT-PCR. The expression level was normalized by internal control and shown its expression relative to control sample (mouse placenta as 1). There was no induction of Mmp 1a nor Mmp 1b after 12 and 72 hr exposure as well as serum starved condition (data not shown). B) Western blotting. Expression of MMP1 after 24 hr exposure was confirmed by western blotting. The serum free culture media from cells were collected and applied to the lanes. Tissue extract from mouse placenta was used as positive control. There was no induction of Mmp 1a nor Mmp 1b after 72 hr exposure (data not shown).
3.3 No induction of Mmp 1a or Mmp 1b in cigarette smoke exposed mouse lungs
qRT-PCR demonstrated no baseline expression nor induction of Mmp 1a and Mmp 1b in cigarette smoke exposed mouse lungs in both short (2 weeks) - and long-term (2 months) exposure studies (Fig. 3A). The same result was seen with the cDNA derived from macrophages of C57BL/J mice exposed to 2 weeks of smoking (data not shown). The AKR mouse strain is known to be the cigarette smoke susceptible strain and the C57BL/J a resistant strain (Guerassimov et al. , 2004). We did not observe any strain difference in Mmp 1a/b up-regulation after 2 weeks of smoke exposure with both strains of mice. The mRNA expression data was confirmed by IHC analyzing for protein expression in smoked exposed lung tissue (Fig. 3B). The primer/probe set and the antibodies exhibited positive results in the mouse placenta samples (positive control) (Balbin, Fueyo, 2001).
Fig. 3.
A) qRT-PCR. Mmp 1a and Mmp 1b expression in lung from cigarette smoke exposed AKR mice were examined by qRT-PCR. The expression level was normalized by internal control and shown its expression relative to control sample (mouse placenta as 1). B) IHC. The expression of MMP1 a/b was analyzed by IHC. Placenta tissue was used as positive control for the staining. The scale bar represents 100 μm. All of the pictures are the same magnification.
3.4 Sequence heat map shows low consensus in the distal 1kbp region
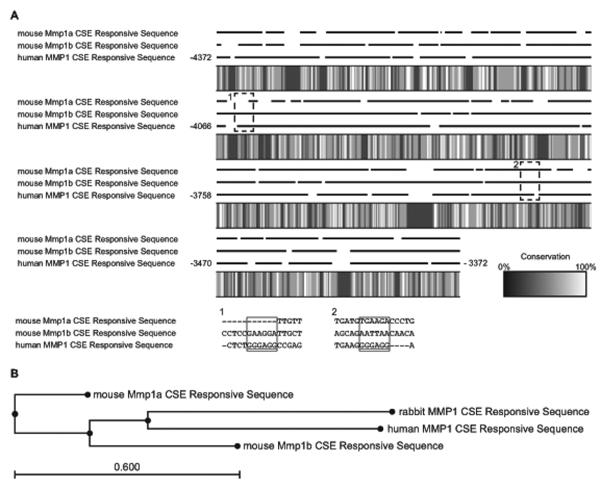
Due to the lack of induction of Mmp 1a and Mmp 1b with cigarette smoke, particular attention was given to analyze the sequence homology of the distal 1kbp region of the promoter located at −4372 to −3372 bp of the sequence (with RefSeq NC_000011.9 setting start codon (ATC) of A as position +1). This CSE responsive region of human MMP1 vs mouse Mmp 1a exhibited low consensus between sequences representing only a 32.9% overlap. Human MMP1 vs mouse Mmp 1b exhibited a 36.06% match, while mouse Mmp 1a vs mouse Mmp 1b had a 41.72% match. It was also noted that there were several regions where no similarity existed between the three genomic sequences in this distal region. These regions included −4055 to −4050 bp (corresponding to the critical cigarette smoke responsive Sp1 site at position −4091 (Mercer, Wallace, 2009) or −3987 (Wallace et al. , 2012)), and −3523 to −3518 bp (corresponding to the Sp1 site at the position −3491 (Mercer, Wallace, 2009) or −3455 (Wallace, Mercer, 2012); Fig. 4A) in the region of the CS responsive element of human MMP1. In comparison to rabbit MMP1, the CSE responsive sequence is known to respond to CSE (Lemaitre et al. , 2011), the mouse Mmp 1a and Mmp 1b regions have less similarity compared with those of human and rabbit (Fig. 4B).
Fig. 4.
A) Sequence Heat Map. The cigarette smoke responsive promoter region of human MMP1 and the corresponding sequences of mouse Mmp 1a and Mmp 1b were aligned with conservation heat map. The dotted rectangle labeled 1 and 2 represented the zoomed sequence in Sp1 binding sites described in the previous publication. Both mouse Mmp 1a and Mmp2 lacked the Sp1 consensus sequence “GGGAGG” in area 1 and 2.
B) Evolutionary tree of Mouse Mmp 1a, Mmp 1b, human MMP1 and rabbit mmp1 CSE Responsive sequence. Human MMP1 and rabbit MMP1, both of which respond to CSE, have closer relationship compared to mouse Mmp 1a and Mmp 1b.
4. Discussion
Multiple reports highlight the importance of MMP1 expression during critical states of human pathology including tumorigenesis and cancer invasion (Egeblad and Werb, 2002, Fingleton, 2003). It is clear that MMP1 is indeed upregulated in patients with diseases such as COPD (Imai, Dalal, 2001) and lung cancer (Joos, He, 2002) and attempts have been and continue to be made to seek out viable MMP1 inhibitors in an effort to provide a therapeutic treatment to many of the diseases aggravated by upregulated MMP1 expression. However, a limitation for this search is not only the specificity of MMP inhibition, but also the difficulty in providing in vivo research on the effects of MMP1 regulation. The research performed in this area is limited to utilizing in vitro models or in other cases, using transgenic mouse models overexpressing human MMP1 (D'Armiento et al. , 1992, Elkington, Shiomi, 2011, George et al. , 2012, Imai et al. , 2007, Kim et al. , 2000, Lemaitre et al. , 2001). These studies can be difficult to validate as the upregulation of a protease that is not natural to the mouse model can lead to multiple adverse affects such as cleavage of non-natural substrates or expression in tissues that do not normally express the enzyme (Overall and Kleifeld, 2006). It is because of this that a search for a viable mouse ortholog to human MMP1 has been of great interest. Recent studies suggest that mouse Mmp 1a and Mmp 1b may indeed provide these orthologs allowing for a more valid approach to in vivo models of MMP1 therapeutic research (Balbin, Fueyo, 2001, Foley, Fanjul-Fernandez, 2013).
In the present study, we compared human MMP1 to the proposed mouse orthologs Mmp 1a and Mmp 1b using sequence mapping and expression analysis under smoke exposure conditions to determine if there was regulation of the orthologs by cigarette smoke. Our goal was to provide evidence on the efficacy of using these orthologs in studies specifically involving cigarette smoke induced MMP1 expression. At first, through chromosome mapping studies of human MMP1 compared to mouse Mmp 1a, it was noted that both the gene location as well as the surrounding genes such as MMP3 and MMP10 coincided closely between the human version and the potential mouse ortholog, Mmp 1a and Mmp 1b. Following this, the gene sequences were then examined in closer detail. Upon analyzing the sequence heat map of the three gene sequences particular attention was taken to the distal 1kbp of the promoter region from −5372bp to −4372bp in human MMP1. This region is necessary for smoke induced expression of MMP1 in small airway epithelial cells (Mercer, Wallace, 2009). Interestingly, this portion of the sequence, represented a very small consensus between the genes of less then 33%. Indeed, mouse Mmp 1a and Mmp 1b lack the ERK binding Sp1 sites that were identified in previous studies to be critical to the MMP1 smoke induction (Mercer, Wallace, 2009). In addition, when compared to the rabbit Mmp1 cigarette smoke-responsive region, the corresponding regions in the mouse Mmp 1a and Mmp 1b genes exhibited more similarity to each other than to the human and rabbit MMP1 which both respond to cigarette smoke.
From here, the mRNA and protein expression in various mouse cells (LLC – lung carcinoma cell (epithelial cell origin), L cells – fibroblast and MH-S – alveolar macrophage) and lung tissue of mice exposed to short term as well as long-term chronic cigarette smoke demonstrated that cigarette smoke did not induce Mmp 1a or Mmp 1b. Since studies demonstrate a clear induction of MMP1 when lung cells are exposed to cigarette smoke (Bulmanski et al. , 2012, Geraghty, Dabo, 2011, Moon, Kim, 2014), this data provides evidence that although in certain studies Mmp 1a may indeed serve as a viable ortholog for human MMP1, it simply cannot be used for studies involving cigarette smoke and the inducible expression of MMP1. It is likely that the lack of induction is a result of the small regions of complete non-consensus between the sequences of the distal 1kbp promoter region, but an alternative possibility could be due to a lack of appropriate signaling molecules in the MMP1 smoke-induced pathway within the mouse species (Geraghty, Dabo, 2011). It is clear from this study that mouse Mmp 1a and Mmp 1b do not act in the same way as human MMP1 when exposed to short or long term cigarette smoke. As Balbin et al. has shown, Mmp 1a and Mmp 1b also exhibit quite different substrate specificities compared to human MMP1 (Balbin, Fueyo, 2001). Taken together, this data suggests that mouse Mmp 1a and Mmp 1b should be considered to be a different MMP species compared to human MMP1. The described studies provide insight to researchers seeking to use these orthologs in COPD and cancer research in which smoke exposure has a crucial element to the pathogenesis, specifically when utilizing rodent in vivo models. In addition, caution should be made towards other molecular families especially in in vivo animal studies, since the orthologous genes in one species are not regulated in a similar fashion as the human molecule.
Acknowledgement
This work was supported by NIH/NHLBI R01HL086936 (JMD). We thank Drs. Monica P. Goldklang and Piotr Sklepkiewicz to providing us the cDNA samples from cigarette smoked mice lungs. The authors thank Ms. Tomoe Shiomi for her technical assistance to the histological analysis and Mr. Denzel Woode for his critical reading of the manuscript.
Footnotes
Conception and design: TS, JMD; Analysis and interpretation: PIC, VA, TS; Drafting the manuscript for important intellectual content: PIC, TS, JMD
References
- Balbin M, Fueyo A, Knauper V, Lopez JM, Alvarez J, Sanchez LM, et al. Identification and enzymatic characterization of two diverging murine counterparts of human interstitial collagenase (MMP-1) expressed at sites of embryo implantation. J Biol Chem. 2001;276:10253–62. doi: 10.1074/jbc.M009586200. [DOI] [PubMed] [Google Scholar]
- Boire A, Covic L, Agarwal A, Jacques S, Sherifi S, Kuliopulos A. PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell. 2005;120:303–13. doi: 10.1016/j.cell.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Bostrom P, Soderstrom M, Vahlberg T, Soderstrom KO, Roberts PJ, Carpen O, et al. MMP-1 expression has an independent prognostic value in breast cancer. BMC Cancer. 2011;11:348. doi: 10.1186/1471-2407-11-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulmanski Z, Brady M, Stoute D, Lallier TE. Cigarette smoke extract induces select matrix metalloproteinases and integrin expression in periodontal ligament fibroblasts. J Periodontol. 2012;83:787–96. doi: 10.1902/jop.2011.110395. [DOI] [PubMed] [Google Scholar]
- Churg A, Wright JL. Proteases and emphysema. Curr Opin Pulm Med. 2005;11:153–9. doi: 10.1097/01.mcp.0000149592.51761.e3. [DOI] [PubMed] [Google Scholar]
- Cohen AB. Interrelationships between the human alveolar macrophage and alpha-1-antitrypsin. J Clin Invest. 1973;52:2793–9. doi: 10.1172/JCI107475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Armiento J, Dalal SS, Okada Y, Berg RA, Chada K. Collagenase expression in the lungs of transgenic mice causes pulmonary emphysema. Cell. 1992;71:955–61. doi: 10.1016/0092-8674(92)90391-o. [DOI] [PubMed] [Google Scholar]
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–74. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- Elkington P, Shiomi T, Breen R, Nuttall RK, Ugarte-Gil CA, Walker NF, et al. MMP-1 drives immunopathology in human tuberculosis and transgenic mice. J Clin Invest. 2011;121:1827–33. doi: 10.1172/JCI45666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanjul-Fernandez M, Folgueras AR, Fueyo A, Balbin M, Suarez MF, Fernandez-Garcia MS, et al. Matrix metalloproteinase Mmp-1a is dispensable for normal growth and fertility in mice and promotes lung cancer progression by modulating inflammatory responses. J Biol Chem. 2013;288:14647–56. doi: 10.1074/jbc.M112.439893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingleton B. Matrix metalloproteinase inhibitors for cancer therapy:the current situation and future prospects. Expert Opin Ther Targets. 2003;7:385–97. doi: 10.1517/14728222.7.3.385. [DOI] [PubMed] [Google Scholar]
- Foley CJ, Fanjul-Fernandez M, Bohm A, Nguyen N, Agarwal A, Austin K, et al. Matrix metalloprotease 1a deficiency suppresses tumor growth and angiogenesis. Oncogene. 2013 doi: 10.1038/onc.2013.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George J, Sun J, D'Armiento J. Transgenic expression of human matrix metalloproteinase-1 attenuates pulmonary arterial hypertension in mice. Clin Sci (Lond) 2012;122:83–92. doi: 10.1042/CS20110295. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Geraghty P, Dabo AJ, D'Armiento J. TLR4 protein contributes to cigarette smoke-induced matrix metalloproteinase-1 (MMP-1) expression in chronic obstructive pulmonary disease. J Biol Chem. 2011;286:30211–8. doi: 10.1074/jbc.M111.238824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenlee KJ, Werb Z, Kheradmand F. Matrix metalloproteinases in lung: multiple, multifarious, and multifaceted. Physiol Rev. 2007;87:69–98. doi: 10.1152/physrev.00022.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerassimov A, Hoshino Y, Takubo Y, Turcotte A, Yamamoto M, Ghezzo H, et al. The development of emphysema in cigarette smoke-exposed mice is strain dependent. Am J Respir Crit Care Med. 2004;170:974–80. doi: 10.1164/rccm.200309-1270OC. [DOI] [PubMed] [Google Scholar]
- Imai K, Dalal SS, Chen ES, Downey R, Schulman LL, Ginsburg M, et al. Human collagenase (matrix metalloproteinase-1) expression in the lungs of patients with emphysema. Am J Respir Crit Care Med. 2001;163:786–91. doi: 10.1164/ajrccm.163.3.2001073. [DOI] [PubMed] [Google Scholar]
- Imai K, Dalal SS, Hambor J, Mitchell P, Okada Y, Horton WC, et al. Bone growth retardation in mouse embryos expressing human collagenase 1. Am J Physiol Cell Physiol. 2007;293:C1209–15. doi: 10.1152/ajpcell.00213.2007. [DOI] [PubMed] [Google Scholar]
- Joos L, He JQ, Shepherdson MB, Connett JE, Anthonisen NR, Pare PD, et al. The role of matrix metalloproteinase polymorphisms in the rate of decline in lung function. Hum Mol Genet. 2002;11:569–76. doi: 10.1093/hmg/11.5.569. [DOI] [PubMed] [Google Scholar]
- Kanamori Y, Matsushima M, Minaguchi T, Kobayashi K, Sagae S, Kudo R, et al. Correlation between expression of the matrix metalloproteinase-1 gene in ovarian cancers and an insertion/deletion polymorphism in its promoter region. Cancer Res. 1999;59:4225–7. [PubMed] [Google Scholar]
- Kim HE, Dalal SS, Young E, Legato MJ, Weisfeldt ML, D'Armiento J. Disruption of the myocardial extracellular matrix leads to cardiac dysfunction. J Clin Invest. 2000;106:857–66. doi: 10.1172/JCI8040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre V, Dabo AJ, D'Armiento J. Cigarette smoke components induce matrix metalloproteinase-1 in aortic endothelial cells through inhibition of mTOR signaling. Toxicol Sci. 2011;123:542–9. doi: 10.1093/toxsci/kfr181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre V, O'Byrne TK, Borczuk AC, Okada Y, Tall AR, D'Armiento J. ApoE knockout mice expressing human matrix metalloproteinase-1 in macrophages have less advanced atherosclerosis. J Clin Invest. 2001;107:1227–34. doi: 10.1172/JCI9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS medicine. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer BA, D'Armiento JM. Emerging role of MAP kinase pathways as therapeutic targets in COPD. Int J Chron Obstruct Pulmon Dis. 2006;1:137–50. doi: 10.2147/copd.2006.1.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer BA, Kolesnikova N, Sonett J, D'Armiento J. Extracellular regulated kinase/mitogen activated protein kinase is up-regulated in pulmonary emphysema and mediates matrix metalloproteinase-1 induction by cigarette smoke. J Biol Chem. 2004;279:17690–6. doi: 10.1074/jbc.M313842200. [DOI] [PubMed] [Google Scholar]
- Mercer BA, Wallace AM, Brinckerhoff CE, D'Armiento JM. Identification of a cigarette smoke-responsive region in the distal MMP-1 promoter. Am J Respir Cell Mol Biol. 2009;40:4–12. doi: 10.1165/rcmb.2007-0310OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon HG, Kim SH, Gao J, Quan T, Qin Z, Osorio JC, et al. CCN1 secretion and cleavage regulate the lung epithelial cell functions after cigarette smoke. Am J Physiol Lung Cell Mol Physiol. 2014;307:L326–37. doi: 10.1152/ajplung.00102.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray GI, Duncan ME, O'Neil P, Melvin WT, Fothergill JE. Matrix metalloproteinase-1 is associated with poor prognosis in colorectal cancer. Nat Med. 1996;2:461–2. doi: 10.1038/nm0496–461. [DOI] [PubMed] [Google Scholar]
- Overall CM, Kleifeld O. Tumour microenvironment - opinion: validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat Rev Cancer. 2006;6:227–39. doi: 10.1038/nrc1821. [DOI] [PubMed] [Google Scholar]
- Pardo A, Selman M. MMP-1: the elder of the family. Int J Biochem Cell Biol. 2005;37:283–8. doi: 10.1016/j.biocel.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Selman M, Montano M, Ramos C, Vanda B, Becerril C, Delgado J, et al. Tobacco smoke-induced lung emphysema in guinea pigs is associated with increased interstitial collagenase. Am J Physiol. 1996;271:L734–43. doi: 10.1152/ajplung.1996.271.5.L734. [DOI] [PubMed] [Google Scholar]
- Tao YS, Ma XY, Chai DM, Ma L, Feng ZZ, Cheng ZN, et al. Overexpression of MMP-1 and VEGF-C is associated with a less favorable prognosis in esophageal squamous cell carcinoma. Onkologie. 2012;35:651–6. doi: 10.1159/000343637. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services . The Health Consequences of Smoking: A Report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta (GA): 2004. [Google Scholar]
- Wallace AM, Mercer BA, He J, Foronjy RF, Accili D, Sandford AJ, et al. Functional characterization of the matrix metalloproteinase-1 cigarette smoke-responsive region and association with the lung health study. Respir Res. 2012;13:79. doi: 10.1186/1465-9921-13-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . WHO report on the global tobacco epidemic, 2008; the MPOWER package. WHO; Geneva: 2008. [Google Scholar]