Abstract
Objective
Pregnancy-associated plasma protein-A (PAPP-A) is a zinc metalloproteinase in the insulin-like growth factor system that is expressed by tissues outside of pregnancy and involved in normal and dysregulated growth. PAPP-A has been implicated in several cancers. However, studies of PAPP-A expression in breast cancer are limited. In this study, we assessed PAPP-A expression in different subtypes of human malignant breast cancer.
Design
Formalin-fixed paraffin-embedded tumor samples from 46 female patients with invasive breast cancer were divided into five defined groups [using markers for HER2, estrogen receptor, progesterone receptor, proliferation] that roughly correlate with molecularly defined subtypes (luminal A, luminal B, luminal/HER2+, HER2+, triple negative). These samples were analyzed for PAPP-A expression by immunohistochemistry.
Results
PAPP-A staining in tumor tissue was detected in 45 of 46 specimens. There was significantly greater extent and intensity of PAPP-A expression in luminal B specimens with high proliferation index than luminal A specimens (P = 0.01). However, there were no differences between specimens positive or negative for HER2 (P = 0.14) or positive and negative for estrogen receptor (P = 0.31).
Conclusion
PAPP-A was detected in almost all breast cancer specimens and a more intense and greater extent of its expression was associated with luminal B specimens compared to luminal A specimens. The role of PAPP-A in breast cancer prognosis, and possibly therapeutics, warrants further investigation.
Keywords: pregnancy-associated plasma protein-A, breast cancer, immunohistochemistry
INTRODUCTION
Pregnancy-associated plasma protein-A (PAPP-A) was originally discovered as one of four proteins found at high levels in late-term plasma of pregnant women [1]. More recently, PAPP-A has been shown to be expressed by multiple tissues and to play additional roles outside of pregnancy [reviewed in 2]. PAPP-A is the founding member of a metzincin subfamily (pappalysins), functioning as an important regulator of local insulin-like growth factor (IGF) availability for receptor binding and activation [2]. IGF receptor signaling strongly influences cancer growth and metastases [3]. However, very little is known about PAPP-A expression in cancer. PAPP-A was found to be elevated in subsets of patients with lung and ovarian cancer [4,5], and overexpression of PAPP-A by a human ovarian cancer cell line was shown to have increased tumor aggressiveness in vivo [6]. Down-regulation of PAPP-A expression decreased lung and ovarian cancer growth in vivo [7,8]. Furthermore, PAPP-A has been identified as a potential target for malignant pleural mesothelioma [9]. Studies of PAPP-A in human breast cancer, subsets of which are IGF-responsive [10], are limited. There is some evidence that PAPP-A plays a role in promoting breast cancer progression [11,12]. However, a recent study suggested that PAPP-A is epigenetically silenced in human breast cancer [13]. In this study, we determined PAPP-A expression by immunohistochemistry in defined subtypes of malignant human breast cancer.
METHODS
Immunohistochemistry (IHC)
Formalin-fixed paraffin-embedded samples from 46 patients with a pathologically proven diagnosis of breast cancer, and who had consented to the use of their tissue in the Mayo Clinic Breast SPORE tissue bank, were selected for this study. Samples were processed as previously described [14,15]. Briefly, paraffin-embedded formalin-fixed tissue blocks were cut at 5 µm and deparaffinized in xylene and rehydrated in a graded series of ethanol. Antigen retrieval was performed with citric acid. Specimens were incubated in methanol with 3% hydrogen peroxide for 30 minutes to block endogenous peroxidase activity then washed with Tris-buffered saline (pH 7.4). Sections were incubated with recombinant anti-human PAPP-A monoclonal antibody [16] 2 µg/ml at room temperature for one hour after blocking for non-discriminant antibody:protein binding (Protein Block, Dako, Carpinteria, CA). After washing, sections were incubated with a secondary antibody and visualized with Novo Red substrate (Vector Laboratories, Burlingame, CA). Negative controls (omission of primary antibody) and positive controls (placenta) were included to ensure quality and specificity of staining. The malignant cells were scored by a pathologist (DWV) based on the percent of positively-stained cells, and the intensity of the staining (weak = 1, medium = 2, strong = 3, intensity of positively-stained cells relative to positively-stained placental controls). The product of the percent of positively-stained cells and the intensity score times 100 was calculated for each specimen and used for comparisons between groups.
Classification
Patient tumors included in this study were divided into five immunohistochemically-defined groups [estrogen receptor (ER), progesterone receptor (PR), HER2, proliferation (Ki67)] that roughly correlate with molecularly-defined subtypes [17]. Specimens that were ER+/PR+/HER2−/Ki67 low (less than 13.5%) were classified as luminal A; specimens that were ER+/PR + or −/HER2−/Ki67 high (greater than or equal to 13.5%) were classified as luminal B, specimens that were ER+/PR+ or −/HER2+ were classified as luminal/HER2+; specimens that were ER−/HER2+ were classified as HER2+, and patients that were ER− (less than 1% nuclear staining)/PR−/HER2− were classified as basal-like/triple negative.
Statistics
The product of the percent of positively-stained cells, the intensity score and 100 was compared between groups using Kruskal-Wallis one-way analysis of variance. Pearson’s chi squared test was used to compare the distributions of the percent of positively-stained cells between the classification groups. These tests were used to determine if our null hypothesis that there was no difference in PAPP-A staining between breast cancer subtypes was correct. JMP 9.0.3 (SAS Institute, Inc. Cary, NC) was used for all tests. Graphs were made with Prism 6 (GraphPad Software, Inc. La Jolla, CA).
RESULTS
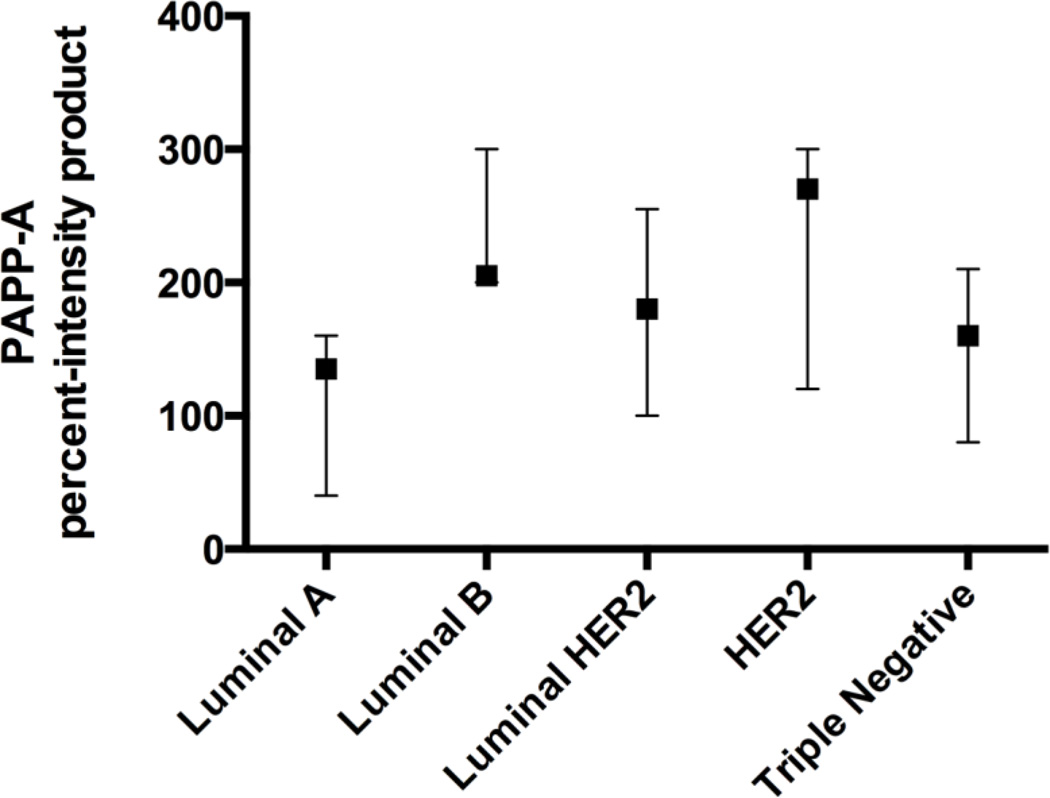
There were 46 specimens available for review, and PAPP-A protein expression (Figure 1 and Tables 1–2) was detected in 45 of these (98%). Representative IHCs are presented in Figure 2. There was a significant difference in the product of the percent of positively-stained malignant cells and the intensity when comparing all groups (P = 0.019). The luminal A specimens had a significantly lower product [median 120, interquartile range (IQR) 25–160] than the luminal B specimens (median 200, IQR 150–278; P = 0.01). There was no significant difference in the product of PAPP-A expression between all HER2− (n = 26; median product 160, interquartile range 95–202.5) and HER2+ patients (n = 20; median product 195, interquartile range 100–300; P = 0.14), between all ER− (n = 18; median product 190, interquartile range 95–300) and ER+ patients (n = 28; median product 160, interquartile range 100–210; P = 0.31), or between grades (I–III; P = 0.52). Almost no staining was detected in non-malignant tissue.
Figure 1. Distribution of PAPP-A expression by group.
The medians and interquartile ranges of the product of percent of positively-stained cells and intensity are graphed by each IHC-defined group of breast cancer specimens.
Table 1.
Patient characteristics
| Age (years; median, interquartile range) | 59 (50–67) | |
| Histology (n, %) | ||
| Infiltrating Ductal Carcinoma | 39 (81%) | |
| Infiltrating Lobular Carcinoma | 4 (8%) | |
| Mixed | 3 (6%) | |
| ER (n, %) | ||
| positive | 29 (63%) | |
| negative | 17 (37%) | |
| HER2 (n, %) | ||
| positive | 20 (43%) | |
| negative | 26 (57%) | |
| Ki67 (%, median, interquartile range) | 25 (9–43) | |
| Grade | ||
| 1 | 7 | |
| 2 | 15 | |
| 3 | 20 | |
| Not reported | 4 | |
| Stage* | ||
| 0 | 2 (4%) | |
| 1 | 19 (40%) | |
| 2 | 13 (27%) | |
| 3 | 6 (13%) | |
| 4 | 8 (17%) | |
Total not equal 100% due to rounding
Table 2.
PAPP-A immunostaining: distribution of results
| ER−/PR− /HER2− |
ER+/PR+ /HER2− /Ki67 low |
ER+/PR+ /HER2− /Ki67 high |
ER+/PR+/ HER2+ |
ER−/PR− /HER2+ |
||
|---|---|---|---|---|---|---|
| n | 7 | 9 | 10 | 9 | 11 | |
| Intensity n (%) | ||||||
| none | 1 (11%) | |||||
| weak | 1 (14%) | 3 (33%) | 1 (11%) | 1 (9%) | ||
| moderate | 4 (57%) | 4 (44%) | 6 (60%) | 3 (33%) | 3 (27%) | |
| strong | 2 (29%) | 1 (11%) | 4 (40%) | 5 (56%) | 7 (64%) | |
| % positive* | 70 (50–90) | 60 (25–80) | 95 (67.5–100) | 70 (50–85) | 90 (60–100) | |
| Product* | 160 (80–210) | 120 (25–160) | 200 (150–278) | 180 (100–255) | 270 (120–300) | |
The medians, with the interquartile ranges in parentheses, are reported in the table for the percent of positively-stained cells and the product of the intensity and percent of positively-stained cells. The product of the percent of positively-stained cells and the intensity score times 100 was calculated for each specimen and used for comparisons between groups.
Figure 2. Examples of PAPP-A immunostaining.
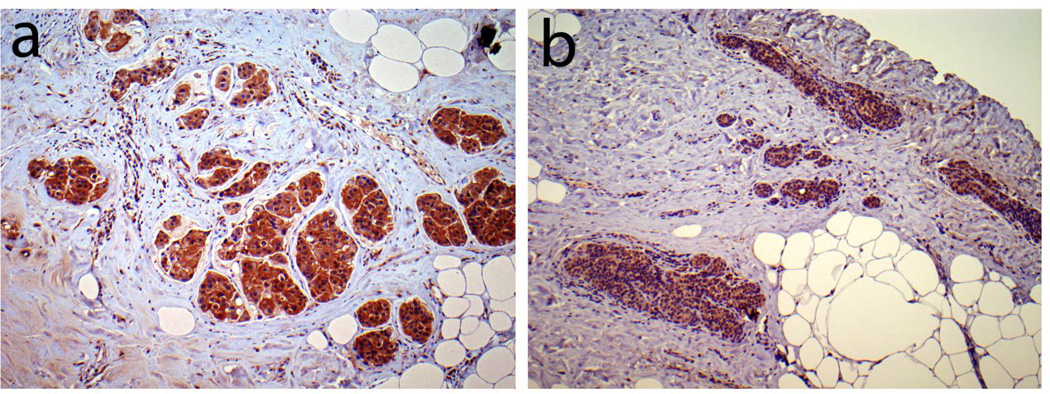
An invasive carcinoma of the breast from the luminal B group with strong and extensive PAPP-A staining of malignant cells is shown (a) next to an invasive carcinoma in a separate specimen from the luminal A group with weak but extensive PAPP-A staining of malignant cells (b) at 100X.
DISCUSSION
Specific PAPP-A immunostaining was found in 45 of 46 individual patient breast cancer specimens, including luminal A, luminal B, luminal/HER2+, HER2+, and triple negative subtypes. PAPP-A was overexpressed in the luminal B group with a high proliferation index when compared to the luminal A group. Although the average highest staining for PAPP-A was observed in HER2 subtypes, there was no statistically significant difference in expression between this and other subtypes due to the wide distribution of staining in this subtype. Thus, PAPP-A is expressed in human breast cancer and may be associated with a more aggressive phenotype, as luminal B is associated with worse survival than luminal A [18].
Very little is known about PAPP-A in human breast cancer. An early study suggested that PAPP-A tumor immunopositivity was associated with early recurrence in patients with stage I breast cancer [11]. It was later reported that the polyclonal antibody in that study recognizes other antigens, in particular haptoglobin-related protein [19]. Therefore, it was unclear whether the immunoreactivity in malignant cells was associated with PAPP-A or other co-purifying proteins during antibody generation. In contrast, the antibody we used for immunostaining is a well-characterized and specific monoclonal antibody [16], which adds confidence to our results. However, larger studies might clarify differences in expression between subtypes of breast cancer.
Our data showing intense immunostaining for PAPP-A protein associated with aggressive forms of breast cancer seemingly conflict with those of Loddo et al. [13]. In their study, loss of PAPP-A through epigenetic silencing was associated with mitotic delay in the genesis of breast cancer. The reason for the differential findings is unclear, but may depend on the stage of breast cancer development and progression. In other studies relevant to PAPP-A and breast cancer, infants exposed to high maternal concentrations of PAPP-A during pregnancy were reported to have an increased future risk of breast cancer development [20], and increased PAPP-A mRNA expression was found in breast cancer cell lines expressing mutant p53 [21].
IGF signaling is involved with tumorigenesis [22], and several studies have shown a correlation between IGF receptor expression and breast cancer aggressiveness [10]. Clinical trials targeting IGF receptor have been disappointing as there is no clear biomarker for patient selection [23]. We did not investigate the expression of IGF receptor or other related markers in this study, although any associations with PAPP-A expression would be interesting. The described role of PAPP-A in regulating local IGF availability for receptor binding and activation and its high degree of expression in breast cancer suggests PAPP-A might be a predictive biomarker for anti-PAPP-A therapies. As PAPP-A can be targeted [24], these investigations should be pursued as well.
In summary, PAPP-A expression was detected in almost all breast cancer specimens. There was more extensive and intense PAPP-A expression in luminal B than in luminal A specimens. The role of PAPP-A in breast cancer and its potential as a therapeutic target warrants further study.
HIGHLIGHTS.
PAPP-A expression was assessed by immunohistochemistry in human breast cancer
Specific staining for PAPP-A was found in almost all breast cancer specimens
The extent and intensity of staining was greater in the more aggressive subtypes
ACKNOWLEDGMENTS
The authors wish to thank Sean C. Harrington for excellent technical assistance and Hanne M. Lucier for help with manuscript submission.
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award number P50CA 116201, and an unrestricted research grant from Ansh Labs (to CAC). The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health. The funding sources had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Aaron S. Mansfield, Email: Mansfield.Aaron@mayo.edu.
Daniel W. Visscher, Email: Visscher.Daniel@mayo.edu.
Steven N. Hart, Email: Hart.Steven@mayo.edu.
Chen Wang, Email: Wang.Chen@mayo.edu.
Matthew P. Goetz, Email: Goetz.Matthew@mayo.edu.
Claus Oxvig, Email: co@mb.au.dk.
Cheryl A. Conover, Email: Conover.Cheryl@mayo.edu.
References
- 1.Lin TM, Galbert SP, Kiefer D, Spellacy WN, Gall S. Characterization of four human pregnancy-associated plasma proteins. Am J Obstet Gynecol. 1974;118:223–236. doi: 10.1016/0002-9378(74)90553-5. [DOI] [PubMed] [Google Scholar]
- 2.Conover CA. Key questions and answers about pregnancy-associated plasma protein- A Trends. Endocrinol Metab. 2012;23:242–249. doi: 10.1016/j.tem.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samani AA, Yakar S, LeRoith D, Brodt P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev. 2007;28:20–47. doi: 10.1210/er.2006-0001. [DOI] [PubMed] [Google Scholar]
- 4.Bulut I, Coskun A, Ciftci A, Cetinkaya E, Altiay G, Caglar T, Gulcan E. Relationship between pregnancy-associated plasma protein-A and lung cancer. Am J Med Sci. 2009;337:241–244. doi: 10.1097/MAJ.0b013e31818967a3. [DOI] [PubMed] [Google Scholar]
- 5.Alexiadis M, Mamers P, Chu S, Fuller PJ. Insulin-like growth factor, insulin-like growth factor-binding protein-4, and pregnancy-associated plasma protein-A gene expression in human granulosa cell tumors. Int J Gynecol Cancer. 2006;16:1973–1979. doi: 10.1111/j.1525-1438.2006.00724.x. [DOI] [PubMed] [Google Scholar]
- 6.Boldt HB, Conover CA. Overexpression of pregnancy-associated plasma protein-A in ovarian cancer cells promotes tumor growth in vivo. Endocrinology. 2011;152:1470–1478. doi: 10.1210/en.2010-1095. [DOI] [PubMed] [Google Scholar]
- 7.Pan H, Hanada S, Zhao J, Mao L, Zhi-Qing Ma M. Protein secretion is required for pregnancy-associated plasma protein-A to promote lung cancer growth in vivo. PLoS ONE. 2012;7:e4799. doi: 10.1371/journal.pone.0048799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanaka Y, Jobayashi H, Suzuki M, Hirashima Y, Kanayama N, Terao T. genetic downregulation of pregnancy-associated plasma protein-A (PAPP-A) by bikunin reduces IGF-I-dependent Akt and ERK1/2 activation and subsequently reduces ovarian cancer cell growth, invasion and metastasis. Int J Cancer. 2004;109:336–347. doi: 10.1002/ijc.11700. [DOI] [PubMed] [Google Scholar]
- 9.Huang J, Tabata S, Kakiuchi S, The Van T, Goto H, Hanibuchi M, Nishioka Y. Identification of pregancy-associated plasma protein A as a migration-promoting gene in malignant pleural mesothelioma cells: a potential therapeutic target. Oncotarget. 2013;4:1172–1184. doi: 10.18632/oncotarget.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Surmacz E. Function of the IGF-1 receptor in breast cancer. J Mammary Gland Biol Neoplasia. 2000;5(1):95–105. doi: 10.1023/a:1009523501499. [DOI] [PubMed] [Google Scholar]
- 11.Kuhajda FP, Eggleston JC. Pregnancy-associated plasma protein A. A clinically significant predictor of early recurrence in stage I breast carcinoma is independent of estrogen receptor status. The American Journal of Pathology. 1985;121:342–348. [PMC free article] [PubMed] [Google Scholar]
- 12.Ryan AJ, Napoletano S, Fitzpatrick PA, Currid CA, O'Sullivan NC, Harmey JH. Expression of a protease-resistant insulin-like growth factor binding protein-4 inhibits tumor growth in a murine model of breast cancer. British J Cancer. 2009;101:278–286. doi: 10.1038/sj.bjc.6605141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loddo M, Andryszkiewicz J, Rodriguez-Acebes S, Stoeber K, Jones A, Dafou D, Apostolidou S, Wollenschlaeger A, Widschwerndter M, Sainsbury R, Tudzarova S, Williams GH. Pregnancy-associated plasma protein A regulates mitosis and is epigenetically silenced in breast cancer. J Pathol. 2014;233:344–356. doi: 10.1002/path.4393. [DOI] [PubMed] [Google Scholar]
- 14.Bayes-Genis A, Conover CA, Overgaard MT, Bailey KR, Christiansen M, Holmes DR, Jr, Virmani R, Oxvig C, Schwartz RS. Pregnancy-associated plasma protein-A and diagnosis of acute coronary syndromes. N Engl J Med. 2001;345:1022–1029. doi: 10.1056/NEJMoa003147. [DOI] [PubMed] [Google Scholar]
- 15.Chen B-K, Leiferman KM, Pittelkow MR, Overgaard MT, Oxvig C, Conover CA. Localization and regulation of pregnancy associated plasma protein-A expression in healing human skin. J Clin Endocrinol Metab. 2003;88:4465–4471. doi: 10.1210/jc.2003-030193. [DOI] [PubMed] [Google Scholar]
- 16.Mikkelsen JH, Steffensen LB, Oxvig C. Development of a recombinant antibody towards PAPP-A for immunohistochemical use in multiple animal species. J Immunol Methods. 2014;404:33–40. doi: 10.1016/j.jim.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, Watson M, Davies S, Bernard PS, Parker JS, Perou CM, Ellis MJ, Nielsen TO. Ki index, HER2 status, and prognosis of patients with luminal B cancer. J Natl Cancer Inst. 2009;101:736–750. doi: 10.1093/jnci/djp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Creighton CJ. The molecular profile of luminal B breast cancer. Biologics. 2012;6:289–297. doi: 10.2147/BTT.S29923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhajda FP, Katumuluwa AI, Pasternack GR. Expression of haptoglobin-related protein and its potential role as a tumor antigen. Proc Natl Acad Sci USA. 1989;86:1188–1192. doi: 10.1073/pnas.86.4.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bukowski R, Chlebowski RT, Thune I, Furberg AS, Hankins GD, Malone FD, D'Alton ME. Birth weight, breast cancer and the potential mediating hormonal environment. PLoS One. 2012;7:e40199. doi: 10.1371/journal.pone.0040199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chander H, Halpern M, Resnick-Silverman L, Manfredi JJ, Germain D. Skp2B overexpression alters a prohibitin-p53 axis and the transcription of PAPP-A, the protease of insulin-like growth factor binding protein 4. PLoS One. 2011;6:e22456. doi: 10.1371/journal.pone.0022456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prager D, Li HL, Asa S, Melmed S. Dominant negative inhibition of tumorigenesis in vivo by human insulin-like growth factor I receptor mutant. Proc Natl Acad Sci U S A. 1994;91:2181–2185. doi: 10.1073/pnas.91.6.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer. 2012;12:159–169. doi: 10.1038/nrc3215. [DOI] [PubMed] [Google Scholar]
- 24.Mikkelsen JH, Resch ZT, Kalra B, Savjani G, Kumar A, Conover CA, Oxvig C. Indirect targeting of IGF receptor signaling in vivo by substrate selective inhibition of PAPP-A proteolytic activity. Oncotarget. 2014;5:1014–1025. doi: 10.18632/oncotarget.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]