Abstract
Background
Elevated blood pressure is the leading modifiable risk factor for cardiovascular disease (CVD) and premature death. The blood pressure waveform consists of discrete hemodynamic components, derived from measured central pressure and flow, which may contribute separately to risk for an adverse outcome. However, pressure-flow measures have not been studied in a large, community-based sample.
Methods and Results
We used proportional hazards models to examine association of incident CVD with forward pressure wave amplitude, mean arterial pressure, and global reflection coefficient derived from wave separation analysis and echocardiography in 2492 participants (mean age 66 ± 9 years, 56% women) in the Framingham Heart Study. During follow up (0.04 – 6.8 years), 149 participants (6%) had a CVD event. In multivariable models adjusting for age, sex, antihypertensive therapy, body mass index, heart rate, total and high density lipoprotein cholesterol concentrations, smoking, and presence of diabetes, forward pressure wave amplitude (HR=1.40; 95% CI: 1.16, 1.67; P=0.0003) was associated with incident CVD whereas mean arterial pressure (HR=1.10; 95% CI: 0.94, 1.29; P=0.25) and global wave reflection (HR=0.93; 95% CI: 0.78, 1.12; P=0.58) were not. After adding systolic blood pressure and carotid-femoral pulse wave velocity to the model, forward pressure wave amplitude persisted as a correlate of events (HR=1.33; 95% CI, 1.05, 1.68; P=0.02).
Conclusions
Higher forward pressure wave amplitude (a measure of proximal aortic geometry and stiffness) was whereas mean arterial pressure and relative wave reflection (correlates of resistance vessel structure and function) were not associated with increased risk for incident CVD.
Keywords: hemodynamic load, pulsatile hemodynamics, forward pressure wave amplitude, cardiovascular disease
Blood pressure is a major risk factor for cardiovascular disease (CVD) that accounts for almost 8 million premature deaths per year worldwide.1, 2 Over the past decades, medications used to treat hypertension and prevent CVD events were designed to reduce mean arterial pressure (MAP). However, this MAP-focused approach may be suboptimal in light of the preponderance of predominant or isolated systolic hypertension, particularly among patients with persistently elevated blood pressure despite treatment.3-5 To assess residual CVD risk associated with persistently elevated blood pressure, investigators have evaluated novel measures of aortic stiffness and hemodynamic load, such as peripheral and central pulse pressure (PP) and aortic pulse wave velocity, which may be predictive of CVD progression and events.6-14 Indeed, we have shown previously that increased carotid-femoral pulse wave velocity (CFPWV) is associated with increased risk for CVD events.6 Yet, little is known about the relative and incremental contributions to CVD risk of the mean and various pulsatile components of blood pressure.
PP plays an important role in the pathogenesis of hypertension, particularly after midlife, and higher PP is related to clinical events.11, 15-18 However, the components of PP that confer higher risk remain unclear. Some have argued that greater wave reflection, as indicated by augmentation index or augmented pressure, is associated with increased risk.7, 19-21 Yet, augmentation index and augmented pressure are composite measures that may be affected by forward and backward waves.22, 23 One must measure pressure and flow in order to separate forward and backward waves and compute the global reflection coefficient, which is the reference standard for assessing wave reflection.24, 25 Systolic blood pressure (SBP) has been proposed as a primary guide to prognosis and therapy; however, SBP provides an aggregate measure of the effects of mean and pulsatile pressure that may potentially overlook components of each. Therefore, knowledge of SBP alone does not establish whether an individual has an abnormality of mean or pulsatile load, which is a distinction that may have treatment implications. Moreover, an earlier Framingham study showed that blood pressure models combining SBP with diastolic blood pressure (DBP) or PP with MAP were superior to any of the four single blood pressure components (SBP, DBP, MAP or PP) considered alone at predicting CVD risk.26
To our knowledge, no prior community-based study has compared the relations to incident CVD of a comprehensive panel of individual mean and pulsatile components of blood pressure derived from an analysis of measured central aortic pressure and flow. We hypothesized that true central forward pressure wave amplitude (FWA), the primary hemodynamic correlate of variability in central and peripheral PP in younger and older individuals,27 would be an important predictor of CVD risk in models that adjusted for standard risk factors, including SBP.
Methods
Participants
The design and selection criteria for the Framingham Offspring study have been detailed previously.28 Participants attending the eighth examination cycle of the Offspring cohort (N=3021; 2005-2008) were eligible for this analysis. Tonometry measurements were first implemented beginning in February 1999 as described previously.29 A more comprehensive assessment of proximal aortic pressure-flow relations was implemented beginning with examination cycle eight of the Offspring cohort. Participants were excluded for the following reasons: prior CVD (n=295); off-site exam with no laboratory data (n=79); incomplete hemodynamic data (n=110); no follow up after examination cycle (n=12); missing covariate data (n=33). Only 45 (1.5%) participants were missing data on covariates or follow up. All protocols were approved by Boston University Medical Center's Institutional Review Board and participants provided written informed consent.
Clinical Evaluation and Definitions
Medical history, physical examination, and electrocardiography were performed routinely at each Framingham Heart Study (FHS) examination.28 Physician-acquired blood pressures represent the mean of two auscultatory measurements obtained on seated participants at the time of the Framingham clinic examination. The physician blood pressures were acquired using a mercury column sphygmomanometer and a standardized protocol with excellent measurement reproducibility. Peripheral PP was calculated as the difference between SBP and DBP. Body mass index was calculated by dividing weight in kilograms by the square of the height in meters. Criteria for diabetes mellitus were a fasting glucose level of 126 mg/dL (7.0 mmol/L) or greater, or the use of medications to treat diabetes. Smoking was defined as regular use of cigarettes in the prior year.
Non-invasive hemodynamics
Hemodynamic data were acquired as previously described.6 Participants were studied in the supine position after a 5-minute rest. Supine auscultatory brachial SBP and DBP at the time of tonometry (referred to as tonometry blood pressures) were obtained using a computer-controlled device. Arterial tonometry with simultaneous electrocardiography was obtained from brachial, radial, femoral, and carotid arteries using a custom tonometer. Next, 2-dimensional echocardiographic images of the left ventricular outflow tract were obtained from a parasternal long axis view followed by pulsed Doppler of the left ventricular outflow tract from an apical 5-chamber view. Tonometric, electrocardiographic, and echocardiographic data were digitized during the primary acquisition and transferred to the core laboratory (Cardiovascular Engineering, Inc., Norwood, MA) for analyses that were performed blinded to clinical data.
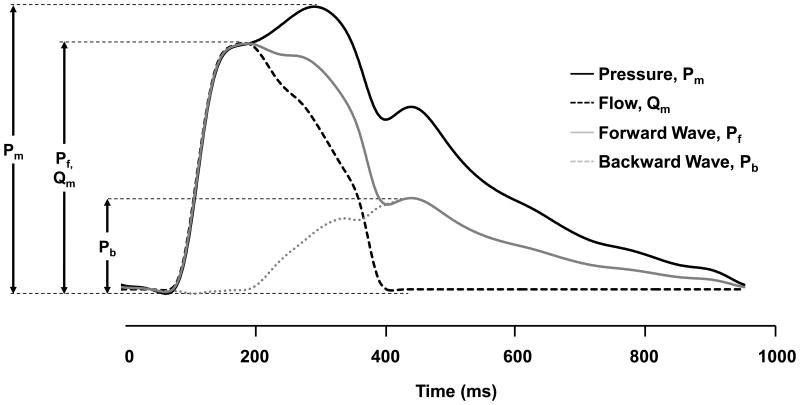
Tonometry waveforms were signal-averaged using the electrocardiographic R-wave as a fiducial point.6 Cuff SBP and DBP obtained at the time of tonometry were used to calibrate the peak and trough of the signal-averaged brachial pressure waveform. DBP and integrated brachial MAP were used to calibrate carotid pressure tracings.30 Calibrated carotid pressure was used as a surrogate for central pressure.30 MAP was calculated by integration of the calibrated brachial pressure waveform. By using measured central pressure and flow, the forward and backward pressure waves were separated as described previously (Figure 1).6 The FWA was defined as the difference between pressure at the foot and at the peak of the forward pressure waveform. The global reflection coefficient was calculated as backward wave amplitude divided by FWA. The primary pressure wave amplitude, a pressure-only surrogate for FWA, was defined as the pressure difference between the foot of the upstroke and the pressure at the first peak or inflection point of the carotid pressure waveform.31
Figure 1.
Central aortic wave separation analysis. Diastolic blood pressure is subtracted from measured pressure (Pm), leaving only the pulsatile component. Flow (Qm) is rescaled by characteristic impedance, Zc, in order to transform units to equivalent mm Hg and the upstrokes of pressure and flow are aligned. After rescaling and alignment, the forward pressure wave (Pf) is the average of Qm and Pm and backward wave (Pb) is the difference between Pm and Pf.
Outcomes
Major CVD events were defined as fatal or nonfatal myocardial infarction, unstable angina (prolonged ischemic episode with documented reversible ST segment changes), heart failure, and ischemic or hemorrhagic stroke. Medical records were obtained for all hospitalizations and physician visits related to CVD during follow up and were reviewed by a committee of three investigators; events were adjudicated following written guidelines. Criteria for these CVD events have been described previously.32, 33 Follow-up evaluations were performed on data acquired through December 31, 2011.
Statistical analyses
The primary goal was to assess whether individual key components of hemodynamic load are associated with increased risk for a first major CVD event adjusting for standard CVD risk factors. Baseline characteristics for the study sample were tabulated. We examined the association between separate components of hemodynamic load (FWA, global reflection coefficient, and MAP) and the time to a first major CVD event by using Cox proportional hazards regression, after confirming that the assumption of proportionality was met. Covariates were selected a priori and included components of the Framingham risk score:34 age, sex, use of antihypertensive therapy, serum total and high density lipoprotein cholesterol concentrations, smoking, body mass index, heart rate, and presence of diabetes mellitus.
Clinically-acquired physician SBP and the components of the pressure waveform (FWA, global reflection coefficient, primary pressure wave amplitude, and MAP) were added individually to the base model. Individual hemodynamic variables that were related to the incidence of CVD events in multivariable Cox models were evaluated further after accounting for physician SBP. In addition, in order to assess whether pulsatile load, input or both related to events, characteristic impedance and peak aortic flow, which are the major correlates of FWA, were entered together in a Cox model adjusting for covariates defined above. Similarly, in order to assess whether FWA or backward wave amplitude or both related to events, FWA and backward wave amplitude were entered together in a Cox model adjusting for covariates defined above. For individual hemodynamic variables that showed statistically significant relations with incident CVD events, we examined effect modification. We tested interactions of three covariates – older age (defined as 65 – 90 vs. 40 – 64), sex, and hypertension treatment status – with hemodynamic variables by incorporating corresponding interaction terms in the analysis. In order to illustrate relations between hemodynamic variables and events, continuous predictor variables were segregated by quartiles (Q1 – Q4), and Kaplan-Meier curves of survival free of a CVD event were constructed. We performed cause-specific hazard analyses in the Cox models, where non-CVD death was a competing (censoring) event; Kaplan-Meier plots were not modified for competing events.
All analyses were performed with SAS version 9.3. For our primary analysis, we evaluated four components of blood pressure (FWA, reflection coefficient, primary pressure wave, and MAP); hence, a 2-sided P < 0.05/4 = 0.0125 was considered statistically significant.
Results
Study exclusion criteria resulted in a sample of 2492 participants (1402 [56%] women). Table S1 presents a comparison of baseline characteristics of included and excluded participants. Baseline characteristics of the study sample are presented in Table 1. During follow-up (0.04 – 6.8, median 5.4 years), 149 of 2492 participants (6%) had a first major CVD event. The most common events were myocardial infarctions (n=50), heart failure (n=46), and stroke (n=38); 13 episodes (9% of all events) were fatal. Figure 1 depicts a representative example of measured pressure and flow and separated forward and backward pressure waveforms. The amplitudes (maximum minus minimum) of these waveforms were used to define the hemodynamic components of blood pressure. Table S2 provides a correlation matrix for standard blood pressure and component hemodynamic measures.
Table 1.
Baseline characteristics of the sample (N=2492).
| Variable | Value |
|---|---|
| Clinical Measures | |
| Age, years | 66 ± 9 |
| Women, N (%) | 1402 (56) |
| Height, cm | 167 ± 10 |
| Weight, kg | 78 ± 17 |
| Body mass index, kg/m2 | 28 ± 5 |
| Physician blood pressure, mm Hg | |
| Systolic | 128 ± 17 |
| Diastolic | 74 ± 10 |
| Pulse | 54 ± 16 |
| Heart rate, beats/min | 62 ± 10 |
| Total cholesterol, mg/dl | 189 ± 37 |
| HDL cholesterol, mg/dl | 58 ± 18 |
| Hypertension treatment, N (%) | 1124 (45) |
| Diabetes, N (%) | 288 (12) |
| Current smoker, N (%) | 219 (9) |
| Hemodynamics measures | |
| True forward pressure wave amplitude, mm Hg | 57 ± 17 |
| Primary pressure wave amplitude, mm Hg | 50 ± 15 |
| Tonometry mean arterial pressure, mm Hg | 98 ± 12 |
| Global reflection coefficient | 0.36 ± 0.07 |
| Characteristic impedance, DSC | 245 ± 97 |
| Peak aortic flow, mL/s | 306 ± 69 |
DSC, dyne × sec/cm5. All values are mean ± standard deviation except as noted.
Cox proportional hazards models for individual components of hemodynamic load are presented in Table 2. After adjusting for standard risk factors, several of the pulsatile blood pressure measures, including physician SBP, true FWA, backward wave, and primary pressure wave amplitude, were associated with increased risk for a first major CVD event (Table 2, left). In contrast, MAP and reflection coefficient were not significantly related to events in risk factor-adjusted models. In a dual model that considered both FWA and backward wave amplitude, FWA (HR=1.32, 95% CI: 1.01, 1.73; P=0.04) was associated with events whereas backward wave amplitude (HR=1.07, 95% CI: 0.84, 1.37; P=0.59) was not. When the single hemodynamic component models were adjusted for physician SBP (Table 2, center) and further adjusted for CFPWV (Table 2, right), the relation between true FWA and CVD events persisted whereas relations for other variables were no longer significant.
Table 2.
Measures of individual hemodynamic components as predictors of a major CVD event (N=2492).
| Hemodynamic measure | Hazard Ratio Including Standard Risk Factors (LCL, UCL) | P | Hazard Ratio Including Standard Risk Factors and Physician SBP (LCL, UCL) | P | Hazard Ratio Including Standard Risk Factors, Physician SBP, and CFPWV (LCL, UCL) | P |
|---|---|---|---|---|---|---|
| Physician SBP | 1.26 (1.07, 1.48) | 0.005 | - | - | - | - |
| Tonometry mean arterial pressure | 1.10 (0.94, 1.29) | 0.25 | 0.92 (0.75, 1.13) | 0.45 | 0.98 (0.79, 1.22) | 0.88 |
| Forward pressure wave amplitude | 1.40 (1.16, 1.67) | <0.001 | 1.32 (1.06, 1.64) | 0.01 | 1.33 (1.05, 1.68) | 0.02 |
| Backward pressure wave amplitude | 1.30 (1.10, 1.53) | 0.002 | 1.20 (0.98, 1.47) | 0.08 | 1.21 (0.97, 1.50) | 0.09 |
| Primary pressure wave amplitude | 1.28 (1.07, 1.52) | 0.007 | 1.17 (0.95, 1.44) | 0.13 | 1.16 (0.94, 1.45) | 0.17 |
| Global reflection coefficient | 0.95 (0.79, 1.14) | 0.58 | 0.93 (0.78, 1.12) | 0.45 | 0.94 (0.78, 1.13) | 0.52 |
Models add hemodynamic variables to the covariates individually, one at a time. In the center, all models also include physician SBP, and on the right, all models are additionally adjusted for carotid femoral pulse wave velocity (CFPWV). LCL, UCL, lower and upper limits of the 95% confidence intervals; SBP, systolic blood pressure. HRs expressed per 1 SD higher value, adjusted for standard risk factors; 149 events (6%) with median of 5.4 years of follow up.
In a dual model that evaluated whether risk associated with FWA was attributable to increased pulsatile load or flow, characteristic impedance (HR=1.36; 95% CI: 1.09, 1.71; P=0.008), but not peak aortic flow (HR=1.36; 95% CI: 0.99, 1.56; P=0.06), was related to events. We did not find significant effect modification of the FWA relation with events by sex (P=0.06), age (P=0.39), or hypertension treatment status (P=0.59). Since the cerebral circulation may be particularly sensitive to loss of wave reflection,35 we performed a sensitivity analysis that excluded stroke as an endpoint (n=38), resulting in 111 events (4.5%) with a median of 5.4 years of follow-up. Results of the primary outcome models were not substantively different in that MAP (P=0.14) and global reflection coefficient (P=0.20) remained not significantly related to outcomes whereas FWA (HR=1.60, 95% CI: 1.29, 1.97; P<0.001) and related variables had moderately higher hazard ratios.
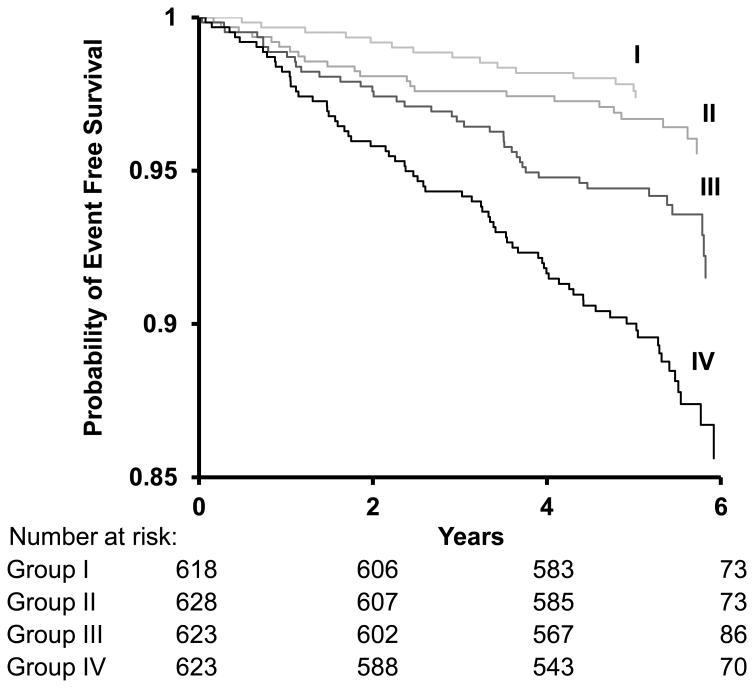
Figure 2 depicts the estimated cumulative probability of remaining free of a first major CVD event in groups defined according to quartiles of FWA. In a model that adjusted for standard risk factors, participants in the highest (≥67 mm Hg) as compared with those of the lowest (<45 mm Hg) FWA group had an adjusted HR of 2.5 (95% CI, 1.4 to 4.6; P=0.002).
Figure 2.
Kaplan-Meier plot of the probability of remaining free of first major CVD event during follow-up by quartiles of forward pressure wave amplitude. Participants are grouped according to quartiles of forward pressure wave amplitude: Group I (<45.0 mm Hg), Group II (45.0 to <55.1 mm Hg), Group III (55.1 to <66.8 mm Hg), and Group IV (≥66.8 mm Hg). CVD events per person for quartile groups of FWA were as follows: first quartile group, 16/618 (2.4%); second quartile group, 23/628 (3.7%); third quartile group, 40/623 (6.4%); and fourth quartile group, 71/623 (11.4%).
Discussion
Principal Findings
We investigated relations of individual mean and pulsatile components of blood pressure with incidence of first-onset CVD events in middle-age and older participants in the community-based Framingham Heart Study. In a model that included standard CVD risk factors, SBP, primary pressure wave, and FWA were associated with increased risk whereas MAP was not, indicating that the pulsatile rather than the steady flow component of blood pressure was associated with increased risk for CVD. The global reflection coefficient was not associated with incident CVD events in the base risk factor model. Similarly, backward wave amplitude was not related to events in a dual model that included FWA, indicating that after considering the effects of FWA, relative wave reflection was not associated with CVD risk. When the base model was further adjusted for physician SBP, the association between higher FWA and CVD risk persisted, indicating that clinic SBP alone does not fully capture blood pressure-related risk. We have demonstrated previously that higher CFPWV was associated with higher CVD risk.6 In the present study, after further adjusting for CFPWV, the relation between FWA and CVD remained, underscoring the complementary nature of these related but distinct measures of aortic function. Additionally, in the dual model, we showed that characteristic impedance, but not peak aortic flow, was associated with elevated risk for CVD events. These data suggest that aortic wall stiffness or a mismatch between aortic diameter and pulsatile flow accounts for the increased risk associated with higher FWA. Thus, our results indicate that the forward pressure wave component of blood pressure and pulsatile load, rather than MAP, is the individual component of blood pressure that is most closely associated with first-onset major CVD events in our middle-aged and older community-based sample.
Relations of Components of Hemodynamic Load with Incident CVD Events
Several studies have evaluated relations between hemodynamic load and risk for CVD and disease-related events,6-13 including studies by Kannel et al.,36, 37 along with many others who have emphasized the prognostic value of SBP.38 Recent studies have shown that higher PP is associated with increased long-term CVD risk and progression, especially in advanced age,16, 18, 39-41 and in patients with heart failure,42 myocardial infarction,43 coronary artery disease,44, 45 and hypertension.15, 46 Although PP is a widely available measure of arterial stiffness and pressure pulsatility, components of PP that account for increased CVD risk remain unknown. In this study, we adjusted our models for physician SBP, a well-accepted CVD risk factor; however, variable amplification between central and peripheral sites may offer variable relations of central and peripheral SBP and PP with CVD events. Yet, SBP and PP — whether central or brachial — represent composite hemodynamic measures. As a result, one cannot be certain which component of blood pressure confers risk when SBP or PP alone is examined.
Other studies that sought to assess the relation between central arterial hemodynamics and CVD events did not directly measure central aortic flow. Instead, they used either a typical flow waveform or a pseudo-flow waveform that was derived from the pressure data using various modeling assumptions.7, 20, 21 Therefore, these studies were able to provide only an estimate of FWA and backward wave amplitude. In contrast, our study assessed the relative contributions to CVD risk of a comprehensive panel of individual components of mean and pulsatile hemodynamic load derived from directly measured central pressure and flow. The utility of absolute as compared to relative measures of wave reflection as markers of CVD risk has been debated.7, 20, 22 However, measures of absolute pressure wave reflection, such as augmentation pressure or backward wave amplitude, are highly correlated with FWA.23 For example, in our cohort, absolute backward wave amplitude was more closely related to FWA (r=0.78, P<0.001) than to the global reflection coefficient (r=0.39, P<0.001). Additionally, pressure-only measures of wave reflection are confounded by ventricular function, which affects the balance between pressure augmentation and flow deceleration.22, 23, 47 In the present study, to assess whether CVD event risk was attributable to increased forward or backward waves, we used a dual model that considered both FWA and backward wave amplitude: FWA remained significant while backward wave amplitude did not, demonstrating that the association between the backward pressure wave and events was attributable to larger forward wave, not greater wave reflection, which is consistent with the findings for reflection coefficient alone.
The primary pressure wave amplitude, which is a pressure-only surrogate for FWA, was not associated with CVD risk after multivariable adjustment. The primary pressure wave amplitude, which is identified by an initial peak or inflection point in the central pressure waveform (Figure 1), may be confounded by variable overlap between forward and backward pressure waves. Depending on timing and shape of the backward wave, the true forward wave may peak before or continue to rise after the inflection point that is used to identify the transition from forward to backward pressure wave in a central pressure waveform. Therefore, primary pressure wave amplitude misclassifies a variable and informative component of the true FWA.
The current study extends prior work and implicates the FWA by demonstrating its role in the adverse effects related to aortic stiffening and increased hemodynamic load with aging, which are not fully captured by SBP alone. SBP represents an aggregate measure of mean and pulsatile load that includes variable contributions from backward pressure waves and only a portion of the forward wave; consequently, SBP fails to capture the full measure of risk associated with increased forward pressure wave pulsatility. Importantly, incident CVD events were not related to wave reflection or components of steady hemodynamic load as they did not provide incremental CVD risk prediction after considering standard CVD risk factors in this community-based sample of middle-aged and older people. Although risk reclassification analysis using candidate hemodynamic measures is beyond the scope of this study, we believe a thorough investigation of the relative prognostic value for individual components of hemodynamic load is an important future direction, which could improve upon current models and standard risk factors for incident CVD.
Potential Paradigm Shift in Hypertension Treatment
The World Health Organization has classified hypertension as the leading cause of preventable death throughout the world.48 A variety of pharmacological agents approved for treatment of hypertension are widely available, included low-cost generics. Yet, control of hypertension is less than ideal, with blood pressure reduced below hypertensive levels in about half of treated patients.3 Although current antihypertensive medications were systematically designed to reduce MAP, we have shown that MAP was not associated with CVD events in our cohort. In addition, drugs that reduce MAP, such as resistance vessel dilators, can increase cardiac output and peak flow, which could increase FWA and limit the reduction in CVD events even though SBP and MAP are reduced. These hypotheses will require testing in suitably designed intervention studies.
Recently, the Eighth Joint National Committee of the National Heart, Lung, and Blood Institute updated the U.S. hypertension management guidelines, which set the target SBP for the population aged 60 years or older to less than 150 mm Hg.49 The relaxed guidelines will presumably reduce the adverse effects of unnecessary antihypertensive medications. However, focusing on the level of SBP alone may incompletely characterize risk. Based on our observations, we posit that a borderline-to-high SBP with normal FWA may not be as risky as a comparable SBP with markedly elevated FWA. Such a distinction may make it possible to ease the guidelines for SBP while still identifying cases that will benefit from more aggressive treatment.
Furthermore, our data suggest a need to better define the effects of existing therapeutic agents on aortic function and FWA. For example, a prior study used a hemodynamic protocol analogous to the one used in the present study and demonstrated that relatively short-term therapy with omapatrilat reduced PP, characteristic impedance, and FWA in middle-aged and older patients with uncomplicated systolic hypertension.50 Similar evaluation of novel and existing therapies would better define the hemodynamic effects of antihypertensive drug therapy and would inform design of clinical trials that could test the potential clinical value of a treatment strategy that targets modification of proximal aortic properties as a primary goal of therapy. Whether current or novel drugs with favorable effects on proximal aortic properties that produce preferential reduction in FWA will be more effective at reducing CVD events will require prospective evaluation in randomized trials.
Limitations
Our study has limitations that should be considered. Our prospective study is observational; therefore, we are unable to demonstrate a causal link underlying the association between increased FWA and increased CVD events. In addition, we cannot discount the possibility of residual confounding by duration or severity of unknown or associated risk factors. Although we adjusted for use of antihypertensive medications and did not find evidence of effect modification by their usage, this potential confounder was highly prevalent in our cohort (45%). Furthermore, during the follow-up period, the cohort had a modest number of events; however, we had adequate power to detect moderate effects. In addition, since the low number of incident CVD events reduces the precision of the estimates, an estimation of risk reclassification was not performed. Finally, since we evaluated participants who were middle-aged and older and predominately white, our results may not be generalizable to younger individuals or different ethnicities.
Conclusion
In summary, we have shown that FWA is the key component of hemodynamic load that is associated with CVD events in a model that adjusts for standard risk factors. Current pharmacological strategies used to treat hypertension focus on reducing hemodynamic load by reducing MAP and may not adequately address a primary cause of blood pressure elevation,10 CVD progression, and associated excess morbidity and mortality. Our data suggest that further research on hemodynamic mechanisms and clinical effects of interventions that focus on proximal aortic properties and pulsatile load is merited.
Supplementary Material
Acknowledgments
From the Framingham Heart Study of the National Heart Lung and Blood Institute of the National Institutes of Health and Boston University School of Medicine. This article is dedicated to the memory of Dr. Joseph A. Vita, MD, an admired and respected friend, colleague and mentor who will be greatly missed.
Funding Sources: This work was supported by the National Heart, Lung and Blood Institute's Framingham Heart Study (Contract No. N01-HC-25195) and by HL076784, AG028321, HL070100, HL060040, HL080124, HL071039, HL077447, HL107385, and 2-K24-HL04334. L.L.C. is also supported by NIH grant 5T32HL094300-05.
Footnotes
Disclosures: G.F.M. is owner of Cardiovascular Engineering, Inc., a company that designs and manufactures devices that measure vascular stiffness. The company uses these devices in clinical trials that evaluate the effects of diseases and interventions on vascular stiffness. The remaining authors report no conflicts.
References
- 1.Fields LE, Burt VL, Cutler JA, Hughes J, Roccella EJ, Sorlie P. The burden of adult hypertension in the United States 1999 to 2000: a rising tide. Hypertension. 2004;44:398–404. doi: 10.1161/01.HYP.0000142248.54761.56. [DOI] [PubMed] [Google Scholar]
- 2.Lawes CMM, Vander Hoorn S, Rodgers A. Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371:1513–1518. doi: 10.1016/S0140-6736(08)60655-8. [DOI] [PubMed] [Google Scholar]
- 3.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ National High Blood Pressure Education Program Coordinating Committee. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 4.Lloyd-Jones DM, Evans JC, Larson MG, O'Donnell CJ, Roccella EJ, Levy D. Differential control of systolic and diastolic blood pressure: factors associated with lack of blood pressure control in the community. Hypertension. 2000;36:594–599. doi: 10.1161/01.hyp.36.4.594. [DOI] [PubMed] [Google Scholar]
- 5.Franklin SS, Jacobs MJ, Wong ND, L'Italien GJ, Lapuerta P. Predominance of isolated systolic hypertension among middle-aged and elderly US hypertensives: analysis based on National Health and Nutrition Examination Survey (NHANES) III. Hypertension. 2001;37:869–874. doi: 10.1161/01.hyp.37.3.869. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weber T, Wassertheurer S, Rammer M, Haiden A, Hametner B, Eber B. Wave reflections, assessed with a novel method for pulse wave separation, are associated with end-organ damage and clinical outcomes. Hypertension. 2012;60:534–541. doi: 10.1161/HYPERTENSIONAHA.112.194571. [DOI] [PubMed] [Google Scholar]
- 8.Russo C, Jin ZZ, Palmieri V, Homma S, Rundek T, Elkind MSV, Sacco RL, Di Tullio MR. Arterial stiffness and wave reflection: sex differences and relationship with left ventricular diastolic function. Hypertension. 2012;60:362–368. doi: 10.1161/HYPERTENSIONAHA.112.191148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Regnault V, Thomas F, Safar ME, Osborne-Pellegrin M, Khalil RA, Pannier B, Lacolley P. Sex difference in cardiovascular risk: role of pulse pressure amplification. J Am Coll Cardiol. 2012;59:1771–1777. doi: 10.1016/j.jacc.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308:875–881. doi: 10.1001/2012.jama.10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glasser SP, Halberg DL, Sands C, Gamboa CM, Muntner P, Safford M. Is pulse pressure an independent risk factor for incident acute coronary heart disease events? the REGARDS Study. Am J Hypertens. 2013;27:555–563. doi: 10.1093/ajh/hpt168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berard E, Bongard V, Ruidavets JB, Amar J, Ferrieres J. Pulse wave velocity, pulse pressure and number of carotid or femoral plaques improve prediction of cardiovascular death in a population at low risk. J Hum Hypertens. 2013;27:529–534. doi: 10.1038/jhh.2013.8. [DOI] [PubMed] [Google Scholar]
- 13.Baba Y, Ishikawa S, Kayaba K, Gotoh T, Kajii E. High pulse pressure is associated with increased risk of stroke in Japanese: the JMS Cohort Study. Blood Press. 2011;20:10–14. doi: 10.3109/08037051.2010.516075. [DOI] [PubMed] [Google Scholar]
- 14.Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, Hwang SJ, Lakatta EG, Laurent S, Maldonado J, Mitchell GF, Najjar SS, Newman AB, Ohishi M, Pannier B, Pereira T, Vasan RS, Shokawa T, Sutton-Tyrell K, Verbeke F, Wang KL, Webb DJ, Willum Hansen T, Zoungas S, McEniery CM, Cockcroft JR, Wilkinson IB. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63:636–646. doi: 10.1016/j.jacc.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blacher J, Staessen JA, Girerd X, Gasowski J, Thijs L, Liu L, Wang JG, Fagard RH, Safar ME. Pulse pressure not mean pressure determines cardiovascular risk in older hypertensive patients. Arch Intern Med. 2000;160:1085–1089. doi: 10.1001/archinte.160.8.1085. [DOI] [PubMed] [Google Scholar]
- 16.Benetos A, Safar M, Rudnichi A, Smulyan H, Richard JL, Ducimetieere P, Guize L. Pulse pressure: a predictor of long-term cardiovascular mortality in a French male population. Hypertension. 1997;30:1410–1415. doi: 10.1161/01.hyp.30.6.1410. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell GF, Vasan RS, Keyes MJ, Parise H, Wang TJ, Larson MG, D'Agostino RB, Kannel WB, Levy D, Benjamin EJ. Pulse pressure and risk of new-onset atrial fibrillation. JAMA. 2007;297:709–715. doi: 10.1001/jama.297.7.709. [DOI] [PubMed] [Google Scholar]
- 18.Vaccarino V, Holford TR, Krumholz HM. Pulse pressure and risk for myocardial infarction and heart failure in the elderly. J Am Coll Cardiol. 2000;36:130–138. doi: 10.1016/s0735-1097(00)00687-2. [DOI] [PubMed] [Google Scholar]
- 19.Janner JH, Godtfredsen NS, Ladelund S, Vestbo J, Prescott E. High aortic augmentation index predicts mortality and cardiovascular events in men from a general population, but not in women. Eur J Prev Cardiol. 2012;20:1005–1012. doi: 10.1177/2047487312449588. [DOI] [PubMed] [Google Scholar]
- 20.Wang KL, Cheng HM, Sung SH, Chuang SY, Li CH, Spurgeon HA, Ting CT, Najjar SS, Lakatta EG, Yin FC, Chou P, Chen CH. Wave reflection and arterial stiffness in the prediction of 15-year all-cause and cardiovascular mortalities: a community-based study. Hypertension. 2010;55:799–805. doi: 10.1161/HYPERTENSIONAHA.109.139964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chirinos JA, Kips JG, Jacobs DR, Brumback L, Duprez DA, Kronmal R, Bluemke DA, Townsend RR, Vermeersch S, Segers P. Arterial wave reflections and incident cardiovascular events and heart failure: MESA (multiethnic study of atherosclerosis) J Am Coll Cardiol. 2012;60:2170–2177. doi: 10.1016/j.jacc.2012.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell GF. Arterial stiffness and hypertension. Hypertension. 2014 doi: 10.1161/hypertensionaha.114.00921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torjesen A, Wang N, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Forward and backward wave morphology and central pressure augmentation in men and women in the Framingham Heart Study. Hypertension. 2014;64:259–265. doi: 10.1161/HYPERTENSIONAHA.114.03371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tartiere JM, Logeart D, Safar ME, Cohen-Solal A. Interaction between pulse wave velocity, augmentation index, pulse pressure and left ventricular function in chronic heart failure. J Hum Hypertens. 2006;20:213–219. doi: 10.1038/sj.jhh.1001965. [DOI] [PubMed] [Google Scholar]
- 25.Hughes AD, Park C, Davies J, Francis D, Mc GTSA, Mayet J, Parker KH. Limitations of augmentation index in the assessment of wave reflection in normotensive healthy individuals. PloS One. 2013;8:e59371. doi: 10.1371/journal.pone.0059371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franklin SS, Lopez VA, Wong ND, Mitchell GF, Larson MG, Vasan RS, Levy D. Single versus combined blood pressure components and risk for cardiovascular disease: the Framingham Heart Study. Circulation. 2009;119:243–250. doi: 10.1161/CIRCULATIONAHA.108.797936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitchell GF, Wang N, Palmisano JN, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS. Hemodynamic correlates of blood pressure across the adult age spectrum: noninvasive evaluation in the Framingham Heart Study. Circulation. 2010;122:1379–1386. doi: 10.1161/CIRCULATIONAHA.109.914507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham Offspring Study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell GF, Guo CY, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Cross-sectional correlates of increased aortic stiffness in the community: the Framingham Heart Study. Circulation. 2007;115:2628–2636. doi: 10.1161/CIRCULATIONAHA.106.667733. [DOI] [PubMed] [Google Scholar]
- 30.Kelly R, Fitchett D. Noninvasive determination of aortic input impedance and external left ventricular power output: a validation and repeatability study of a new technique. J Am Coll Cardiol. 1992;20:952–963. doi: 10.1016/0735-1097(92)90198-v. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43:1239–1245. doi: 10.1161/01.HYP.0000128420.01881.aa. [DOI] [PubMed] [Google Scholar]
- 32.Kannel WB, Wolfe PA, Garrison RJ. Monograph Section 34: Some Risk Factors Related to the Annual Incidence of Cardiovascular Disease and Death in Pooled Repeated Biennial Measurements: Framingham Heart Study, 30 Year Follow-up. Spingfiled, MA: National Technical Information Service; 1987. pp. 1–459. [Google Scholar]
- 33.Frankel DS, Vasan RS, D'Agostino RB, Sr, Benjamin EJ, Levy D, Wang TJ, Meigs JB. Resistin, adiponectin, and risk of heart failure: the Framingham Offspring Study. J Am Coll Cardiol. 2009;53:754–762. doi: 10.1016/j.jacc.2008.07.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Cholesterol Education Program Expert Panel on Detection Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 35.Mitchell GF, van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson O, Garcia M, Aspelund T, Harris TB, Gudnason V, Launer LJ. Arterial stiffness, pressure and flow pulsatility and brain structure and function: The age, gene/environment susceptibility--Reykjavik study. Brain. 2011;134:3398–3407. doi: 10.1093/brain/awr253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kannel WB, Schwartz MJ, Mcnamara PM. Blood Pressure and Risk of Coronary Heart Disease: the Framingham Study. Dis Chest. 1969;56:43–52. doi: 10.1378/chest.56.1.43. [DOI] [PubMed] [Google Scholar]
- 37.Kannel WB, Dawber TR, Mcgee DL. Perspectives on systolic hypertension: the Framingham Study. Circulation. 1980;61:1179–1182. doi: 10.1161/01.cir.61.6.1179. [DOI] [PubMed] [Google Scholar]
- 38.Kocemba J, Kawecka-Jaszcz K, Gryglewska B, Grodzicki T. Isolated systolic hypertension: pathophysiology, consequences and therapeutic benefits. J Hum Hypertens. 1998;12:621–626. doi: 10.1038/sj.jhh.1000676. [DOI] [PubMed] [Google Scholar]
- 39.Benetos A, Gautier S, Labat C, Salvi P, Valbusa F, Marino F, Toulza O, Agnoletti D, Zamboni M, Dubail D, Manckoundia P, Rolland Y, Hanon O, Perret-Guillaume C, Lacolley P, Safar ME, Guillemin F. Mortality and cardiovascular events are best predicted by low central/peripheral pulse pressure amplification but not by high blood pressure levels in elderly nursing home subjects: the PARTAGE (predictive values of blood pressure and arterial stiffness in institutionalized very aged population) Study. J Am Coll Cardiol. 2012;60:1503–1511. doi: 10.1016/j.jacc.2012.04.055. [DOI] [PubMed] [Google Scholar]
- 40.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: A “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 41.Domanski MJ, Davis BR, Pfeffer MA, Kastantin M, Mitchell GF. Isolated systolic hypertension: prognostic information provided by pulse pressure. Hypertension. 1999;34:375–380. doi: 10.1161/01.hyp.34.3.375. [DOI] [PubMed] [Google Scholar]
- 42.Domanski MJ, Mitchell GF, Norman JE, Exner DV, Pitt B, Pfeffer MA. Independent prognostic information provided by sphygmomanometrically determined pulse pressure and mean arterial pressure in patients with left ventricular dysfunction. J Am Coll Cardiol. 1999;33:951–958. doi: 10.1016/s0735-1097(98)00679-2. [DOI] [PubMed] [Google Scholar]
- 43.Mitchell GF, Moye LA, Braunwald E, Rouleau JL, Bernstein V, Geltman EM, Flaker GC, Pfeffer MA. Sphygmomanometrically determined pulse pressure is a powerful independent predictor of recurrent events after myocardial infarction in patients with impaired left ventricular function. Circulation. 1997;96:4254–4260. doi: 10.1161/01.cir.96.12.4254. [DOI] [PubMed] [Google Scholar]
- 44.Jankowski P, Kawecka-Jaszcz K, Czarnecka D, Brzozowska-Kiszka M, Styczkiewicz K, Loster M, Kloch-Badelek M, Wilinski J, Curylo AM, Dudek D Aortic Blood Pressure and Survival Study Group. Pulsatile but not steady component of blood pressure predicts cardiovascular events in coronary patients. Hypertension. 2008;51:848–855. doi: 10.1161/HYPERTENSIONAHA.107.101725. [DOI] [PubMed] [Google Scholar]
- 45.Franklin SS, Khan SA, Wong ND, Larson MG, Levy D. Is pulse pressure useful in predicting risk for coronary heart disease? the Framingham Heart Study. Circulation. 1999;100:354–360. doi: 10.1161/01.cir.100.4.354. [DOI] [PubMed] [Google Scholar]
- 46.Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Pede S, Porcellati C. Ambulatory pulse pressure: a potent predictor of total cardiovascular risk in hypertension. Hypertension. 1998;32:983–988. doi: 10.1161/01.hyp.32.6.983. [DOI] [PubMed] [Google Scholar]
- 47.Fok H, Guilcher A, Li Y, Brett S, Shah A, Clapp B, Chowienczyk P. Augmentation pressure is influenced by ventricular contractility/relaxation dynamics novel mechanism of reduction of pulse pressure by nitrates. Hypertension. 2014;63:1050–1055. doi: 10.1161/HYPERTENSIONAHA.113.02955. [DOI] [PubMed] [Google Scholar]
- 48.World Health Organization. World Health Statistics 2013. [Accessed May 14, 2014]; http://www.who.int/gho/publications/world_health_statistics/EN_WHS2013_Full.pdf?ua=1.
- 49.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, Lefevre ML, Mackenzie TD, Ogedegbe O, Smith SC, Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT, Jr, Narva AS, Ortiz E. Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the eighth joint national committee (JNC 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 50.Mitchell GF, Izzo JL, Lacourciere Y, Ouellet JP, Neutel J, Qian CL, Kerwin LJ, Block AJ, Pfeffer MA. Omapatrilat reduces pulse pressure and proximal aortic stiffness in patients with systolic hypertension: results of the conduit hemodynamics of omapatrilat international research study. Circulation. 2002;105:2955–2961. doi: 10.1161/01.cir.0000020500.77568.3c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.