Abstract
Background
The degree to which people with schizophrenia show awareness of cognitive dysfunction and whether this neurocognitive insight affects treatment use or outcome is understudied. We aimed to examine neurocognitive insight among a treatment-seeking sample of patients with psychotic disorders, and whether neurocognitive insight affected treatment utilization or outcome.
Method
69 individuals with schizophrenia-spectrum disorders enrolled in a trial comparing Compensatory Cognitive Training (CCT) to standard pharmacotherapy. Participants with objective cognitive impairment were identified and grouped into “intact” vs. “impaired” neurocognitive insight groups. These groups were then compared via ANCOVA on three treatment utilization variables and six post-treatment cognitive/functional variables.
Results
43 participants demonstrated objective cognitive impairment. Among those individuals, 31 were considered to have intact neurocognitive insight and 12 showed impaired neurocognitive insight. These two groups did not differ on CCT attendance, satisfaction with the intervention, or self-reported cognitive strategy use at post-treatment. There were significant treatment group by neurocognitive insight group interactions for verbal memory and functional capacity outcomes, such that individuals with impaired neurocognitive insight who received treatment performed better than those who did not receive treatment.
Conclusions
Even among individuals who self-select into a cognitive treatment study, many show minimal awareness of cognitive dysfunction. Impaired neurocognitive insight, however, was not associated with decreased treatment utilization, and was associated with positive treatment outcomes in some cognitive domains as well as functional capacity. As cognitive training treatments become increasingly available, impaired neurocognitive insight need not be a barrier to participation.
Keywords: Cognitive remediation, cognition, awareness, psychosis, functional capacity
1. Introduction
Cognitive impairment is a central feature of schizophrenia, affects everyday functioning, and limits benefit from psychiatric rehabilitation (Green, 1996; Harding et al., 2008; McGurk et al., 2004; Velligan et al., 1997; Walsh et al., 2003). Cognitive training or remediation is an intervention to improve cognition in this population; the most recent meta-analysis of 2,104 participants demonstrated effect sizes of 0.45 on cognition and 0.42 on functioning, with no evidence that treatment approach or duration affected cognitive outcome (Wykes et al., 2011).
Awareness of cognitive impairment, or neurocognitive insight, may moderate treatment adherence and effectiveness, but few studies have examined these questions. One recent study demonstrated that, contrary to expectation, higher rates of cognitive complaints were associated with lower treatment utilization (Gooding et al., 2012). Another study found that cognitive complaints generally decreased from baseline to post-treatment (Lecardeur et al., 2009).
Given the limited literature in this area, the current study examined awareness of cognitive dysfunction among participants in a randomized controlled trial of cognitive training, and whether awareness was related to treatment utilization or outcome. We hypothesized that (1) participants with impaired neurocognitive insight would demonstrate poorer treatment attendance, lower treatment satisfaction, and less strategy use at post-treatment than those with intact neurocognitive insight, and (2) impaired neurocognitive insight would negatively affect treatment outcome as measured by cognitive and functional capacity performance.
2. Method
2.1 Participants
Participants included 69 outpatient adults with a DSM-IV (American Psychiatric Association, 1994) primary psychotic disorder who enrolled in a study of Compensatory Cognitive Training (CCT) (for further details, see Table 1 and Twamley et al., 2012). This study was approved by the UCSD Institutional Review Board and all participants provided written informed consent.
Table 1.
Demographic and clinical features of the full sample (n=69) and the cognitively impaired sample (n=43)
| Full sample | Cognitively impaired | |
|---|---|---|
| Mean (SD) or % | Mean (SD) or % | |
| Age, years | 46.3 (9.7) | 48.7 (8.1) |
| Education, years | 12.9 (1.7) | 12.8 (1.7) |
| Duration of illness, years | 23.3 (12.3) | 25.8 (10.1) |
| % Male | 65.2 | 67.4 |
| % Caucasian | 76.8 | 72.1 |
| % Hispanic ethnicity | 17.4 | 18.6 |
| % African American | 11.6 | 16.3 |
| % Schizophrenia | 53.6 | 55.8 |
| % Prescribed antipsychotic medication | 94.0 | 90.2 |
| % Living independently | 80.6 | 78.0 |
| % Never married | 55.9 | 41.8 |
| PANSS positive symptoms total | 16.7 (6.3) | 16.5 (6.4) |
| PANSS negative symptoms total | 15.0 (5.7) | 16.4 (6.1) |
| HAMD 1-17 total | 11.7 (7.0) | 11.4 (7.1) |
Note. HAMD=Hamilton Depression Rating Scale; PANSS=Positive and Negative Syndrome Scale
2.2 Procedures
Participants completed a baseline assessment and were randomly assigned to standard pharmacotherapy plus CCT or to standard pharmacotherapy (SP) alone. A neuropsychological, clinical, and functional battery was administered at baseline and 3 months (immediate post-treatment) by blinded raters. The 12-week CCT intervention emphasized compensatory strategies in four cognitive domains: prospective memory, attention, learning and memory, and executive functioning. The procedures and main outcomes of the randomized controlled trial are reported elsewhere (Twamley et al., 2012).
2.3 Measures
Premorbid intellectual functioning was measured with the American National Adult Reading Test (ANART; Grober and Sliwinski, 1991). CCT-targeted cognitive domains and measures included:
-
1
Prospective memory: Memory for Intentions Screening Test total score (Raskin, 2004)
-
2
Attention: Wechsler Adult Intelligence Scale, third edition (WAIS-III) Digit Span total score (Wechsler, 1997a)
-
3
Verbal learning and memory: Hopkins Verbal Learning Test-Revised (HVLT-R) total immediate recall and total delayed recall (Brandt and Benedict, 2001); Wechsler Memory Scale, third edition (WMS-III) Logical Memory total immediate recall and total delayed recall (Wechsler, 1997b)
-
4
Executive Functioning: Wisconsin Card Sorting Test (WCST-64) total errors (Kongs et al., 2000); Trail making test, part B minus part A (Reitan, 1992)
Non-CCT-targeted domains and measures included:
-
5
Processing speed: WAIS-III Digit Symbol total correct and Symbol Search total correct (Wechsler, 1997a), Trail making test, part A (Reitan, 1992)
-
6
Working memory: WAIS-III Letter-Number Sequencing total correct (Wechsler, 1997a)
-
7
Visual learning and memory: Brief Visuospatial Memory Test–Revised (BVMT-R) total immediate recall and total delayed recall (Benedict, 1997)
Psychiatric symptoms were measured with the Positive and Negative Syndrome Scale (PANSS; Kay et al., 1987) and the Hamilton Depression Rating Scale (HAMD; Hamilton, 1960). Functional capacity was measured with the UCSD Performance-Based Skills Assessment (UPSA; Patterson et al., 2001; total score), a role-play measure that assesses skills in various everyday activities.
Self-reported frequency of cognitive problems was measured with the Cognitive Problems subscale of the Cognitive Problems and Strategies Assessment (CPSA), created for the study. The subscale contains thirty statements (e.g., “I have trouble staying focused during conversations”) rated by the respondent as occurring rarely/never (0 points), sometimes (1 point), often (2 points), and always (3 points). The CPSA also has a 30-item subscale measuring cognitive strategy use.
2.4 Analyses
All continuous variables were normally distributed. To identify participants with objective cognitive impairment, a global deficit score (GDS) was calculated for each participant using thirteen baseline cognitive t-scores that were assigned a numerical degree of deficit on a scale from 0 (t-score ≥40; no deficit) to 5 (t-score ≤19; severe deficit) in five point decrements in the t-score, and then averaged. The recommended cutoff of GDS ≥0.50 was used to indicate cognitive impairment (Heaton et al., 2007).
Latent profile analysis (LPA) was used to group cases based on participants’ similarities on two continuous observed variables: baseline GDS and CPSA problems total score. In contrast to other techniques like cluster analysis or median split, LPA is a multivariate statistical approach that generates testable models whose goodness of fit can be analyzed. For the descriptive fit indices, lower values are considered better fit for the Akaike Information Criterion (AIC), Bayesian Information Criterion (BIC), and Adjusted BIC; higher values indicate better fit for Entropy. The Lo-Mendell-Rubin Test (LMRT) of significance indicates whether a model has a statistically better fit than a lower-order model using a cutoff of <.05 for significance.
The main hypotheses were tested using the resulting groups from the LPA. To examine treatment utilization, the intact vs. impaired neurocognitive insight groups were compared via t-tests for independent samples on adherence (percent of CCT classes attended), satisfaction (overall rating of the CCT class on a 1–10 scale), and post-treatment cognitive strategy use (post-treatment mean CPSA strategies). To evaluate whether neurocognitive insight affected treatment outcome, analysis of covariance (ANCOVA) was conducted using two dichotomous independent variables: treatment group (CCT versus SP) and neurocognitive insight group (intact versus impaired), and six continuous dependent variables: immediate post-treatment MIST total raw score, WAIS-III digit span total score, HVLT-R total recall raw score, HVLT-R delayed recall raw score, WCST-64 total errors raw score, and UPSA total raw score. Baseline scores were entered as covariates, and the dependent variables only included CCT-targeted cognitive domains to reduce Type I error. Omega squared was calculated as a measure of effect size.
3. Results
Among all 69 participants, 43 (62.3%) were classified as cognitively impaired (GDS ≥0.5) while 26 (37.7%) were classified as cognitively intact. Because awareness of cognitive impairment necessarily requires impaired cognition, the remainder of the analyses included only the 43 cognitively impaired participants.
The LPA demonstrated that on all of the descriptive fit indices, the 2-class model was preferred, though the LMRT test was not significant (Table 2). According to this LPA, 31 participants demonstrated “intact neurocognitive insight” (mean GDS=0.95; CPSA mean total=27.15), and 12 participants demonstrated “impaired neurocognitive insight” (mean GDS=2.29; CPSA mean total=38.02). The intact neurocognitive insight participants had significantly higher levels of education by one year, estimated premorbid IQ by 8.5 points, less severe positive symptoms by 5.9 points, and less severe depressive symptoms by 5.7 points (all ps≤0.017).
Table 2.
Descriptive and statistical fit indices
| Cognitively Impaired (n=43) | 1 class | 2 classes | 3 classes |
|---|---|---|---|
| AIC | 450.21 | 442.00 | 448.00 |
| BIC | 457.25 | 454.33 | 465.61 |
| Adjusted BIC | 444.72 | 432.40 | 434.29 |
| Entropy | N/A | 0.84 | 0.45 |
| LMRT | N/A | 0.30 | 0.50 |
Note. Favorable values are indicated in bold font. AIC=Akaike Information Criterion; BIC=Bayesian Information Criterion; LMRT=Lo-Mendell-Rubin Test of significance
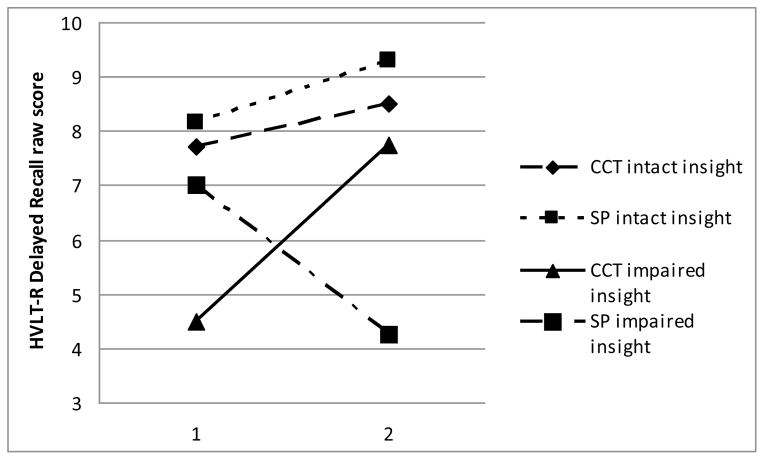
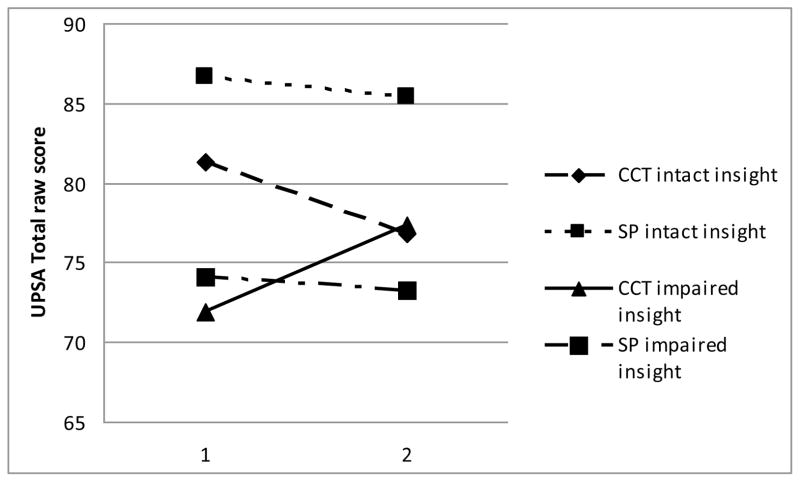
There were no statistically significant group differences on the treatment utilization variables, including CCT attendance, satisfaction with the intervention, or self-reported strategy use at post-treatment (all ps>0.187; Table 3). It is possible that high satisfaction ratings in both groups resulted in a restricted range and non-significant results. With regard to treatment outcome, the 2×2 ANCOVA yielded two statistically significant interactions between treatment group and neurocognitive insight group (Table 4): HVLT-R delayed recall (Figure 1) and UPSA total score (Figure 2). These interactions remained significant when GDS was added as an additional covariate, suggesting that the effects were not simply due to lower initial performance in the impaired neurocognitive insight group.
Table 3.
Group differences in treatment utilization variables
| n | Intact Neurocognitive Insight Mean (SD) |
Impaired Neurocognitive Insight Mean (SD) |
t | df | p | |
|---|---|---|---|---|---|---|
| CCT attendance (%) | 20 | 52.5 (41.9) | 69.1 (42.0) | −0.84 | 18 | 0.411 |
| Satisfaction (1–10) | 10 | 9.3 (1.0) | 8.3 (1.2) | 1.44 | 8 | 0.187 |
| Mean self-reported strategy use at post-treatment | 8 | 1.8 (0.7) | 1.4 (1.0) | 0.66 | 6 | 0.533 |
Table 4.
Interaction effects of treatment group and insight group on cognitive and functional outcomes
| Dependent variable | interaction F | df | p | % variance accounted for (omega squared) | description of effect size |
|---|---|---|---|---|---|
| MIST total score | 0.043 | 1 | 0.838 | 0 | no effect |
| WMS-III digit span total | 0.315 | 1 | 0.580 | 0 | no effect |
| HVLT-R total recall | 3.708 | 1 | 0.066 | 5.5 | medium effect |
| HVLT-R delayed recall | 9.776 | 1 | 0.005 | 11.4 | large effect |
| WCST-64 total errors | 0.031 | 1 | 0.863 | 0 | no effect |
| UPSA total score | 6.085 | 1 | 0.022 | 9.2 | large effect |
Note. HVLT-R=Hopkins Verbal Learning Test, revised; MIST=Memory for Intentions Screening Test; UPSA=UCSD Performance Based Skills Assessment; WCST-64=Wisconsin Card Sorting Test 64 card version; WMS-III=Wechsler Memory Scale, third edition
Figure 1.
Baseline and post-treatment scores by group on HVLT-R delayed recall
Figure 2.
Baseline and post-treatment scores by group on UPSA total score
4. Discussion
Taken together, these results indicate that among individuals with schizophrenia-spectrum disorders who self-selected into a cognitive training study, approximately two-thirds demonstrated impaired cognition and one-third showed poor awareness of that impairment. The percentage of individuals with impaired neurocognitive insight was lower than in previous reports (28% in this sample, compared to 54% in Burton et al., under review and 50% in Medalia and Thysen, 2008), which could reflect the treatment-seeking nature of patients who self-selected into this cognitive training study. Impaired neurocognitive insight may be more prevalent in the general clinical population, and may differentially affect treatment adherence in clinical settings; further research is needed. Despite some participants’ lack of neurocognitive insight, they were no different from those with intact neurocognitive insight in terms of treatment utilization, and they demonstrated good treatment outcomes in the domains of verbal memory and functional capacity. In short, we found no evidence that unawareness of cognitive impairment is a barrier to participation in or ability to benefit from cognitive training.
The limitations of this study must be considered. The cognitively impaired sample size of 43 is small and limits power to detect significant group differences. To preserve power, we did not correct for multiple comparisons or use additional covariates. However, although the neurocognitive insight groups significantly differed on some variables, the correlations between these factors and the dependent variables were modest and mostly not significant, supporting their exclusion as covariates. The psychometric properties of the CPSA also remain under investigation. Nevertheless, replication of these results in a larger sample would be beneficial to support and extend our conclusions.
Acknowledgments
Role of the funding source
This work was supported by the Brain and Behavior Research Foundation, National Alliance for Research on Schizophrenia and Depression (award to E.W.T.), and by the National Institutes of Health (T32MH019934 to C.Z.B.)
Footnotes
Contributors
Authors Burton and Twamley designed the study. Author Burton managed the literature search, conducted the statistical analyses, and wrote the first draft of the manuscript. Author Twamley contributed to and has approved the final manuscript.
Conflict of interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 1994. [Google Scholar]
- Benedict RHB. Professional Manual. Psychological Assessment Resources; Florida: 1997. Brief Visuospatial Memory Test – Revised. [Google Scholar]
- Brandt J, Benedict RHB. Professional Manual. Psychological Assessment Resources; Florida: 2001. Hopkins Verbal Learning Test – Revised. [Google Scholar]
- Gooding AL, Saperstein A, Rivera Mindt M, Medalia A. Predictors of treatment utilisation at cognitive remediation groups for schizophrenia: The roles of neuropsychological, psychological and clinical variables. Neuropsychol Rehabil. 2012;22:516–531. doi: 10.1080/09602011.2012.665583. [DOI] [PubMed] [Google Scholar]
- Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Grober E, Sliwinski M. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. J Clin Exp Neuropsychol. 1991;13:933–949. doi: 10.1080/01688639108405109. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding B, Torres-Harding S, Bond GR, Salyers MP, Rollins AL, Hardin T. Factors associated with early attrition from psychosocial rehabilitation programs. Community Ment Health J. 2008;44:283–288. doi: 10.1007/s10597-008-9128-9. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Miller WS, Taylor MJ, Grant I. Revised comprehensive norms for an expanded Halstead-Reitan Battery: Demographically adjusted neuropsychological norms for African American and Caucasian adults (HRB) Psychological Assessment Resources; Florida: 2007. [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kongs SK, Thompson LL, Iverson GL, Heaton RK. Wisconsin Card Sorting Test – 64 card version (WCST-64) Psychological Assessment Resources; Florida: 2000. [Google Scholar]
- Lecardeur L, Stip E, Giguere M, Blouin G, Rodriguez JP, Champagne-Lavau M. Effects of cognitive remediation therapies on psychotic symptoms and cognitive complaints in patients with schizophrenia and related disorders: a randomized study. Schizophr Res. 2009;111:153–158. doi: 10.1016/j.schres.2009.03.029. [DOI] [PubMed] [Google Scholar]
- McGurk SR, Mueser KT, Walling DW, Harvey PD, Meltzer HY. Cognitive functioning predicts outpatient service utilization in schizophrenia. Ment Health Serv Res. 2004;6:185–188. doi: 10.1023/b:mhsr.0000036491.58918.71. [DOI] [PubMed] [Google Scholar]
- Medalia A, Thysen J. Insight into neurocognitive dysfunction in schizophrenia. Schizophr Bull. 2008;34:1221–1230. doi: 10.1093/schbul/sbm144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson TL, Moscona S, McKibbin CL, Hughs T, Jeste DV. UCSD performance-based skills assessment (UPSA): Development of a new measure of everyday functioning for severely mentally ill adults. Schizophr Bull. 2001;27:235–245. doi: 10.1093/oxfordjournals.schbul.a006870. [DOI] [PubMed] [Google Scholar]
- Raskin S. Memory for Intentions Screening Test [Abstract] J Int Neuropsychol Soc. 2004;10(Suppl 1):110. [Google Scholar]
- Reitan RM. Trail Making Test: Manual for Administration and Scoring. Reitan Neuropsychology Laboratory; Arizona: 1992. [Google Scholar]
- Twamley EW, Vella L, Burton CZ, Heaton RK, Jeste DV. Compensatory Cognitive Training for psychosis: Effects in a randomized controlled trial. J Clin Psychiatry. 2012;73:1212–1219. doi: 10.4088/JCP.12m07686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velligan DI, Mahurin RK, Diamond PL, Hazleton BC, Eckert SL, Miller AL. The functional significance of symptomatology and cognitive function in schizophrenia. Schizophr Res. 1997;25:21–31. doi: 10.1016/S0920-9964(97)00010-8. [DOI] [PubMed] [Google Scholar]
- Walsh EG, Wu B, Mitchell JB, Berkmann LF. Cognitive function and acute care utilization. J Gerontol B Psychol Sci Soc Sci. 2003;58:S38–S49. doi: 10.1093/geronb/58.1.s38. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale – Third Edition (WAIS-III) The Psychological Corporation; Texas: 1997a. [Google Scholar]
- Wechsler D. Wechsler Memory Scale – Third Edition (WMS-III) The Psychological Corporation; Texas: 1997b. [Google Scholar]
- Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry. 2011;168:472–485. doi: 10.1176/appi.ajp.2010.10060855. [DOI] [PubMed] [Google Scholar]