SUMMARY
Phospholipase C β (PLCβ) enzymes are dramatically activated by heterotrimeric G proteins. Central to this response is the robust autoinhibition of PLCβ by an X–Y linker region within its catalytic core and by the Hα2′ helix in the C-terminal extension of the enzyme. The molecular mechanism of each and their mutual dependence are poorly understood. Herein it is shown that distinct regions within the X–Y linker have specific roles in regulating activity. Most importantly, an acidic stretch within the linker stabilizes a lid that occludes the active site, consistent with crystal structures of variants lacking this region. Inhibition by the Hα2′ helix is independent of the X–Y linker and likely regulates activity by limiting membrane interaction of the catalytic core. Full activation of PLCβ thus requires multiple independent molecular events induced by membrane association of the catalytic core and by the binding of regulatory proteins.
INTRODUCTION
Phospholipase C (PLC) enzymes hydrolyze the inner membrane lipid phosphatidylinositol-4,5-bisphosphate (PIP2) to produce inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG) in response to diverse cellular stimuli. IP3 promotes the release of intracellular Ca2+, and DAG, together with increased Ca2+, activates protein kinase C (PKC) (Gresset et al., 2012; Kadamur and Ross, 2013). PLCβ enzymes thereby contribute to numerous processes, including chemotaxis (Li et al., 2000; Tang et al., 2011), neurological function (Han et al., 2006; Kim et al., 1997), and opioid sensitivity (Bianchi et al., 2009; Bonacci et al., 2006; Mathews et al., 2008). The physiological role of PLCβ is best characterized in the cardiovascular system, where dysregulation of activation or expression can result in hypertrophy (Filtz et al., 2009; Grubb et al., 2012) and heart failure (Mende et al., 1998; Woodcock et al., 2010). Pathways for PLCβ activation involve direct interactions with the heterotrimeric G protein subunits Gαq and Gβγ (Camps et al., 1992a; Camps et al., 1992b; Jiang et al., 1994; Paterson et al., 1995; Smrcka et al., 1991; Smrcka and Sternweis, 1993) and with the Rac family of small molecular weight G proteins (Camps et al., 1990; Illenberger et al., 1997; Illenberger et al., 2003a). These interactions increase the rate of PIP2 hydrolysis up to ~60-fold.
PLCβ enzymes are comprised of an N-terminal pleckstrin homology (PH) domain, four tandem EF hand repeats, a catalytic TIM barrel domain (split by an inhibitory X–Y linker), a C2 domain, and a ~400 amino acid extension (Gresset et al., 2012) which is unique to the PLCβ family. Its amino terminus contains the proximal C-terminal domain (CTD) which houses the primary Gαq binding site (the Hα1/Hα2 helical elbow) (Waldo et al., 2010) and the Hα2′ autoinhibitory helix (Lyon et al., 2011). An unconserved CTD linker connects the proximal and distal CTD, the latter of which forms an extended coiled coil domain that contributes to Gαq binding and serves as the primary membrane binding determinant of the enzyme (Ilkaeva et al., 2002; Jenco et al., 1997; Kim et al., 1996; Lyon et al., 2013; Park et al., 1993; Singer et al., 2002)(Figure 1A). Both Rac and Gβγ are thought to activate PLCβ via direct interaction with the catalytic core (Guo et al., 2003; Illenberger et al., 2003a; Jezyk et al., 2006; Runnels and Scarlata, 1999; Sankaran et al., 1998), which is thought help orient the catalytic core of the enzyme with respect to the membrane where its substrate is found (Drin et al., 2006; Han et al., 2011; Illenberger et al., 2003b).
Figure 1.
The PLCβ X–Y linker and proximal C-terminal domain (CTD) are highly conserved elements that regulate basal activity. (A) Primary structure. Numbers above the diagram correspond to domain boundaries. In the sequence alignment (human PLCβ1 (AAF86613), PLCβ2 (NP_004564), and PLCβ3 (NP_000923)), residues in the acidic stretch are shown in red, and the C-terminal ends of X–Y linker internal deletions in PLCβ3 are noted below. The primary Gαq binding site in the proximal CTD is shown in blue. (B) Crystal structure of the Gαq–PLCβ3-Δ892Δacid complex. AlF4−-activated Gαq is shown as a gray surface and the domains of PLCβ3 are colored as in (A). The observed N- and C-termini of PLCβ3-Δ892Δacid and Gαq are labeled N and C, and N′ and C′, respectively. Dashed lines correspond to disordered loops. (C) View of the PLCβ3 catalytic core (PDB entry 3OHM) and proximal CTD from the perspective of the membrane plane. PLCβ3 is colored as in (A). Circled minus signs indicate the relative position of the acidic stretch in the X–Y linker. See also Supplemental Figures 1,2.
Two autoinhibitory elements have been identified in PLCβ, the X–Y linker within the TIM barrel domain (Hicks et al., 2008) and the Hα2′ helix in the C-terminal extension (Lyon et al., 2011) (Figure 1B,C). The X–Y linker varies in sequence and length among PLCβ isoforms, but can be subdivided into an unconserved N-terminal region, a highly acidic stretch of 10–15 residues, and a conserved C-terminal region of ~15 residues that occludes the active site in all PLCβ crystal structures determined to date (Hicks et al., 2008; Jezyk et al., 2006; Lyon et al., 2013; Waldo et al., 2010) (Supplemental Figure 1). Deletion or cleavage of the X–Y linker increases basal activity in PLCβ and other PLC proteins, consistent with a common mode of inhibition within the PLC family (Hicks et al., 2008; Schnabel and Camps, 1998; Zhang and Neer, 2001). Displacement of the ordered part of the linker has been proposed to occur via electrostatic repulsion between the acidic stretch of the linker and the negatively charged inner leaflet of the membrane in a form of interfacial activation (Hicks et al., 2008; Waldo et al., 2010).
The Hα2′ helix binds to a highly conserved cleft between the TIM barrel and C2 domains of the catalytic core, in close proximity to the active site and the ordered region of the X–Y linker (Figure 1C). Mutations within the catalytic core–Hα2′ interface decrease the melting point of the enzyme as measured by differential scanning fluorimetry (DSF), increase basal activity, and decrease the efficacy of Gαq activation, whose binding is proposed to displace Hα2′ from the catalytic core (Lyon et al., 2011). The Hα2′ binding site on the catalytic core is in close proximity to the ordered part of the X–Y linker, separated only by the Tα5 helix of the TIM barrel domain, an element implicated in Gβγ-mediated activation (Bonacci et al., 2005; Kuang et al., 1996; Sankaran et al., 1998). Thus, there are several possible models for the molecular basis underlying autoinhibition by Hα2′. The first is for Hα2′ to dampen the overall dynamics of the catalytic core, inhibiting displacement the X–Y linker. A second is for residues in the proximal CTD preceding Hα2′, including those that bind Gαq, to interact with and stabilize the X–Y linker in the absence of Gαq. A third is for Hα2′ to act independently of the X–Y linker, such as by preventing the catalytic core from interacting productively with the membrane.
Herein, protease protection and activity assays, DSF, and X-ray crystallography were used to determine how distinct regions within the X–Y linker and the proximal CTD contribute to autoinhibition of PLCβ and whether they act independently of each other. The ability of these regions to influence activation by heterotrimeric G proteins was also assessed. Deletion of the acidic stretch within the X–Y linker decreases thermal stability of the enzyme, increases basal activity, and decreases activation by heterotrimeric G proteins. Consistent with these observations, crystal structures of PLCβ3 lacking the acidic stretch, yet still retaining the C-terminal region of the X–Y linker, exhibit an open active site, allowing co-crystallization with IP3. In contrast, the Hα2′ helix dampens basal activity via a mechanism independent of the X–Y linker, and serves to prevent full activity until Gαq is bound. This finding could explain the basis for synergistic activation of the enzyme by Gαq and Gβγ (Philip et al., 2010; Rebres et al., 2011).
RESULTS
Hα2′ docking does not alter X–Y linker proteolysis
Hα2′ may regulate PLCβ activity and facilitate displacement of the X–Y linker by modulating the conformational dynamics of the catalytic core. Disruption of the interactions between Hα2′ and the catalytic core significantly decreases the melting point (Tm) of PLCβ3 (Lyon et al., 2011). Therefore, the proteolytic sensitivity of two human PLCβ3 C-terminal truncations was compared: PLCβ3-Δ847, which corresponds to the catalytic core, and PLCβ3-Δ892, which contains the catalytic core and the proximal CTD, including Hα2′. PLCβ3-Δ847 and PLCβ3-Δ892 were incubated with trypsin or clostripain and analyzed by SDS-PAGE (Supplemental Figure 2). The individual bands and the total proteolytic reactions were analyzed by MALDI-TOF and tandem mass spectrometry (MS/MS) to determine the molecular weights and sequences of the proteolytic fragments (Supplemental Table 1). MS/MS analysis showed that PLCβ3-Δ847 and PLCβ3-Δ892 have the same major cleavage sites after residues Arg483 and Arg545. These positions are within the disordered N-terminal region of the X–Y linker, with Arg545 immediately preceding the acidic stretch. Therefore, the presence of Hα2′ seems to have no effect on the susceptibility of cleavage sites in PLCβ3. The acidic stretch, the C-terminal ordered region of the X–Y linker, and proximal CTD contain predicted trypsin and clostripain sites. However, no significant proteolysis was detected within these regions. This result is consistent with Hα2′ stably bound to the catalytic core in solution, and, unexpectedly, that the acidic stretch, although not ordered in crystal structures, is likewise protected.
The X–Y linker and Hα2′ regulate basal activity independently
To examine the dependence of autoinhibition by the X–Y linker on Hα2′, internal deletions were created within the X–Y linker in the background of both PLCβ3-Δ847 and PLCβ3-Δ892 and their basal activities compared. Deletions were designed considering the published crystal structures of PLCβ (Hicks et al., 2008; Jezyk et al., 2006; Lyon et al., 2013; Lyon et al., 2011; Waldo et al., 2010) and the observed protease cleavage sites. Homology modeling of the resulting variants (Roy et al., 2010; Zhang, 2008) indicated these changes were unlikely to perturb the structure of the catalytic core. The Δ471-559 deletion (denoted by Δdisorder) removes the unconserved and disordered N-terminal region of the X–Y linker. Δ471-569 (Δacid) removes both the N-terminal region and the acidic stretch. Δ471-581 (Δall) removes the entire X–Y linker, leaving enough residues to form a stable loop between the Tβ4 strand and Tα4 helix. With the exception of PLCβ3-Δ847Δacid, which did not express, all variants were purified to homogeneity. To test whether the disordered loop linking the C-terminus of the catalytic core and Hα2′ plays a role in autoinhibition, the Δ859-863 deletion (denoted as ΔLINPI) was created in the background of PLCβ3-Δ892. This removes the Gαq binding site and considerably shortens the loop.
PLCβ3-Δ847Δdisorder exhibited ~10-fold lower basal activity relative to PLCβ3-Δ847 (2.6 ± 0.5 vs. 30.0 ± 3.5 nmol IP3/min/nmol PLCβ3-Δ847, respectively). In contrast, PLCβ3-Δ847Δall was activated ~30-fold relative to PLCβ3-Δ847 (980 ± 92 nmol IP3/min/nmol, P≤0.0001) (Figure 2A, Table 1). Similar trends were observed in the background of PLCβ3-Δ892 (i.e. in the presence of bound Hα2′). PLCβ3-Δ892Δdisorder had ~4-fold reduced basal activity relative to PLCβ3-Δ892 (0.6 ± 0.09 nmol IP3/min/nmol PLCβ3 variant), whereas PLCβ3-Δ892Δall had ~100 fold higher activity (250 ± 37 nmol IP3/min/nmol PLCβ3 variant). PLCβ3-Δ892Δacid, which lacks the acidic stretch but retains the ordered C-terminal region, had even higher activity (320 ± 26 nmol IP3/min/nmol PLCβ3-Δ892Δacid, P≤0.0001 compared to PLCβ3-Δ892). PLCβ3-Δ892ΔLINPI exhibited no defect in basal activity. Altogether, these data provided two surprising results. The first is that deletion of the N-terminal region in the X–Y linker leads to loss of activity, suggesting that the length of the linker plays a role in autoinhibition. The second is that removal of the N-terminal region and acidic stretch (Δacid) has greater activity than removal of the entire X–Y linker (Δall). Independently of the X–Y linker deletions, Hα2′ repressed basal activity, as each PLCβ3-Δ892 variant had lower specific activity than its PLCβ3-Δ847 counterpart.
Figure 2.
Basal activity and DSF measurements of PLCβ3 variants. (A) Deletions within the X–Y linker, but not the Gαq binding site, alter basal activity. Increased activity is observed when the acidic stretch or entire linker is removed. Data represent at least five experiments performed in duplicate. (B) Addition of 5 mM IP3 to hyperactive PLCβ3 variants increases their thermal stability (see Supplemental Figure 3A). Tm values were determined by monitoring the increase in fluorescence of ANS as a function of temperature. See Supplemental Table 2. Data represent at least five experiments performed in triplicate. (C) Deletions including the acidic stretch show decreased thermal stability compared to variants containing intact X–Y linkers. The Tm of each PLCβ3 variant was determined with respect to PLCβ3-Δ847, used as a control on each plate (Supplemental Table 3). PLCβ3-Δ892 and its variants have higher thermal stability relative to PLCβ3-Δ847 variants due to Hα2′. Data represents at least four experiments performed in triplicate. In panels B and C, * = P≤0.5, ** = P≤0.01, *** = P≤0.001, **** =P≤0.0001.
Table 1.
Basal Activity of PLCβ3 variants
| Variant | Basal specific activity ± S.E.M. (nmol IP3/min/nmol PLCβ3) (n) | Fold increase relative to PLCβ3-Δ847 or PLCβ3-Δ892 |
|---|---|---|
| PLCβ3-Δ847 | 30. ± 3.5 (9) | 1 |
| -disorder | 2.6 ± 0.5 (7) | 0.08 |
| -all | 980 ± 91 (10) | 33 |
| PLCβ3-Δ892 | 2.3 ± 0.3 (9) | 1 |
| -disorder | 0.6 ± 0.09 (7) | 0.3 |
| -acid | 320 ± 26 (5) | 140 |
| -all | 250 ± 37 (10) | 110 |
| -ΔLINPI | 2.0 ± 0.4 (5) | 0.9 |
Data represents 5–10 individual experiments measured in duplicate, from at least two purifications.
The X–Y linker regulates access to the active site
The X–Y linker and Hα2′ may both regulate access of ligands to the active site. To test whether deletion of Hα2′ or of regions within the X–Y linker facilitate ligand binding in solution, DSF was used to detect the direct binding of IP3. The Tm values of PLCβ3-Δ892, PLCβ3-Δ847, and the most active linker deletion variants (PLCβ3-Δ892Δacid, PLCβ3-Δ892Δall, and PLCβ3-Δ847Δall) were determined in the presence or absence of 5 mM IP3. PLCβ3-Δ847, and PLCβ3-Δ892 did not show any significant change in thermal stability in response to IP3, consistent with their active sites being blocked, impairing ligand binding (Figure 2B, Supplemental Figure 3A). However, the Tm of PLCβ3-Δ847Δall was significantly increased by 2.5 °C in the presence of IP3, and by 3 °C for PLCβ3-Δ892Δacid and PLCβ3-Δ892Δall (Figure 2B, Supplemental Table 2). These results are consistent with the idea that deletions including the acidic stretch facilitate ligand binding in the active site. The presence of Hα2′ seems to have little effect on substrate access because similar ΔTm values were observed for PLCβ3-Δ892Δall vs. PLCβ3-Δ847Δall, and PLCβ3-Δ847 vs. PLCβ3-Δ892.
Deletion of the acidic stretch within the X–Y linker decreases thermal stability
Based on the above results, PLCβ3 variants lacking the acidic stretch or the entire X–Y linker have significantly increased basal activity and are able to bind IP3, potentially due to loss of the active site blockade. DSF was used to determine whether deletions within the X–Y linker alter the Tm values of the apo enzymes. PLCβ3-Δ847 and PLCβ3-Δ847Δdisorder exhibited similar Tm values, consistent with deletion of a disordered region. The Tm of PLCβ3-Δ847Δall, however, was decreased relative to PLCβ3-Δ847 by 4 °C (P ≤ 0.0001) (Figure 2C, Supplemental Table 3), reflecting a loss of stabilizing interactions between the catalytic core and the acidic stretch and/or the C-terminal region of the X–Y linker.
PLCβ3-Δ892 had a 6 °C higher Tm than PLCβ3-Δ847 due to the presence of Hα2′, consistent with prior reports (Lyon et al., 2013), and the Tm of PLCβ3-Δ892Δdisorder was similar. However, PLCβ3-Δ892Δacid and PLCβ3-Δ892Δall both had significantly decreased Tm values (−3 °C and −4 °C, respectively), suggesting the acidic stretch is primarily responsible for the destabilization observed in the PLCβ3-Δ847 and PLCβ3-Δ892 variants. The most likely explanation would be via direct interactions with the catalytic core. PLCβ3-Δ892ΔLINPI exhibited no change in thermal stability.
Deletion of the X–Y linker diminishes fold activation by heterotrimeric G proteins
Activation of PLCβ3 by Gαq and Gβγ are hypothesized to involve interfacial activation (Hicks et al., 2008; Waldo et al., 2010), and Hα2′ displacement is proposed to be an additional step in activation by Gαq (Lyon et al., 2011). Little is known regarding the mechanisms of activation by Gβγ, although it is independent of the proximal and distal CTDs (Bonacci et al., 2005; Jenco et al., 1997; Kuang et al., 1996; Lee et al., 1993). To identify the roles of specific regions within the X–Y linker and the proximal CTD in activation by heterotrimeric G proteins, liposome-based activity assays were used to measure Gαq and Gβγ activation of the PLCβ3-Δ847 and PLCβ3-Δ892 variants.
Gαq can only activate the PLCβ3-Δ892 variants as they retain the proximal CTD (Waldo et al., 2010). PLCβ3-Δ892 was activated ~17-fold by the addition of Gαq to 40 ± 3.9 nmol IP3/min/nmol PLCβ3 (Lyon et al., 2013; Lyon et al., 2011) (Figure 3A, Table 2), and loss of the Gαq binding site in PLCβ3-Δ892ΔLINPI completely eliminated activation. PLCβ3-Δ892Δdisorder, PLCβ3-Δ892Δacid, and PLCβ3-Δ892Δall were all activated by Gαq, but with varying efficacy (Table 2, Supplemental Figure 3B). PLCβ3-Δ892Δdisorder was activated ~60-fold by Gαq to a maximum specific activity of 37 ± 3.9 nmol IP3/min/nmol PLCβ3 variant. In contrast, Gαq addition only increased activity by ~3–4 fold for PLCβ3-Δ892Δacid and PLCβ3-Δ892Δall to ~1000 nmol IP3/min/nmol PLCβ3. The differences in efficacy cannot be attributed to defects in binding, as there were no significant differences in EC50 values (Table 2). Instead, changes in efficacy were due to differences in basal activities. PLCβ3-Δ892Δacid and PLCβ3-Δ892Δall both exhibited ~30-fold higher maximal Gαq-stimulated activity than PLCβ3-Δ892 and PLCβ3-Δ892Δdisorder, again demonstrating a prominent role of the acidic stretch in autoinhibition.
Figure 3.
Heterotrimeric G protein activation of PLCβ3 variants. The activity of PLCβ3 variants was measured at 30 °C for 5 min at increasing concentrations of AlF4−-activated Gαq or Gβ1γ2. (A) Gαq activates PLCβ3-Δ892 3–60 fold. As expected, loss of the primary Gαq binding site (PLCβ3-Δ892ΔLINPI) abolishes Gαq activation. PLCβ3-Δ892Δdisorder has the lowest basal activity, and thus shows the greatest fold increase, whereas PLCβ3-Δ892Δacid and PLCβ3-Δ892Δall variants are weakly activated (Table 2, Supplemental Figure 3B). Data represents at least five experiments in duplicate. (B) Gβ1γ2-mediated activation of PLCβ3 variants. Gβ1γ2 robustly activates PLCβ3Δdisorder variants due to their low basal activity, whereas PLCβ3Δacid and PLCβ3Δall variants are activated only 3–5 fold. Deletion of the Gαq binding site in PLCβ3-Δ892ΔLINPI had no effect on Gβ1γ2 activation (Supplemental Figure 3C, D, Table 2). Data represent at least four experiments performed in duplicate.
Table 2.
Heterotrimeric G protein activation of PLCβ3 variants
| Variant | Gαq-dependent maximum specific activity (nmol IP3 min−1 nmol PLCβ3 −1) (n) | Maximum fold increase in activity over basal | Gαq EC50 (nM) | Gβγ-dependent maximum specific activity (nmol IP3 min−1 nmol PLCβ3−1) (n) | Maximum fold increase in activity over basal | Gβγ EC50 (nM) |
|---|---|---|---|---|---|---|
| PLCβ3-Δ892 | 40 ± 4 (11) | 17 | 700 ± 115 | 56 ± 2 (10) | 24 | 102 ± 23 |
| -disorder | 37 ± 4 (10) | 63 | 2180 ± 650 | 39 ± 1 (11) | 56 | 54 ± 10 |
| -acid | 1050 ± 43 (5) | 3 | 155 ± 21 | 1110 ± 28 (6) | 3 | 25 ± 3 |
| -all | 1070 ± 29 (5) | 4 | 78 ± 17 | 970 ± 48 (5) | 4 | 15 ± 5 |
| -ΔLINPI | ND | ND | ND | 55 ± 4 (4) | 28 | 66 ± 8 |
| PLCβ3-Δ847 | ND | ND | ND | 114 ± 6 (5) | 4 | 8 ± 3 |
| -disorder | ND | ND | ND | 56 ± 4 (5) | 22 | 330 ± 6 |
| -all | ND | ND | ND | 4900 ± 250 (9) | 5 | 140 ± 30 |
Data represents individual experiments measured in duplicate from at least two purifications. ND, not determined.
PLCβ3-Δ892 and its variants were all activated by Gβ1γ2 with similar trends to those observed with Gαq (Supplemental Figure 3C,D) with the exception of PLCβ3-Δ892ΔLINPI, which was comparable toPLCβ3-Δ892 (Figure 3B, Table 2). PLCβ3-Δ892 was activated 25-fold by Gβ1γ2, to a maximum specific activity of 56 ± 2.2 nmol IP3/min/nmol PLCβ3 variant. PLCβ3-Δ892Δdisorder was activated 56-fold, whereas PLCβ3-Δ892Δacid and PLCβ3-Δ892Δall were only activated ~3- and 4-fold, respectively, to ~1000 nmol IP3/min/nmol PLCβ3 variant. PLCβ3-Δ847 was activated ~4-fold, due to loss of autoinhibition by Hα2′. PLCβ3-Δ847Δdisorder was activated ~19-fold to 57 ± 3.1, and PLCβ3-Δ847Δall was activated ~5-fold to 4,900 ± 250 nmol IP3/min/nmol PLCβ3 variant. As in the case for Gαq, the EC50 values for Gβ1γ2 activation were comparable between variants (Table 2). Strikingly, PLCβ3-Δ847 variants have significantly higher Gβ1γ2-dependment maximum specific activities compared to their PLCβ3-Δ892 counterparts, again demonstrating that Hα2′ is a potent inhibitor of PLCβ3 activity, even under saturating concentrations of Gβ1γ2.
Deletion of the acidic stretch of the X–Y linker disorders the active site lid
To determine the, crystal structures were determined of the apo Gαq–PLCβ3-Δ892Δ complex and of IP3 in complex with Gαq–PLCβ3-Δ892Δ and Gαq–PLCβ3-Δ892Δ (Table 3). Gαq was included to facilitate crystallization, but because Hα2′ is bound to the catalytic core via in trans crystal contacts in these structures (Lyon et al., 2013; Waldo et al., 2010), they are thought to represent the basal state of each PLCβ3-Δ892 variant. The Gαq–PLCβ3-Δ892Δacid (Figure 1B) and Gαq–PLCβ3-Δ892Δall structures are very similar to that of Gαq–PLCβ3-Δ882 (RMSD = 0.4 Å2 for observed Cα atoms) (Figure 4A). The largest conformational differences occur at the C-terminus of Hα2′ and in some loops of the PH domain, likely due to slight differences in crystal packing. Thus, deletions within the X–Y linker do not perturb the overall structure of the complex.
Table 3.
Data collection and refinement statistics
| Gαq–PLCβ3-Δ892Δacid | Gαq–PLCβ3-D892Δacid ·IP3 | Gαq–PLCβ3-Δ892 Δall·IP3 | |
|---|---|---|---|
| Data collection | |||
| Space group | C121 | C121 | C121 |
| Cell dimensions | |||
| a, b, c (Å) | 200.6, 88.8, 92.2 | 201.9, 89.2, 92.6 | 205.9, 89.9, 93.3 |
| α, β, γ (°) | 90, 101, 90 | 90, 102, 90 | 90, 102, 90 |
| Resolution (Å) | 30.0–3.00 (3.05–3.00)* | 29.4–3.28 (3.34–3.30)* | 30.0–3.40 (3.46–3.40)* |
| Rsym or Rmerge | 0.100 (0.527) | 0.221 (0.480) | 0.142 (0.444) |
| I/sI | 14.3 (1.8) | 9.52 (3.3) | 7.7 (2.1) |
| Completeness (%) | 99.8 (97.2) | 85.6 (64.6) | 96.1 (95.9) |
| Redundancy | 5.1 (3.9) | 3.7 (2.7) | 3.3 (2.1) |
| Refinement | |||
| Resolution (Å) | 30.0–3.0 | 29.4–3.30 | 30.0–3.40 |
| No. reflections | 31,965 | 21,289 | 22,003 |
| Rwork/Rfree | 0.195/0.240 | 0.208/0.267 | 0.212/0.277 |
| No. atoms | |||
| Protein | 8,684 | 8,646 | 8,621 |
| Ligand/ion | 63 | 71 | 61 |
| B-factors | |||
| Protein | 59.57 | 73.74 | 85.73 |
| Ligand/ion | 32.65 | 68.45 | 86.23 |
| R.m.s. deviations | |||
| Bond lengths (Å) | 0.004 | 0.005 | 0.005 |
| Bond angles (°) | 0.887 | 0.930 | 1.016 |
Values in parentheses are for highest-resolution shell.
Data sets from two regions of a single crystal were merged for the Gαq–PLCβ3-Δ892Δacid structure.
Figure 4.
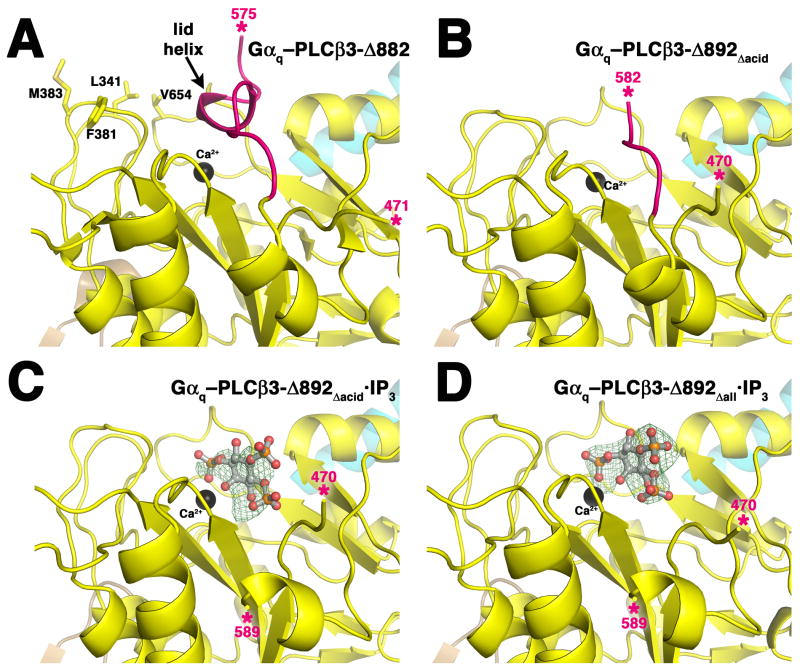
Structural characterization of PLCβ3 variants. PLCβ3 variants are colored as in Figure 1A, and the observed ends of the X–Y linker are marked with pink asterisks. The Hα2′ helix is shown in cyan. (A) Active site of the Gαq–PLCβ3-Δ882 complex (PDB entry 3OHM). Residues Leu341, Phe381, Met383, and Val654 form the hydrophobic ridge, which anchors the active site at the membrane for catalysis (Essen et al., 1997; Lyon and Tesmer, 2013). The lid helix at the C-terminal end of the X–Y linker physically occludes the active site. (B) The active site of the Gαq–PLCβ3-Δ892Δacid complex is open. Residues that would form the lid helix are present in this variant, but deletion of the acidic stretch leads to their disorder. (C) Active site of the Gαq–PLCβ3-Δ892Δacid·IP3 complex. Electron density is observed for most atoms of the ligand, as seen in a 3σ |Fo|-|Fc| omit map (green wire cage). Electron density for the X–Y linker is not observed until residue 589. (D) Active site of the Gαq–PLCβ3-Δ892Δall·IP3 complex. Strong 3σ |Fo|-|Fc| omit map density is observed for the entire IP3 molecule. Deletion of the entire X–Y linker in this variant has no effect on the position of the bound ligand. See also Supplemental Figure 4.
In all previously reported PLCβ structures, part of the ordered C-terminal region of the X–Y linker (generally human PLCβ3 residues 575–584) folds into a short 310 helix (the lid helix) that occludes the active site (Hicks et al., 2008) (Supplemental Figure 1). Based on the structure of IP3 and analogs bound in the active site of PLCδ (Essen et al., 1996; Essen et al., 1997), this helix would prevent substrate binding. However, in the Gαq–PLCβ3-Δ892Δacid structure, only residues 582-584 are observed (Figure 4B). The best explanation is that deletion of the acidic stretch eliminates docking of the lid helix, because modeling indicates there is a sufficient number of residues in the remaining X–Y linker to fully form the lid helix structure.
Because the Tm values of PLCβ3-Δ892Δacid and PLCβ3-Δ892Δall were increased by IP3, these variants were co-crystallized with IP3.In the resulting structures, strong density for IP3 was observed in their active sites permitting modeling of the ligand (Figure 4C,D, Table 3). These structures confirm that PLCβ and PLCδ, and likely all PLC enzymes, recognize the PIP2 substrate in a similar way (Gresset et al., 2012). As in PLCδ (Essen et al., 1996; Essen et al., 1997), conserved basic residues in the active site recognize the phosphate groups of IP3, with the 1- and 4-phosphates forming the majority of contacts. IP3 binds the active site such that the 1-phosphate and 2-hydroxyl coordinates the catalytic Ca2+ atom, although in a slightly different orientation than PLCδ (Supplemental Figure 4A). PLCβ3-Ser619 and Arg646 form hydrogen bonds with the 4-phosphate and the 3-hydroxyl group of IP3, respectively, and Tyr648 stacks with the inositol ring (Supplemental Figure 4B). Based on the PLCδ–IP3 structure, additional contacts are likely mediated by water molecules that are not observed due to lower resolution. In these structures, interpretable electron density for the linker is not observed until residue 589, even though PLCβ3-Δ892Δacid contains linker residues starting at 569 and PLCβ3-Δ892Δall at 582. The fact that these structures are technically “Hα2′-bound” reinforces the conclusion from the DSF experiments (Supplemental Table 2) that Hα2′ does not control ligand accessibility of the active site.
Discussion
Under normal cellular conditions PLCβ activity is very low, and direct interactions with Gαq, Gβγ, or Rac proteins dramatically enhance the rate of PIP2 hydrolysis (Lyon and Tesmer, 2013). The low basal activity of PLCβ enzymes has thus far been attributed to two structural elements: the X–Y linker of the TIM barrel domain, and the Hα2′ helix of the proximal CTD, as perturbation of either element increases basal activity. The results herein reconfirm these elements are essential for maintaining the basal activity of PLCβ, and demonstrate that they inhibit activity through independent mechanisms. The presence of Hα2′ decreases maximal activity of PLCβ3-Δ892 under every condition tested, and all results reported suggest this region inhibits activity independently of the X–Y linker. Protease protection assays indicate Hα2′ does not alter the susceptibility of the enzyme to proteolysis, even within the X–Y linker, a known proteolytically sensitive site (Supplemental Table 1, Supplemental Figure 2)(Schnabel and Camps, 1998). Furthermore, this assay showed the region of the X–Y linker spanning the acidic stretch to its C-terminus, and the proximal CTD are both protected from proteolysis in solution, even though these regions contain protease consensus sites and crystal structures suggest these elements are largely unstructured. Such protection is likely conferred via interactions of these or adjacent residues with the catalytic core, consistent with the observation that deletion of the acidic stretch of the X–Y linker decreases the Tm of the catalytic core (Figure 2C, Supplemental Table 3). It was also hypothesized that the disordered region preceding Hα2′ in the proximal CTD, which would be topologically constrained due to interactions of Hα2′ with the catalytic core, could contribute to regulation of basal activity through interactions with the X–Y linker. The most likely and conserved element to mediate this interaction is the LINPI motif, which forms the primary Gαq binding site (Waldo et al., 2010). The ΔLINPI deletion, however, had no effect in any assay except Gαq activation (Figure 3A, Table 2, Supplemental Figure 3B). Truncating the loop by five amino acids, via this deletion, also has no effect. Thus, regulation of basal activity by the proximal CTD seems to depend entirely on the interaction of Hα2′ with the catalytic core. We hypothesize this interaction somehow prevents the active site from achieving optimal interactions with lipid bilayers.
The X–Y linker varies in length among PLCβ isoforms, but in each it has an unconserved N-terminal region and two conserved elements: an acidic stretch and a C-terminal region that forms the active site lid helix. The studies described herein demonstrate specific and, in some cases, unanticipated roles for each of these elements (Hicks et al., 2008). Deletion of the N-terminal region decreased basal activity (Figure 2A, Table 1), possibly due to decreased conformational flexibility, which may hinder ejection of the lid helix when the catalytic core approaches the membrane. In contrast, any deletion including the acidic stretch dramatically increased basal activity, decreased the Tm, and facilitated IP3 binding (Figure 2, Table 1, Supplemental Table 2). These results are consistent with the acidic region regulating access to the active site. Similar trends were reported for deletions within the X–Y linker of human PLCβ2 (Hicks et al., 2008): deletions within PLCβ2 encompassing the majority of the acidic stretch or the entire X–Y linker (human PLCβ2 residues 470-515 and 470-524, respectively) had increased basal activity compared to wild type. In this same study, deletion of just the ordered portion of the X–Y linker (residues 516–535) also elevated basal activity, but not to the same extent as deletions including the acidic stretch. All of these results support a model wherein the acidic stretch somehow maintains the structural integrity of the lid helix, which is insufficient to inhibit basal activity on its own.
Maximal Gαq or Gβγ-stimulated activity requires the release of autoinhibition mediated by the X–Y linker and Hα2′. Deletion of the acidic stretch or entire X–Y linker in PLCβ3 decreased the efficacy of G protein activation whether or not Hα2′ was present (Figure 3, Table 2), just as deletion of the acidic stretch or the entire X–Y linker in PLCβ2 decreased fold activation by heterotrimeric G proteins and Rac1 (Hicks et al., 2008). Interestingly, even though Gβγ is not believed to interact with or displace Hα2′, it promoted comparable maximal PLCβ3-Δ892 activity as Gαq (Figure 3, Table 2). Thus, interfacial activation by Gαq in the context of this assay system may simply be less efficient than Gβγ, possibly reflecting heterogeneously palmitoylated Gαq (Lyon et al., 2013) or the distal CTD, which also interacts with Gαq, was absent in the variants tested in this study (Kim et al., 1996; Lee et al., 1993; Wu et al., 1993) (Ilkaeva et al., 2002; Lyon et al., 2013; Singer et al., 2002). In every case, Hα2′ acted as brake on Gβγ activation, because none of the PLCβ3-Δ892 variants reached the same maximum specific activity exhibited by PLCβ3-Δ847Δall, which lacks all known autoinhibitory elements.
Thus, Hα2′ dampens both basal and G protein stimulated activity independently of the X–Y linker, and may underlie the synergistic activation of PLCβ3 by Gαq and Gβγ subunits (Philip et al., 2010; Rebres et al., 2011). The residual 3–4 fold activation mediated by Gαq in the context of PLCβ3-Δ892Δall could reflect displacement of the Hα2′ helix. However, it is not entirely clear what is responsible for the residual 3–4 fold activation induced by Gβγ in the context of PLCβ3-Δ847Δall, which has no known autoinhibitory elements remaining. This may be due to membrane-induced conformational changes within the PLCβ active site. Alternatively, it may reflect a shift in equilibrium between soluble and membrane bound enzyme, or the existence of other yet to be determined inhibitory elements.
Prior work hypothesized that the acidic stretch of the X–Y linker serves to drive repulsive interactions with the membrane, ejecting the lid helix from the active site (Hicks et al., 2008). The studies presented here are instead consistent with a mechanism wherein the acidic stretch acts as a clasp to stabilize the lid helix by interacting with highly conserved basic residues in close proximity to the active site. Evidence for such an interaction is seen in the structure of the Rac1–PLCβ2 complex (PDB entry 2FJU (Jezyk et al., 2006)), wherein the last two residues of the acidic stretch form salt bridges with conserved lysine and arginine side chains on the TIM barrel (Figure 5). Interestingly, the Tα5 helix, which interacts with Hα2′ in the basal state, contains two of these conserved lysine residues. Deletion of the acidic stretch breaks this clasp, leading to disordering of the lid helix, as suggested by the crystal structure of Gαq–PLCβ3-Δ892Δacid (Figure 4B), which lacks electron density for the lid helix. During catalysis, the clasp could be displaced by electrostatic repulsion between the acidic stretch and the negatively charged surface of the membrane, or by competition between the acidic stretch and the membrane for the basic patch on the catalytic core.
Figure 5.
A model of PLCβ autoinhibition by the X–Y linker. Under basal conditions, the lid helix at the C-terminal end of the X–Y linker blocks access to the active site. The acidic stretch interacts with conserved solvent-exposed basic residues on one face of the TIM barrel domain. In this model, based on PDB entry 2FJU, the last two residues of the PLCβ2 acidic stretch, Glu512 and Glu513, interact with conserved lysines and arginines. Breaking the electrostatic interactions between the TIM barrel domain and the acidic stretch, such as by interactions with the cell membrane, leads to loss of structure in the lid helix, increasing activity. Meanwhile, the Hα2′ helix remains bound to the catalytic core, acting as a brake on PLCβ activity via an independent mechanism until displaced by the binding of Gαq.
A complete picture of the regulation of PLCβ activity will only be obtained after consideration of the contributions by the distal CTD, a key membrane-binding determinant. How the distal CTD influences the roles various regions of the X–Y linker is currently unknown. There is strong evidence from cryo-electron microscopy that the PLCβ3 distal CTD interacts transiently with the catalytic core in solution, which could alter membrane partitioning, further occlude the active site, or even interact with the linker via its own conserved basic region (Lyon et al., 2013). A better molecular understanding of how Gβγ binds to and activates PLCβ will also be essential, and will be the subject of future studies.
MATERIALS AND METHODS
Protein expression, purification, and mutagenesis
N-terminally His-tagged human PLCβ3 C-terminal truncations comprising residues 10-847 (PLCβ3-Δ847) and 10-892 (PLCβ3-Δ892), and X–Y linker deletion variants were expressed in baculovirus-infected High Five cells and purified as described previously (Lyon et al., 2013). Internal deletions were introduced via QuikChange Site-Directed Mutagenesis (Stratagene) and sequenced over the open reading frame. Efforts to express and purify PLCβ3-Δ847Δacid were unsuccessful despite several independent attempts at production and amplification of viruses in Sf9 or High Five cell lines, expression trials in both of these lines, and variation of time and temperature. We therefore concluded this variant is toxic, which is consistent with the hypothesis that this should have been the most active variant based on the trends of the PLCβ3-Δ892 variants (Table I). Mouse Gαq (residues 7-359) was expressed and purified as previously described (Lyon et al., 2013).
Bovine Gβ1 and N-terminally His-tagged Gγ2 baculoviruses were co-infected into High Five cells and harvested after 40–48 h. Cells were resuspended in Buffer A (20 mM HEPES pH 8, 100 mM NaCl, 10 mM β-mercaptoethanol, 0.1 mM EDTA, 0.1 mM leupeptin, and 0.1 mM phenylmethylsulfonyl fluoride (PMSF)) and lysed by dounce on ice. The lysate was centrifuged, the membrane pellets were homogenized by dounce in Buffer A with 1% (w/v) sodium cholate, and stirred at 4 °C for 1 h to solubilize the proteins. The slurry was centrifuged again, the supernatant filtered, diluted 2-fold with Buffer A, and applied to a Ni-NTA column (Qiagen) equilibrated with Buffer A. The column was washed with 10 column volumes of Buffer A with 10 mM imidazole pH 8.0 and 0.2% cholate, followed by 10 column volumes of Buffer A with 10 mM imidazole pH 8.0 and 10 mM CHAPS. Gβ1γ2 was eluted with Buffer A with 250 mM imidazole pH 8.0 and 10 mM CHAPS. The sample was diluted 5-fold with Buffer B (20 mM HEPES pH 8.0, 1 mM MgCl2, 2 mM DTT, and 1 mM CHAPS), and applied to a MonoQ column pre-equilibrated with Buffer B. Gβ1γ2 was then eluted with a gradient of 0–500 mM NaCl. Peaks containing Gβ1γ2 were pooled, concentrated, and further purified over tandem S200 columns equilibrated with 20 mM HEPES pH 8.0, 50 mM NaCl, 1 mM MgCl2, 2 mM DTT, and either 1 mM or 10 mM CHAPS.
Protease Protection Assays
Clostripain (0.01 U/25 μg PLCβ3) and trypsin (0.05 U/25 μg PLCβ3) were used to digest PLCβ3-Δ892 or PLCβ3-Δ847 at room temperature and assessed at various time points to identify optimal conditions, which was determined to be 2 h. To estimate the molecular weights of the major degradation products with MALDI TOF mass spectrometry, 50 μL reactions containing 25 μg PLCβ3-Δ892 or PLCβ3-Δ847, at final concentrations of 4.96 μM and 5.23 μM, respectively, were digested for 2 h at room temperature in buffer containing 20 mM HEPES pH 8, 200 mM NaCl, and 2 mM DTT. Clostripain reactions were quenched by the addition of 1 mM tosyllysine chloromethylketone, and trypsin reactions by 10 mM PMSF. To map the proteolytic sites, reactions were quenched by the addition of 4x SDS-PAGE sample buffer, and 10 μL samples were separated by 10% SDS-PAGE. The bands were excised and analyzed by the Proteomics Resource at the Fred Hutchinson Cancer Research Center. Peptides were solubilized and divided into two sets, one of which was subjected to further proteolysis by thermolysin. All samples (trypsin, clostripain, trypsin and thermolysin, and clostripain and thermolysin) were analyzed by tandem MS/MS to determine the identity of residues within each band. This data was correlated with the MALDI-TOF data of intact proteolytic fragments (Supplemental Table 2).
DSF
Tm values for PLCβ3 variants were determined as previously described (Lyon et al., 2011), with the modifications that PLCβ3 samples were used at a concentration of 0.2 mg/mL and all samples contained 5 mM CaCl2. For assessment of IP3 binding, a final concentration of 5 mM IP3 was added to each reaction.
PLCβ3 activity assays
Basal, Gαq- and Gβ1γ2-stimulated PLCβ3 activity was quantified by measuring the amount of free [3H]-IP3 released from [3H]-PIP2-containing liposomes. Basal assays and Gαq-stimulated assays were performed as previously described (Ghosh and Smrcka, 2004; Lyon et al., 2011). Gβ1γ2-mediated activation assays were carried out similarly, with the addition of 65 μM CHAPS to the reaction. Increasing amounts of Gβ1γ2 were added to the PLCβ3 variants, incubated for 10–15 min, and reactions initiated by addition of liposomes and transfer to 30 °C for 5 min. Reactions were quenched as previously described (Ghosh and Smrcka, 2004; Lyon et al., 2011). PLCβ3-Δ892, PLCβ3-Δ892ΔLINPI, and PLCβ3-Δ847Δdisorder were assayed at 10 ng/μL, PLCβ3-Δ892Δdisorder at 12 or 18 ng/μl, PLCβ3-Δ892Δacid and PLCβ3-Δ892Δall at 0.5 ng/μL, PLCβ3-Δ847 at 4 ng/μl, and PLCβ3-Δ847Δall at 0.1 ng/μL.
Crystallization and structure determination of Gαq–PLCβ3-892 variant complexes
Gαq was pre-activated and Gαq–PLCβ3-Δ892Δacid and Gαq–PLCβ3-Δ892Δall complexes were formed, purified, and concentrated as previously described (Lyon et al., 2013). For Gαq–PLCβ3-Δ892Δacid, hanging drops contained 0.7 μl of Gαq–PLCβ3-Δ892Δacid at 5.2 mg/mL and 0.7 μL well solution containing 100 mM BisTris pH 6.0, 200 mM NaCl, and 5% (w/v) PEG 3350. Crystals were harvested in 20 mM HEPES pH 8.0, 400 mM NaCl, 2 mM DTT, 0.9 mM CaCl2, 5 mM MgCl2, 10 mM NaF, 30 μM AlCl3, 50 μM GDP, 100 mM BisTris pH 6.0, 10% (w/v) PEG 3350, and 30% (v/v) 1,3-butanediol. For co-crystallization with IP3, Gαq–PLCβ3-Δ892Δacid and Gαq–PLCβ3-Δ892Δall were incubated ~30 min on ice with 5 mM IP3 (Cayman Chemical). For Gαq–PLCβ3-Δ892Δacid·IP3, hanging drops contained 0.7 μL of protein complex and 0.7 μL of well solution containing 100 mM BisTris pH 6.5, 200 mM NaCl, and 8% (w/v) PEG 3350. Crystals were harvested in 20 mM HEPES pH 8.0, 400 mM NaCl, 2 mM DTT, 0.9 mM CaCl2, 5 mM MgCl2, 10 mM NaF, 30 μM AlCl3, 50 μM GDP, 100 mM BisTris pH 6.5, 10 mM IP3, 10% (w/v) PEG 3350, and 30% (v/v) 1.3-butanediol. For Gαq–PLCβ3-Δ892Δall·IP3, hanging drops contained 0.7 μL of protein complex at 8.8 mg/mL mixed with 0.7 μL well solution containing 100 mM MES pH 6.25, 200 mM NaCl, and 5% (w/v) PEG 3350. Crystals were harvested in 20 mM HEPES pH 8.0, 400 mM NaCl, 2 mM DTT, 0.9 mM CaCl2, 5 mM MgCl2, 10 mM NaF, 30 μM AlCl3, 50 μM GDP, 100 mM MES pH 6.0, 10 mM IP3, 7% (w/v) PEG 3350 and 30% (v/v) glycerol. All crystals were frozen on nylon loops in liquid N2 for data collection.
Diffraction data was collected at the Advanced Photon Source at LS-CAT beamlines 21-ID-F and 21-ID-D at wavelengths of 0.9787 Å and 0.9792 Å, respectively, from single crystals maintained at 110 K. Data sets were processed using HKL2000 (Otwinowski and Minor, 1997) and phases determined by molecular replacement using the 3OHM structure as a search model (Waldo et al., 2010). Models were refined in REFMAC (Winn et al., 2003), using two TLS groups: one for Gαq, GDP, Mg2+, and AlF4− molecules, and one for PLCβ3, Ca2+, and IP3 if present. Stereochemical correctness of the structures was assessed using MolProbity (Davis et al., 2007). In the final Gαq–PLCβ3-Δ892Δacid structure, 95.9% of residues were in favored region of the Ramachandran plot, 3.9% in allowed regions, and 0.3% in disallowed regions. Density was observed for Gαq residues 37-352 and PLCβ3-Δ892Δacid residues 12-91, 96-470, and 582-882. In the final Gαq–PLCβ3-Δ892Δacid·IP3 structure, 94.2% of residues were in the favored region of the Ramachandran plot, 4.5% in allowed regions, and 1.3% in disallowed regions. Density was observed for Gαq residues 35-352 and PLCβ3-Δ892Δacid residues 12-91, 96-470, and 589-882. In the final Gαq–PLCβ3-Δ892Δall·IP3 structure, 91.2% of residues were in the favored region of the Ramachandran plot, 6.9% in the allowed regions, and 1.9% in disallowed regions. Density was observed for Gαq residues 36-352 and PLCβ3-Δ892Δall residues 12-91, 97-470, and 589-881.
Accession codes
Coordinates and diffraction intensities for Gαq–PLCβ3-Δ892Δacid, Gαq–PLCβ3-Δ892Δacid·IP3, and Gαq–PLCβ3-Δ892Δall·IP3 are deposited with the Protein Data Bank as entries 4QJ3, 4QJ4, and 4QJ5, respectively.
Statistical methods
All statistical analyses used analysis of variance (ANOVA) with Tukey’s post test as implemented in Prism (version 6.0).
Figures
Structural images were generated using PymolX11Hybrid (http://www.pymol.org/). Figures were created using Adobe Photoshop and Illustrator.
Supplementary Material
Supplemental Figure 1, related to Figures 1 and 4. The C-terminus of the X–Y linker has a conserved structure in all published crystal structures of PLCβ homologs: (A) S. officinales PLC21 (PDB entry 3QR0), (B) L. pealei PLC21 PDB entry 3QR1, (C) PLCβ2 catalytic core (PDB entry 2ZKM), (D) the Rac–PLCβ2 complex (PDB entry 2FJU), (E) the Gαq–PLCβ3-Δ882 complex (PDB entry 3OHM), and (F) Gαq in complex with full length PLCβ3 (PDB entry 4GNK). The most C- terminal residues of the X–Y linker forms a short helix (the “lid helix”) that occludes the active site. The active site Ca2+ atom is shown as a black sphere, the disordered region of the X–Y linker is shown as a dashed line, and the first observed amino acid on either end of the disordered region is labeled on each structure.
Supplemental Figure 2, related to Figure 1, Tables 1 and 2. SDS-PAGE analysis of PLCβ3 trypsin digests. One representative experiment is shown. 25 μg of PLCβ3-Δ892 or PLCβ3-Δ847 were incubated with 0.05 U trypsin in 50 μl reactions at room temperature for 0.5, 1, 2, and 4 h, and overnight. The samples were quenched upon the addition of 4x SDS-PAGE sample buffer, and separated by10% SDS-PAGE. Bands identified at the 2 h time point (PLCβ3-Δ847 bands A-F and PLCβ3-Δ892 bands G-K) were excised from the SDS polyacrylamide gel for fragment identification by MALDI-TOF and tandem MS/MS. See Supplemental Table 1.
Supplemental Figure 3, related to Figures 2 and 3. Determination of Tm values and heterotrimeric G protein activation of PLCβ3 variants. (A) DSF curves demonstrating stabilization by IP3. PLCβ3-Δ847 variants at 0.2 mg/mL were incubated with 5 mM CaCl2 and 200 μM ANS ± 5 mM IP3. Representative curves are shown for PLCβ3-Δ847 (gray and red circles) and PLCβ3-Δ892Δall (orange and yellow inverted triangles). PLCβ3-Δ847Δall was stabilized (right-shifted) in the presence of IP3. (B) Representative Gαq activation curves of PLCβ3-Δ892. Gαq-stimulated activity of PLCβ3-Δ892 variants was measured at increasing Gαq concentrations by quantifying free 3H- IP3 released from 3H-PIP2 liposomes after 5 min at 30 °C. PLCβ3-Δ892 (blue circles) and its variants PLCβ3-Δ892Δdisorder (green squares), PLCβ3-Δ892Δacid (pink diamonds), and PLCβ3-Δ892Δall (purple inverted triangles) are all activated at least 3-fold by Gαq, but with no significant change in EC50 values. Loss of the acidic stretch or the entire X–Y linker in PLCβ3-Δ892Δacid and PLCβ3-Δ892Δall significantly decreased the efficacy of activation. Deletion of the primary Gαq binding site in PLCβ3-Δ892ΔLINPI (light blue triangles) abrogates Gαq activation. (C) Representative Gβ1γ2 activation curves for PLCβ3-Δ892 variants. Gβ1γ2- stimulated activation of all PLCβ3 variants was carried out as described for Gαq activation assays. PLCβ3-Δ892 (blue circles) and its variants PLCβ3-Δ892Δdisorder (green squares) and PLCβ3-Δ892ΔLINPI (light blue triangles) were activated at least 24-fold by Gβ1γ2, whereas PLCβ3- Δ892Δacid (pink diamonds) and PLCβ3-Δ892Δall (purpled inverted triangles) were only activated 3-fold. (D) Representative Gβ1γ2 activation curves for PLCβ3-Δ847 variants. Similar trends were observed for PLCβ3-Δ847 (red circles) and its variants PLCβ3-Δ847Δdisorder (orange squares) and PLCβ3-Δ847Δall (yellow inverted triangles). The variants show little change in EC50 values.
Supplemental Figure 4, related to Figure 4. PLCβ3 and PLCδ differ only slightly in their mode of IP3 binding. (A) Superposition of the IP3 bound structures of PLCδ (PDB entry 1DJX) and Gαq–PLCβ3- Δ892Δall. Ca2+ and IP3 from PLCδ are shown as a slate blue sphere and sticks, respectively, whereas those from PLCβ3-Δ892Δall are shown as a black spheres and gray sticks, respectively. In the PLCδ·IP3 structure, the ligand is bound slightly deeper in the active site (downward in this view), and more interactions are made with the 4- and 5-phosphate groups. In contrast, the IP3 molecule in the Gαq–PLCβ3-Δ892Δall structure interacts most intimately with the active site Ca2+ atom, and no interactions are observed between the protein and the 5- phosphate. (B) Detailed interactions of IP3 in the active site of Gαq–PLCβ3-Δ892Δall.
Supplemental Table 1, related to Figure 1, Tables 1 and 2. PLCβ3-Δ847 and PLCβ3-Δ892 Trypsin Fragments
Supplemental Table 2, related to Figure 2, Table 1. Stabilization of PLCβ3 variants in the presence of IP3
Supplemental Table 3, related to Figure 2, Table 1. Stability of PLCβ3 variants
HIGHLIGHTS.
Distinct regions of the X–Y linker play distinct roles in autoinhibition.
Loss of the acidic stretch increases activity and allows ligand binding.
Hα2′ helix prevents full activation of PLCβ3 independent of the X–Y linker.
Acidic stretch in the linker autoinhibits activity by binding the PLCβ core.
Acknowledgments
We thank K. T. Homan, M. R. Nance, and V. M. Tesmer (University of Michigan, Ann Arbor, Michigan, U.S.A.) for production of purified Gβ1γ2. We thank W. Clay Brown for advice and assistance in optimizing protein expression (High Throughput Lab, Life Sciences Institute, University of Michigan, Ann Arbor, Michigan, U.S.A.). This work was supported by US National Institutes of Health (NIH) grants HL086865 and HL071818 (J.J.G.T.) and an American Heart Association Post-Doctoral Fellowship 13POST1637009 (A.M.L.). Use of the Advanced Photon Source at Argonne National Laboratory was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under contract no. DE-AC02-06CH11357. Use of LS-CAT Sector 21 was supported by the Michigan Economic Development Corporation and Michigan’s Technology Tri-Corridor (grant 085P100817). This work used the Cell and Molecular Biology Core of the Michigan Diabetes Research and Training Center, supported by DK20572. This research was supported in part by the NIH through the University of Michigan Cancer Center Support Grant (P30 CA-46592).
Footnotes
The authors report no conflicts of interest.
AUTHOR CONTRIBUTIONS
A.M.L. and J.J.G.T. designed the overall experimental approach. A.M.L, J.A.B., and T.D.M. cloned, expressed, and purified all PLCβ3 variants, Gαq and Gβ1γ2. A.M.L. and J.A.B. carried out proteolysis assays, A.M.L. carried out all activity-based assays, and A.M.L., J.A.B., and T.D.M. carried out DSF assays. A.M.L. crystallized and solved the Gαq–PLCβ3 structures. A.M.L. and J.J.G.T. wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bianchi E, Norcini M, Smrcka A, Ghelardini C. Supraspinal Gβγ-dependent stimulation of PLCβ originating from G inhibitory protein-mu opioid receptor-coupling is necessary for morphine induced acute hyperalgesia. J Neurochem. 2009;111:171–180. doi: 10.1111/j.1471-4159.2009.06308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonacci TM, Ghosh M, Malik S, Smrcka AV. Regulatory interactions between the amino terminus of G-protein βγ subunits and the catalytic domain of phospholipase Cβ2. J Biol Chem. 2005;280:10174–10181. doi: 10.1074/jbc.M412514200. [DOI] [PubMed] [Google Scholar]
- Bonacci TM, Mathews JL, Yuan C, Lehmann DM, Malik S, Wu D, Font JL, Bidlack JM, Smrcka AV. Differential targeting of Gβγ-subunit signaling with small molecules. Science. 2006;312:443–446. doi: 10.1126/science.1120378. [DOI] [PubMed] [Google Scholar]
- Camps M, Carozzi A, Schnabel P, Scheer A, Parker PJ, Gierschik P. Isozyme-selective stimulation of phospholipase C-β2 by G protein βγ-subunits. Nature. 1992a;360:684–686. doi: 10.1038/360684a0. [DOI] [PubMed] [Google Scholar]
- Camps M, Hou C, Sidiropoulos D, Stock JB, Jakobs KH, Gierschik P. Stimulation of phospholipase C by guanine-nucleotide-binding protein βγ subunits. Eur J Biochem. 1992b;206:821–831. doi: 10.1111/j.1432-1033.1992.tb16990.x. [DOI] [PubMed] [Google Scholar]
- Camps M, Hou CF, Jakobs KH, Gierschik P. Guanosine 5′-[γ-thio]triphosphate-stimulated hydrolysis of phosphatidylinositol 4,5-bisphosphate in HL-60 granulocytes. Evidence that the guanine nucleotide acts by relieving phospholipase C from an inhibitory constraint. Biochem J. 1990;271:743–748. doi: 10.1042/bj2710743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis IW, Leaver-Fay A, Chen VB, Block JN, Kapral GJ, Wang X, Murray LW, Arendall WB, 3rd, Snoeyink J, Richardson JS, et al. MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 2007;35:W375–383. doi: 10.1093/nar/gkm216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drin G, Douguet D, Scarlata S. The pleckstrin homology domain of phospholipase Cβ transmits enzymatic activation through modulation of the membrane-domain orientation. Biochemistry. 2006;45:5712–5724. doi: 10.1021/bi052317n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essen LO, Perisic O, Cheung R, Katan M, Williams RL. Crystal structure of a mammalian phosphoinositide-specific phospholipase Cδ. Nature. 1996;380:595–602. doi: 10.1038/380595a0. [DOI] [PubMed] [Google Scholar]
- Essen LO, Perisic O, Katan M, Wu Y, Roberts MF, Williams RL. Structural mapping of the catalytic mechanism for a mammalian phosphoinositide-specific phospholipase C. Biochemistry. 1997;36:1704–1718. doi: 10.1021/bi962512p. [DOI] [PubMed] [Google Scholar]
- Filtz TM, Grubb DR, McLeod-Dryden TJ, Luo J, Woodcock EA. Gq- initiated cardiomyocyte hypertrophy is mediated by phospholipase Cβ1b. FASEB J. 2009;23:3564–3570. doi: 10.1096/fj.09-133983. [DOI] [PubMed] [Google Scholar]
- Ghosh M, Smrcka AV. Assay for G protein-dependent activation of phospholipase C β using purified protein components. Methods Mol Biol. 2004;237:67–75. doi: 10.1385/1-59259-430-1:67. [DOI] [PubMed] [Google Scholar]
- Gresset A, Sondek J, Harden TK. The phospholipase C isozymes and their regulation. Subcell Biochem. 2012;58:61–94. doi: 10.1007/978-94-007-3012-0_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb DR, Luo J, Yu YL, Woodcock EA. Scaffolding protein Homer 1c mediates hypertrophic responses downstream of Gq in cardiomyocytes. FASEB J. 2012;26:596–603. doi: 10.1096/fj.11-190330. [DOI] [PubMed] [Google Scholar]
- Guo Y, Philip F, Scarlata S. The Pleckstrin homology domains of phospholipases C-β and -δ confer activation through a common site. J Biol Chem. 2003;278:29995–30004. doi: 10.1074/jbc.M301438200. [DOI] [PubMed] [Google Scholar]
- Han DS, Golebiewska U, Stolzenberg S, Scarlata SF, Weinstein H. A dynamic model of membrane-bound phospholipase Cβ2 activation by Gβγ subunits. Mol Pharmacol. 2011;80:434–445. doi: 10.1124/mol.111.073403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SK, Mancino V, Simon MI. Phospholipase Cβ 3 mediates the scratching response activated by the histamine H1 receptor on C-fiber nociceptive neurons. Neuron. 2006;52:691–703. doi: 10.1016/j.neuron.2006.09.036. [DOI] [PubMed] [Google Scholar]
- Hicks SN, Jezyk MR, Gershburg S, Seifert JP, Harden TK, Sondek J. General and versatile autoinhibition of PLC isozymes. Mol Cell. 2008;31:383–394. doi: 10.1016/j.molcel.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilkaeva O, Kinch LN, Paulssen RH, Ross EM. Mutations in the carboxyl-terminal domain of phospholipase Cβ 1 delineate the dimer interface and a potential Gαq interaction site. J Biol Chem. 2002;277:4294–4300. doi: 10.1074/jbc.M109612200. [DOI] [PubMed] [Google Scholar]
- Illenberger D, Schwald F, Gierschik P. Characterization and purification from bovine neutrophils of a soluble guanine-nucleotide-binding protein that mediates isozyme-specific stimulation of phospholipase C β2. Eur J Biochem. 1997;246:71–77. doi: 10.1111/j.1432-1033.1997.t01-1-00071.x. [DOI] [PubMed] [Google Scholar]
- Illenberger D, Walliser C, Nurnberg B, Diaz Lorente M, Gierschik P. Specificity and structural requirements of phospholipase C-β stimulation by Rho GTPases versus G protein βγ dimers. J Biol Chem. 2003a;278:3006–3014. doi: 10.1074/jbc.M208282200. [DOI] [PubMed] [Google Scholar]
- Illenberger D, Walliser C, Strobel J, Gutman O, Niv H, Gaidzik V, Kloog Y, Gierschik P, Henis YI. Rac2 regulation of phospholipase C-β 2 activity and mode of membrane interactions in intact cells. J Biol Chem. 2003b;278:8645–8652. doi: 10.1074/jbc.M211971200. [DOI] [PubMed] [Google Scholar]
- Jenco JM, Becker KP, Morris AJ. Membrane-binding properties of phospholipase C-β1 and phospholipase C-β2: role of the C-terminus and effects of polyphosphoinositides, G-proteins and Ca2+ Biochem J. 1997;327(Pt 2):431–437. doi: 10.1042/bj3270431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezyk MR, Snyder JT, Gershberg S, Worthylake DK, Harden TK, Sondek J. Crystal structure of Rac1 bound to its effector phospholipase C-β2. Nat Struct Mol Biol. 2006;13:1135–1140. doi: 10.1038/nsmb1175. [DOI] [PubMed] [Google Scholar]
- Jiang H, Wu D, Simon MI. Activation of phospholipase C β 4 by heterotrimeric GTP-binding proteins. J Biol Chem. 1994;269:7593–7596. [PubMed] [Google Scholar]
- Kadamur G, Ross EM. Mammalian phospholipase C. Annu Rev Physiol. 2013;75:127–154. doi: 10.1146/annurev-physiol-030212-183750. [DOI] [PubMed] [Google Scholar]
- Kim CG, Park D, Rhee SG. The role of carboxyl-terminal basic amino acids in Gqα-dependent activation, particulate association, and nuclear localization of phospholipase C-β1. J Biol Chem. 1996;271:21187–21192. doi: 10.1074/jbc.271.35.21187. [DOI] [PubMed] [Google Scholar]
- Kim D, Jun KS, Lee SB, Kang NG, Min DS, Kim YH, Ryu SH, Suh PG, Shin HS. Phospholipase C isozymes selectively couple to specific neurotransmitter receptors. Nature. 1997;389:290–293. doi: 10.1038/38508. [DOI] [PubMed] [Google Scholar]
- Kuang Y, Wu Y, Smrcka A, Jiang H, Wu D. Identification of a phospholipase C β2 region that interacts with Gβγ. Proc Natl Acad Sci U S A. 1996;93:2964–2968. doi: 10.1073/pnas.93.7.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Shin S, Hepler J, Gilman A, Rhee S. Activation of phospholipase C-β2 mutants by G protein αq and βγ subunits. J Biol Chem. 1993;268:25952–25957. [PubMed] [Google Scholar]
- Li Z, Jiang H, Xie W, Zhang Z, Smrcka AV, Wu D. Roles of PLC-β2 and -β3 and PI3Kγ in chemoattractant-mediated signal transduction. Science. 2000;287:1046–1049. doi: 10.1126/science.287.5455.1046. [DOI] [PubMed] [Google Scholar]
- Lyon AM, Dutta S, Boguth CA, Skiniotis G, Tesmer JJ. Full-length Gαq-phospholipase C-β3 structure reveals interfaces of the C-terminal coiled-coil domain. Nat Struct Mol Biol. 2013;20:355–362. doi: 10.1038/nsmb.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon AM, Tesmer JJ. Structural Insights into Phospholipase C-β Function. Mol Pharmacol. 2013;84:488–500. doi: 10.1124/mol.113.087403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon AM, Tesmer VM, Dhamsania VD, Thal DM, Gutierrez J, Chowdhury S, Suddala KC, Northup JK, Tesmer JJ. An autoinhibitory helix in the C-terminal region of phospholipase C-β mediates Gαq activation. Nat Struct Mol Biol. 2011;18:999–1005. doi: 10.1038/nsmb.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews JL, Smrcka AV, Bidlack JM. A novel Gβγ-subunit inhibitor selectively modulates μ-opioid-dependent antinociception and attenuates acute morphine-induced antinociceptive tolerance and dependence. J Neurosci. 2008;28:12183–12189. doi: 10.1523/JNEUROSCI.2326-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mende U, Kagen A, Cohen A, Aramburu J, Schoen FJ, Neer EJ. Transient cardiac expression of constitutively active Gαq leads to hypertrophy and dilated cardiomyopathy by calcineurin-dependent and independent pathways. Proc Natl Acad Sci U S A. 1998;95:13893–13898. doi: 10.1073/pnas.95.23.13893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- Park D, Jhon DY, Lee CW, Ryu SH, Rhee SG. Removal of the carboxyl-terminal region of phospholipase C-β1 by calpain abolishes activation by Gαq. J Biol Chem. 1993;268:3710–3714. [PubMed] [Google Scholar]
- Paterson A, Boyer JL, Watts VJ, Morris AJ, Price EM, Harden TK. Concentration of enzyme-dependent activation of PLC-β1 and PLC-β2 by Gα11 and βγsubunits. Cell Signal. 1995;7:709–720. doi: 10.1016/0898-6568(95)00039-r. [DOI] [PubMed] [Google Scholar]
- Philip F, Kadamur G, Silos RG, Woodson J, Ross EM. Synergistic activation of phospholipase C-β3 by Gαq and Gβγ describes a simple two-state coincidence detector. Curr Biol. 2010;20:1327–1335. doi: 10.1016/j.cub.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebres RA, Roach TI, Fraser ID, Philip F, Moon C, Lin KM, Liu J, Santat L, Cheadle L, Ross EM, et al. Synergistic Ca2+ responses by Gαi- and Gαq-coupled G-protein-coupled receptors require a single PLCβ isoform that is sensitive to both Gβγ and Gαq. J Biol Chem. 2011;286:942–951. doi: 10.1074/jbc.M110.198200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Kucukural A, Zhang Y. I-TASSER: a unified platform for automated protein structure and function prediction. Nat Protoc. 2010;5:725–738. doi: 10.1038/nprot.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runnels LW, Scarlata SF. Determination of the affinities between heterotrimeric G protein subunits and their phospholipase C-β effectors. Biochemistry. 1999;38:1488–1496. doi: 10.1021/bi9821519. [DOI] [PubMed] [Google Scholar]
- Sankaran B, Osterhout J, Wu D, Smrcka AV. Identification of a structural element in phospholipase C β2 that interacts with G protein βγ subunits. J Biol Chem. 1998;273:7148–7154. doi: 10.1074/jbc.273.12.7148. [DOI] [PubMed] [Google Scholar]
- Schnabel P, Camps M. Activation of a phospholipase Cβ2 deletion mutant by limited proteolysis. Biochem J. 1998;330(Pt 1):461–468. doi: 10.1042/bj3300461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer AU, Waldo GL, Harden TK, Sondek J. A unique fold of phospholipase C-β mediates dimerization and interaction with Gαq. Nature structural biology. 2002;9:32–36. doi: 10.1038/nsb731. [DOI] [PubMed] [Google Scholar]
- Smrcka AV, Hepler JR, Brown KO, Sternweis PC. Regulation of polyphosphoinositide-specific phospholipase C activity by purified Gq. Science. 1991;251:804–807. doi: 10.1126/science.1846707. [DOI] [PubMed] [Google Scholar]
- Smrcka AV, Sternweis PC. Regulation of purified subtypes of phosphatidylinositol-specific phospholipase Cβ by G protein α and βγ subunits. J Biol Chem. 1993;268:9667–9674. [PubMed] [Google Scholar]
- Tang W, Zhang Y, Xu W, Harden TK, Sondek J, Sun L, Li L, Wu D. A PLCβ/PI3Kγ-GSK3 signaling pathway regulates cofilin phosphatase slingshot2 and neutrophil polarization and chemotaxis. Dev Cell. 2011;21:1038–1050. doi: 10.1016/j.devcel.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldo GL, Ricks TK, Hicks SN, Cheever ML, Kawano T, Tsuboi K, Wang X, Montell C, Kozasa T, Sondek J, et al. Kinetic scaffolding mediated by a phospholipase C-β and Gq signaling complex. Science. 2010;330:974–980. doi: 10.1126/science.1193438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn MD, Murshudov GN, Papiz MZ. Macromolecular TLS refinement in REFMAC at moderate resolutions. Methods Enzymol. 2003;374:300–321. doi: 10.1016/S0076-6879(03)74014-2. [DOI] [PubMed] [Google Scholar]
- Woodcock EA, Grubb DR, Iliades P. Potential treatment of cardiac hypertrophy and heart failure by inhibiting the sarcolemmal binding of phospholipase Cβ1b. Curr Drug Targets. 2010;11:1032–1040. doi: 10.2174/138945010791591331. [DOI] [PubMed] [Google Scholar]
- Wu D, Jiang H, Katz A, Simon MI. Identification of critical regions on phospholipase C-β 1 required for activation by G-proteins. J Biol Chem. 1993;268:3704–3709. [PubMed] [Google Scholar]
- Zhang W, Neer EJ. Reassembly of phospholipase C-β2 from separated domains: analysis of basal and G protein-stimulated activities. J Biol Chem. 2001;276:2503–2508. doi: 10.1074/jbc.M003562200. [DOI] [PubMed] [Google Scholar]
- Zhang Y. I-TASSER server for protein 3D structure prediction. BMC bioinformatics. 2008;9:40. doi: 10.1186/1471-2105-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1, related to Figures 1 and 4. The C-terminus of the X–Y linker has a conserved structure in all published crystal structures of PLCβ homologs: (A) S. officinales PLC21 (PDB entry 3QR0), (B) L. pealei PLC21 PDB entry 3QR1, (C) PLCβ2 catalytic core (PDB entry 2ZKM), (D) the Rac–PLCβ2 complex (PDB entry 2FJU), (E) the Gαq–PLCβ3-Δ882 complex (PDB entry 3OHM), and (F) Gαq in complex with full length PLCβ3 (PDB entry 4GNK). The most C- terminal residues of the X–Y linker forms a short helix (the “lid helix”) that occludes the active site. The active site Ca2+ atom is shown as a black sphere, the disordered region of the X–Y linker is shown as a dashed line, and the first observed amino acid on either end of the disordered region is labeled on each structure.
Supplemental Figure 2, related to Figure 1, Tables 1 and 2. SDS-PAGE analysis of PLCβ3 trypsin digests. One representative experiment is shown. 25 μg of PLCβ3-Δ892 or PLCβ3-Δ847 were incubated with 0.05 U trypsin in 50 μl reactions at room temperature for 0.5, 1, 2, and 4 h, and overnight. The samples were quenched upon the addition of 4x SDS-PAGE sample buffer, and separated by10% SDS-PAGE. Bands identified at the 2 h time point (PLCβ3-Δ847 bands A-F and PLCβ3-Δ892 bands G-K) were excised from the SDS polyacrylamide gel for fragment identification by MALDI-TOF and tandem MS/MS. See Supplemental Table 1.
Supplemental Figure 3, related to Figures 2 and 3. Determination of Tm values and heterotrimeric G protein activation of PLCβ3 variants. (A) DSF curves demonstrating stabilization by IP3. PLCβ3-Δ847 variants at 0.2 mg/mL were incubated with 5 mM CaCl2 and 200 μM ANS ± 5 mM IP3. Representative curves are shown for PLCβ3-Δ847 (gray and red circles) and PLCβ3-Δ892Δall (orange and yellow inverted triangles). PLCβ3-Δ847Δall was stabilized (right-shifted) in the presence of IP3. (B) Representative Gαq activation curves of PLCβ3-Δ892. Gαq-stimulated activity of PLCβ3-Δ892 variants was measured at increasing Gαq concentrations by quantifying free 3H- IP3 released from 3H-PIP2 liposomes after 5 min at 30 °C. PLCβ3-Δ892 (blue circles) and its variants PLCβ3-Δ892Δdisorder (green squares), PLCβ3-Δ892Δacid (pink diamonds), and PLCβ3-Δ892Δall (purple inverted triangles) are all activated at least 3-fold by Gαq, but with no significant change in EC50 values. Loss of the acidic stretch or the entire X–Y linker in PLCβ3-Δ892Δacid and PLCβ3-Δ892Δall significantly decreased the efficacy of activation. Deletion of the primary Gαq binding site in PLCβ3-Δ892ΔLINPI (light blue triangles) abrogates Gαq activation. (C) Representative Gβ1γ2 activation curves for PLCβ3-Δ892 variants. Gβ1γ2- stimulated activation of all PLCβ3 variants was carried out as described for Gαq activation assays. PLCβ3-Δ892 (blue circles) and its variants PLCβ3-Δ892Δdisorder (green squares) and PLCβ3-Δ892ΔLINPI (light blue triangles) were activated at least 24-fold by Gβ1γ2, whereas PLCβ3- Δ892Δacid (pink diamonds) and PLCβ3-Δ892Δall (purpled inverted triangles) were only activated 3-fold. (D) Representative Gβ1γ2 activation curves for PLCβ3-Δ847 variants. Similar trends were observed for PLCβ3-Δ847 (red circles) and its variants PLCβ3-Δ847Δdisorder (orange squares) and PLCβ3-Δ847Δall (yellow inverted triangles). The variants show little change in EC50 values.
Supplemental Figure 4, related to Figure 4. PLCβ3 and PLCδ differ only slightly in their mode of IP3 binding. (A) Superposition of the IP3 bound structures of PLCδ (PDB entry 1DJX) and Gαq–PLCβ3- Δ892Δall. Ca2+ and IP3 from PLCδ are shown as a slate blue sphere and sticks, respectively, whereas those from PLCβ3-Δ892Δall are shown as a black spheres and gray sticks, respectively. In the PLCδ·IP3 structure, the ligand is bound slightly deeper in the active site (downward in this view), and more interactions are made with the 4- and 5-phosphate groups. In contrast, the IP3 molecule in the Gαq–PLCβ3-Δ892Δall structure interacts most intimately with the active site Ca2+ atom, and no interactions are observed between the protein and the 5- phosphate. (B) Detailed interactions of IP3 in the active site of Gαq–PLCβ3-Δ892Δall.
Supplemental Table 1, related to Figure 1, Tables 1 and 2. PLCβ3-Δ847 and PLCβ3-Δ892 Trypsin Fragments
Supplemental Table 2, related to Figure 2, Table 1. Stabilization of PLCβ3 variants in the presence of IP3
Supplemental Table 3, related to Figure 2, Table 1. Stability of PLCβ3 variants