Abstract
Background and Purpose
Norrin and its receptor Frizzled 4 have important roles in the blood-brain barrier (BBB) development. This study is to investigate a potential role and mechanism of Norrin/Frizzled 4 on protecting BBB integrity after subarachnoid hemorrhage (SAH).
Methods
One hundred and seventy-eight male Sprague-Dawley rats were used. SAH model was induced by endovascular perforation. Frizzled 4 small interfering RNA (siRNA) was injected intracerebroventricularly 48 hours before SAH. Norrin was administrated intracerebroventricularly 3 hours after SAH. SAH grade, neurologic scores, brain water content, Evans blue extravasation, western blots and immunofluorescence were employed to study the mechanisms of Norrin and its receptor regulation protein TSPAN12, as well as neurological outcome.
Results
Endogenous Norrin and TSPAN12 expression were increased after SAH, and Norrin was colocalizated with astrocytes marker GFAP in cortex. Exogenous Norrin treatment significantly alleviated neurobehavioral dysfunction, reduced brain water content and Evans blue extravasation, promoted β-catenin nuclear translocation and increased Occludin, VE-Cadherin and ZO-1 expressions. These effects were abolished by Frizzled 4 siRNA pretreated before SAH.
Conclusions
Norrin protected BBB integrity and improved neurological outcome after SAH, and the action of Norrin seemed mediated by Frizzled 4 receptor activation which promoted β-catenin nuclear translocation, which then enhanced Occludin, VE-Cadherin and ZO-1 expression. Norrin might have potential to protect BBB after SAH.
Keywords: Subarachnoid hemorrhage, Norrin, Blood-brain barrier, Frizzled-4, β-catenin
Introduction
The pathophysiology of subarachnoid hemorrhage (SAH) and other stroke events involve the cerebral vascular neural network which includes arterial and venous systems as well as neuronal cells and other support cells and matrix1. Within this vascular neural network, blood-brain barrier (BBB) damage contributes to brain edema after SAH2. Therefore, to improve clinical outcomes of SAH patients, it may be important to develop new therapies against BBB disruption3.
Norrin, encoded by Norrie Disease Protein gene, is a secreted small molecule protein which highly expressed in embryo development to regulate angiogenesis4. Recent studies demonstrated that Norrin is also essential to BBB formation5. Norrin activates the Frizzled 4 receptor, which is also the receptor for canonical Wnt/β-catenin pathway6. The canonical Wnt/β-catenin pathway is reported to exhibit neuroprotective and angiogenesis actions7.
However, it is unclear whether endogenous Norrin is induced and whether Norrin/Frizzled 4 will be protective against BBB disruption after SAH. This study investigated a potential role and mechanisms of Norrin and its receptor in the protection of BBB in a rat model of SAH (Supplemental Figure I).
Materials and Methods
Animals
One hundred and seventy-eight male adult Sprague-Dawley rats (Harlan, Indianapolis, IN) weighting 300-350 g were used in the present study. All experimental protocols were approved by the Institutional Animal Care and Use Committee of Loma Linda University.
Experimental Design
The experiment was designed as follows.
Experiment I (Supplemental Figure II)
To determine the time course of Norrin and TSPAN12 after SAH, twenty rats were randomly assigned into five groups: Sham (n = 4), SAH 6 hours (n = 4), SAH 12 hours (n = 4), SAH 24 hours (n = 4), and SAH 72 hours (n=4). Western blots were used to detect the protein expression of Norrin and TSPAN12 in ipsilateral/left hemisphere of each group. Double immunohistochemistry staining of Norrin and GFAP were also performed in at 12 hours after SAH(n = 1).
Experiment II (Supplemental Figure II)
For outcome evaluation, Sixty-four rats were randomly divided into six groups: Sham (n = 13), SAH (n = 7), SAH + Vehicle (5 ul sterile saline8) (n = 15), SAH + 5 ng/ul9 r-Norrin (25 ng in 5 ul sterile saline) (n = 8), SAH + 25 ng/ul r-Norrin (125 ng in 5 ul sterile saline) (n = 7) and SAH + 50 ng/ul r-Norrin (250 ng in 5 ul sterile saline)(n = 14). Vehicle or r-Norrin was injected intracerebroventricularly 3 hours after SAH onset. Neurologic Scores, including Modified Garcia Test and Beam Balance Test, SAH Grading Scores and Brain Water Content were assessed at 24 and 72 hours after SAH in all groups (n = 6). Evans Blue Extravasation was evaluated at 24 hours after SAH in Sham, SAH + Vehicle and SAH + 50 ng/ul r-Norrin groups (n = 5). Double immunohistochemistry staining of ZO-1 and vWF were also performed in Sham, SAH, and SAH + 50 ng/ul r-Norrin groups (n = 2).
Experiment III (Supplemental Figure II)
Nine rats were randomly assigned into three groups: Sham (n = 3), SAH + 500 pmol Scrambled siRNA (in 5 ul sterile saline) (n = 3), SAH + 500 pmol Frizzled 4 siRNA (in 5 ul sterile saline) (n = 3). Scrambled siRNA or Frizzled 4 siRNA was intracerebroventricularly injected 48 hours before SAH10. Western blots of ipsilateral/left hemisphere were conducted at 48 hours after injection in all groups.
Eighty rats were randomly divided into five groups for mechanism study: Sham (n = 10), SAH + Vehicle (n = 11), SAH + 50 ng/ul r-Norrin (250 ng in 5 ul sterile saline)(n = 11), SAH + 50 ng/ul r-Norrin + 500 pmol Scrambled siRNA (in 5 ul sterile saline) (n = 24) and SAH + 50 ng/ul r-Norrin + 500 pmol Frizzled 4 siRNA (in 5 ul sterile saline) (n = 24). Scrambled siRNA or Frizzled 4 siRNA was intracerebroventricularly injected 48 hours before SAH modeling10. Modified Garcia Test and Brain Water Content and were performed at 24 hours after SAH in SAH + r-Norrin+ Scrambled siRNA and SAH + r-Norrin+ Frizzled 4 siRNA groups (n = 6), so did Evans Blue Extravasation assessment (n = 5). The data of these three tests in Sham, SAH + Vehicle and SAH + r-Norrin groups were shared with Experiment II. Then, Evans Blue Fluorescence of ipsilateral/left cortex were also performed at 24 hours after SAH in Sham, SAH, SAH + r-Norrin and SAH + r-Norrin + Frizzled 4 siRNA groups(n = 1). Western blots of ipsilateral/left hemisphere were conducted at 24 hours after SAH in all groups (n = 10, 5 rats for total protein extraction and other 5 rats for nucleic and cytoplasmic protein extraction).
SAH Model
SAH rat model was induced by endovascular perforation as previously described11. All animals were transorally intubated after induced anesthesia with 5% isoflurane in 70/30% medical air/oxygen, and a small rodent respirator (Harvard Apparatus, Holliston, MA) was used to maintain an adequate respiration. Anesthesia was then maintained with 3% isoflurane in 70/30% medical air/oxygen. The external carotid artery was identified and transected distally with a 3 mm stump. A 4-0 sharpened monofilament nylon suture was advanced into the internal carotid artery through the external carotid artery until resistance was felt (at 18-20 mm) and then was pushed 5 mm further to penetrate the bifurcation of the anterior and middle cerebral artery. The suture was then withdrawn and the internal carotid artery was reperfused to produce SAH. Sham-operated rats underwent the same procedure except the suture was withdrawn without perforation after feeling resistance.
Intracerebroventricular (ICV) Injection
Intracerebroventricular injection procedure was performed as reported previously12. A small burr hole was drilled on the skull according to the following coordinates relative to bregma: 1.5 mm posterior; 1.0 mm lateral. The needle of 10 μl Hamilton syringe (Microliter 701; Hamilton Company, Reno, NV) was stereotactically inserted into the left lateral ventricle through the burr hole 4.0 mm below the horizontal plane of bregma. Five microliters of r-Norrin (R&D system, Minneapolis, MN) in sterile saline were infused at a rate of 0.5μl/min 3 hours after SAH induction, while 500 pmol/5μl Frizzled 4 or scrambled siRNA (Invitrogen, Grand Island, NY) were infused at the same rate at 48 hours before SAH modeling. Frizzled 4 siRNA is a pool of three different siRNA duplexes in order to improve the knockdown efficiency. All Frizzled 4 siRNA sequences are provided in 5′→3′orientation:
(I) Sense: CCG UUC UCA UCC AAG AGG GAC UUA A
Antisense: UUA AGU CCC UCU UGG AUG AGA ACG G
(II) Sense: GGC ACU CUU UCG GUA UUC UGA AGA U
Antisense: AUC UGC AGA AUA CCG AAA GAG UGC C
(III) Sense: CCU AUU UGG UGA UUG GAA CUC UAU U
Antisense: AAU AGA GUU CCA AUC ACC AAA UAG G
The syringe was left in situ for an additional 10 min before slowly removing. In Experiment I, the Sham group rats were subjected the same procedure without inserting the needle.
Neurological Outcome Assessment
Neurological deficits were evaluated at 24 and 72 hours after SAH using an 18-point score system named Modified Garcia Scale and another 4-points score system named Beam Balance Test in the outcome study13. Modified Garcia assessment consisted of six tests covering spontaneous activity, spontaneous movement of four limbs, forepaw outstretching, climbing, body proprioception, and response to whisker stimulation (3-18 points). For beam balance test, the rats were placed on a beam to be observed their walking distance within 1 min (0-4 points). The mean of neurologic score was evaluated by two blinded observers for grading.
SAH Grade Assessment
An 18-point SAH severity grading system was used as previously described14. The basal cistern was divided into six segments that can be scored from 0 to 3 according to the amount of subarachnoid blood clot. A total score was calculated by adding the scores from six segments (0-18 points). Animals received a score less than 8 should be excluded from the study.
Brain Water Content
The brains were quickly separated into the left and right cerebral hemispheres, cerebellum, and brain stem and weighed (wet weight). Then, the brain samples were dried in an oven at 105° C for 72 h and weighed again (dry weight). The percentage of the water content was calculated as [(wet weight–dry weight)/wet weight]×100%8.
Evans Blue Extravasation and Fluorescence
Evans Blue Extravasation was performed as reported previously13. At 24 hours post-operation, the Evans blue dye (2%, 5 ml/kg) (Sigma-Aldrich, St. Louis, MO) was injected and administered over 2 min into the left femoral vein, where it was allowed to circulate for 60 min. Under anesthesia, the rats were sacrificed by an intracardial perfusion with phosphate-buffered solution (PBS). After that the brains were removed and divided into the left and right cerebral hemispheres for homogenate. The brain samples were weighed, homogenized in saline, and centrifuged at 15,000 g for 30 minutes. Next, an equal volume of trichloroacetic acid was added to the resultant supernatant. The samples were then incubated overnight at 4° C and centrifuged at 15,000 g for 30 minutes. The resultant supernatant was then spectrophotometrically quantified for the extravasated Evans blue dye at 615 nm.
For Evans Blue Fluorescence, PBS was replaced by 4% paraformaldehyde after PBS intracardial perfusion. Then the brains were removed to be prepared for coronal brain sections (10 μm) as same as Immunofluorescence Staining. And red autofluorescence of Evans Blue was observed on the slides using excitation and emission filters for rhodamine fluorescence15 (Olympus OX51, Tokyo, Japan).
Western-Blotting
Western blot was performed as reported previously16. The protein extracted from left hemisphere (perforation side) was used for western blot analysis. Membrane proteins were extracted by NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce Biotechnology, Rockford, IL), following instruction manual. Equivalent total, nucleic or cytoplasmic protein amounts (30μg) were loaded in each lane of SDS-PAGE gels. After gel electrophoresis, protein was transferred onto a nitrocellulose membrane, which was then blocked by blocking buffer for 2 hours at room temperature. Following primary antibodies were diluted to incubate with the membrane under gentle agitation at 4°C overnight: anti-Norrin, anti-TSPAN12 (Abcam, Cambridge, MA), anti-β-catenin, anti-ZO-1, anti-Occludin, anti-VE-Cadherin (Santa Cruz Biotechnology, Santa Cruz, CA). β-actin was used as an internal loading control by using anti-β-actin primary antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Appropriate secondary antibodies were incubated with the nitrocellulose membrane for 2 hours at room temperature. Chemiluminescent detection was performed to identify the immune bands with the kit (ECL Plus; Amersham Bioscience, Arlington Heights, IL). Data was analyzed by densitometry with Quantity One 4.6.2(Bio-Rad Laboratories, Berkeley, CA).
Immunofluorescence Staining
Immunofluorescence staining for brain was performed on fixed frozen section as previously described17. Twelve hours or twenty-four hours after SAH, rats were deeply anesthetized and transcardially perfused with phosphate-buffered solution (PBS) and 10% formalin. Rats' brains were rapidly isolated and postfixed in 10% formalin for 24 hours and then in 30% sucrose for 3 days. Coronal brain sections (10 μm) were obtained with the help of cryostat (Leica CM3050S-3-1-1, Bannockburn, IL) and permeabilized with 0.3% Triton X-100 in PBS for 30 min. Sections were blocked with 5% donkey serum for 1 hour and incubated at 4° C overnight with primary antibodies: anti-ZO-1 (Abcam, Cambridge, MA) and anti-von Willebrand factor (vWF) (Millipore, Temecula, CA), anti-Norrin(Abcam, Cambridge, MA) and anti-Glial Fibrillary Acidic Protein(GFAP) (Santa Cruz Biotechnology, Santa Cruz, CA) followed by fluorescein isothiocyanate (FITC)- and Texas Red-conjugated secondary antibodies (Jackson Immunoresearch, West Grove, PA) for 2 hours at room temperature. The colocalization of ZO-1 with the marker of endothelial cells vWF and the colocalization of Norrin with the marker of astrocytes GFAP were examined by fluorescent microscope (Olympus OX51, Tokyo, Japan).
Statistical Analysis
One-way analysis of variance (ANOVA) followed by Tukey's multiple comparisons test was used for different groups' comparison. Chi-square tests were used for behavior score analyses. Data were showed as mean ± SD. P < 0.05 was considered statistical difference.
Results
Time Course of Endogenous Norrin and TSPAN12 Expression after SAH
SAH blood clots were cleared from the basal cisterns as time passed, but significant hemorrhage remained 72 hours after SAH. Western blot analysis demonstrated a significant elevation of Norrin in ipsilateral hemisphere after SAH except at 24 hours, with two peaks at 12 hours and 72 hours after SAH (Figure 1A, 1B). TSPAN12 was significantly increased at all four time points after SAH (Figure 1A, 1C). Furthermore, double immunohistochemistry staining at the first peak of Norrin(12 hours) after SAH showed that Norrin expression was colocalizated with GFAP(Figure 1D) in left/ipsilateral cortex.
Figure 1.
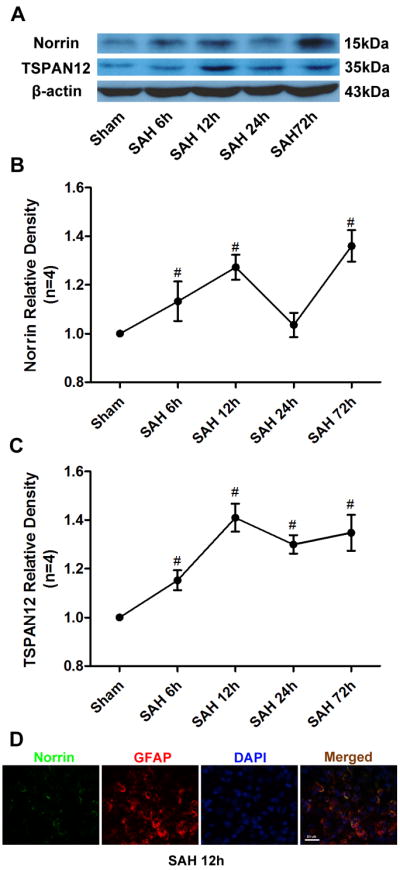
Time course of endogenous Norrin and TSPAN12 expression after SAH. (A) Representative western blot bands of Norrin and TSPAN12 time course from ipsilateral hemisphere after SAH. Quantitative analyses of (B) Norrin and TSPAN12 (C) time course from ipsilateral hemisphere after SAH. (D) Representative immunohistochemistry staining slices of Norrin and GFAP at 12 hours after SAH. GFAP= Glial Fibrillary Acidic Protein. Relative densities of each protein have been normalized against the sham group. n=4 #: vs. Sham P<0.05.
Exogenous Recombinant Norrin(r-Norrin) Treatment
Comparisons of SAH grading score revealed no significant differences among the groups either at 24 or 72 hours after SAH (Supplemental Figure IIIA, IIIB). None of the sham-operated rats died, and 6 rats died within 24 hours after SAH due to severe hemorrhagic volume, 1 rat died during intracerebralventricular injection.
Compared with sham group, the rats from the SAH-, Vehicle- and r-Norrin 5 ng/ul groups showed significant neurologic impairment on Modified Garcia Test both at 24 and 72 hours after SAH, but not after r-Norrin 25 ng/ul and 50 ng/ul treatments (Figure 2A, 2B). Further analysis showed that both r-Norrin 25 ng/ul and 50 ng/ul treatment significantly improved neurobehavioral outcomes 72 hours after SAH (Figure 2B), while only 50 ng/ul treatment was effective at 24 hours after SAH when compared with the sham or vehicle groups (Figure 2A).
Figure 2.
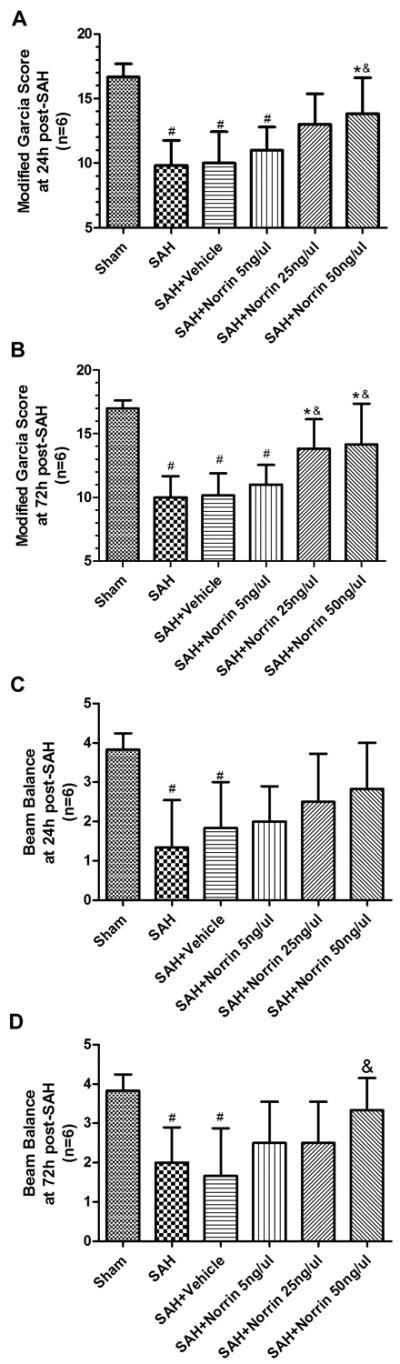
Effects of exogenous Norrin treatment on neurobehavioral outcomes after SAH. Modified Garcia test results of each group at 24 hours (A) and 72 hours (B) after SAH. Beam balance test results of each group at 24 hours (C) and 72 hours (C) after SAH. n = 6 #: vs. Sham P < 0.05 *: vs. SAH P < 0.05 &: vs. SAH + Vehicle P<0.05.
For Beam Balance Test, the rats from both SAH and vehicle groups also showed significant neurobehavioral dysfunction when compared to sham group, but not any of the r-Norrin treatment groups (Figure 2C, 2D). Only r-Norrin 50 ng/ul treatment significantly alleviated neurological impairment at 72 hours after SAH (Figure 2D).
Both SAH and vehicle groups showed increased brain water content at 24 hours after SAH in both hemispheres at 24 hours (Figure 3A) and in ipsilateral hemisphere at 72 hours after SAH (Figure 3B). r-Norrin 50 ng/ul treatment significantly reduced brain water content while 25 ng/ul treatment only reduced brain water content at contralateral hemisphere at 24 and ipsilateral at 72 hours after SAH (Figure 3A, 3B). Overall, high dosage of r-Norrin (50 ng/ul) was the most effective treatment and it was used for the rest of the experiments.
Figure 3.
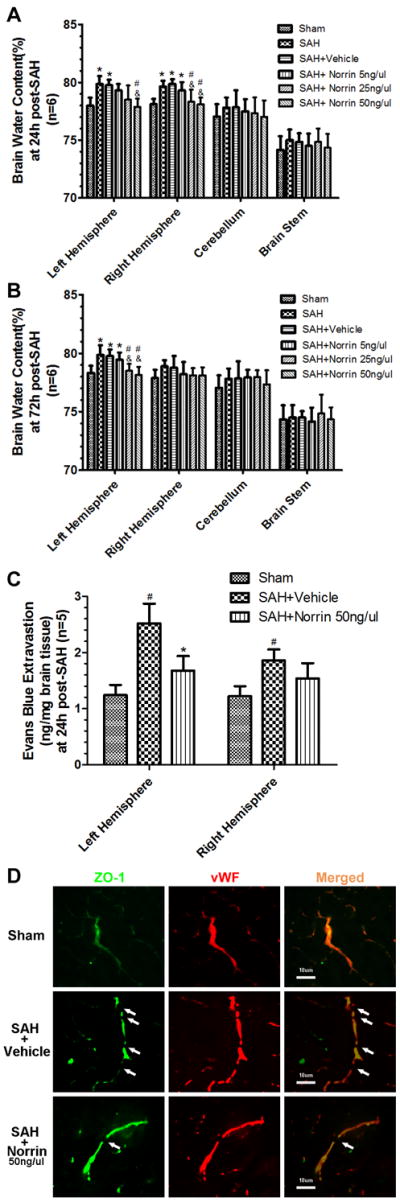
Effects of exogenous Norrin treatment on BBB after SAH. Brain water content assessment at 24 hours (A) and 72 hours (B) after SAH (n=6). (C) Evans blue extravasation evaluation at 24 hours after SAH (n=5). (D) Representative immunohistochemistry staining slices of ZO-1 and vWF at 24 hours after SAH. Arrow indicates the breakdown of continuous endothelia cell layer. vWF = von Willebrand factor. #: vs. Sham P<0.05 *: vs. SAH P<0.05 &: vs. SAH+Vehicle P<0.05.
We used Evans Blue Extravasation to evaluate the BBB integrity after SAH. The results showed that there was more Evans Blue dye leaking out of vessel in both hemispheres at 24 hour after SAH. The r-Norrin treatment significantly reduced Evans blue leakage in ipsilateral hemisphere (Figure 3C). Although there was no significant difference between SAH and r-Norrin treatment groups in contralateral hemisphere, no significant difference between sham and r-Norrin treatment groups were identified either, indicating r-Norrin effectively reduced Evans Blue dye leakage (Figure 3C). In immunohistochemical staining, continuous endothelial cells (vWF) and ZO-1 in sham rats were shown. However, vWF and ZO-1 structures broke up at 24 hours after SAH, and r-Norrin treatment effectively reduced those damages (Figure 3D).
Specific Inhibition of Frizzled 4 Receptor Expressions before Norrin Treatment
Comparisons of SAH grading score revealed no significant differences among the groups at 24 hours after SAH (Supplemental Figure IIIC). None of the sham-operated rats died, 6 rats died after SAH modeling and 2 rats died after intracerebralventricularly injection
Frizzled 4 expression was measured by western blot and no difference was observed in scrambled siRNA pretreatment group when compared with sham, but Frizzled 4 siRNA pretreatment significantly inhibited Frizzled 4 receptor expression in ipsilateral hemisphere at 48 hours after siRNA injection (Supplemental Figure IVA, IVB).
Frizzled 4 siRNA pretreatment sufficiently abolished the protective effective of r-Norrin as shown in Modified Garcia Test (Figure 4A), Brain Water Content (Figure 4B), Evans Blue Extravasation (Figure 4C) and Evans Blue Fluorescence in left cortex(Figure 4D) when compared to r-Norrin treatment group.
Figure 4.
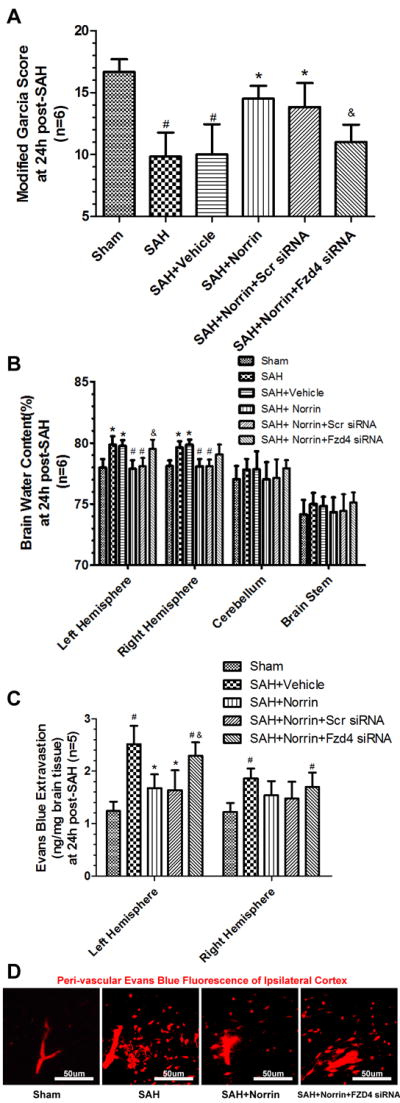
Pretreated animals with Frizzled 4 siRNA reversed the effects of Norrin treatment after SAH. The Modified Garcia test (A) results (n=6), (B) Brain water content (n=6) and (C) Evans blue extravasation results (n=5) (D) Representive Evans blue fluorenscence in ipsilateral/left cortex after using pretreated Frizzled 4 siRNA in Norrin treatment groups after SAH. Scr = Scrambled; Fzd4 = Frizzled 4. #: vs. Sham P<0.05 *: vs. SAH+Vehicle P<0.05 &: vs. SAH+Scr siRNA P<0.05.
Expressions of Endothelial Junction Proteins and β-Catenin after Treatment
The expression of Occludin, VE-Cadherin and ZO-1 were significantly reduced at 24 hours after SAH (Figure 5A-D), while nucleic β-catenin levels increased (Figure 6A, 6D) even though the total and cytoplasmic levels remained (Figure 6A-C). r-Norrin preserved the expression levels of Occludin, VE-Cadherin and ZO-1, and increased the nuclear portion of β-catenin levels when compared with sham and vehicle groups (Figure 5A-D, 6A-D).
Figure 5.
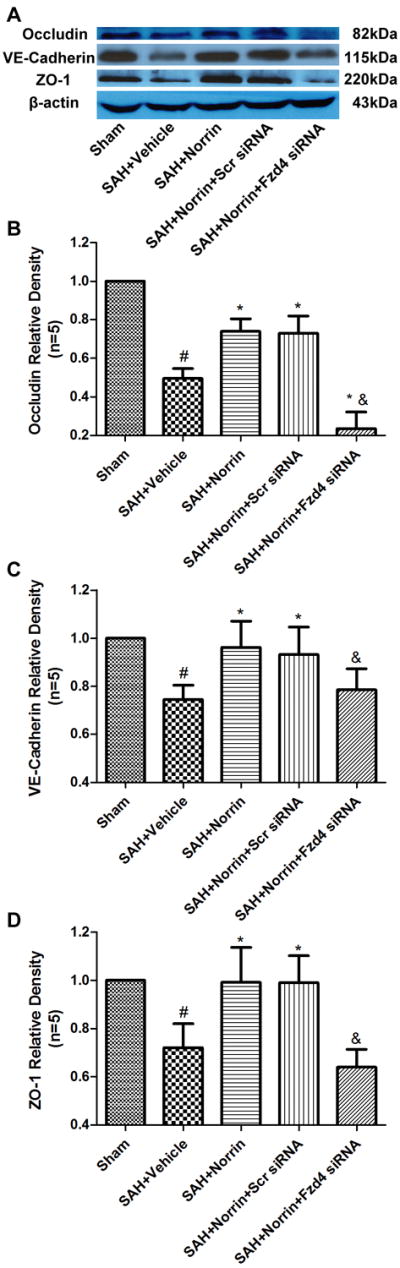
Changes of endothelial junction proteins expression after treatment at 24 hours post-SAH. Representative bands (A) and quantitative analysis of Occludin (B), VE-Cadherin (C), ZO-1 (D) expression in ipsilateral hemisphere of brain specimen at 24 hours after SAH. Relative densities of each protein have been normalized against the sham group. Scr = Scrambled; Fzd4 = Frizzled 4. n=5 #: vs. Sham P<0.05 *: vs. SAH+Vehicle P<0.05 &: vs. SAH+Scr siRNA P<0.05.
Figure 6.
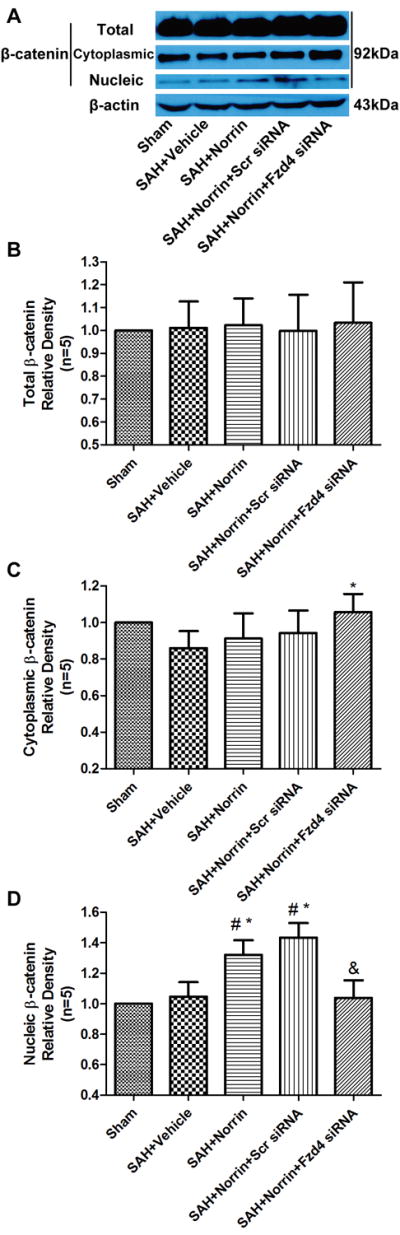
Changes of β-catenin expression after treatment at 24 hours post-SAH. Representative bands (A) and quantitative analysis of β-catenin expression in total (B), cytoplasmic (C) and nucleic (D) in ipsilateral hemisphere of brain specimen at 24 hours after SAH. Relative densities of each protein have been normalized against the sham group. Scr = Scrambled; Fzd4 = Frizzled 4. n=5 #: vs. Sham P<0.05 *: vs. SAH+Vehicle P<0.05 &: vs. SAH+Scr siRNA P<0.05.
Frizzled 4 siRNA pretreatment decreased the expression levels of Occludin, VE-Cadherin and ZO-1 compared to r-Norrin treatment group, while scrambled siRNA did not show those effects (Figure 5A-D). Frizzled 4 siRNA pretreatment but not scrambled siRNA decreased the nucleic β-catenin levels when compared to SAH + r-Norrin group (Figure 6A, 6D).
Discussion
The present study demonstrated that endogenous Norrin was increased in brain tissues with two peaks at 12 and 72 hours post-SAH. The essential Norrin supporting protein TSPAN12 18 was also increased up to 72 hours after SAH. Recombinant Norrin protein alleviated neurologic impairment and BBB disruption, which were associated with β-catenin translocation from cytoplasmic into nuclear and an increase of Occludin, VE-cadherin and ZO-1 protein expressions. Furthermore, blockage of Frizzled 4 receptor by siRNA at 48 hours before SAH eliminated these protective effects of Norrin, prevented β-catenin translocation, and reduced those three endothelial junction proteins Occludin, VE-cadherin and ZO-1 expressions. Taking together, these observations suggested that Norrin may be a protective factor against BBB disruption after SAH.
Previous studies suggested that Norrin is essential to BBB development and maintaince5, 19, even though its underlying mechanisms are unclear. Endothelial tight junction proteins such as Occludin and ZO-1, and endothelial adherent proteins like VE-cadherin, are important to BBB integrity 20. It has been suggested that canonical β-catenin translocation binds to TCF/TLF and initiates downstream protein transcription21. The only reported receptor of Norrin is Frizzled 46, 22, which happens to be the same receptor of canonical β-catenin pathway21. In addition, this pathway has been suggested to promote angiogenesis and protect BBB7, 19, 21. The observations of this study are consistent with previous reports that both neurological dysfunction and BBB disruption were reduced by exogenous recombinant Norrin, whereas the action of Norrin was eliminated by inhibition of Frizzled 4 receptor. This observation indicated that the protective effect of Norrin may be mediated by Frizzled 4 receptor, which in turn promoted β-catenin nuclear translocation, enhanced Occludin, ZO-1 and VE-Cadherin transcription and expression.
Norrin is highly expressed in embryo development, but barely detected in adult rodent animals23. In addition, Norrin was thought to be expressed only in cerebellum and retina in CNS19. This might be caused by a regulate protein TSPAN12, which could specifically enhance Norrin/Frizzled 4 pathway, but not Wnt/Frizzled 418 in those brain regions. However, there are contradictory evidences such as TSPAN12 increased in colon cancer microenvironment24. In the current study, TSPAN12 was observed increased in ipsilateral hemisphere of SAH rats.
As mentioned above, another contradictory issue is the potential therapeutic function of either Norrin/Frizzled 4 pathway or Wnt/Frizzled pathways18 in brain tissues after SAH. Wnt protein is widely expressed and non-specifically affected, which is difficult to manipulate and could cause high bone mass, even cancer, if Wnt is enhanced; or osteoporosis and heart failure if Wnt is inhibited21. On the other hand, Norrin is a autocrine/paracrine protein with low abundance in normal adults, which has local effect toward surrounding cells25. An increase of TSPAN12 in CNS could prominently enhance the protective effects of Norrin but not Wnt18. Furthermore, the molecule weight of Norrin is relatively low, which may has more potential BBB permeability after peripheral administration and easy to be delivered into CNS26, but pharmaceutical and pharmacokinetic studies of Norrin treatment are still needed in the future studies.
This study has limitations that it is focused on the Norrin/Frizzled 4 pathways but not designed to study β-catenin translocation in details. β-catenin translocation which enhances junction protein transcription was well established in other studies27-29. In addition, Norrin was believed to be secreted by astrocyte, and affecting surrounding cells30, 31, which consist with the present study. But it cannot be ruled out other unknown mechanisms of Norrin, thus in our future studies, other particular cell-location and machanisms of Norrin are still needed to be clarified.
In conclusion, this study demonstrated for the first time that endogenous Norrin elevation occurred after SAH in brain tissues, and extraneous recombinant Norrin protected BBB and improved neurological outcome, mediated possibly by Frizzled 4 which may in turn promoted β-catenin nuclear translocation, and then enhanced junction proteins expression in the brain. Recombinant Norrin might be a promising treatment option for BBB protection after SAH.
Supplementary Material
Acknowledgments
Sources of Funding: This research was supported by the National Institutes of Health grants NS081740 and NS084921 to John H Zhang, the National Basic Research Program of China (973 Program) 2014CB541600 and the National Natural Science Foundation of China grant 81220108009 to Hua Feng.
Footnotes
Disclosures: None
References
- 1.Zhang JH, Badaut J, Tang J, Obenaus A, Hartman R, Pearce WJ. The vascular neural network--a new paradigm in stroke pathophysiology. Nat Rev Neurol. 2012;8:711–716. doi: 10.1038/nrneurol.2012.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen S, Feng H, Sherchan P, Klebe D, Zhao G, Sun X, et al. Controversies and evolving new mechanisms in subarachnoid hemorrhage. Prog Neurobiol. 2014;115:64–91. doi: 10.1016/j.pneurobio.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med. 2013;19:1584–1596. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohlmann A, Tamm ER. Norrin: Molecular and functional properties of an angiogenic and neuroprotective growth factor. Progress in retinal and eye research. 2012;31:243–257. doi: 10.1016/j.preteyeres.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Rattner A, Zhou Y, Williams J, Smallwood PM, Nathans J. Norrin/frizzled4 signaling in retinal vascular development and blood brain barrier plasticity. Cell. 2012;151:1332–1344. doi: 10.1016/j.cell.2012.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smallwood PM, Williams J, Xu Q, Leahy DJ, Nathans J. Mutational analysis of norrin-frizzled4 recognition. J Biol Chem. 2007;282:4057–4068. doi: 10.1074/jbc.M609618200. [DOI] [PubMed] [Google Scholar]
- 7.Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA. Wnt/beta-catenin signaling is required for cns, but not non-cns, angiogenesis. Proc Natl Acad Sci U S A. 2009;106:641–646. doi: 10.1073/pnas.0805165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki H, Hasegawa Y, Kanamaru K, Zhang JH. Mechanisms of osteopontin-induced stabilization of blood-brain barrier disruption after subarachnoid hemorrhage in rats. Stroke. 2010;41:1783–1790. doi: 10.1161/STROKEAHA.110.586537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seitz R, Hackl S, Seibuchner T, Tamm ER, Ohlmann A. Norrin mediates neuroprotective effects on retinal ganglion cells via activation of the wnt/beta-catenin signaling pathway and the induction of neuroprotective growth factors in muller cells. J Neurosci. 2010;30:5998–6010. doi: 10.1523/JNEUROSCI.0730-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salahpour A, Medvedev IO, Beaulieu JM, Gainetdinov RR, Caron MG. Local knockdown of genes in the brain using small interfering rna: A phenotypic comparison with knockout animals. Biol Psychiatry. 2007;61:65–69. doi: 10.1016/j.biopsych.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 11.Chen S, Ma Q, Krafft PR, Chen Y, Tang J, Zhang J, et al. P2×7 receptor antagonism inhibits p38 mitogen-activated protein kinase activation and ameliorates neuronal apoptosis after subarachnoid hemorrhage in rats. Crit Care Med. 2013;41:e466–474. doi: 10.1097/CCM.0b013e31829a8246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki H, Hasegawa Y, Chen W, Kanamaru K, Zhang JH. Recombinant osteopontin in cerebral vasospasm after subarachnoid hemorrhage. Ann Neurol. 2010;68:650–660. doi: 10.1002/ana.22102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krafft PR, Caner B, Klebe D, Rolland WB, Tang J, Zhang JH. Pha-543613 preserves blood-brain barrier integrity after intracerebral hemorrhage in mice. Stroke. 2013;44:1743–1747. doi: 10.1161/STROKEAHA.111.000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sugawara T, Ayer R, Jadhav V, Zhang JH. A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. J Neurosci Methods. 2008;167:327–334. doi: 10.1016/j.jneumeth.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marzal M, Guerra-Giraldez C, Paredes A, Cangalaya C, Rivera A, Gonzalez AE, et al. Evans blue staining reveals vascular leakage associated with focal areas of host-parasite interaction in brains of pigs infected with taenia solium. PLoS One. 2014;9:e97321. doi: 10.1371/journal.pone.0097321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma Q, Chen S, Hu Q, Feng H, Zhang JH, Tang J. Nlrp3 inflammasome contributes to inflammation after intracerebral hemorrhage. Ann Neurol. 2014;75:209–219. doi: 10.1002/ana.24070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma Q, Huang B, Khatibi N, Rolland W, 2nd, Suzuki H, Zhang JH, et al. Pdgfr-alpha inhibition preserves blood-brain barrier after intracerebral hemorrhage. Ann Neurol. 2011;70:920–931. doi: 10.1002/ana.22549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Junge HJ, Yang S, Burton JB, Paes K, Shu X, French DM, et al. Tspan12 regulates retinal vascular development by promoting norrin- but not wnt-induced fzd4/beta-catenin signaling. Cell. 2009;139:299–311. doi: 10.1016/j.cell.2009.07.048. [DOI] [PubMed] [Google Scholar]
- 19.Engelhardt B, Liebner S. Novel insights into the development and maintenance of the blood-brain barrier. Cell Tissue Res. 2014;355:687–699. doi: 10.1007/s00441-014-1811-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 21.Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, et al. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol. 2008;183:409–417. doi: 10.1083/jcb.200806024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu Q, Wang Y, Dabdoub A, Smallwood PM, Williams J, Woods C, et al. Vascular development in the retina and inner ear: Control by norrin and frizzled-4, a high-affinity ligand-receptor pair. Cell. 2004;116:883–895. doi: 10.1016/s0092-8674(04)00216-8. [DOI] [PubMed] [Google Scholar]
- 23.Ye X, Smallwood P, Nathans J. Expression of the norrie disease gene (ndp) in developing and adult mouse eye, ear, and brain. Gene Expr Patterns. 2011;11:151–155. doi: 10.1016/j.gep.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Planutis K, Planutiene M, Holcombe RF. A novel signaling pathway regulates colon cancer angiogenesis through norrin. Scientific reports. 2014;4:5630. doi: 10.1038/srep05630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ye X, Wang Y, Nathans J. The norrin/frizzled4 signaling pathway in retinal vascular development and disease. Trends Mol Med. 2010;16:417–425. doi: 10.1016/j.molmed.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohlmann A, Merkl R, Tamm ER. Focus on molecules: Norrin. Exp Eye Res. 2012;102:109–110. doi: 10.1016/j.exer.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 27.Ramirez SH, Fan S, Dykstra H, Rom S, Mercer A, Reichenbach NL, et al. Inhibition of glycogen synthase kinase 3beta promotes tight junction stability in brain endothelial cells by half-life extension of occludin and claudin-5. PLoS One. 2013;8:e55972. doi: 10.1371/journal.pone.0055972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ueno M. Molecular anatomy of the brain endothelial barrier: An overview of the distributional features. Curr Med Chem. 2007;14:1199–1206. doi: 10.2174/092986707780597943. [DOI] [PubMed] [Google Scholar]
- 29.Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and ve-cadherin in the control of vascular permeability. J Cell Sci. 2008;121:2115–2122. doi: 10.1242/jcs.017897. [DOI] [PubMed] [Google Scholar]
- 30.Braunger BM, Tamm ER. The different functions of norrin. Adv Exp Med Biol. 2012;723:679–683. doi: 10.1007/978-1-4614-0631-0_86. [DOI] [PubMed] [Google Scholar]
- 31.Ke J, Harikumar KG, Erice C, Chen C, Gu X, Wang L, et al. Structure and function of norrin in assembly and activation of a frizzled 4-lrp5/6 complex. Genes Dev. 2013;27:2305–2319. doi: 10.1101/gad.228544.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


