Abstract
The Microsporidium, Anncaliia algerae, an obligate intracellular parasite, has been identified as an opportunistic human pathogen but treatment has not been evaluated for infections with this organism. Albendazole, an anti-tubulin polymerization drug used against parasitic worm infections, has been the medication of choice used to treat some microsporidial infections affecting humans, with varying results ranging from clearing infection (Encephalitozoon) to resistance (Enterocytozoon). This study illustrates the effect of albendazole treatment on A. algerae infection in Rabbit Kidney (RK13) cells and Human Fetal Lung (HFL-1) fibroblasts. Albendazole appears to have an attenuating effect on A. algerae infection and albendazole’s IC50 in RK13 cells is 0.1μg/ml. Long-term treatment inhibits up to 98% of spore production, but interrupting treatment re-establishes the infection without new exposure to the parasite as supported by microscopic observations. The parasite’s Beta-Tubulin gene was purified, cloned, and sequenced. Five of the six specific amino acids, associated with benzimidazole sensitivity, are conserved in A. algerae. These findings suggest that A. algerae is sensitive to albendazole; however, the organism is not completely cleared from cultures.
Keywords: Benzimidazole effects, drug treatment, electron microscopy, Microsporidia, pathology
MICROSPORIDIA are obligate intracellular parasitic protists. There are over 1200 species in more than 170 genera that infect almost all major animal groups including humans (Becnel et al. 2014). Among these, some of the human infecting genera include Vittaforma, Anncaliia, Brachiola, Pleistophora, Encephalitozoon (Enc.), Enterocytozoon (Ent.), and Nosema (Bryan et al. 1991; Cali et al. 1998; Franzen et al. 2006; Weber and Bryan 1994). The most diagnostically important feature of microsporidial parasites is the production of environmentally resistant spores that contain a coiled polar filament used to infect the host cells (Bryan et al. 1991; Cali and Takvorian 2002; Takvorian et al. 2005). Microsporidial spores range in size from 1 to 15μm in length and produce an intracellular developmental cycle that can be divided into two main stages within the host cell: proliferative and sporogonic.
Anncaliia algerae, previously known as Brachiola algerae, is a microsporidium originally described in mosquitoes as Nosema algerae (Vavra and Undeen 1970), and it subsequently has been identified as a human opportunistic pathogen, with the majority of cases leading to the death of the patient (Cali et al. 2004; Cali et al. 2010; Coyle et al. 2004; Field et al. 2012; Watts et al. 2012; Watts et al. 2014). Its opportunistic and life-threatening characteristics are a cause for increasing concern among physicians who treat the immuno-compromised community, including transplant recipients and patients with rheumatoid arthritis or AIDS. However, no specific treatment for A. algerae infections has been reported. Albendazole, an anti-tubulin polymerization drug used to treat helminthic infections (Lacey 1988, 1990; Edlind et al. 1996; Katiyar et al. 1994), has been one of the drugs of choice used successfully against microsporidial infections caused by Encephalitozoon spp. (Conteas et al. 2000; Edlin et al. 1996; De Groote et al. 1995; Didier 1997, 2005; Didier and Weiss 2006; Fournier et al. 2000; Franssen 1995; Joste et al. 1996; Lecuit et al. 1994; Weber et al. 1994); but it has been less effective against infections with Enterocytozoon spp. (Akiyoshi et al. 2007; Conteas et al. 2000; Didier 1997, 2005; Didier and Weiss 2006) and Vitaforma spp. (Franzen and Salzberger 2008).
The current study examines the anti-microsporidial pathological effects of albendazole on A. algerae infections and analyzes the Beta-tubulin gene sequence segment highly suggestive of benzimidazole sensitivity. Additionally, the efficacy of the drug was evaluated by calculating the drug’s half maximal inhibitory concentration (IC50) in infected RK13 cells.
MATERIALS AND METHODS
Cell culture
Rabbit Kidney (RK-13) cells and Human Fetal Lung (HFL-1) fibroblasts were purchased from the American Type Culture Collection (ATCC CCL-37 and CCL-153). RK-13 cells were grown in minimal essential medium (MEM) supplemented with 7% fetal bovine serum (FBS), 1% Penicillin-Streptomycin (PS), and 1% Fungizone. HFL-1 cells were grown in F-12K medium supplemented with 10% FBS, 1% PS, and 1% Fungizone. All media and supplements were purchased from Gibco Life Technologies. Both RK13 and HFL-1 cells were maintained in 50ml Falcon tissue culture flasks (Becton-Dickinson) at 37 °C in 5% CO2. During experiments 37 °C cells were subcultured and maintained at 32 °C for the duration of the procedure as per Lowman et al. (2000). Cell culture media was replaced twice per week.
Parasite spore collection and purification
Anncaliia algerae was propagated in RK13 cell cultures. Anncaliia algerae was provided by Dr. Albert H. Undeen from his original isolate and has been maintained in our laboratory for approximately 20 years. The parasite spores were collected from the culture media, concentrated by centrifugation, and stored in sterile distilled water at 4 °C. Spores used in these experiments were counted with a hemocytometer (three times/sample and averaged), diluted in appropriate supplemented media, then introduced to 70% confluent cell cultures. During the study, cell cultures were exposed to approximately 2-4×107 parasite spores for 2 h then rinsed with media to remove free spores and incubated with the parasite for 2-3 days then fresh media containing albendazole was added. The parasite spores from these cultures were concentrated and counted when media was replaced.
Drug assay
Albendazole (Sigma Cat#: 54965-21-8) was diluted in dimethyl sulfoxide (DMSO) to a 10 mg/ml stock solution, kept at -20 °C, and further diluted with appropriate media to the different concentrations used during the experiments. The starting concentration, 5 μg/ml, was based on treatment reports of other microsporidial parasites (Didier 1997; Franssen et al. 1995). The concentrations used in this study were selected from dilutions of the starting dose (5 μg/ml) and optimized for both infection and cell viability. Control cultures were maintained in mock media containing DMSO at the same concentrations used for albendazole treatment. Both albendazole and mock media were maintained continuously for the time required by each experiment.
Light microscopy
Live imaging of control and target samples was performed weekly directly from the culture flasks in which the cells were kept during the study. Images were taken with an Inverted Phase Contrast Microscope (Nikon Diaphot 300), and analyzed using the computer software SPOT Advanced (SPOT Imaging Solutions, Sterling Heights, MI). Fixed culture samples were imaged and analyzed with a Zeiss Axiovert 200M motorized epifluorescence or a Zeiss LSM780 confocal scanning microscope (Zeiss, Thornwood, NY). Samples were fixed in 100% methanol and stained with 20% Giemsa solution (EMD Millipore# R03055, Billerica, MA.). Stained samples were mounted with Permount Mounting Medium (Fisher Cat# SP15-100, Pittsburgh, PA).
Electron microscopy
Samples were prepared and analyzed following the published standard protocol in Cali et al. (2004). Thin sections were stained with lead citrate and uranyl acetate then viewed with a TECNAI-12 Transmission Electron Microscope (FEI, Hillsboro, OR).
Half maximal inhibitory concentration (IC50)
RK13 cells were seeded on glass coverslips in 6-well plates and incubated for 3 days at 32 °C, then infected with 5.5 × 106 spores per well and incubated for an additional 3 days. After rinsing the infected cultures, mock media or media supplemented with albendazole was added and maintained for 5 more days. At the end of the treatment, samples were fixed, stained, mounted, and imaged as described above. A total of 500 cells were counted per sample, in both controls and drug treated.
DNA isolation, ß-tubulin gene amplification, and cloning
DNA was extracted from Anncaliia algerae spores then purified from samples using standard methods, phenol/chloroform extraction and ethanol precipitation. The ß-Tubulin sequence segment was amplified by polymerase chain reaction (PCR) using the degenerate primers BTUBf (5’-GCC TGC AGG NCA RTG YGG NAA YCA-3’) and BTUBr (5’-GGC CTC AGT RAA YTC CAT YTC RTC CAT-3’) obtained from the published data by Franzen and Salzberger (2008). PCR fragments were ligated into the vector pCR4-TOPO and cloned into TOP10 chemically competent cells using the Invitrogen TOPO TA Cloning Kit (Invitrogen K4575-02), according to the protocol provided. Plasmids were isolated from positive clones and sequenced using vector directed primers T7 and M13 in a capillary sequencer (Applied Biosystems model 3130xl at the University of Medicine and Dentistry of New Jersey). The obtained sequence was translated using the EMBOSS Transeq tool and aligned using ClustalW sequence alignment tool (EMBL-EBI website). The sequence obtained in our experiments was compared with published data from albendazole sensitive and resistant organisms (NCBI-PubMed).
Statistical analysis
Parasite spores were counted using a hemocytometer. The data obtained was plotted as log10 values of the original counts. Infection percentages were calculated for each condition by dividing the number of infected cells by the number uninfected cells counted from three experimental trials. Plotted data was normalized to the control values (Bacchi et al. 2002; Bacchi et al. 2003; Coyle et al. 1998; Didier 1997; Franssen et al. 1995; Zou et al. 2001). Standard errors were calculated with the KaleidaGraph software (Synergy Software; Reading, PA) using the Student t-test for unpaired data with unequal variance function.
RESULTS
Microscopic Analysis of RK13 cells
The infected cell cultures were maintained in normal media for three days post exposure (PE) then treated with albendazole for 40 days. No normal developmental progression of the proliferative/sporogonic stages or spores was observed as compared to the control (infected, mock treated) flasks. The experiment was terminated at 40 days since <1% of RK13 cells remained in the control flasks due to parasite infection, while >60% of cells remained in the albendazole treated cell cultures demonstrating the effect of the lack of parasite development or drug toxicity. Both uninfected and infected RK13 cells had similar morphological appearance after exposure to albendazole; they all exhibited a rounder morphology. At 8 days post exposure, 90% of the RK13 control cells were filled with elongated developing parasites and newly formed spores. Infected cell cultures that were treated with albendazole for 6 days, did not contain the elongated proliferative parasites observed in control cells. In addition, very few spores were observed in or outside the treated host cells (Fig. 1). Parallel experiments with three albendazole doses (1.25, 0.93 and 0.63 μg/ml) produced similar results. In addition, we verified by electron microscopy that the intracellular parasite stages had changes in their internal structure. The untreated infection resulted in production of many proliferative stages containing ER and diplokaryotic nuclei, several actively dividing (Fig. 2A). The drug treated developmental stages had homogeneously granular cytoplasm and lacked ER (Fig. 2B, 2C). Furthermore, the nuclei were clearly degenerate having separated nuclear membranes and loss of nuclear density, as compared to the control samples (Fig. 2A). The spores collected from the 40 days exposure were concentrated and counted. Spore production in the albendazole treated cultures was 98% less than in control flasks (Fig. 3).
Fig. 1.
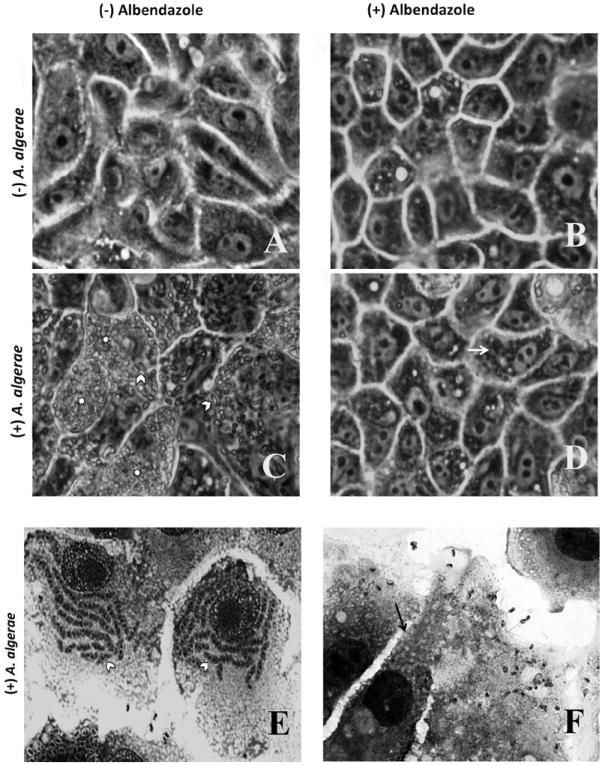
Light microscopic comparison of RK13 cell cultures with or without Anncaliia algerae and with or without albendazole treatment. (A-D) Phase contrast images of live RK13 cell cultures. A. RK13 control cells, B. RK13 cells with albendazole added to culture media, these cells appear less elongated than control cells. C. Anncaliia algerae infected RK13 cells containing large quantities of organisms (*) and elongated developing parasitic stages (arrow head). D. Anncaliia algerae infected RK13 cell cultures treated with albendazole. These cells contain few elongated organisms (arrow) indicating the reduced parasite burden due to inhibition of growth by the drug. These cells also appear less elongated than cells in A or C. E-F Fixed and Giemsa stained RK 13 cell cultures. E. Anncaliia algerae infected RK13 cells containing large quantities of elongated developing parasite stages (arrow head) F. Anncaliia algerae infected RK13 cells treated with albendazole. These cells contain few identifiable parasite spores (arrow) and no elongated developing parasite stages.
Fig. 2.
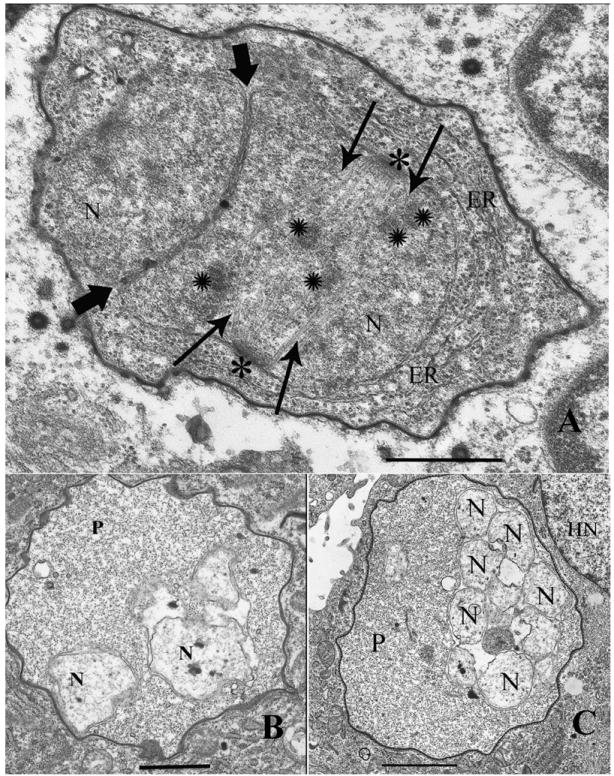
Electron microscopic observations of A. algerae development in control RK13 cultures and in albendazole treated infected cells. A. Infected (Control) RK13 cell with A. algerae developing in its cytoplasm. The typical proliferative stage of the parasite contains endoplasmic reticulum (ER), nuclei (N) in a diplokaryotic arrangement with tightly abutted nuclear membranes (bold arrowheads). The diplokaryon is undergoing karyokinesis, which is evident due to the presence of nuclear spindle plaques (*), spindle microtubules (arrows), and chromosomes (✺). Magnification bar = 600nm. B. Albendazole treated RK13 cell infected with an A. algerae proliferative (P) stage developing in its cytoplasm. The parasite has a homogeneous granular cytoplasm, lacking ER or any other defining structure. The nuclei (N) are clearly degenerate having separated; they are no longer in its characteristic diplokaryotic arrangement. The nuclear membranes are degenerate and the entire parasite cell is much less dense when compared to the parasite in figure 2A. Magnification bar = 1.2 μm. C. Albendazole treated RK13 cell infected with an A. algerae proliferative (P) stage developing in close proximity to the host cell nucleus (HN). This aberrant parasite lacks ER, has a homogeneous granular cytoplasm, and contains multiple degenerate nuclei (N), indicating that the drug treatment has severely disrupted its developmental cycle. Magnification bar = 1.7 μm.
Fig. 3.
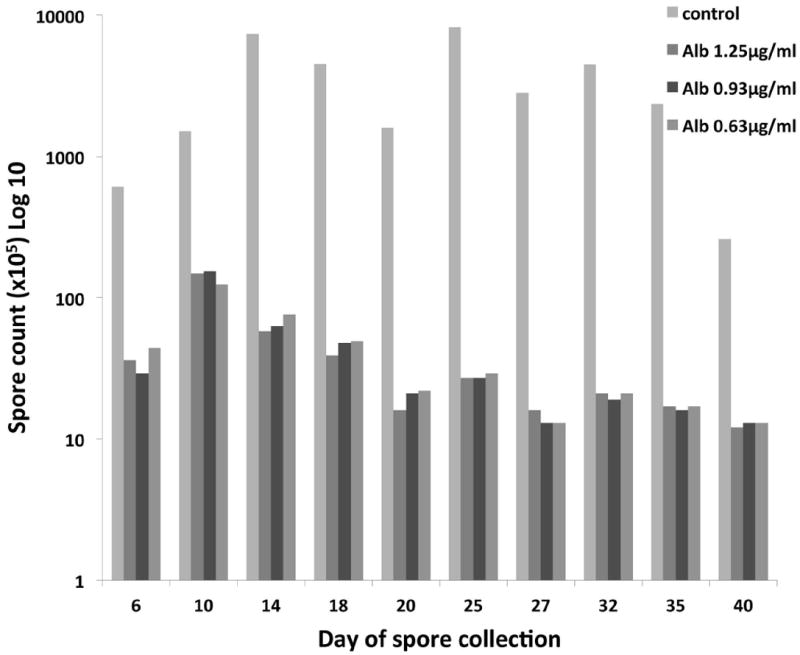
Analysis of parasite spore output and levels of RK13 host cell infection. Anncaliia algerae spore counts from collected culture media during 40 days of treatment with different concentrations of albendazole indicate the drug can decrease new spore production by as much as 98% when compared to untreated (control) infected cells.
To determine the viability of the spores collected during albendazole treatment, we introduced them to uninfected RK13 cells. For this experiment, cell cultures were inoculated with 5 × 106 of these A. algerae spores. Normally, control cultures will develop observable infection in <3 days. The albendazole treated spores required 11 days post exposure before infection was observed. However, when infection was observed the typical elongated developing stages and spores formed. Spores collected from cell cultures treated with albendazole for five weeks, only produced infection in 2% of the cells while spores exposed to one week of albendazole treatment produced infection in 28% of the cells. At the same inoculum dosage with untreated spores, 75% of the cultured cells were infected (Fig. 4A).
Fig. 4.
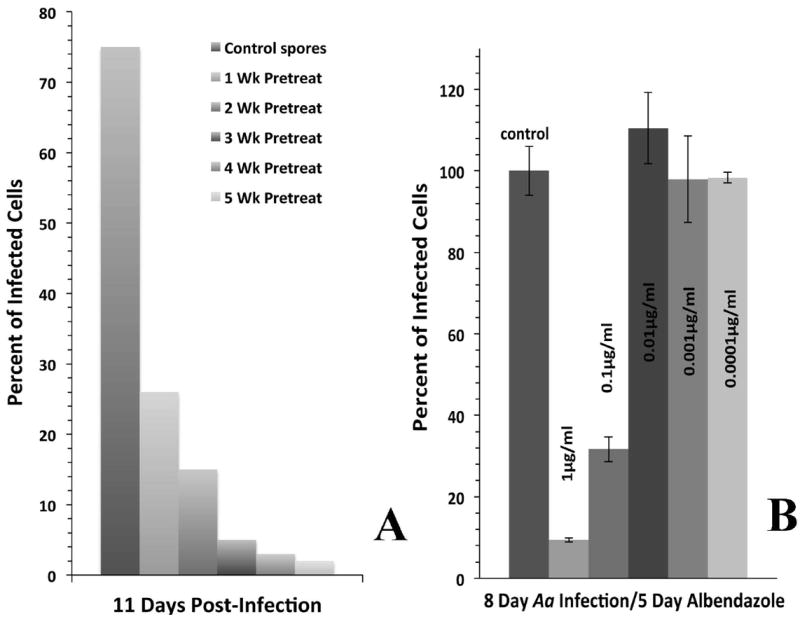
A. Anncaliia algerae spores that were exposed to albendazole treatment for 1-5 weeks were still capable of infecting RK13 cell cultures. One effect of the drug treatment was varying/dose exposure induced delays in the onset of infection when compared to the infection levels achieved with untreated spores. B. Albendazole’s IC50 was analyzed from the number of infected cells in fixed samples from three replicate experiments. Three days post infection RK13 cell cultures were treated with 1.0, 0.1, 0.01, 0.001, or 0.0001 μg/ml of albendazole for 5 days. Data gathered from treated cultures was calculated normalized to the untreated control cultures. At 1.0 μg/ml, only 10% of cells were infected; and in any dose lower than 0.01 μg/ml, the percent of infected cells was higher or equal to the control cultures. Albendazole’s IC50 is 0.1μg/ml, since at this dose 35% of the cells were infected.
IC50 analysis
The IC50 was calculated to be 0.1 μg/ml of albendazole for A. algerae in RK13 cells. At eight days post exposure, 32% of the RK13 cells were infected as compared to the control cultures. In any doses lower than 0.1 μg/ml of albendazole, the cultures had the same level of infection as the control cultures (Fig. 4B).
Microscopic analysis of HFL-1 cells
Since A. algerae is well documented as a human pathogen, Human Fetal Lung (HFL-1) fibroblast cell line was cultured and infected with A. algerae and also treated with albendazole to compare its inhibitory effects. In the infected untreated control fibroblast cells the parasite developed normally and produced massive amounts of spores (Fig. 5). These results were similar to those observed in the untreated A. algerae infected RK13 cells. In the albendazole treated fibroblast cells, the drug had an inhibitory effect on the parasite. Infected fibroblast cultures showed no normal progression in parasite development. At eight days post-exposure, the albendazole treated fibroblast cells did not contain any elongated proliferative parasites and very few spores were visible (Fig. 5E-F). The fibroblast cells were more sensitive to albendazole; therefore, the dose concentrations used for the RK13 cells were decreased to minimize cell toxicity. For these experiments, 3-day-old fibroblast cell cultures were inoculated with 3 × 107 A. algerae spores/flask. Cultures were incubated with the parasite for 2 h, rinsed, and incubated for and additional 70 days with supplemented media containing 0.1, 0.15, or 0.25 μg/ml of albendazole. Similar results were observed with all the albendazole doses tested in parallel.
Fig. 5.
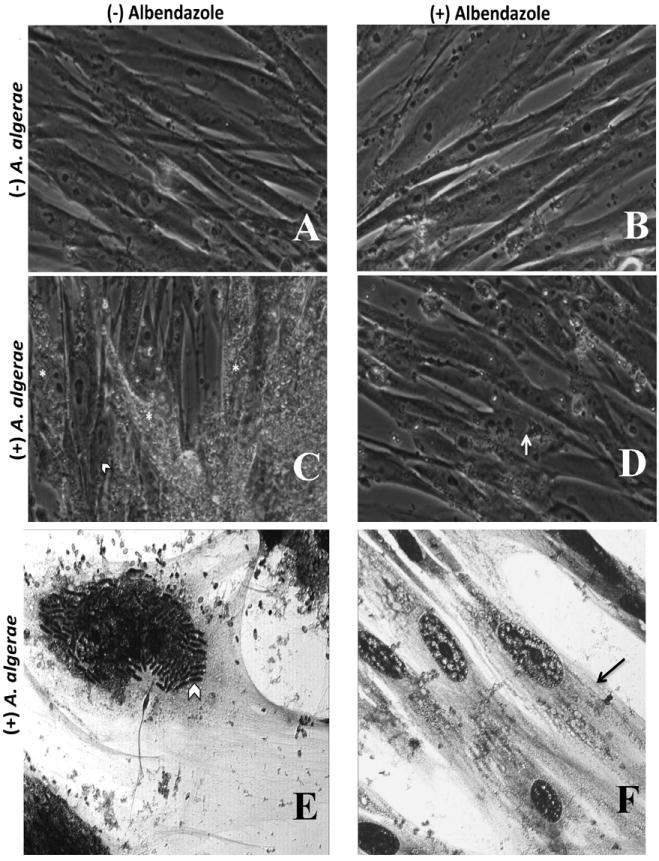
Light microscopic observations of HFL-1 (human fibroblast) cell cultures with or without A. algerae, and with or without albendazole treatment. (A-D) Phase contrast images of live HFL-1cell cultures. A. Control HFL-1 cells have typical fibroblast morphology. B. HFL-1 cells with albendazole added to culture media appear to have the same morphological features as the untreated control cells. C. Anncaliia algerae infected HFL-1 cells contain large quantities of organisms (*) and elongated developing parasite stages (arrow head). D. Anncaliia algerae infected HFL-1 cells treated with albendazole contain few spores and no elongated developing organisms (arrow) indicating the reduced parasite burden due to inhibition of growth by the drug. (E, F) Fixed and Giemsa stained HFL-1cell cultures. E. Anncaliia algerae infected HFL-1cells containing large quantities of elongated developing parasite stages (arrow head). Large numbers of spores are visible in the extracellular space. F. Anncaliia algerae infected HFL-1 cells treated with albendazole. These cells contain few identifiable parasite spores (arrow) and no visible elongated developing parasite stages.
After treating the cell cultures with albendazole for 70 days, the treatment was stopped in an effort to determine if the parasite would reestablish the infection without new parasite exposure. The parasite was able to re-infect the cultures but the onset of infection was delayed from the usual 3 days to 24 days. There after, the number of infected cells grew exponentially reaching 60% by 34 days (Fig. 6).
Fig. 6.
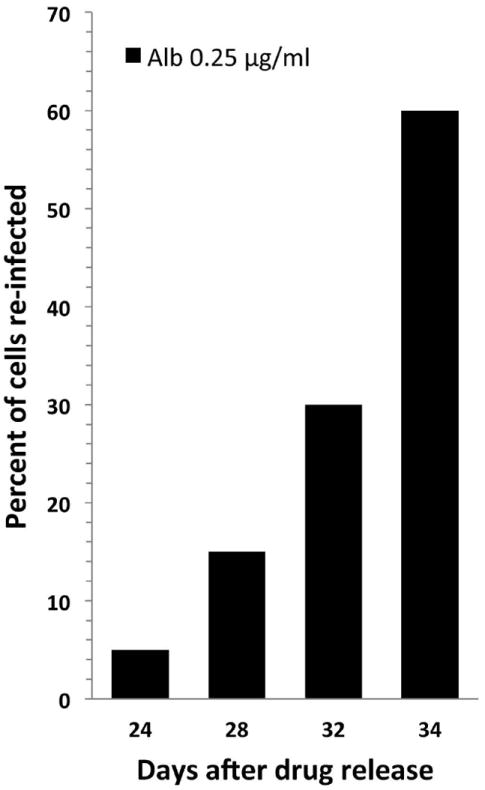
Analysis of parasite re-infection of HFL-1 host cells after cessation of albendazole treatment. Anncaliia algerae infected HFL-1 cells were maintained in 0.25μg/ml albendazole supplemented culture media for 70 days. After removal of albendazole from the culture media, infected cells were identified 24 days post-cessation of treatment and the percentage of re-infection continued to rapidly increase through the end of the experiment at day 34, indicating infection can reoccur but the rate of parasite development was slower and the onset of infection was delayed from the usual 3 days to 24 days.
Anncaliia algerae ß-tubulin Sequence Analysis
The results of the sequence analysis from A. algerae are presented in figure 7. The Clustal W alignment of the ß-tubulin amino acid sequence from A. algerae is compared to the sequences of microsporidia sensitive and resistant to albendazole. The obtained A. algerae sequence contains five amino acids linked to benzimidazole sensitivity. However, Proline6 replaced Histidine6 and the remaining five amino acids associated with sensitivity are conserved.
Fig. 7.

Analysis of the ß-tubulin amino acid sequences suggests Anncaliia algerae is sensitive to albendazole.
Six amino acids of the ß-tubulin amino acid sequence have been reported to be associated with Benzimidazole sensitivity, (Boxed areas) (His6, Ala165, Phe167, Glu198, Phe200, and Arg241; numbering based on the Saccharomyces cerevisiae sequence, GenBank accession no. V01296). Furthermore, changes to Glu198 and/or Phe200 are highly indicative of Benzimidazoles resistance, but in Anncaliia algerae 5 of the 6 amino acids, including Glu198 and Phe200, are conserved with the sensitive organisms. For this Partial ClustalW alignment of the ß-tubulin amino acid sequences, we used organisms that were found to be sensitive to albendazole: Enc. cuniculi (GenBank accession no. NM_001040955), Enc. intestinalis (GenBank accession no. AF297876), and Enc. hellem (GenBank accession no. L47271). And organisms found to be resistant to albendazole: Ent. bieneusi (GenBank accession no. DQ242640), V. corneae (GenBank accession no. EU031749.1), and Homo sapiens (GenBank accession no. NM_178014).
DISCUSSION
Anncaliia algerae infections can be attenuated and new spore production inhibited with the application of albendazole, with long-term treatment (30-70 days). Light and EM analysis of the effect of the drug on parasite development demonstrates the disruption of developmental stages (Fig.2B and 2C). Propagation and treatment of A. algerae in two cell types derived from different hosts and cultured under different conditions resulted in similar inhibitory effects from the drug. The analysis of the Beta-tubulin gene sequence from A. algerae demonstrated a highly conserved alignment with sequences from other microsporidia sensitive to benzimidazoles (Fig. 7). Together, these data suggest that A. algerae is sensitive to albendazole.
Previous in vitro studies on albendazole treatment of Enc. hellem, Enc. intestinalis, and. Enc. cuniculi infections have reported that albendazole treatment of infected cell cultures, inhibited the normal development, drastically reduced the production and release of new spores, and caused abnormalities to the developing parasite (Blanshard et al. 1993; Colbourn et al. 1994; Didier 1997; Katiyar and Edlind 1997). These data support our observations with A. algerae, which demonstrate similar inhibitory effects on spore production and parasite malformations. Unlike the findings reported by Katiyar and Edlind (1997) on Enc. intestinalis infections, we did not achieve complete spore clearing from our infected cell cultures. Instead, some viable spores were able to cause reinfection but the infection was attenuated compared to untreated inoculum. It is probable that most of the treated inoculum were not viable, resulting in minimal infectivity.
Studies performed on human infecting microsporidia and other organisms have linked six amino acids from the Beta-tubulin gene sequence (Histidine6, Alanine165, Phenylalanine167, Glutamic Acid198, Phenylalanine200, and Arginine241; based on the Saccharomyces cerevisiae sequence) to an organism’s sensitivity to benzimidazoles (including albendazole). Some of these studies have demonstrated that His6, Ala165, Phe167, and Arg241 have little predictive value, but changes to the amino acid positions specific to Glu198 and/or Phe200 have been highly indicative of resistance to this drug (Akiyoshi, et al. 2007; Franzen and Salzberger 2008; Katiyar et al. 1994; Lacey 1988; Li et al. 1996; Thomas, et al. 1985).
In the current study, A. algerae appears to belong in the group of organisms sensitive to the drug. The sequence we obtained, although not a 100% match, has five of the six target amino acids (including Glu198 and Phe200 which could have indicated resistance) conserved among microsporidia that are sensitive to benzimidazoles. The change in A. algerae’s sequence from His6 to Pro6 may not play a significant role in its sensitivity to albendazole because Histidine6 is conserved in both sensitive and resistant species. Although further investigation needs to be done in this area, these molecular data support our culture and morphology findings (Fig. 7).
Very little is known on how to treat A. algerae infections, and the information gathered so far has been from eight reported case studies, with six fatalities. The treatment given to these patients had a very tough regime that included a variety of drugs; in four cases Albendazole was part of this regime. Out of these four cases, two patients survived and two patients died but the deaths were not linked directly to the A. algerae infection (Coyle et al. 2004; Field et al. 2012; Cali et al. 2010; Watts et al. 2012 and 2014).
The results obtained in this study demonstrate the benefit of long-term cell culture studies. Figure 4 indicates that A. algerae exposed to 5 weeks of albendazole treatment in culture resulted in a dramatic reduction of spore viability when the collected spores were used to inoculate new untreated cultures. And it also helped with extended cell survival, >60% of the infected and treated cells were left in the cultures at the end of the 30 and 70 day experiments.
Together, our molecular, culture, and morphology findings suggest that A. algerae is sensitive to albendazole; and provides information useful in the treatment of human infections.
Acknowledgments
We would like to thank Dr. Nihal Altan-Bonnet for her invaluable assistance with sequencing and confocal imaging. This work was supported by NIH Grant R25 60825-06 from the IMSD Minority grant and NIH Grant AI31788 from the National Institute of Allergy and Infectious Diseases.
Footnotes
We dedicate this paper to Dr. Visvesvara at the time of his retirement, to acknowledge his contributions to the fields of culture, development, and treatment of amoebae and microsporidia.
LITERATURE CITED
- Akiyoshi D, Weiss L, Feng X, Williams B, Keeling P, Zhang Q, Tzipori S. Analysis of the Beta-Tubulin genes from Enterocytozoon bieneusi isolates from a Human and Rhesus Macaque. J Eukaryot Microbiol. 2007;54:38–41. doi: 10.1111/j.1550-7408.2006.00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchi C, Yarlett N, Weiss LM. Polyamine metabolism in the Microsporidia. Biochem Soc Trans. 2003;31:420–423. doi: 10.1042/bst0310420. [DOI] [PubMed] [Google Scholar]
- Bacchi JC, Weiss LM, Lane S, Frydman B, Valasinas A, Reddy V, Sun JS, Marton LJ, Khan IA, Moretto M, Yarlett N, Wittner M. Novel synthetic polyamines are effective in the treatment of experimental Microsporidiosis, an opportunistic AIDS-associated infection. Antimicrob Agents Chemother. 2002;46:55–61. doi: 10.1128/AAC.46.1.55-61.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becnel JJ, Takvorian PM, Cali A. Checklist of available generic names for Microsporidia with type species and type hosts. In: Weiss LM, Becnel J, editors. Microsporidia: Pathogens of Opportunity. Wiley Press; 2014. [Google Scholar]
- Bigliardi E, Riparbelli MG, Selmi MG, Lanzarini P, Corona S, Gatti S, Scaglia M, Sacchi L. Mechanisms of microsporidial cell division: ultrastructural study on Encephalitozoon hellem. J Eukaryot Microbiol. 1998;45:347–51. doi: 10.1111/j.1550-7408.1998.tb04547.x. [DOI] [PubMed] [Google Scholar]
- Blanshard C, Ellis DS, Dowell SP, Tovey G, Gazzard BG. Electron microscopic changes in Enterocytozoon bieneusi following treatment with albendazole. J Clin Pathol. 1993;46:898–902. doi: 10.1136/jcp.46.10.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanshard C, Ellis DS, Tovey D, Dowell SP, Gazzard BG. Treatment of intestinal microsporidiosis with albendazole in patients with AIDS. AIDS. 1992;6:311–313. doi: 10.1097/00002030-199203000-00009. [DOI] [PubMed] [Google Scholar]
- Blanshard C, Peacock C, Ellis D, Gazzard B. Treatment of intestinal microsporidiosis with albendazole. VII International Conference on AIDS: Science challenging AIDS; Florence, Italy. 1991. Clin.Sci. Trials. [Google Scholar]
- Bryan RT, Cali A, Owen RL, Spencer HC. Microsporidia: opportunistic pathogens in patients with AIDS. Field & Wood Medical Publishers; New York: 1991. [PubMed] [Google Scholar]
- Bulla LAJ, Cheng TC. Biology of the Microsporidia. New York, NY: Plenum Press; 1976. Comparative Pathobiology. [Google Scholar]
- Cali A, Neafie R, Weiss LM, Ghosh K, Vergara RB, Gupta R, Takvorian PM. Human vocal cord infection with the microsporidium Anncaliia algerae. J Eukaryot Microbiol. 2010;57:562–567. doi: 10.1111/j.1550-7408.2010.00510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cali A, Neafie RC, Takvorian PM. Microsporidiosis. In: Meyers WM, editor. Topics on the Pathology of Protozoan and Invasive Arthropod Diseases. Armed Forces Institute of Pathology; 2011. [Google Scholar]
- Cali A, Takvorian PM, Lewin S, Rendel M, Sian CS, Wittner M, Tanowitz HB, Keohane E, Weiss LM. Brachiola vesicularum, N. G., N. Sp., a new microsporidium associated with AIDS and myositis. J Eukaryot Microbiol. 1998;45:240–251. doi: 10.1111/j.1550-7408.1998.tb04532.x. [DOI] [PubMed] [Google Scholar]
- Cali A, Weiss LM, Takvorian PM. Brachiola algerae Spore membrane systems, their activity during extrusion, and a new structural entity, the Multilayered Interlaced Network, associated with the polar tube and the sporoplasm. J Eukaryot Microbiol. 2002;49:164–174. doi: 10.1111/j.1550-7408.2002.tb00361.x. [DOI] [PubMed] [Google Scholar]
- Cali A, Weiss LM, Takvorian PM. An analysis of the microsporidian genus Brachiola, with comparisons of Human and Insect isolates of Brachiola algerae. J Eukaryot Microbiol. 2004;51:678–685. doi: 10.1111/j.1550-7408.2004.tb00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cali A, Weiss LM, Takvorian PM. A review of the development of two types of human skeletal muscle infections from microsporidia associated with pathology in invertebrates and cold-blooded vertebrates. Folia Parasitol. 2005;52:51–61. doi: 10.14411/fp.2005.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbourn NI, Hollister WS, Curry A, Canning EU. Activity of albendazole against Encephalitozoon cuniculi in vitro. Europ J Protistol. 1994;30:211–220. [Google Scholar]
- Conteas CN, Berlin OGW, Ash LR, Pruthi JS. Therapy for human gastrointestinal microsporidiosis. Am Trop Med Hyg. 2000;63:121–127. doi: 10.4269/ajtmh.2000.63.121. [DOI] [PubMed] [Google Scholar]
- Costa SF, Weiss LM. Drug treatment of microsporidiosis. Drug Resist Updat. 2000;3:384–399. doi: 10.1054/drup.2000.0174. [DOI] [PubMed] [Google Scholar]
- Coyle C, Kent M, Tanowitz HB, Wittner M, Weiss LM. TNP-470 is an effective antimicrosporidial agent. J Infect Dis. 1998;177:515–518. doi: 10.1086/517390. [DOI] [PubMed] [Google Scholar]
- Coyle CM, Weiss LM, Rhodes LV, Cali A, Takvorian PM, Brown DF, Visvesvara GS, Xiao L, Naktin J, Young E, Gareca M, Colasante G, Wittner M. Fatal myositis due to the microsporidian Brachiola algerae, a mosquito pathogen. N Engl J Med. 2004;351:42–7. doi: 10.1056/NEJMoa032655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degroote MA, Visvesvara G, Wilson ML, Pieniazek NJ, Slemenda SB, Dasilva AJ, Leitch GJ, Bryan RT, Reves R. Polymerase chain reaction and culture confirmation of disseminated Encephalitozoon cuniculi in a patient with AIDS: successful therapy with albendazole. J Infect Dis. 1995;171:1375–1378. doi: 10.1093/infdis/171.5.1375. [DOI] [PubMed] [Google Scholar]
- Didier ES. Effects of albendazole, fumagillin, and Tnp-470 on Microsporidial replication in vitro. Antimicrob Agents Chemother. 1997;41:1541–1546. doi: 10.1128/aac.41.7.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didier ES. Microsporidiosis: An emerging and opportunistic infection in humans and animals. Acta Trop. 2005;94:61–76. doi: 10.1016/j.actatropica.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Didier ES, Rogers LB, Brush AD, Wong S, Traina-Dorge V, Bertucci D. Diagnosis of disseminated microsporidian Encephalitozoon hellem infection by PCR-Southern analysis and successful treatment with albendazole and fumagillin. J Clin Microbiol. 1996;34:947–52. doi: 10.1128/jcm.34.4.947-952.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didier ES, Weiss LM. Microsporidiosis: current status. Curr Opin Infect Dis. 2006;19:485–492. doi: 10.1097/01.qco.0000244055.46382.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlind TD, Katiyar SK, Visvesvara G, Li J. Evolutionary origins of Microsporidia and basis for benzimidazole sensitivity: an update. J Eukaryot Microbiol. 1996;43:109S. doi: 10.1111/j.1550-7408.1996.tb05029.x. [DOI] [PubMed] [Google Scholar]
- Field AS, Paik JY, Stark D, Qiu MR, Morey A, Plit ML, Canning EU, Glanville AR. Myositis due to the microsporidian Anncaliia (Brachiola) algerae in a lung transplant recipient. Transpl Infect Dis. 2012;14:169–76. doi: 10.1111/j.1399-3062.2012.00724.x. [DOI] [PubMed] [Google Scholar]
- Fournier S, Liquory L, Sarfati C, David-Quaknine F, Derouin F, Decazes JM, Molina JM. Disseminated infection due to Encephalitozoon cuniculi in a patient with AIDS: case report and review. HIV Med. 2000;1:155–161. doi: 10.1046/j.1468-1293.2000.00022.x. [DOI] [PubMed] [Google Scholar]
- Franssen FFJ, Lumeij JT, Van Knapen F. Susceptibility of Encephalitozoon cuniculi to several drugs in vitro. Antimicrob Agents Chemother. 1995;39:1265–1268. doi: 10.1128/aac.39.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzen C, Nassonova ES, Scholmerich J, Issi IV. Transfer of the members of the genus Brachiola (Microsporidia) to the genus Anncaliia based on ultrastructural and molecular data. J Eukaryot Microbiol. 2006;53:26–35. doi: 10.1111/j.1550-7408.2005.00066.x. [DOI] [PubMed] [Google Scholar]
- Franzen C, Salzberger B. Analysis of the Beta-Tubulin gene from Vittaforma corneae suggests benzimidazole resistance. Antimicrob Agents Chemother. 2008;52:790–793. doi: 10.1128/AAC.00928-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh K, Weiss LM. Microsporidia 2006: IWOP-9. J Euk Microbiol. 2006;53:S52–S54. doi: 10.1111/j.1550-7408.2006.00171.x. [DOI] [PubMed] [Google Scholar]
- Joste NE, Rich JD, Busam KJ, Schwartz DA. Autopsy verification of Encephalitozoon intestinalis (microsporidiosis) eradication following albendazole therapy. Arch Pathol Lab Med. 1996;120:199–203. [PubMed] [Google Scholar]
- Katiyar SK, Edlind TD. In vitro susceptibilities of the AIDS-associated microsporidian Encephalitozoon intestinalis to albendazole, its sulfoxide metabolite, and 12 additional benzimidazole derivatives. Antimicrob Agents Chemother. 1997;41:2729–32. doi: 10.1128/aac.41.12.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katiyar SK, Gordon VR, McLaughlin GL, Edlind TD. Antiprotozoal activities of benzimidazoles and correlations with beta-tubulin sequence. Antimicrob Agents Chemother. 1994;38:2086–90. doi: 10.1128/aac.38.9.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey E. The role of the cytoskeletal protein, tubulin, in the mode of action and mechanism of drug resistance to benzimidazoles. Int J Parasitol. 1988;18:885–936. doi: 10.1016/0020-7519(88)90175-0. [DOI] [PubMed] [Google Scholar]
- Lacey E. Mode of action of benzimidazoles. Parasitol Today. 1990;6:112–115. doi: 10.1016/0169-4758(90)90227-u. [DOI] [PubMed] [Google Scholar]
- Lecuit M, Oksenhendler E, Sarfati C. Use of albendazole for disseminated microsporidian infection in a patient with AIDS. Clin Infect Diseases. 1994;19:332–333. doi: 10.1093/clinids/19.2.332. [DOI] [PubMed] [Google Scholar]
- Lee RC, Williams BA, Brown AM, Adamson ML, Keeling PJ, Fast NM. Alpha- and beta-tubulin phylogenies support a close relationship between the microsporidia Brachiola algerae and Antonospora locustae. J Eukaryot Microbiol. 2008;55:388–92. doi: 10.1111/j.1550-7408.2008.00348.x. [DOI] [PubMed] [Google Scholar]
- Li J, Katiyar SK, Hamelin A, Visvesvara GS, Edlind TD. Tubulin genes from AIDS-associated microsporidia and implications for phylogeny and benzimidazole sensitivity. Mol Biochem Parasitol. 1996;78:289–95. doi: 10.1016/s0166-6851(96)02628-x. [DOI] [PubMed] [Google Scholar]
- Lowman PM, Takvorian PM, Cali A. The effects of elevated temperatures and various time-temperature combinations on the development of Brachiola (Nosema) algerae N. Comb. in mammalian cell culture. J Euk Microbiol. 2000;47:221–34. doi: 10.1111/j.1550-7408.2000.tb00041.x. [DOI] [PubMed] [Google Scholar]
- Monaghan SR, Rumney RL, Vo NT, Bols NC, Lee LE. In vitro growth of microsporidia Anncaliia algerae in cell lines from warm water fish. In Vitro Cell Dev Biol Anim. 2011;47:104–13. doi: 10.1007/s11626-010-9366-3. [DOI] [PubMed] [Google Scholar]
- Ridoux O, Drancourt M. In vitro Susceptibilities of the Microsporidia Encephalitozoon cuniculi, Encephalitozoon hellem, and Encephalitozoon intestinalis to albendazole and Its sulfoxide and sulfone metabolites. Antimicrob Agents Chemother. 1998;42:3301–3303. doi: 10.1128/aac.42.12.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takvorian PM, Weiss LM, Cali A. The early events of Brachiola algerae (Microsporidia) infection: spore germination, sporoplasm structure, and development within host cells. Folia Parasitol. 2005;52:118–129. doi: 10.14411/fp.2005.015. [DOI] [PubMed] [Google Scholar]
- Thomas JH, Neff NF, Botstein D. Isolation and characterization of mutations in the beta-tubulin gene of Saccharomyces cerevisiae. Genetics. 1985;111:715–34. doi: 10.1093/genetics/111.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavra J, Undeen AH. Nosema algerae n. sp. (Cnidospora, Microsporida) a pathogen in a laboratory colony of Anopheles stephensi Liston (Diptera, Culicidae) J Protozool. 1970;17:240–249. doi: 10.1111/j.1550-7408.1970.tb02365.x. [DOI] [PubMed] [Google Scholar]
- Watts MR, Chan RC, Cheong EY, Brammah S, Clezy KR, Tong C, Marriott D, Webb CE, Chacko B, Tobias V, Outhred AC, Field AS, Prowse MV, Bertouch JV, Stark D, Reddel SW. Anncaliia algerae microsporidial myositis. Emerg Infect Dis. 2014;20:185–91. doi: 10.3201/eid2002.131126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts MR, Chan RC, Cheong EY, Prowse MV, Bertouch JV, Field AS, Stark D, Brammah S, Clezy KR, Tong CW, Outhred AC, Tobias V, Reddel SW. Survival versus a fatal outcome in two cases of Anncaliia algerae microsporidial myositis. Neuromuscular Disorders. 2012;22:855. [Google Scholar]
- Weber R, Bryan RT. Microsporidial infections in immunodeficient and immunocompetent patients. Clin Infect Dis. 1994;19:517–21. doi: 10.1093/clinids/19.3.517. [DOI] [PubMed] [Google Scholar]
- Weber R, Bryan RT, Schwartz DA, Owen RL. Human microsporidial infections. Clin Microbiol Rev. 1994;7:426–61. doi: 10.1128/cmr.7.4.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss LM, Michalakakis E, Coyle CM, Tanowitz HB, Wittner M. The in vitro activity of albendazole against Encephalitozoon cuniculi. J Eukaryot Microbiol. 1994;41:65S. [PubMed] [Google Scholar]
- Wittner M. The Microsporidia and Microsporidiosis. Washington, D. C.: ASM Press; 1999. [Google Scholar]
- Zou Y, W Z, Sirisoma, Woster PM, Casero RA, Weiss LM, Rattendi D, Lane S, Bacch CJ. Novel alkylpolyamine analogues that possess both antitrypanosomal and antimicrosporidial activity. Bioor Med Chem Lett. 2001;11:1613–1617. doi: 10.1016/s0960-894x(01)00315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


