Abstract
Background
The Chicago Classification (CC) of esophageal motility disorders, utilizing an algorithmic scheme to analyze clinical high-resolution manometry (HRM) studies, has gained acceptance worldwide.
Purpose
This 2014 update, CC v3.0, developed by the International HRM Working Group, incorporated the extensive clinical experience and interval publications since the prior (2011) version.
Key results
CC v3.0 utilizes a hierarchical approach, sequentially prioritizing: 1) disorders of esophagogastric junction (EGJ) outflow (achalasia subtypes I–III and EGJ outflow obstruction), 2) major disorders of peristalsis (absent contractility, distal esophageal spasm, hypercontractile esophagus), and 3) minor disorders of peristalsis characterized by impaired bolus transit. EGJ morphology, characterized by the degree of overlap between the lower esophageal sphincter and the crural diaphragm and baseline EGJ contractility are also part of CC v3.0. Compared to the previous CC version, the key metrics of interpretation, the integrated relaxation pressure (IRP), the distal contractile integral (DCI), and the distal latency (DL) remain unchanged, albeit with much more emphasis on DCI for defining both hypo- and hypercontractility. New in CC v3.0 are: 1) the evaluation of the EGJ at rest defined in terms of morphology and contractility, 2) ‘fragmented’ contractions (large breaks in the 20-mmHg isobaric contour), 3) ineffective esophageal motility (IEM), and 4) several minor adjustments in nomenclature and defining criteria. Absent in CC v3.0 are contractile front velocity (CFV) and small breaks in the 20-mmHg isobaric contour as defining characteristics.
Conclusion
CC v3.0 is an updated analysis scheme for clinical esophageal HRM recordings developed by the International HRM Working Group.
Keywords: High resolution manometry, achalasia, ineffective esophageal motility, dysphagia, esophageal motility disorders
INTRODUCTION
The Chicago Classification (CC) categorizes esophageal motility disorders in high-resolution manometry (HRM) depicted with color pressure topography plots, also known as Clouse plots in honor of Ray E. Clouse (1951–2007), their key innovator [1]. The first major version of the CC was published in 2009 after the inaugural meeting of the International HRM Working Group in San Diego in 2008 [2]. The next major update followed from a meeting of the International HRM Working Group in Ascona in 2011 and was endorsed by numerous international motility and gastroenterology societies [3]. Since then, the clinical application of HRM has increased rapidly as have the number of relevant clinical and investigational publications. Consequently, an expanded International HRM Working Group met in Chicago in conjunction with DDW 2014 to formulate the CC v3.0. Current plans are for future versions (with appropriate version numbers) to be organized in conjunction with and endorsed by the American Neurogastroenterology and Motility Society (ANMS), the European Society of Neurogastroenterology and Motility (ESNM), and other motility societies that elect to become involved to coincide with intervals of the anticipated 3-year cycle of DDW being hosted in Chicago, depending on the perceived need at the time. This article summarizes the deliberations emanating from the 2014 Chicago meeting of the International HRM Working Group to formulate the second major update of the CC of esophageal motility disorders, version 3.0.
INTERPRETING THE HRM STUDY
The primary objective of the CC is to apply standardized HRM metrics to categorize esophageal motility disorders in patients with non-obstructive dysphagia and/or esophageal chest pain. The evaluation scheme is based on the analysis of ten 5-ml swallows performed in supine position and the Classification is intended for patients without prior surgery affecting the esophagus or the esophagogastric junction (EGJ). Several proposals were put forth to the International HRM Working Group pertinent to expanding and/or modifying this mandate. Some of these were ultimately accepted and some not. In broad terms, the proposed modifications were:
Simplifying HRM metrics and making technology-specific adjustments to normative values
Incorporating metrics pertinent to EGJ morphology and tone
Simplifying the criteria for disorders (and abnormalities) of peristalsis
Clarifying the diagnostic criteria for achalasia and EGJ outflow obstruction
Incorporating metrics pertinent to upper esophageal sphincter (UES) disorders
Incorporating physiological challenges as adjuncts to the manometric evaluation
The major points of discussion as well as the outcome of those discussions are summarized as follows.
Simplifying metrics and technology-specific adjustments to normative values
The basic measurements utilized in prior versions of the CC are the integrated relaxation pressure (IRP), distal contractile integral (DCI), distal latency (DL), and the contractile front velocity (CFV). Several issues were raised pertinent to the clinical experience of applying these:
A median rather than a mean cutoff value among test swallows for the IRP is more logical
Cutoffs defining abnormality for some measures, especially the IRP, are technology-specific
Localization of the contractile deceleration point (CDP), used in the measurement of DL and CFV, can be difficult
The CFV is of unclear relevance
Hypercontractility occasionally uniquely affects the LES and not the distal esophagus
With respect to the IRP, although the metric has served well to identify EGJ outflow obstruction, it was unanimously agreed that utilizing a median rather than a mean value among test swallows would minimize the impact of one or more outlier values that might otherwise skew the result (e.g. due to cough during measurement period). Another important consideration is that the cutoff for the upper limit of normal is technology-specific ranging from a low value of 15 mmHg for the Sierra design transducers to as high as 28 mmHg for the Unisensor design (Table S1) [4–9]. A correlate of this observation is that the diagnostic accuracy for detecting EGJ outflow obstruction for each device varies and, to date, has only been rigorously analyzed for the Sierra design. The variability evident in Table S1 also emphasizes the peril of being overly rigid in the application of cutoff values.
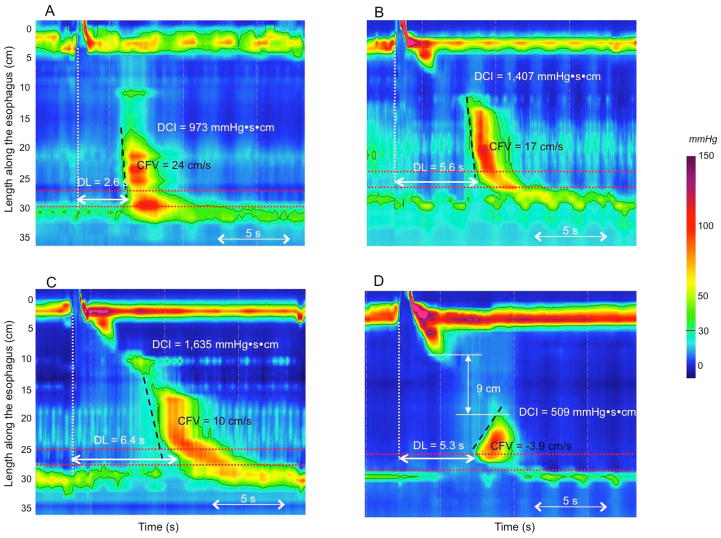
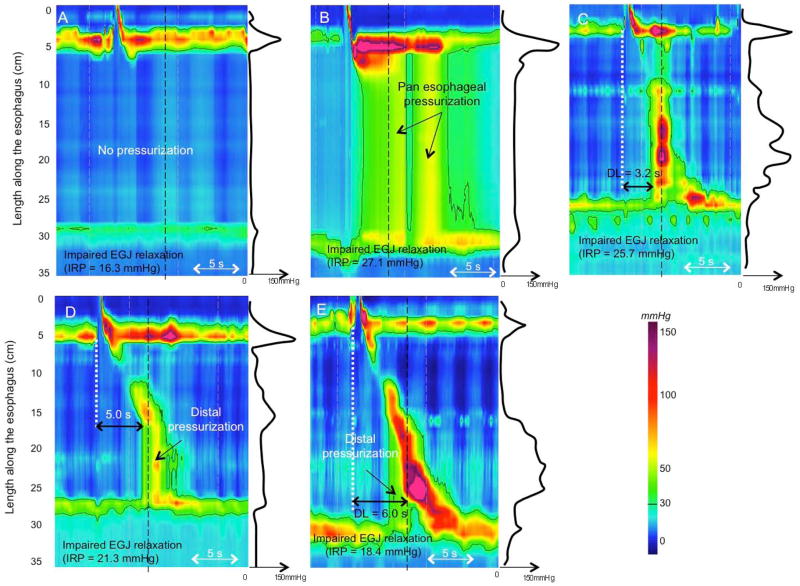
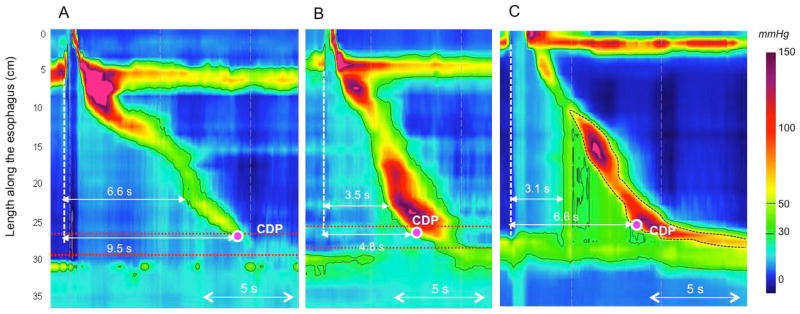
The definition of distal esophageal spasm (DES) in the CC depends on identifying premature contractions, defined by the DL and physiologically representing an attenuation of deglutitive inhibition. The DL is measured as the interval from UES relaxation to the CDP [10]; a value less than 4.5 s defines a premature contraction. Hence, the CDP is a key landmark in the assessment of the contraction pattern. On Clouse plots, the CDP represents the inflexion point in the contractile front propagation velocity in the distal esophagus [11]. After the CDP, propagation velocity slows signifying the termination of esophageal peristalsis and the onset of ampullary emptying, which is mechanistically very distinct, amounting to the reconstitution of the contracted LES [12]. However, the CDP can be difficult to localize in instances of atypical peristaltic architecture or compartmentalized pressurization (Figure 1). Hence, there are two added caveats for localizing the CDP: 1) to deal with problems of atypical peristaltic architecture an added criterion is that the CDP must be localized to within 3 cm of the LES [13], and 2) in instances of compartmentalized pressurization the CDP needs to be localized along an isobaric contour line of greater magnitude than the compartmentalized intrabolus pressure.
Figure 1.
The CDP represents the inflexion point in the contractile front propagation velocity in the distal esophagus. However, two inflexion points can sometimes be identified in instances of atypical peristaltic architecture (Panels A and B). Depending of the location of the inflexion point, DL measured from UES relaxation to the CDP might be either normal (>4.5 s) or reduced (upper location, Panel B). Adding the stipulation that the CDP must be localized to within 3 cm of the proximal margin of the LES (area between the 2 red dotted lines) prevents miscategorization in Panels A and B. In instances of compartmentalized pressurization (Panel C) the CDP is localized along a pressure above the intra-bolus pressure (50 mmHg isobaric contour on Panel C), not to the moment of compartmentalized pressurization.
With the definition of DES predicated on contractile latency rather than contractile velocity, the issue also arises of what relevance remains for the CFV. Comparison between the CFV and the DL clearly demonstrated that a rapid CFV lacks specificity for spasm, often instead identifying fragmented peristaltic contractions with atypical architecture (Figure 2) [10]. Among 67 patients with at least 20% of rapid contractions with normal latency, Pandolfino et al. showed that this abnormality was associated with a variety of conditions: EGJ outflow obstruction (n=7, 11%), weak peristalsis (n=41, 61%), hypertensive peristalsis (n=5, 7%) and even normal peristalsis (n=14, 21%) [10]. These observations led the Working Group to conclude that the CFV should not be used as a defining metric in CC v3.0.
Figure 2.
Lack of relevance of CFV in the identification of DES. The CFV can be elevated (>9 cm/s) with a reduced DL (<4.5 s) (Panel A, a patient with symptoms of dysphagia and chest pain). However, in cases with a normal DL, an elevated CFV might be seen as the consequence of atypical contraction architecture (patients without dysphagia in Panels B and C) or a large break in the contractile front (Panel D, patient with IEM and a negative CFV value on this swallow).
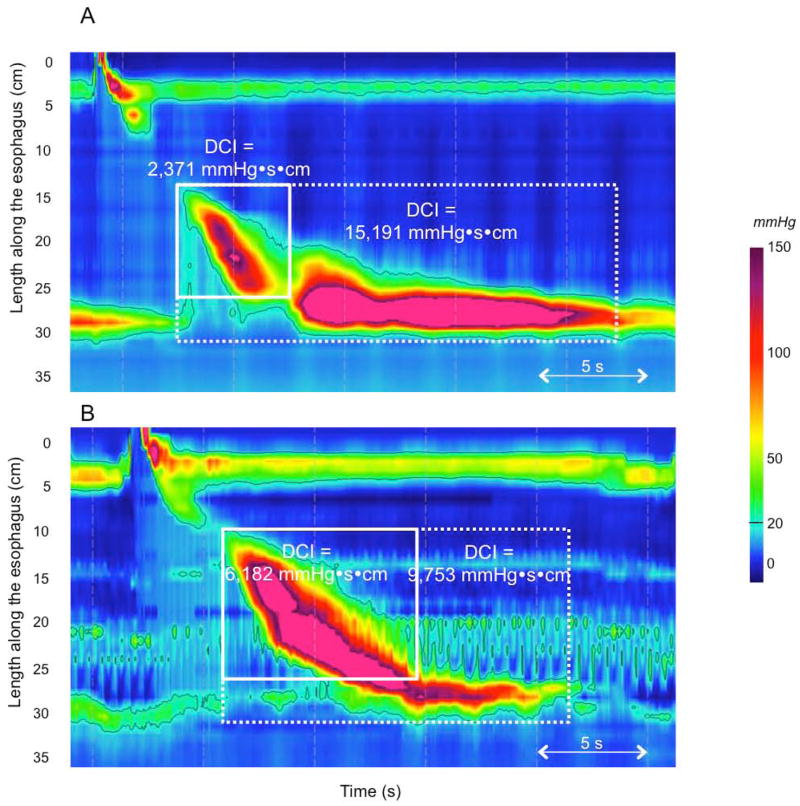
Distal esophageal contractile vigor is summarized using the DCI. This metric applies an algorithm to quantify the contractile pressure exceeding 20 mmHg for the region spanning from the transition zone to the proximal aspect of the LES. As such, it encompasses the space-time domain of the 2nd and 3rd contractile segments defined by Clouse [14] and provides a single number summarizing contractile vigor in this region. The DCI was initially devised to differentiate hypercontractility (jackhammer) from normal, but has subsequently been adapted to identify hypocontractile conditions as well. With respect to hypercontractility, there are clearly instances in which this phenomenon involves the LES as well as the 2nd and 3rd contractile segments and others in which it seemingly only involves the LES (Figure 3). In some of these cases, excluding the LES from the measurement domain might then fail to detect the abnormality. However, the Working Group was averse to the proposal of introducing a new metric to quantify post-deglutitive LES contractility in the absence of any clinical data demonstrating the significance of such a distinction. Rather, it was proposed to simply incorporate the LES into the DCI measurement domain in these instances and use the existing cutoff value (8,000 mmHg) to define hypercontractility. The rationale was that the degree to which the normal LES pressure exceeds 20 mmHg is sufficiently slight such that its ‘normal’ contribution to the DCI would be minimal.
Figure 3.
Normal esophageal contraction followed by hyperconctractility of the LES (Panel A). Including the LES (the 4th contractile segment defined by Clouse) in the DCI measurement (white dashed box) result in a diagnosis of hypercontractility. In the case of borderline DCI (Panel B) including LES in the DCI measurement also results in the diagnosis of hypercontractility.
Revised metrics used in the Chicago Classification v3.0 are summarized in Table 1 and normal values for currently available devices are given in Supplementary Materials (Tables S1 and S2).
Table 1.
Pressure topography metrics utilized in the Chicago Classification v3.0. Unless otherwise specified, pressures are referenced to atmospheric. Metrics are listed alphabetically.
| Pressure Topography Metrics | |
|---|---|
| CDP (time, position)* Contractile Deceleration Point |
Inflection point along the 30 mmHg isobaric contour (or pressure greater than intrabolus pressure in instances of compartmentalized pressurization) at which propagation velocity slows, demarcating peristalsis from ampullary emptying. The CDP must be localized within 3 cm of the proximal margin of the LES |
| DCI (mmHg·s·cm)† Distal Contractile Integral |
Amplitude x duration x length (mmHg·s·cm) of the distal esophageal contraction exceeding 20 mmHg from the transition zone to the proximal margin of the LES (Clouse, 2nd and 3rd contractile segments) § |
| DL (s)* Distal Latency |
Interval between UES relaxation and the CDP |
| IRP (mmHg)† Integrated Relaxation Pressure |
Mean of the 4s of maximal deglutitive relaxation in the 10-s window beginning at UES relaxation. Contributing times can be contiguous or non-contiguous (eg interrupted by diaphragmatic contraction). Referenced to gastric pressure. |
CD= crural diaphragm; EGJ= esophagogastric junction; UES= upper esophageal sphincter
Normal values are independent of the manometric hardware utilized
Normal values are dependent on the specific manometric hardware utilized
In instances of suspected hypercontractility involving the LES, or even restricted to the LES, the DCI box should include the LES (Clouse, 4th contractile segment) as well
Metrics pertinent to EGJ morphology and tone
Absent from prior versions of the CC were any characterization of EGJ morphology or contractility. However, despite the absence of conventions, several publications pertinent to reflux pathophysiology have utilized HRM and devised metrics to characterize both EGJ morphology and phasic contractions. Hence, the Working Group agreed on the significance of the issue and on establishing basic conventions for characterizing the EGJ in CC v3.0 (Table 2).
Table 2.
Pressure topography baseline EGJ morphology and tone derived from an overview of the study.
| EGJ
| ||
|---|---|---|
| EGJ morphology | Type I: | Complete overlap of CD and LES components with single peak on the spatial pressure variation plot |
| Type II: | Double-peaked pressure zone with the inter-peak nadir pressure greater than gastric pressure and a separation of 1–2 cm between peaks. This can vary or be present intermittently in which case the report should mention the range of observed LES-CD separation | |
| Type IIIa: | Double-peaked pressure zone with the inter-peak nadir pressure less than or equal to gastric pressure, but the pressure inversion point remains at the CD level. The range of observed LES-CD separation is reported | |
| Type IIIb: | Double-peaked pressure zone with the inter-peak nadir pressure equal to gastric pressure and the pressure inversion point at the LES level. The range of observed LES-CD separation is reported | |
|
| ||
| LES-CD separation | The EGJ pressure profile on a spatial pressure variation plot at peak inspiration can be a single or double peak. If a single peak, the LES-CD separation is 0; if a double peak, LES-CD separation is the axial distance between peaks | |
|
| ||
| Inspiratory EGJ pressure | Average of maximal inspiratory EGJ pressure reached during 3 respiratory cycles, ideally in a quiescent portion of recording, free of swallows | |
|
| ||
| Expiratory EGJ pressure | Average EGJ pressure midway between adjacent inspirations for 3 respiratory cycles, ideally in a quiescent portion of recording, free of swallows | |
EGJ morphology
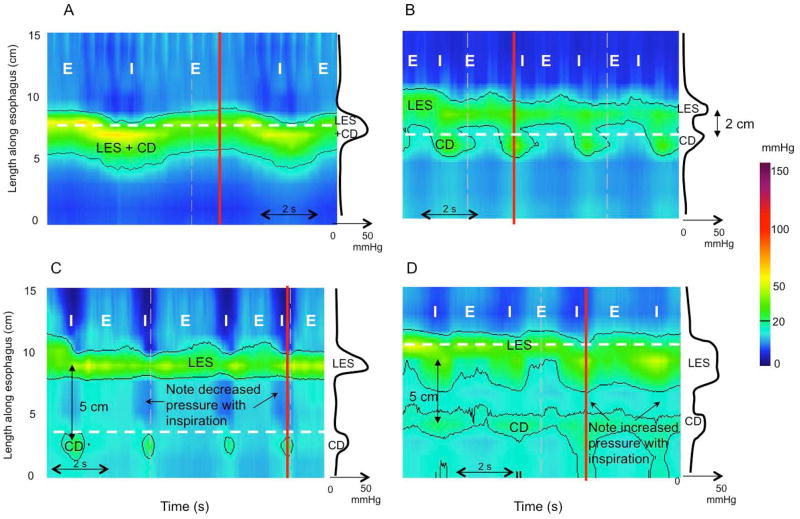
With HRM and Clouse plots, the relative localization of the two contractile elements of the EGJ, the LES and the crural diaphragm (CD) can be distinguished. Based on observations in control subjects and gastroesophageal reflux disease (GERD) patients, Pandolfino et al. described three EGJ subtypes [15] (Figure 4). With type I EGJ morphology, there is complete overlap of the CD and LES on the Clouse plot and a single pressure peak evident at inspiration on the associated spatial pressure variation plot. With type II EGJ morphology, the LES and CD are spatially separated such that there is a double-peaked pressure profile on the spatial pressure variation plot at inspiration, but the nadir pressure between peaks does not decline to gastric pressure and the separation between the LES and CD peaks is 1–2 cm. With type III EGJ morphology, the inspiratory spatial pressure variation plot exhibits >2cm separation between the LES and CD peaks with the nadir pressure between them equal to or less than gastric pressure; with type IIIa the pressure inversion point remains at the CD level, while in type IIIb it is located at the LES level. In terms of the relationship between manometric EGJ morphology and hiatus hernia, clearly there would be no radiographically evident hiatus hernia with type I EGJ morphology and a persistent hernia with EGJ separation of 2 cm or more. Between those extremes, and in the absence of any data on the subject, one would speculate that a hernia would be variably diagnosed depending on the interpreter and the radiographic criteria applied.
Figure 4.
sophagogastric junction (EGJ) morphology subtypes. For each panel the instantaneous spatial pressure variation plot corresponding to the red line on the pressure topography plot is illustrated by the black line to the right. The two main EGJ components are the LES and CD, which cannot be independently quantified when they are superimposed as with a type I EGJ (Panel A). The respiratory inversion point (RIP), shown by the white horizontal dashed line, lies near the proximal margin of the EGJ. During inspiration (I) EGJ pressure increases, whereas it decreases during expiration (E). Type II EGJ pressure morphology is illustrated in Panel B. Note the 2 peaks on the instantaneous spatial pressure variation plot; the nadir pressure between the peaks is greater than the intra gastric pressure. The RIP is at the level of the CD. Panels C and D correspond to type III EGJ pressure morphology defined as the presence of 2 peaks of the instantaneous spatial pressure variation plot with the nadir pressure between the peaks equal to or less than intragastric pressure. The RIP is at the CD with type IIIa (Panel C) whereas it is at the level of the LES in IIIb (Panel D).
EGJ morphology has been shown to be an important determinant of EGJ barrier function in that spatial separation between LES and CD facilitates gastroesophageal reflux [16]. It is also important to note that the separation between LES and CD may fluctuate in the course of a prolonged HRM study because reflux preferentially occurs when the EGJ assumes type II or III morphology [16]. Hence, the Working Group concluded that LES-CD separation has physiological relevance and should be reported as the both the morphologic subtype and the range of LES-CD separation observed throughout the study.
EGJ tone
In HRM recordings there is a strong phasic component to EGJ pressure during normal respiration that is attributable to CD contraction at inspiration. Hence, a robust measure of EGJ tone needs to account for the effects of respiratory variation. The simplest way to accomplish this is to measure both inspiratory and expiratory EGJ pressure averaged over 3–5 respiratory cycles, referenced to intragastric pressure. For each respiratory cycle, inspiratory EGJ pressure is defined as the maximal pressure occurring at inspiration and expiratory pressure as the maximal pressure occurring at the midpoint between adjacent inspirations. Using that approach, Pandolfino et al. reported that the strongest single predictor of clinical category (esophagitis, abnormal pH monitoring study, control) was the magnitude of inspiratory augmentation observed [15]. The emphasis on inspiratory as opposed to expiratory EGJ pressure is also supported by preliminary work with 3D-HRM, demonstrating that the hiatus and CD dominate over the intrinsic LES in defining EGJ barrier function [17]. Preliminary data utilizing inspiratory muscle training as an effective therapeutic intervention in GERD also support this contention [18].
An alternative approach to summarizing EGJ barrier function would be to develop a metric analogous to the DCI only applied to the EGJ. Such a metric would then incorporate EGJ length and respiratory variability. Along that line, Hoshino et al. described the LES pressure integral, in essence simply measuring the ‘DCI’ at 20-mmHg isobaric contour of the EGJ complex for a 10-s window devoid of swallows [19]. The LES pressure integral was found to be significantly lower in patients with pathological esophageal acid exposure than in patients with normal esophageal acid exposure (p <0.05). A LES pressure integral <400 mmHg·s·cm had a sensitivity of 79% and a specificity of 54% in predicting abnormal esophageal acid exposure. More recently, the Chicago group proposed to use the EGJ contractile integral (EGJ-CI) to quantify EGJ contractility in HRM [20]. The EGJ-CI was also determined using the DCI tool, but for a duration of 3 consecutive respiratory cycles with the isobaric contour threshold set at 2 mmHg above the gastric pressure. The ‘DCI’ value was then divided by the duration of the three respiratory cycles (in seconds) yielding EGJ-CI units of mmHg·cm. The median (IQR) EGJ-CI was 39 (25–55) mmHg-cm in controls versus 18 (8–30) in GERD patients characterized by abnormal pH-impedance studies (p <0.05). Finally, the Saint Louis group proposed a slightly different calculation of the EGJ-CI [21] not normalized to time, referenced to intragastric pressure, and excluding the CD component in instances of EGJ type III morphology. Using ROC analysis, a threshold of 122 mmHg·s·cm had a sensitivity of 61% and specificity of 61% in predicting abnormal esophageal acid exposure. A value <122 mmHg·s·cm was also associated with good outcome after antireflux surgery in 75% of patients versus 43% after medical treatment.
Conclusions of the HRM Working Group
The Working Group considered the existing data on application of the EGJ-CI metric and concluded that, although promising, further work was necessary to standardize the methodology of calculation. Hence, it was concluded that the simplest assessment of EGJ pressure was an average of inspiratory and expiratory values for 3 normal respiratory cycles, ideally in a quiescent portion of the recording, remote from either spontaneous or test swallows in order to exclude the effect of the post-deglutitive contraction. The inspiratory EGJ pressure is the mean of maximal inspiratory EGJ pressures reached during inspiration, and the expiratory EGJ pressure is the average EGJ pressure midway between inspirations; normative values are reported in Supplementary Materials Table S3.
Simplifying the criteria for disorders (and abnormalities) of peristalsis
In prior versions of the CC, the assessment of peristaltic function was based on scoring ten 5-ml swallows performed in the supine position in terms of ‘integrity’, contraction pattern, and intrabolus pressure pattern. The scoring of ‘integrity’ relied heavily on the identification of large (>5 cm in length) or small (2–5 cm in length) breaks in the 20-mmHg isobaric contour with the DCI being used primarily to identify hypercontractility, which was categorized as a contraction pattern. The Working Group had several proposals for simplifying and clarifying the evaluation of peristalsis in CC v3.0:
The measure of contractile vigor should be independent of contraction pattern (breaks)
The utility of separately counting weak and failed contractions was questioned
The significance of hypertensive peristaltic contractions (DCI 5,000–8,000 mmHg·s·cm) was questioned
The significance of detecting small 20-mmHg isobaric contour breaks was questioned
Contraction vigor
Although an ineffective contraction was originally defined in conventional manometry on the basis of low amplitude peristalsis, this criterion was not used to define weak peristalsis in prior versions of the CC. Hence, some contractions with normal or even high DCI were categorized as ‘weak’ because of small or large breaks in the 20-mmHg isobaric contour, while contractions with low DCI might be considered normal as long as the 20-mmHg isobaric contour was intact. Consequently, when correlating conventional line tracing analysis and Clouse plot analysis, Xiao et al. demonstrated that swallows scored as ineffective on conventional line tracing potentially corresponded to a mixture of intact contractions (a contraction without a break in the 20-mmHg isobaric contour), weak contractions with small or large breaks, and failed contractions on Clouse plots [22]. In brief, although breaks in the 20-mmHg isobaric contour might correspond to ineffective swallows, they were non-specific.
To clarify the distinction between contractile vigor and the contraction pattern, the Working Group elected to separate these concepts and base the evaluation of contractile vigor entirely on the DCI, using cutoff values of 100 mmHg·s·cm for failed peristalsis and 450 mmHg·s·cm for weak peristalsis. The value for weak peristalsis was derived directly from the Xiao paper (positive percent agreement in predicting ineffective swallows of 83% and negative percent agreement 90% in a validation sample of 100 patients) while the threshold for failed peristalsis represented a convenient compromise between proposed values ranging from 50 to 150 mmHg·s·cm. Both failed and weak peristaltic contractions are ineffective. At the other extreme of contractile vigor, it was accepted to keep the cutoff for hypercontractility at 8,000 mmHg·s·cm, but to eliminate the ‘hypertensive’ designation for contractions with DCI between 5,000 and 8,000 mmHg·s·cm because it has no apparent clinical significance.
Contraction pattern
The Working Group opined that breaks in the 20-mmHg isobaric contour be considered under the heading of ‘contraction pattern’. However, since small breaks (<3 cm) in the 20-mmHg isobaric contour are frequently encountered in normal subjects [23], the Working Group proposed that these be considered normal. On the other hand, large breaks (>5 cm) in the 20-mmHg isobaric contour are significantly more common in patients with dysphagia than in controls (14 vs 4%, p=0.02) [24] and these are still scored in CC v3.0. However, to complete the separation between the concepts of contractile vigor and contraction pattern, the term of ‘fragmented’ was adopted to characterize a contraction with a large break in the 20-mmHg isobaric contour, but normal or elevated DCI (>450 mmHg·s·cm) [25]. Similarly, a premature contraction must have both a DL <4.5 s and a DCI >450 mmHg·s·cm to qualify; otherwise it would be considered failed and fall under the category of ‘ineffective contractions’.
Intrabolus pressure pattern
The patterns of intrabolus pressure identified in CC v3.0 remain as previously defined. Intrabolus pressure is scored using the 30-mmHg isobaric contour and abnormal pressurization corresponds to regions of esophageal pressurization to >30 mmHg. Intrabolus pressure is ‘panesophageal’ if it spans from the EGJ to the UES, ‘compartmentalized’ if it extends from the deglutitive contractile front to the EGJ and ‘EGJ pressurization’ if restricted to zone between the LES and CD in conjunction with hiatal hernia.
Summary
The updated classifications and associated criteria for deglutitive contractile vigor, contraction pattern, and intrabolus pressure are summarized in Table 3, with key illustrative examples depicted in Figure 5.
Table 3.
Characterization of esophageal contractility. Contraction pattern is not scored for ineffective swallows (DCI <450 mmHg·s·cm)
| Contraction Vigor | |
|---|---|
| Failed | DCI <100 mmHg·s·cm |
| Weak | DCI >100 mmHg·s·cm, but <450 mmHg·s·cm |
| Ineffective | Failed or Weak |
| Normal | DCI >450 mmHg·s·cm but <8,000 mmHg·s·cm |
| Hypercontractile | DCI ≥8,000 mmHg·s·cm |
| Contraction Pattern | |
| Premature | DL <4.5 s |
| Fragmented | Large break (>5 cm length) in the 20-mmHg isobaric contour with DCI >450 mmHg·s·cm |
| Intact | Not achieving the above diagnostic criteria |
| Intrabolus Pressure Pattern (30 mmHg isobaric contour referenced to atmospheric) | |
| Panesophageal pressurization | Uniform pressurization of >30 mmHg extending from the UES to the EGJ |
| Compartmentalized | Pressurization of >30 mmHg extending from the contractile |
| esophageal pressurization | front to the EGJ |
| EGJ pressurization | Pressurization restricted to zone between the LES and CD in conjunction with LES-CD separation |
| Normal | No bolus pressurization >30 mmHg |
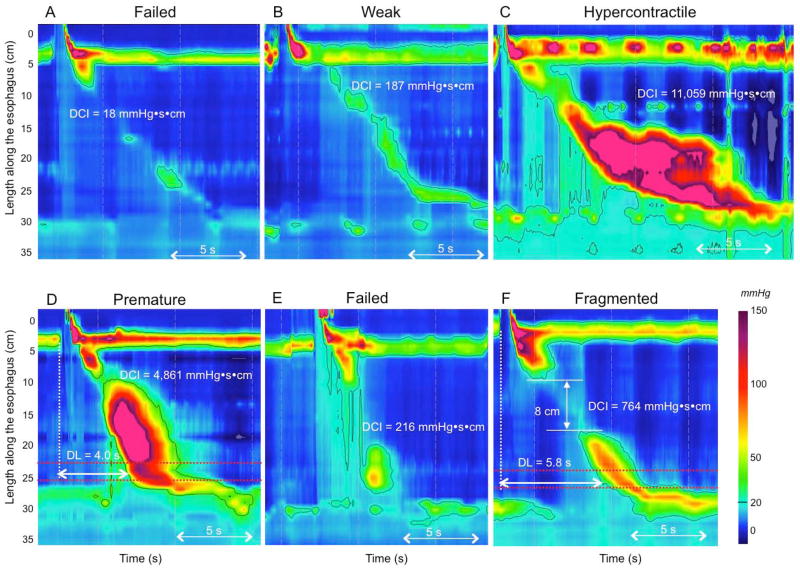
Figure 5.
Contractile vigor and contraction pattern. Contractile vigor is assessed using the DCI: a contraction with a DCI <100 mmHg·s·cm is failed (Panel A); a contraction with a DCI >100 but <450 mmHg·s·cm is weak (Panel B); and a hypercontractile swallow is defined as a DCI >8,000 mmHg·s·cm (Panel C). Premature contraction is defined as a distal latency (DL) <4.5 s (Panel D). A weak contraction (DCI <450 mmHg·s·cm) with a reduced distal latency is considered failed (Panel E). A contraction with a normal DCI (450–8,000 mmHg·s·cm) and a break >5 cm is a fragmented contraction (Panel F).
THE CHICAGO CLASSIFICATION v3.0
Achalasia and EGJ outflow obstruction
The first assessment of esophageal motility in the CC is whether or not EGJ outflow obstruction is present as defined by the IRP. Disorders of EGJ outflow obstruction are further subdivided into achalasia subtypes and EGJ outflow obstruction (Figure 6). Three subtypes of achalasia were defined in the previous iteration of the CC, differentiated by the patterns of non-peristaltic esophageal pressurization that accompanied the elevated IRP: type I achalasia was characterized by 100% failed contractions and no esophageal pressurization; type II achalasia was defined by panesophageal pressurization occurring with at least 20% of swallows; and type III achalasia was defined by the presence of preserved fragments of distal peristalsis or premature contractions for at least 20% of the swallows in the 2012 Chicago Classification [26]. Finally, EGJ outflow obstruction was defined by an elevated IRP with some instances of intact or weak peristalsis such that the criteria of achalasia were not met with the caveats that EGJ outflow obstruction might be due to achalasia, wall stiffness consequent from an infiltrative disease, or a manifestation of hiatal hernia. It was also acknowledged that an isolated elevated IRP value could be an artifact and evidence of bolus pressurization proximal to the EGJ in conjunction with an elevated IRP bolsters its functional significance. However, without a validated metric to quantify bolus pressurization, the Working Group was hesitant to require the added criterion.
Figure 6.
Disorders associated with EGJ outflow obstruction. Impaired EGJ relaxation is evident by an IRP >15 mmHg. Instantaneous pressure along the black dashed line is represented by the black line on the right side of each panel. In type I achalasia, there is no esophageal contraction and no esophageal pressurization (Panel A). Type II achalasia is characterized by panesophageal pressurization and absence of a peristaltic contraction (Panel B). Note that pressurization corresponds to homogeneous pressure along the spatial pressure variation plot. In type III achalasia, there are at least 20% premature contractions, defined as DL <4.5 s. (Panel C). Note the multiple peaks corresponding to contractions along the spatial pressure variation plot. EGJ outflow obstruction may represent achalasia (Panel D); it might also be the consequence of a mechanical obstruction (Panel E) such as a distal esophageal stenosis.
Update in Chicago Classification v3.0
The Working Group had several proposals for clarifying the definitions of achalasia and EGJ outflow obstruction in CC v3.0:
Use a median rather than a mean cutoff value among test swallows for the IRP
Use a lower IRP cutoff for type I achalasia as suggested by a regression tree model
Consider that panesophageal pressurization with ≥20% of swallows in conjunction with 100% failed contractions be pathognomonic for type II achalasia, irrespective of IRP
Clarify the definition of type III achalasia, clearly distinguishing it from EGJ outflow obstruction with some preserved peristalsis
Emphasize the heterogeneity of conditions potentially causing EGJ outflow obstruction
Using a median rather than a mean IRP value was noncontroversial as many members of the Working Group had more or less already adopted a practice of ignoring outlier IRP values and this simply formalizes that process. However, the proposal to selectively modify the IRP cutoff value depending on diagnosis ran against the overarching principle of streamlining and simplifying the CC. The Working Group preferred to keep the same IRP threshold for all achalasia subtypes, but to leave some flexibility in interpretation when certain combinations of contractility are observed. For instance, due to the relevance of the regression tree model analysis [27], the Working Group suggested to add a qualifier to the diagnosis of absent contractility: in instances of 100% failed contractions, a diagnosis of achalasia should be considered if there is a borderline median IRP value or if there is evidence of esophageal pressurization. Another pertinent observation relevant to defining type III achalasia was that after treatment with pneumatic dilation or Heller myotomy, peristalsis that was not observed prior to treatment can be observed [28]. This led to the hypothesis that remnants of peristalsis are more common than previously thought and were simply being obscured by esophageal pressurization in the pre-treatment studies. Hence, in CC v3.0 the Working Group restricted the definition of Type III achalasia to premature contractions, eliminating the possibility that it be defined by remnants of esophageal peristalsis.
Finally, EGJ outflow obstruction is defined by an elevated median IRP with some instances of intact or weak peristalsis such that the criteria of achalasia are not met. This has several potential etiologies including incompletely expressed achalasia, early achalasia, esophageal wall stiffness consequent from an infiltrative disease or cancer, vascular obstruction of the distal esophagus, or a manifestation of a sliding or paraesophageal hiatal hernia. Hence this finding should prompt further investigation such as with endoscopic ultrasound or CT to clarify its etiology. The revised definitions of achalasia and EGJ outflow obstruction are detailed in Table 4.
Table 4.
The Chicago Classification of esophageal motility v3.0
| ACHALASIA and EGJ OUTFLOW OBSTRUCTION | CRITERIA |
|---|---|
| Type I achalasia (classic achalasia) | Elevated median IRP (>15 mmHg†), 100% failed peristalsis |
| (DCI <100 mmHg) | |
| Premature contractions with DCI values less than 450 mmHg·s·cm satisfy criteria for failed peristalsis | |
| Type II achalasia (with esophageal compression) | Elevated median IRP (>15 mmHg†), 100% failed peristalsis, panesophageal pressurization with ≥20% of swallows |
| Contractions may be masked by esophageal pressurization and DCI should not be calculated | |
| Type III achalasia (spastic achalasia) | Elevated median IRP (>15 mmHg†), no normal peristalsis, premature (spastic) contractions with DCI >450 mmHg·s·cm with ≥20% of swallows |
| May be mixed with panesophageal pressurization | |
| EGJ outflow obstruction | Elevated median IRP (>15 mmHg†), sufficient evidence of peristalsis such that criteria for types I-III achalasia are not met* |
| MAJOR DISORDERS of PERISTALSIS | (Not encountered in normal subjects) |
| Absent contractility | Normal median IRP, 100% failed peristalsis |
| Achalasia should be considered when IRP values are borderline and when there is evidence of esophageal pressurization | |
| Premature contractions with DCI values less than 450 mmHg·s·cm meet criteria for failed peristalsis | |
| Distal esophageal spasm | Normal median IRP, ≥20% premature contractions with DCI >450 mmHg·s·cm †. Some normal peristalsis may be present. |
| Hypercontractile esophagus (jackhammer) | At least two swallows with DCI >8,000 mmHg·s·cm †§ |
| Hypercontractility may involve, or even be localized to, the LES | |
| MINOR DISORDERS OF PERISTALSIS | (Characterized by contractile vigor and contraction pattern) |
| Ineffective esophageal motility (IEM) | ≥50% ineffective swallows |
| Ineffective swallows can be failed or weak (DCI<450 mmHg·s·cm) | |
| Multiple repetitive swallow assessment may be helpful in determining peristaltic reserve | |
| Fragmented peristalsis | ≥50% fragmented contractions with DCI > 450 mmHg·s·cm |
| NORMAL ESOPHAGEAL MOTILITY | Not fulfilling any of the above classifications |
Cutoff value dependent on the manometric hardware; this is the cutoff for the Sierra device
Potential etiologies: early achalasia, mechanical obstruction, esophageal wall stiffness, or manifestation of hiatal hernia
Hypercontractile esophagus can be a manifestation of outflow obstruction as evident by instances in which it occurs in association with an IRP greater than the upper limit of normal
Major disorders of peristalsis
Major motility disorders are defined as motility patterns other than achalasia or EGJ outflow obstruction that are not encountered in control subjects. In the prior iteration of the CC, this amounted to three entities: DES defined as normal mean IRP and ≥20% of premature contractions; hypercontractile (or jackhammer) esophagus defined as at least one swallow with DCI ≥8,000 mmHg·s·cm; and absent peristalsis defined as normal mean IRP and 100% of swallows with failed peristalsis.
Update in Chicago Classification v3.0
The Working Group strongly supported the use of the criteria described above. However, in some cases, slight modifications were proposed for CC v3.0:
Modify the requirement for hypercontractile esophagus to ≥20% of swallows with a DCI >8,000 mmHg·s·cm
Recognize the occurrence of hypercontractile LES
Substitute the term ‘absent contractility’ for ‘aperistalsis’ or ‘absent peristalsis’ to differentiate the entity from other clinical scenarios in which peristalsis is absent (eg achalasia)
Advise caution in distinguishing absent contractility from type I achalasia
The definition of hypercontractile esophagus (jackhammer esophagus) was based on the occurrence of at least one swallow with DCI >8,000 mmHg·s·cm [29]. However, it has subsequently become apparent that this disorder is heterogeneous and might occur along with other abnormalities, such as EGJ outflow obstruction, gastroesophageal reflux disease, or eosinophilic esophagitis. Furthermore, with one Working Group member’s observation of an 8,000 mmHg·s·cm DCI occurring in a control subject, the threshold of one swallow meeting that criterion was deemed insufficient and of uncertain relevance. Hence, the international HRM Working Group proposed to define jackhammer esophagus as the occurrence of ≥20% of swallows with a DCI >8,000 mmHg·s·cm and normal latency. Another caveat that has come to light is that hypercontractility can involve the LES or even be restricted to the LES (Figure 3). Hence, as discussed earlier, expanding the DCI measurement to include the EGJ in such instances is warranted.
Finally, the Working Group proposed to change the terminology of ‘absent peristalsis’ to ‘absent contractility’. The definition remained unchanged (normal EGJ relaxation and 100% failed peristalsis). This modification was noncontroversial as the intent was simple to clearly distinguish the entity from absent peristalsis as might occur with achalasia or DES. Also along those lines, the difficulty of distinguishing absent contractility from type I achalasia in the setting of a ‘borderline’ IRP was acknowledged. Based on the classification and regression tree analysis, type I achalasia should be considered in with IRP values in the 10–15 mmHg with the Sierra system [27] and, in general, esophageal pressurization should also alert one to the possibility of achalasia. The revised definitions of the major disorders of peristalsis are detailed in Table 4.
Minor disorders of peristalsis
The clinical significance of minor motility disorders continues to be actively debated. It was the strong feeling of the Working Group that ‘overly classifying’ these was counterproductive as it distracted attention from the importance of identifying the major disorders. In the prior version of the CC, five ‘peristaltic abnormalities’ were recognized: weak peristalsis with large peristaltic breaks; weak peristalsis with small peristaltic breaks; frequent failed peristalsis; rapid contractions with normal latency; and hypertensive peristalsis.
Update in Chicago Classification v3.0
As already mentioned, the prior classification for ‘peristaltic abnormalities’ encountered significant dissatisfaction in the clinical community because of its complexity and unclear relevance. Hence, the Working Group proposed major simplifications to this category. There were a multitude of proposals for modification in CC v3.0, the essence of which were:
Rename the category ‘minor disorders of peristalsis’
Eliminate small breaks (2–5 cm) in the 20-mmHg isobaric contour as a criterion of abnormality
Eliminate rapid CFV (>9 cm/s) as a criterion of abnormality
Eliminate the designation of ‘hypertensive peristalsis’ (DCI 5,000–8,000 mmHg·s·cm)
Adopt the ‘ineffective esophageal motility’ (IEM) designation popularized in conventional manometry using HRM criteria
Eliminate ‘frequent failed peristalsis’ as a distinct diagnostic entity
Incorporate new data from studies of multiple repetitive swallows into the criteria for IEM
As previously mentioned, small breaks in the 20-mmHg isobaric contour are frequently observed in control subjects, making these of unclear significance. Similarly, a substantial number of controls can exhibit rapid contractions with normal latency on the basis of atypical features of their topographic ‘fingerprints’. However, neither of these conditions has recognized clinical significance leading the Working Group to conclude that they should be considered variations of normal. Finally, the relevance of hypertensive peristalsis is not widely accepted; its definition, based on a mean DCI among 10 swallows being >5,000 mmHg·s·cm, was inconsistent with the general scheme of the CC that is otherwise based on the individual scoring of swallows. Furthermore, this value has significant overlap with control subjects. Therefore, while these contraction abnormalities may be descriptively reported when encountered, their clinical significance remains unclear and CC v3.0 does not regard them as abnormal.
A strong sentiment of the Working Group was that CC v3.0 adopt the terminology ‘ineffective esophageal motility’ (IEM) popularized in conventional manometry. The unifying feature of swallows contributing to the diagnosis of IEM is poor bolus transit in the distal esophagus. Using conventional manometry, IEM was defined by 50% or more ineffective swallows, which were in turn defined as contractions exhibiting amplitudes <30 mmHg at pressure sensors positioned 3 or 8 cm above the LES [30]. This approximates a DCI threshold of 450 mmHg·s·cm in HRM with Clouse plots [22]. Thus, it has been proposed to define ineffective swallows in CC v3.0 by a DCI <450 mmHg·s·cm with ≥50% ineffective swallows constituting IEM. No distinction need be made between failed swallows and weak swallows, thereby eliminating the former designation of ‘frequent failed peristalsis’.
Another development relevant to the diagnosis of IEM is the use of multiple rapid swallowing (MRS) as a supplemental test. MRS consists of administering five 2-ml water swallows separated by 2–3 s intervals, too brief a period to allow significant peristaltic progression. MRS profoundly inhibits the esophageal body and LES and is normally followed by an esophageal contraction of augmented amplitude. Using conventional manometry, half of patients with IEM normalized esophageal contraction amplitude after MRS [31], a phenomenon labeled ‘peristaltic reserve.’ Applying this concept to HRM, the DCI of the contraction that followed MRS was compared to the average DCI of the prior 10 test swallows in controls and in a cohort of GERD patients prior to fundoplication [32]. The DCI ratio (DCI after MRS/ average DCI of the 10 swallows) was greater than 1 in 78% of controls, 64% of patients without dysphagia after fundoplication, 44% of patients with early dysphagia after fundoplication and 11% of patients with late dysphagia after fundoplication (p<0.02). Further, a DCI ratio >0.85 had a sensitivity of 67% and a specificity of 64% in segregating patients developing late postoperative dysphagia from those with no postoperative dysphagia. Thus, the DCI ratio might reflect the peristaltic reserve and help predict the occurrence of postoperative dysphagia after antireflux surgery. The Working Group acknowledged the utility of MRS in patients with IEM to evaluate the peristaltic reserve, but was not convinced that it should yet be incorporated into the CC.
The remaining entity under the category of minor disorders of peristalsis pertains to large breaks in the 20-mmHg isobaric contour. Even though the significance of small breaks in the 20-mmHg isobaric contours was discounted, large breaks (>5 cm) might well be clinically relevant. Hence, the Working Group proposed to define ‘fragmented peristalsis’ as ≥50% fragmented contractions with the added stipulation of not meeting IEM criteria. The new definitions of the minor disorders of peristalsis are detailed in Table 4.
NEXT STEPS
The Chicago Classification is an evolving process. Version 3.0 takes into account interval publications since 2012 and the worldwide clinical experience of the experts in the field. Updating classification every 3 years is the goal of the HRM Working Group since this is required to maintain a classification that takes relevant new developments in the field into account.
Although initially an objective of CC v3.0, pharyngeal and UES functions are still not included in the CC v3.0. Recent publications suggest the utility of combined impedance-HRM, but not HRM by itself, in predicting the risk of aspiration in patients with oropharyngeal swallowing disorders [33–35]. Impedance measurement might also complement the analysis of esophageal function in patients without significant pressure abnormalities or to evaluate the impact of pressure abnormalities on bolus flow [36, 37] and might also be incorporated into future versions of the CC. Similarly, performing HRM in alternative conditions such as upright posture [8, 38] or introducing swallow challenges into the study such as free drinking [38] or a test meal, [40] to trigger motility abnormalities may improve the diagnostic yield of the study. Finally, post-surgical conditions are not addressed in the CC v3.0. In brief, although substantially matured since its inception, the CC necessarily is a work in progress and much remains to be addressed in future versions.
CONCLUSION
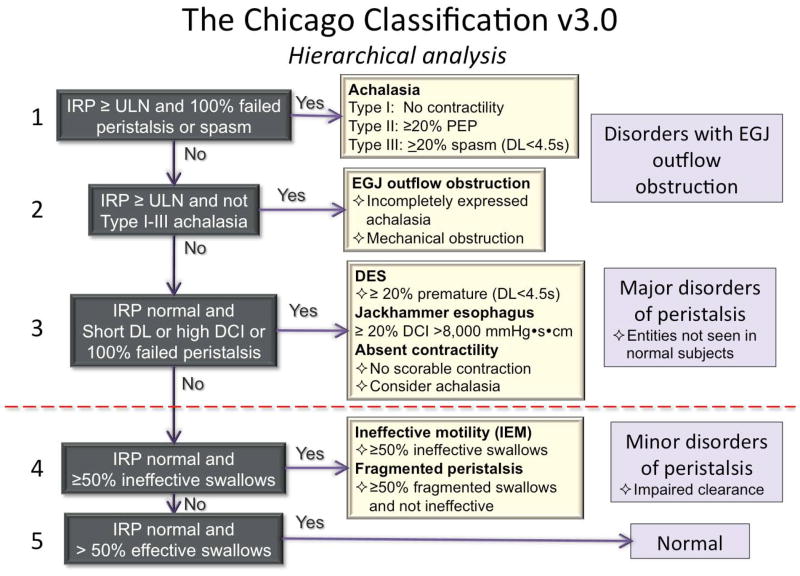
The CC v3.0 incorporates recent advances in the understanding of esophageal motility disorders imaged in HRM with pressure topography plots. Compared to the previous version, the evaluation of the EGJ at rest is now defined in terms of morphology and contractility. The key metrics of interpretation, the IRP, DCI, and DL remain unchanged, albeit with much more emphasis on DCI compared to prior CC versions. Absent in CC v3.0 are CFV, small breaks in the 20-mmHg isobaric contour, and hypertensive contractions (DCI 5,000–8,000 mmHg·s·cm) as defining characteristics. New in CC v3.0 are fragmented contractions (large breaks in the 20-mmHg isobaric contour), IEM, and several minor adjustments in nomenclature and defining criteria, all summarized in Table 4. Figure 7 illustrates a hierarchical, algorithmic approach to the clinical application of CC v3.0
Figure 7.
Hierarchical algorithm for the interpretation of HRM studies with CC v3.0.
Supplementary Material
Key messages.
The Chicago Classification (CC) categorizes esophageal motility disorders utilizing high resolution manometry (HRM) imaged with pressure topography plots
This update, CC v3.0, was developed by the International HRM Working Group through an international consensus process
CC v3.0 utilizes a hierarchical approach, sequentially prioritizing: 1) disorders of esophagogastric junction (EGJ) outflow, 2) other major disorders of peristalsis, and 3) minor disorders of peristalsis
Disorders of EGJ outflow obstruction are characterized by a median integrated relaxation pressure above the limit of normal. These disorders are divided into achalasia subtypes (I, II, and III) and EGJ outflow obstruction
Major motility disorders (never found in control subjects) apart from those characterized by EGJ outflow obstruction are absent contractility, distal esophageal spasm and jackhammer esophagus
Minor motility disorders, characterized by impaired esophageal bolus transit, are ineffective esophageal motility and fragmented peristalsis
Acknowledgments
The CC v3.0 were reviewed by and endorsed by The American Neurogastroenterology and Motility Society, The European Neurogastroenterology and Motility Society, and The Latin American Society of Neurogastroenterology.
The Esophageal HRM Working Group, as a part of the International Gastrointestinal Motility and Function Working Group, is grateful for a long-term educational award from United European Gastroenterology to support the dissemination of the Chicago Classification version 3.0.
The May 2014 meeting of the HRM working Group in Chicago was supported by unrestricted educational grants from Given Imaging and Sandhill Scientific Instruments.
Peter J Kahrilas and John E Pandolfino were supported by R01 DK079902 (JEP) and R01DK56033 (PJK) from the US Public Health Service.
Abbreviations
- CC
Chicago Classification
- CDP
contractile deceleration point
- CFV
contractile front velocity
- CD
crural diaphragm
- DCI
distal contractile integral
- DL
distal latency
- DES
distal esophageal spasm
- EGJ
esophagogastric junction
- EGJ-CI
EGJ contractile integral
- GERD
gastroesophageal reflux disease
- IEM
ineffective esophageal motility
- IRP
integrated relaxation pressure
- LES
lower esophageal sphincter
- UES
upper esophageal sphincter
Footnotes
No competing interests:
Peter J Kahrilas, AJPM Smout, Guy Boeckxstaens, Shobna Bhatia, Minhu Chen, Daniel Cisternas, Ian J Cook, Kerry Dunbar, Geoffrey Hebbard, Ikuo Hirano, Richard H Holloway, David Katzka, Meiyun Ke, Anthony Lembo, Ravinder K Mittal, Jeff Peters, Joel Richter, Nathalie Rommel, Renato Salvador, Stuart Spechler, Jan Tack, Radu Tutuian, Miguel Valdovinos, Yinglian Xiao
COMPETING INTERESTS
Albert J Bredenoord: Given Imaging: research funding; Medical Measurement Systems: educational and research funding
Mark Fox: Given imaging: advisory board, consulting and educational; Sandhill Scientific: educational; Medical Measurement Systems: educational.
C Prakash Gyawali: Given imaging, educational
John E Pandolfino: Given imaging; consulting and educational
Serhat Bor: Medical Measurement Systems: educational and research funding
DO Castell: Sandhill Scientific Instruments; consultant and educational
Jeffrey L Conklin: Given imaging: consulting and educational
Sabine Roman: Given imaging: advisory board, consulting and educational
Phil Katz: Torax: consultant; Prizer Consumer: consultant
Jutta Keller: Given imaging: research funding, consulting and educational; Standard Imaging: consulting and educational
Taher Omari: Sandhill Scientific Instruments: consulting and research grant; Medical Measurement Systems: educational.
Edoardo Savarino: Given imaging; advisory board, consulting and educational
Felice Schnoll-Sussman: Given imaging; advisory board, consulting and educational
Daniel Sifrim: Given imaging; advisory board. Sandhill Scientific Instruments: research grant.
Rami Sweis: Given imaging; consulting and educational
Radu Tutuian: Sandhill Scientific Instruments: educational; Medical Measurement Systems: educational.
Marcelo F Vela: Given Imaging, Consulting
Frank Zerbib: Given imaging; advisory board, consulting, and educational.
AUTHOR CONTRIBUTIONS
PJK organized and chaired the 2014 HRM Working Group meeting in Chicago, drafted the manuscript, organized the revision of the manuscript, and approved the final version.
AJB, MF, CPG, JEP, SR, and AJPMS served as Core Members of the HRM Working Group, organized subsections of the 2014 HRM Working Group meeting in Chicago, pre-reviewed the manuscript before circulation to the Working Group as a whole, finalized the document with PJK, and approved the final version.
Working Group Members (see author list) participated in the 2014 HRM Working Group meeting in Chicago, provided commentary on the manuscript, helped finalize the document with PJK, and approved the final version.
References
- 1.Gyawali CP. High resolution manometry: the Ray Clouse legacy. Neurogastroenterol Motil. 2012 Mar 24;(Suppl 1):2–4. doi: 10.1111/j.1365-2982.2011.01836.x. [DOI] [PubMed] [Google Scholar]
- 2.Pandolfino JE, Fox MR, Bredenoord AJ, Kahrilas PJ. High-resolution manometry in clinical practice: utilizing pressure topography to classify oesophageal motility abnormalities. Neurogastroenterol Motil. 2009;21:796–806. doi: 10.1111/j.1365-2982.2009.01311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bredenoord AJ, Fox M, Kahrilas PJ, et al. Chicago classification criteria of esophageal motility disorders defined in high resolution esophageal esophageal pressure topography (EPT) Neurogastroenterol Motil. 2012;24 (Suppl 1):57–65. doi: 10.1111/j.1365-2982.2011.01834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bogte A, Bredenoord AJ, Oors J, Siersema PD, Smout AJ. Normal values for esophageal high-resolution manometry. Neurogastroenterol Motil. 2013;25:762–e579. doi: 10.1111/nmo.12167. [DOI] [PubMed] [Google Scholar]
- 5.Shi Y, Xiao Y, Peng S, Lin J, Xiong L, Chen M. Normative data of high-resolution impedance manometry in the Chinese population. J Gastroenterol Hepatol. 2013;28:1611–1615. doi: 10.1111/jgh.12285. [DOI] [PubMed] [Google Scholar]
- 6.Ghosh SK, Pandolfino JE, Rice J, Clarke JO, Kwiatek M, Kahrilas PJ. Impaired deglutitive EGJ relaxation in clinical esophageal manometry: a quantitative analysis of 400 patients and 75 controls. Am J Physiol. 2007;293:G878–885. doi: 10.1152/ajpgi.00252.2007. [DOI] [PubMed] [Google Scholar]
- 7.Niebisch S, Wilshire CL, Peters JH. Systematic analysis of esophageal pressure topography in high-resolution manometry of 68 normal volunteers. Dis Esoph. 2013;26:651–660. doi: 10.1111/dote.12027. [DOI] [PubMed] [Google Scholar]
- 8.Sweis R, Anggiansah A, Wong T, Kaufman E, Obrecht S, Fox M. Normative values and inter-observer agreement for liquid and solid bolus swallows in upright and supine positions as assessed by esophageal high-resolution manometry. Neurogastroenterol Motil. 2011;23:509–e198. doi: 10.1111/j.1365-2982.2011.01682.x. [DOI] [PubMed] [Google Scholar]
- 9.Weijenborg PW, Kessing BF, Smout AJ, Bredenoord AJ. Normal values for solid-state esophageal high-resolution manometry in a European population; an overview of all current metrics. Neurogastroenterol Motil. 2014 May;26(5):654–9. doi: 10.1111/nmo.12314. [DOI] [PubMed] [Google Scholar]
- 10.Pandolfino JE, Roman S, Carlson D, et al. Distal esophageal spasm in high-resolution esophageal pressure topography: defining clinical phenotypes. Gastroenterology. 2011;141:469–475. doi: 10.1053/j.gastro.2011.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pandolfino JE, Leslie E, Luger D, Mitchell B, Kwiatek MA, Kahrilas PJ. The contractile deceleration point: an important physiologic landmark on oesophageal pressure topography. Neurogastroenterol Motil. 2010;22:395–400. doi: 10.1111/j.1365-2982.2009.01443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin Z, Yim B, Gawron A, Imam H, Kahrilas PJ, Pandolfino JE. The four phases of esophageal bolus transit defined using high resolution impedance manometry and fluoroscopy. Am J Physiol. 2014;307:G437–44. doi: 10.1152/ajpgi.00148.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin Z, Pandolfino JE, Xiao Y, et al. Localizing the contractile deceleration point (CDP) in patients with abnormal esophageal pressure topography. Neurogastroenterol Motil. 2012;24:972–975. doi: 10.1111/j.1365-2982.2012.01959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clouse RE, Alrakawi A, Staiano A. Intersubject and interswallow variability in topography of esophageal motility. Dig Dis Sci. 1998;43(9):1978–85. doi: 10.1023/a:1018838710214. [DOI] [PubMed] [Google Scholar]
- 15.Pandolfino JE, Kim H, Ghosh SK, Clarke JO, Zhang Q, Kahrilas PJ. High-resolution manometry of the EGJ: an analysis of crural diaphragm function in GERD. Am J Gastroenterol. 2007;102:1056–1063. doi: 10.1111/j.1572-0241.2007.01138.x. [DOI] [PubMed] [Google Scholar]
- 16.Bredenoord AJ, Weusten BL, Timmer R, Smout AJ. Intermittent spatial separation of diaphragm and lower esophageal sphincter favors acidic and weakly acidic reflux. Gastroenterology. 2006;130:334–340. doi: 10.1053/j.gastro.2005.10.053. [DOI] [PubMed] [Google Scholar]
- 17.Nicodème F, Pandolfino JE, Lin Z, Xiao Y, Escobar G, Kahrilas PJ. Adding a radial dimension to the assessment of esophagogastric junction relaxation: validation studies of the 3D-eSleeve. Am J Physiol. 2012;303:G275–280. doi: 10.1152/ajpgi.00063.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nobre e Souza MÂ, Lima MJ, Martins GB, Nobre RA, Souza MH, de Oliveira RB, dos Santos AA. Inspiratory muscle training improves antireflux barrier in GERD patients. Am J Physiol. 2013;305:G862–G867. doi: 10.1152/ajpgi.00054.2013. [DOI] [PubMed] [Google Scholar]
- 19.Hoshino M, Sundaram A, Mittal SK. Role of the lower esophageal sphincter on Acid exposure revisited with high-resolution manometry. J Am Coll Surg. 2011;213:743–750. doi: 10.1016/j.jamcollsurg.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Nicodème F, Pipa-Muniz M, Khanna K, Kahrilas PJ, Pandolfino JE. Quantifying esophagogastric junction contractility with a novel HRM topographic metric, the EGJ-Contractile Integral: normative values and preliminary evaluation in PPI non-responders. Neurogastroenterol Motil. 2014;26:353–360. doi: 10.1111/nmo.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gor P, Li Y, Munigala S, Patel A, Bolkhir A, Gyawali CP. High resolution manometry (HRM) interogation of esophagogastric junction (EGJ) barrier function predicts esophageal acid expsoure and symptomatic outcome. Gastroenterology. 2014;146:S-884. [Google Scholar]
- 22.Xiao Y, Kahrilas PJ, Kwasny MJ, et al. High-Resolution Manometry Correlates of Ineffective Esophageal Motility. Am J Gastroenterol. 2012;107:1647–1654. doi: 10.1038/ajg.2012.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar N, Porter RF, Chanin JM, Gyawali CP. Analysis of Intersegmental Trough and Proximal Latency of Smooth Muscle Contraction Using High-Resolution Esophageal Manometry. J Clin Gastroenterol. 2012;46:375–381. doi: 10.1097/MCG.0b013e31823d3403. [DOI] [PubMed] [Google Scholar]
- 24.Roman S, Lin Z, Kwiatek MA, Pandolfino JE, Kahrilas PJ. Weak peristalsis in esophageal pressure topography: classification and association with dysphagia. Am J Gastroenterol. 2011;106:349–356. doi: 10.1038/ajg.2010.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Porter R, Kumar N, Drapekin J, Gyawali CP. Fragmented smooth muscle contraction segments on high resolution manometry: A marker of esophageal hypomotility. Neurogastroenterol Motil. 2012;24:763–8. doi: 10.1111/j.1365-2982.2012.01930.x. [DOI] [PubMed] [Google Scholar]
- 26.Pandolfino JE, Kwiatek MA, Nealis T, Bulsiewicz W, Post J, Kahrilas PJ. Achalasia: A New Clinically Relevant Classification by High-Resolution Manometry. Gastroenterology. 2008;135:1526–1533. doi: 10.1053/j.gastro.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin Z, Kahrilas PJ, Roman S, Boris L, Carlson D, Pandolfino JE. Refining the criterion for an abnormal Integrated Relaxation Pressure in esophageal pressure topography based on the pattern of esophageal contractility using a classification and regression tree model. Neurogastroenterol Motil. 2012;24:e356–363. doi: 10.1111/j.1365-2982.2012.01952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roman S, Kahrilas PJ, Mion F, et al. Partial recovery of peristalsis after myotomy for achalasia; more the rule than the exception. JAMA Surg. 2013;148:157–164. doi: 10.1001/2013.jamasurg.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roman S, Pandolfino JE, Chen J, Boris L, Luger D, Kahrilas PJ. Phenotypes and clinical context of hypercontractility in high resolution pressure topography (EPT) Am J Gastroenterol. 2012;107:37–45. doi: 10.1038/ajg.2011.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blonski W, Vela M, Safder A, Hila A, Castell DO. Revised criterion for diagnosis of ineffective esophageal motility is associated with more frequent dysphagia and greater bolus transit abnormalities. Am J Gastroenterol. 2008;103:699–704. doi: 10.1111/j.1572-0241.2007.01593.x. [DOI] [PubMed] [Google Scholar]
- 31.Fornari F, Bravi I, Penagini R, Tack J, Sifrim D. Multiple rapid swallowing: a complementary test during standard oesophageal manometry. Neurogastroenterol Motil. 2009;21:718–e741. doi: 10.1111/j.1365-2982.2009.01273.x. [DOI] [PubMed] [Google Scholar]
- 32.Shaker A, Stoikes N, Drapekin J, Kushnir V, Brunt LM, Gyawali CP. Multiple rapid swallow responses during esophageal high-resolution manometry reflect esophageal body peristaltic reserve. Am J Gastroenterol. 2013;108:1706–1712. doi: 10.1038/ajg.2013.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Omari TI, Dejaeger E, van Beckevoort D, et al. A method to objectively assess swallow function in adults with suspected aspiration. Gastroenterology. 2011;140:1454–1463. doi: 10.1053/j.gastro.2011.02.051. [DOI] [PubMed] [Google Scholar]
- 34.Omari TI, Kritas S, Cock C, et al. Swallowing dysfunction in healthy older people using pharyngeal pressure-flow analysis. Neurogastroenterol Motil. 2014;26:59–68. doi: 10.1111/nmo.12224. [DOI] [PubMed] [Google Scholar]
- 35.Omari TI, Papathanasopoulos A, Dejaeger E, et al. Reproducibility and Agreement of Pharyngeal Automated Impedance Manometry With Videofluoroscopy. Clin Gastroenterol Hepatol. 2011;9:862–867. doi: 10.1016/j.cgh.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 36.Lin Z, Imam H, Nicodème F, et al. Flow time through esophagogastric junction derived during high-resolution impedance-manometry studies: a novel parameter for assessing esophageal bolus transit. Am J Physiol. 2014 May 22; doi: 10.1152/ajpgi.00119.2014. pii: ajpgi.00119.2014. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rommel N, Van Oudenhove L, Tack J, Omari TI. Automated impedance manometry analysis as a method to assess esophageal function. Neurogastroenterol Motil. 2014 May;26(5):636–45. doi: 10.1111/nmo.12308. [DOI] [PubMed] [Google Scholar]
- 38.Xiao Y, Read A, Nicodème F, Roman S, Kahrilas PJ, Pandolfino JE. The effect of a sitting vs supine posture on normative esophageal pressure topography metrics and Chicago Classification diagnosis of esophageal motility disorders. Neurogastroenterol Motil. 2012;24:e509–516. doi: 10.1111/j.1365-2982.2012.02001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daum C, Sweis R, Kaufman E, et al. Failure to respond to physiologic challenge characterizes esophageal motility in erosive gastro-esophageal reflux disease. Neurogastroenterol Motil. 2011;23:517–e200. doi: 10.1111/j.1365-2982.2011.01669.x. [DOI] [PubMed] [Google Scholar]
- 40.Sweis R, Anggiansah A, Wong T, Brady G, Fox M. Assessment of esophageal dysfunction and symptoms during and after a standardized test meal: development and clinical validation of a new methodology utilizing high-resolution manometry. Neurogastroenterol Motil. 2014;26:215–228. doi: 10.1111/nmo.12252. [DOI] [PubMed] [Google Scholar]
- 41.Kessing BF, Weijenborg PW, Smout AJ, Hillenius S, Bredenoord AJ. Water-perfused esophageal high-resolution manometry; normal values and validation. Am J Physiol. 2014 Mar;306(6):G491–5. doi: 10.1152/ajpgi.00447.2013. [DOI] [PubMed] [Google Scholar]
- 42.Pandolfino JE, Ghosh SK, Zhang Q, Jarosz A, Shah N, Kahrilas PJ. Quantifying EGJ morphology and relaxation with high-resolution manometry: a study of 75 asymptomatic volunteers. Am J Physiol. 2006;290:G1033–1040. doi: 10.1152/ajpgi.00444.2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.