Introduction
Allergic rhinitis (AR) is an IgE-mediated disease with symptoms of rhinorrhea, nasal obstruction, nasal itching and sneezing upon exposure to aeroallergens. In the USA, approximately $11.2 billion was spent on AR treatments in 2005.1 Preschool children and young adults with AR are at increased risk for asthma later in life.2,3 Early exposure to traffic related air pollutants, specifically diesel exhaust particles (DEP), may enhance risk for aeroallergen sensitization and development of allergic disorders in childhood.4,5 No prospective study has defined the exact relationship between early DEP exposure and percutaneous aeroallergen sensitization, especially the predictive value of early sensitization, during the first three years of life and the subsequent development of childhood AR. To address these gaps, we conducted the Cincinnati Childhood Allergy and Air Pollution Study (CCAAPS) birth cohort to test the hypothesis that childhood DEP exposure is associated with development of aeroallergen sensitization and allergic disease.6 In addition, we examined the predictive utility aeroallergen wheal area on AR at age four.
Percutaneous skin prick testing (SPT) is the most practical method for determining allergen specific IgE-mediated mast cell degranulation especially in young children7. The importance of quantifying the SPT response is widely appreciated.8 The SPT wheal diameter predicts probability of a positive controlled food challenge test in patients with a history of food allergy and may reduce the need for oral food challenges in comparison to in vitro IgE testing.9,10 Asymptomatic birch-sensitized adults with a wheal diameter ≥4 mm area are at increased risk for later development of clinical allergy (nasal, ocular or respiratory symptoms).11 Positive SPT to outdoor allergens in adolescence has been shown to be associated with persistent AR.12 Cat sensitization is associated with asthma and hay fever in young adults.13 Our second hypothesis is SPT wheal area during the first three years of childhood is a continuous predictor for the future development of AR at age four.
Methods
Study Design and location
The CCAAPS was approved by the University of Cincinnati Institutional Review Board. Infants born within the Greater Cincinnati /Northern Kentucky area were identified from birth records. Infants living less than 400 meters or greater than 1,500 meters from a major road (>1000 trucks traveling per day) were eligible to participate. Parents were informed of the study and signed an informed consent approved by the University of Cincinnati Institutional Review Board.14 If at least one parent reported naso-ocular symptoms and/or dyspnea on exposure to pollens, animals, bedrooms or exercise, they underwent skin prick testing to 15 regionally relevant aeroallergens including: white oak, American elm, maple mix, eastern red cedar, meadow fescue, timothy, short ragweed, cat, dog, house dust mite mix (Dermatophagoides farinae, and Dermatophagoides pteronyssinus), German cockroach and four mold allergens (Alternaria alternata, Aspergillus fumigatus, Penicillium species mix and Cladosporium species) (ALK-Abelló Inc.).14 Infants of these symptomatic and SPT positive, parents were enrolled during the first year of life and constituted the ongoing CCAAPS cohort.
Clinical visits
Eligible children were enrolled and beginning at age one, trained clinical staff administered questionnaires to parents regarding the child's medical history over the previous year and a home environmental history questionnaire. Children were examined and skin tested to the same 15 regional aeroallergens used in parental screening as well as cow's milk and hen's egg (ALK-Abelló Inc.). The questionnaires, physical examination, and SPT were repeated annually until age four.
Health outcomes
At the clinical visits, parents were queried about the presence of rhinitis symptoms using the ISAAC question, “In the past 12 months, has your child ever had a problem with sneezing, or a runny, or a blocked nose when he/she did not have a cold or flu?”15,16 One of the primary CCAAPS outcome, AR at age four, was defined as a positive parental response to the ISAAC question and a positive SPT to one or more of fifteen aeroallergens at age four. Aeroallergen sensitization at all ages was defined as a positive SPT to one or more of fifteen aeroallergens. Children who did not have allergic rhinitis (including non-allergic rhinitis, atopic but asymptomatic and non-atopic, asymptomatic children) served as comparison group for this study.
Quantitative Skin Prick Testing
At annual visits, SPT was performed using bifurcated needle (Duo-tip, Lincoln Diagnostics) with a drop of allergen extract (ALK-Abelló Inc). Histamine dihydrochloride (10 mg/ml) and 50% glycerinated human serum albumin-saline control were used as positive and negative controls, respectively (Hollister-Stier Laboratories LLC). Skin tests were interpreted after fifteen minutes with a positive SPT defined as a wheal of 3 mm greater than the negative saline control. The circumferences of all positive wheal reactions to histamine, saline and to each allergen were traced with a pen. The ink tracing was transferred to labeled grid paper to create a permanent record and scanned as true image files, for analysis in AutoCAD (Autodesk, Inc.). The ink circumferences of the wheal reactions were traced in AutoCAD, allowing calculation of the enclosed area by planimetry. The wheal area was used as a continuous independent variable.
Traffic exposure estimation
The methodology of estimation of individual exposure to DEP has been previously published17-19 Harvard–type Impactors (Air Diagnostics and Engineering) were used to collect fine particulate matter (diameter ≤ 2.5 μm, PM2.5) on 37-mm membrane Teflon filters (nominal pore size, 1 μm) and 37-mm quartz filters. The elemental carbon concentration of PM2.5 was determined from the quartz filters (via the thermal optical transmittance technique) and the Teflon filters (via the reflectance technique). Using the optical transmittance technique, the quartz filters were used to determine the organic carbon concentrations of PM2.5. Teflon filters were also analyzed for PM2.5 mass by gravimetric analysis and for 38 elements using x-ray fluorescence to develop a diesel signature profile.18,20 The elemental carbon attributable to traffic was determined by using the multivariable receptor model UNMIX and by the chemical mass balance model. This elemental carbon attributable to traffic was used as an estimate of DEP exposure. The estimate for DEP was further validated with additional analysis identifying subfractions of elemental and organic carbon.19 DEP exposure was estimated using a land-use regression model that included wind direction, elevation, length of bus route and truck intensity within 300 m of sampling site and applied to all locations where the child spent eight or more hours during their first year of life.21
Indoor home assessments and measurements of allergens and endotoxin
Before age one, the CCAAPS infant's home was visited by trained research staff.22 Each room in the home was evaluated for visible mold, water damage and general state of repair. Settled house dust was collected from the infant's primary activity room and analyzed for endotoxin, β- glucan, Fel d1 (cat), Can f1 (dog), Der p1 (house dust mite) and Bla g1 (cockroach) allergens as described before.23-28
Covariates
The CCAAPS study had previously identified risk factors of AR at age one and three including: ethnicity (non-African-American, African American), gender (female, male), annual household income (> $20,000, ≤ $20,000), breastfeeding duration (months), number of children in the home (≥ 2 children, <2 children), and season of birth.26 Hair cotinine levels at age two was measured and included in this study as an objective marker of second hand smoke exposure during early childhood.29 These covariates were evaluated along with DEP, endotoxin and indoor allergen levels.
Data analysis
A detailed description and rationale of the statistical plan is provided online (eMethods). Prior to bivariate comparison, continuous exposure covariates (endotoxin, β-glucan, Fel d1, Can f1, Der p1, and Bla g1) were analyzed by a general additive model to show turning points (i.e., changes in direction) in the smooth plot indicating changes from a linear category threshold.30 High DEP was defined as exposure above the 66th percentile as previously described.28 Logistic regression was performed to determine if the 17 allergen wheal areas at ages one, two, and three were associated with AR at age four, after adjusting for multiple testing using the Holms-Sidak test Logistic regression was used to determine associations between covariates an AR at age four. Any independent predictor variable associated with AR (p <0.20) were further investigated in a multivariable age-stratified model.
Each age-specific multivariable model was reduced by “all subsets” method of selection to include variables that improved model fit.31 The final simplified regression model was achieved by removing an independent variable if the log likelihood ratio did not decrease significantly and/or the remaining variable coefficients did not change by >20%. Additional stratified analyses were performed using same methodology. To confirm findings, any allergen wheal area that improved the final age-stratified regression model fit were summed and then analyzed for associations to AR. All analyses were performed using SAS 9.3 software (SAS Institute Inc).
Result
Subjects
Seven hundred and sixty two children were enrolled in the CCAAPS study. Of these, 638 children returned for clinical evaluation at age four; rhinitis symptom and skin testing data were available for 634 (83%) children. Table 1 shows the demographic characteristics of children in the study and associations to AR. Only ethnicity (p=0.18) and the presence of two or more children in the home during year one (p=0.14) met criteria for evaluation in the multivariate model. Due to previous associations with AR at age three in this cohort, breastfeeding duration and season of birth were examined in the multivariable model.26
Table 1. Characteristics of CCAAPS children.
Baseline comparison categories are indicated first, followed by categories associated with increased risk of AR. Because incomplete parental response, the sum of response to each covariable do not always total 634.
| Covariates | Frequency (%) | Allergic Rhinitis (AR) n=139 | No, or other phenotypes than AR n=495 | OR (95% CI) |
|---|---|---|---|---|
|
| ||||
| Gender: | ||||
| Female | 290 (45.7) | 58 (20.0) | 232 (80.0) | 1 |
| Male | 344 (54.3) | 81 (23.6) | 263 (76.5) | 1.23; (0.84, 1.80) |
|
| ||||
| Ethnicity: | ||||
| Non-African-American | 492 (77.6) | 102 (20.7) | 390 (79.3) | 1 |
| African-American | 142 (22.4) | 37 (26.1) | 105 (73.9) | 1.35; (0.87, 2.07)a |
|
| ||||
| Mother's Education: | ||||
| College graduate | 307 (50.0) | 74 (24.1) | 233 (75.9) | 1 |
| Some college/trade | 307 (50.0) | 63 (20.5) | 244 (79.5) | 0.81; (0.55, 1.19) |
|
| ||||
| Father's Education | ||||
| Some college/trade | 425 (69.3) | 93 (21.9) | 332 (78.1) | 1 |
| HS diploma or less | 188 (30.7) | 44 (23.4) | 144 (76.6) | 1.09; (0.72, 1.63) |
|
| ||||
| Household Income: | ||||
| ≥ $20 K | 502 (82.3) | 109 (21.7) | 393 (78.3) | 1 |
| < $20 K | 108 (17.7) | 28 (25.9) | 80 (74.1) | 1.26; (0.77, 2.02) |
|
| ||||
| Season of Birth: | ||||
| Winter | 209 (33.0) | 45 (21.5) | 164 (78.5) | 1 |
| Spring | 140 (22.1) | 29 (20.7) | 111 (79.3) | 0.95; (0.56, 1.60) |
| Summer | 138 (21.8) | 32 (23.2) | 106 (76.8) | 1.10; (0.65, 1.84) |
| Autumn | 147 (23.2) | 33 (22.5) | 114 (77.6) | 1.06; (0.63, 1.75) |
|
| ||||
| Breastfeeding Duration (months): | ||||
| ≥4 | 289 (45.6) | 66 (22.8) | 223 (77.2) | 1 |
| <4 | 345 (54.4) | 73 (21.2) | 272 (78.8) | 0.91; (0.62, 1.32) |
|
| ||||
| Children in the home at 12 mo | ||||
| ≥ 2 children | 197 (31.1) | 36 (18.3) | 161 (81.7) | 1 |
| < 2 children | 437 (68.9) | 103 (23.6) | 334 (76.4) | 1.38; (0.91, 2.13) |
|
| ||||
| Stays in daycare-like facility for ≥ 8 hours during 1st year. | ||||
| No | 399 (64.2) | 89 (22.3) | 310 (77.7) | 1 |
| Yes | 223 (35.9) | 48 (21.5) | 175 (78.5) | 0.96; (0.64, 1.42) |
|
| ||||
| No. colds reported at age 1 | ||||
| < 7 colds | 581 (91.6) | 125 (21.5) | 456 (78.5) | 1 |
| ≥ 7 colds | 53 (8.4) | 14 (26.4) | 39 (73.6) | 1.31; (0.67, 2.44) |
P<0.2
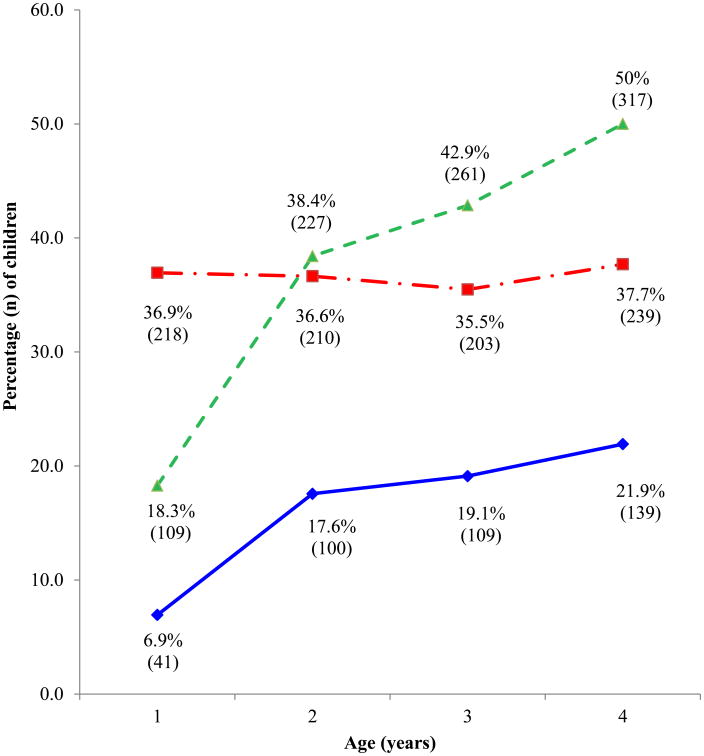
The prevalence estimates of AR, aeroallergen sensitization and rhinitis are shown in Figure 1. Allergic rhinitis prevalence increased annually from 7.0% at age one to 21.9% at age four. When looking at the components of AR, aeroallergen sensitization prevalence increased annually from 18.3% at age one to 50% at age four, whereas the prevalence of rhinitis symptoms remained relatively constant.
Figure 1. Prevalence of allergic rhinitis, aeroallergen sensitization and rhinitis during the first four years of life among the 634 subjects that had complete clinical and skin test data at year four.
Allergic rhinitis prevalence increased during the four years of the study. While rhinitis symptoms remained stable during the study, the prevalence of aeroallergen sensitization increased.
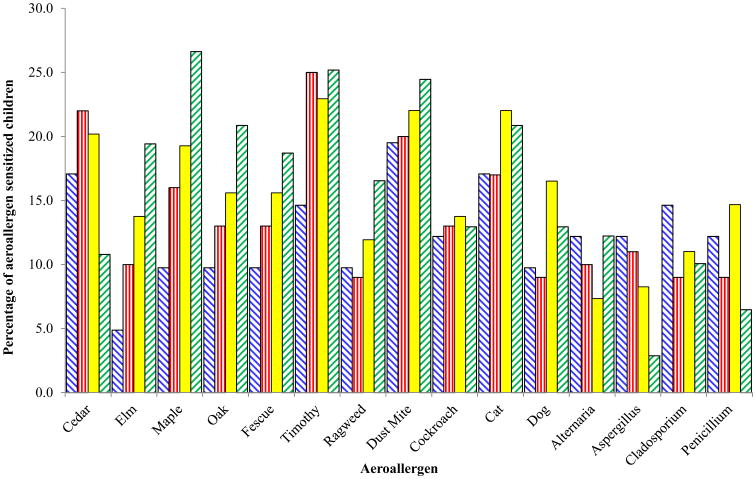
The percentage and absolute number of AR children sensitized to each aeroallergen at each age is shown in Figures 2 and E1, respectively. Timothy was the most prevalent positive SPT response at ages two (25%) and three (22.9%), and second (25.2%) only to maple (26.6%) at age four. Dust mite and cat were the most common indoor aeroallergen sensitizations in all four years. To determine how the magnitudes of these sensitivities at each year are associated with AR at age four, the wheal areas were examined as continuous measurements.
Figure 2. Percentage of aeroallergen sensitivities among allergic rhinitis children at ages one to four.

Unadjusted analyses
The number of allergens associated with AR (p <.20 for the multivariate model inclusion) increased annually, from none in year one, to four (maple, timothy, cat and Alternaria) and six (elm, maple, fescue, timothy, dog and Penicillium) allergens in years two and three, respectively (Table E1). The environmental exposure covariates measured at age one and their unadjusted associations to AR at age four are shown in Table 2. The factors from the settled house dust samples that met inclusion criteria included low (p= 0.1), medium (p= 0.04) and high (p=0.11) levels of endotoxin; medium (p=0.17) and high (p=0.17) β-glucan; and low, medium and high Fel d1 exposure (p=0.03 each). Low levels of hair cotinine at 2 years of age was not significantly associated with AR at 4 years of age (P =0.16).
Table 2. The frequency, unadjusted odds ratios and 95% confidence intervals of covariates and their association with allergic rhinitis (AR).
Baseline comparison categories are indicated first, followed by categories associated with increased risk of AR. Because incomplete parental response, the sum of response to each covariable do not always total 634.
| Covariates | Frequency (%) | Allergic Rhinitis (AR) n=139 | No, or other phenotypes than AR n=495 | OR (95% CI) |
|---|---|---|---|---|
|
| ||||
| Endotoxin (EU/mg dust) | ||||
| <230 | 567 (89.4) | 124 (21.9) | 443 (78.1) | 1.00; (1.00, 1.01)a |
| 230-640 | 57 (9.0) | 13 (22.8) | 44 (77.2) | 0.99; (0.98,1.00)b |
| ≥ 640 | 10 (1.6) | 2 (20.0) | 8 (80.0) | 1.02; (1.00, 1.05)a |
|
| ||||
| β-glucan (μg/g dust) | ||||
| <60 | 401 (63.3) | 90 (22.4) | 311 (77.6) | 1.01; (1.0, 1.02) |
| 60-170 | 159 (25.1) | 35 (22.0) | 124 (78.0) | 0.99; (0.97,1.01)a |
| ≥33.12 | 74 (11.7) | 14 (18.9) | 60 (81.1) | 1.01; (1.0, 1.02)a |
|
| ||||
| Fel d1 (μg/ml) | ||||
| <4.1 | 113 (17.8) | 17 (15.0) | 96 (85.0) | 1.97; (1.07,3.79)b |
| 4.1-148.4 | 337 (53.2) | 75 (22.3) | 262 (77.7) | 0.42; (0.19,0.90)b |
| ≥ 148.4 | 184 (29.0) | 47 (25.5) | 137 (74.5) | 1.48; (1.05 2.08)b |
|
| ||||
| Der p1 (μg/ml) | ||||
| < 54.6 | 485 (76.5) | 106 (21.9) | 379 (78.1) | 1.07; (0.92, 1.24) |
| ≥ 54.6 | 149 (23.5) | 33 (22.2) | 116 (77.9) | 0.79; (0.53, 1.16) |
|
| ||||
| Can f1 (μg/ml) | ||||
| < 0.74 | 295 (46.5) | 67 (22.7) | 228 (77.3) | 0.99; (0.06, 20.56) |
| 0.74-9.03 | 147 (23.2) | 33 (22.5) | 114 (77.6) | 1.02; (0.07, 12.56) |
| 9.03-221.4 | 149 (23.5) | 31 (20.8) | 118 (79.2) | 0.93; (0.49, 1.79) |
| ≥ 221.4 | 43 (6.8) | 8 (18.6) | 35 (81.4) | 1.07; (0.69, 1.65) |
|
| ||||
| Bla g1 (μg/ml) | ||||
| < 0.07 | 612 (96.5) | 133 (21.7) | 479 (78.3) | 0.96; (0.62, 1.46) |
| ≥ 0.07 | 22 (3.5) | 6 (27.3) | 16 (72.7) | 1.14; (0.86, 1.56) |
|
| ||||
| Age 2 Cotinine (ng/mg hair) | ||||
| <0.11 | 447 (70.5) | 94 (21.0) | 353 (79.0) | 1.39; (0.88, 2.20)a |
| 0.11-0.67 | 160 (25.2) | 37 (23.1) | 123 (76.9) | 0.87; (0.66, 1.13) |
| >0.67 | 27 (4.23) | 8 (29.6) | 19 (70.4) | -- |
|
| ||||
| Age 1 DEP Exposure (μg/m3) | ||||
| ≤ 0.32 | 423 (66.7) | 101 (23.9) | 322 (76.1) | 1 |
| > 0.32 | 211 (33.3) | 38 (18.0) | 173 (82.0) | 0.70; (0.46, 1.05) |
P<0.2
P<0.05
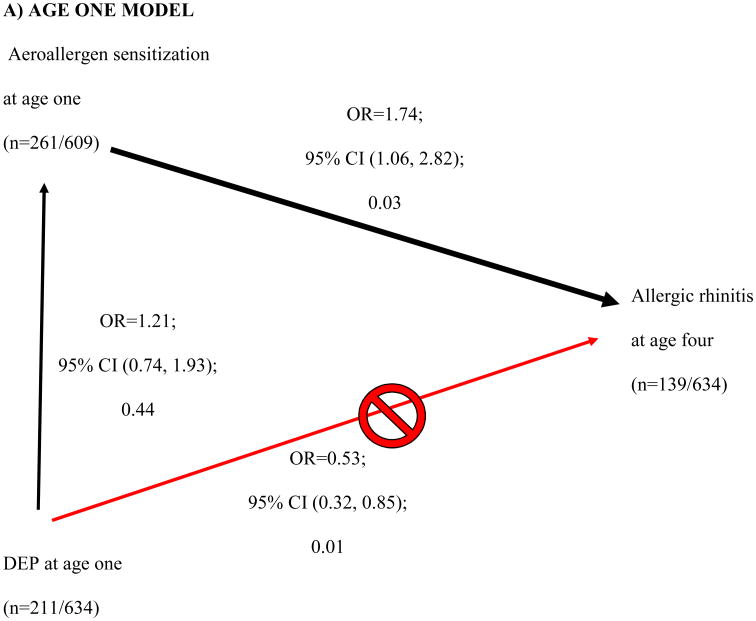
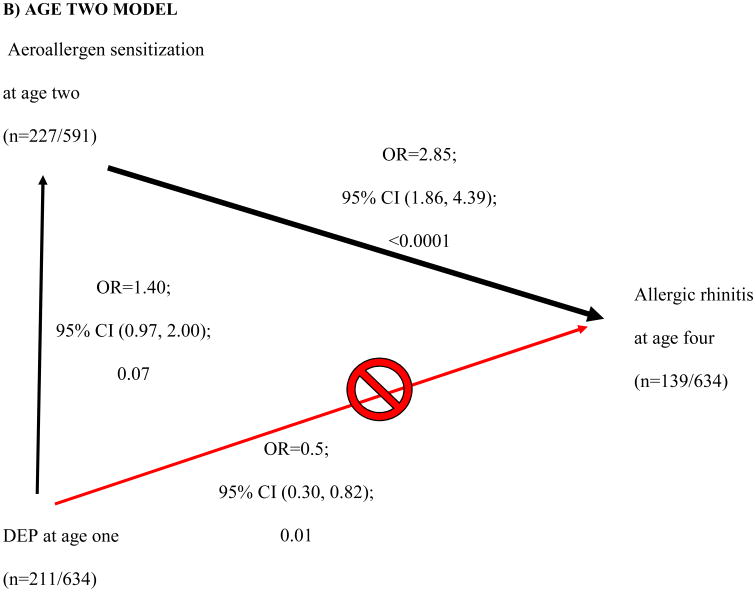
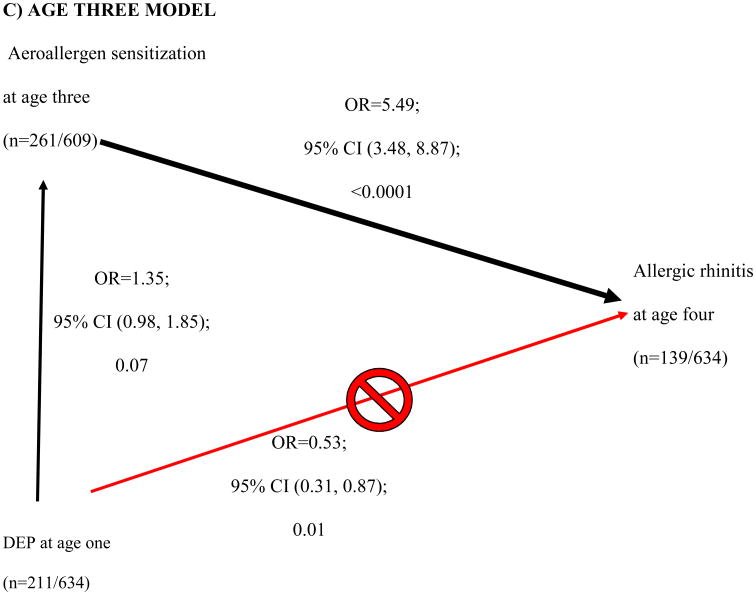
High DEP exposure at age one showed a protective effect for AR at age four (Table 2, p=0.09). This finding led us to examine the relationship between DEP and the other covariates. Further analysis revealed that DEP was borderline associated with aeroallergen sensitization at 2 (OR 1.40; 95% CI [0.97, 2.00]; 0.07) and 3 years of age (OR 1.35; 95% CI [0.98, 1.85]; 0.07) (vertical black arrows, Fig 3B and C). Sensitization at ages one, two and three was significantly associated with AR at age four (downward diagonal black arrow, Figure 3A-C). As DEP is related to aeroallergen sensitization (the main risk factor of interest in this study), the aeroallergen sensitization is an intermediate state in the development of AR. Therefore DEP was removed from the adjusted multivariate analysis.
Figure 3. Directed Acyclic Graph of showing how diesel exhaust particle (DEP) exposure in age one (≥66th tile, < 66th tile) confounds aeroallergen sensitization at age one and allergic rhinitis (AR) at age four.
DEP affects aeroallergen sensitivity at earlier ages, which successively are associated with AR at age four (A-C). DEP appears to be protective of AR at age four (red line in A-C). However, DEP is actually increasing the likelihood of aeroallergen sensitivity at ages two and three, which then increases the risk of AR (black lines in B and C). The age one multivariable model (A) included ethnicity, breastfeeding duration, Fel D1 (low, medium, and high). The age two multivariable model (B) included ethnicity, breastfeeding duration, children in the home at age one. The age multivariable model (C) included breastfeeding duration at age three. Breastfeeding duration, and breastfeeding duration*ethnicity interaction at age one were further evaluated to assess previous age three findings.
Adjusted Analyses
Table 3 shows the results of multivariable regression at each age. At age one, none of the 15 aeroallergen wheal areas were significantly associated with AR at age four. At age two, the final model included the following aeroallergen wheal areas: timothy (aOR 1.06; 95% CI, 1.02-1.11; p=0.01) and Alternaria (aOR 1.07; 95% CI, 1.00-1.15; p=0.04). The covariates in the final age two multivariable model included breastfeeding duration, Fel D1, and season of birth. At age three, the following aeroallergen wheal areas were included: maple (aOR 1.04; 95% CI, 1.00-1.09; p=0.08), fescue (aOR 1.08; 95% CI, 1.02-1.15; p=0.02), dog (aOR 1.06; 95% CI, 1.01-1.12; p=0.03), and Penicillium (aOR 1.14; 95% CI, 1.06-1.23; p=0.001). The covariates in the final age three multivariable model included ethnicity and number of children in the home at age one.
Table 3. Profile likelihood adjusted odds ratio (aOR) and 95% confidence intervals of allergen wheal area regression models at ages two and three associated to allergic rhinitis at age four.
Breastfeeding duration (months), Fel D1 level in settled house dust and season of birth covariables were included in age two model. In the age three model, and the combined ages two and three model, breastfeeding duration (months), Fel D1 level in settled house dust, number of children in the home at age one and ethnicity covariables were included.
| Age Two | Age Three | Combined ages two and three | ||
|---|---|---|---|---|
| Allergen Wheal Area aOR; 95% CI; P-value | Allergen Wheal Area aOR; 95% CI; P-value | Allergen Wheal Area aOR; 95% CI; P-value | ||
| Aeroallergens | Timothy | 1.06; (1.02, 1.11); 0.01 | -- | -- |
| Alternaria | 1.07; (1.00, 1.15); 0.04 | -- | 1.11; (1.03, 1.21); 0.01 | |
| Maple | -- | 1.04; (1.00, 1.09); 0.08 | 1.05; (1.00, 1.10); 0.047 | |
| Fescue | -- | 1.08; (1.02, 1.15); 0.02 | 1.07; (1.01, 1.15); 0.03 | |
| Dog | -- | 1.06; (1.01, 1.12); 0.03 | 1.05; (1.00, 1.11); 0.08 | |
| Penicillium | -- | 1.14; (1.06, 1.23); 0.001 | 1.13; (1.05, 1.22); 0.002 |
All the informative allergen wheal areas (p<0.2) at ages two and three were included into a single regression model. Alternaria at age two (OR 1.11; 95% CI, 1.03-1.21; p=0.01), maple at age three (OR 1.05; 95% CI, 1.00-1.10; p=0.047), fescue at age three (OR 1.08; 95% CI, 1.02-1.15; p=0.03), dog at age three (OR 1.06; 95% CI, 1.01-1.12; p=0.08) and Penicillium at age three (OR 1.14; 95% CI, 1.06-1.23; p=0.002) wheal areas were significantly associated with AR at age four (right column of Table 3).
We further investigated ages two and three to determine if the wheal area association differed among the strata of children who were aeroallergen sensitized but asymptomatic, or among those with early AR (Tables E2 and E3, respectively). Among aeroallergen sensitized but asymptomatic children, elm wheal area at age three met criteria (p < .2) for further analysis (p=0.1; Table E2), this effect was significant in a multivariable model (aOR 1.08; 95% CI, 1.03-1.21; p=0.01; Table E4). Among early AR children, Alternaria wheal area at age two met criteria (p < .2) for further analysis (p=0.002; Table E3), and this gained significance in a multivariable model (aOR 1.19; 95% CI, 1.05-1.48; p=0.03; Table E4).
Comparison of aeroallergen-specific wheal area models to summed aeroallergen wheal area models
After showing that specific allergen wheal areas were associated with AR, we asked if the summed wheal area of all aeroallergens better fit the data than allergen-specific wheal areas. In the final age two multivariable model, the sum of all 15 aeroallergen wheal areas was substituted for the timothy and Alternaria wheal areas. The allergen-specific wheal area model was significantly superior to the sum of all wheal areas of 15 aeroallergens (difference in log likelihood ratio of -36.8). Similarly at age three, the sum of all 15 aeroallergen wheal areas was substituted for maple, fescue, dog and Penicillium. Again, the allergen-specific wheal area model was significantly superior to the total of all aeroallergen wheal areas (difference in log likelihood ratio of -50.7).
Sum of allergen wheal areas in final regression model (informative allergen wheal areas)
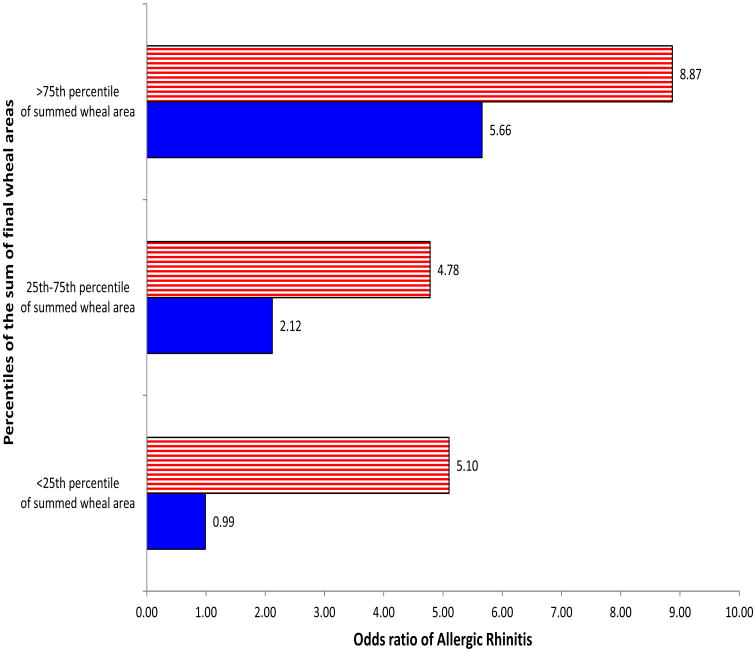
To further evaluate these findings, the sums of informative aeroallergen wheal areas at each age were examined (Figure 4). At ages two and three, the sum of informative allergen wheal areas showed a significant linear relationship to AR at age four (OR 1.06; 95% CI, 1.03-1.10; p<0.001) and (OR 1.07; 95% CI, 1.02-1.14; p=0.02), respectively. At age two, we compared the interquartile range group and the high 75th percentile group to SPT negative children and a significant association was seen with AR (OR 2.12; 95% CI, 1.07-4.07; p=0.001) and (OR 5.66; 95% CI, 2.12-15.92; p<0.001), respectively (Figure 4). Similarly at age three the 25th percentile, interquartile range and 75th percentile children had elevated risk of AR compared to SPT negative children (OR 5.10; 95% CI, 2.47-10.48; p<0.0001), (OR 4.78; 95% CI, 2.65-8.60; p<0.0001) and (OR 8.87; 95% CI, 4.04-20.26; p<0.0001), respectively.
Figure 4. Odds ratios of developing allergic rhinitis at age four by percentiles of sum of informative allergen wheal areas* from multivariable regression model at ages one, two and three.

*Allergen wheal areas that were included in the final multivariable model for the corresponding year were:
Year 2: Timothy, Alternaria.
Year 3: Maple, Fescue, Dog, Penicillium.
Disussion
This study of high-risk CCAAPS children showed AR prevalence increased annually to 21.9% by age four. This is the first investigation of AR of high-risk children at age four. This percentage is higher than previously reported in population cohorts. The Isle of Wight general population-based study found the prevalence of rhinitis at age four to be 17.9%. Another UK population-based study at age five reported a prevalence of 12.1% for current rhinoconjunctivitis, a phenotype suggestive of AR.32,33 Unlike our study, neither of the latter studies included SPT positivity as part of the AR case definition. Our AR case definition better reflects the operational clinical criteria used by physicians to diagnose symptomatic children attending allergy clinics.
Aeroallergen sensitization at age two is a risk factor for AR, but not all sensitized children at age two have the same risk for AR at age four. Our results show that increases in the size of the wheal area of timothy grass allergen (Phleum pratense) and Alternaria at age two was significantly associated with AR. These results indicate that every one mm2 increase in the wheal area of timothy and Alternaria increased the risk of AR by 6% and 7%, respectively. Regression models utilizing only timothy and Alternaria wheal areas were superior to models that totaled the wheal area of all aeroallergens. After summation of these wheal areas, a significant linear relationship persisted, and those with largest timothy-Alternaria wheal areas (i.e. greater than 75th percentile) had an almost six-fold increased risk of AR compared to children that were timothy-Alternaria negative. At age three for every one mm2 increase in the wheal area of maple, fescue grass, dog and Penicillium, there was a 4%, 6%, 8%, and 14% increase in risk of AR at age four, respectively. Similar to age two, a significant linear relationship was observed when the wheal areas of these four allergens at age three were summed to AR at age four. Also similar to age two, regression models at age three containing only maple, fescue, dog and Penicillium wheal areas were superior to models that used the summed wheal area of all aeroallergens tested. In comparison to SPT negative children, the smallest (25th percentile) summed wheal area, the interquartile range of the summed area, and the largest summed wheal area all conferred significantly increased risk of AR at age four. This suggests that the magnitude of wheal reactions of select aeroallergens at specific ages best models the odds of developing AR. The stratified analysis suggests we are not simply detecting early AR, but also increased risk among atopic, asymptomatic children.
Allergen skin tests, or in vitro specific IgE tests, are essential for clinical evaluation of AR as well as for defining the AR phenotype in epidemiologic studies. A recent study showed that case definitions of “hay fever” and “current rhinitis” based on questionnaires cannot be accurately measured or determined.34 Recently, house dust mite sensitization at ages one and two was associated with wheezing in 12 year old children.35 The observed odds ratios were large and had wide confidence intervals, likely the result of dichotomizing the SPT result rather than measuring the wheal area. In our study, the continuous wheal area rather than the dichotomous response provided greater statistical power.36,37 Studies using binary SPT results may imprecisely estimate the effect of the SPT.
We showed that DEP exposure at age one was positively associated with aeroallergen sensitization at ages two and three. At first glance, DEP exposure was inversely associated with AR, suggesting a protective effect on AR (upward diagonal red arrow in Figure 3A-C), however this contradicts the postulated adjuvant effects of DEP.38,39 After adjusting for other covariates, DEP at age 1 showed a borderline association with aeroallergen sensitization at ages 2 and 3, though this was not statistically significant (P=0.07). Aeroallergen sensitization was then associated with AR (downward diagonal black arrow in Figure 3B-C). Directed acyclic graphs identify pathways to explain the relationship between independent and dependent variables.40 We are the first to utilize and take advantage of directed acyclic graphs ability to identify pathways to explain the relationship between DEP exposure, aeroallergen sensitization and AR. Although we detected a trend that DEP exposure enhanced the likelihood of aeroallergen sensitization at age two (p=0.07) and three (p=0.07), a retrospective power calculation demonstrated that we were underpowered (only powered at 46% at age 2 and 57% at age 3) to detect an association. Our finding provides epidemiologic support for work by Diaz-Sanchez and others suggesting that DEP exposure may enhance sensitization to aeroallergens.38 Future studies investigating the relationship between DEP exposure and other allergic disorders (ex. allergic asthma, allergic eczema) with a positive SPT as a component of the definitions should account for aeroallergen sensitization as an intermediate phenotype.
The large sample size and use of aeroallergen wheal areas are strengths of this study. Wheal area allows calculation of the mean diameter, which could be utilized in clinical practice beginning as early as age two. The reduced error with the wheal area compared to binary SPT values may improve model precision. If confirmed, this method could allow for more accurate risk prediction at each year for future AR. Poor parental recall limited our ability to study seasonal AR symptoms. However, our AR definition has been used in other similar birth cohorts.2 Another potential limitation was that parental sensitization was not analyzed, but previous studies and unpublished data from our group showed no association to child sensitization.14 Our DEP was an estimate and there maybe error associated with our measurement however our group and other groups have used land-use regression in estimating fine-particulate matter exposure.41,42 As CCAAPS is a high-risk cohort, our findings may not be applicable to the general population.
In summary, in children of symptomatic and aeroallergen sensitized parents, the size of specific aeroallergen wheal areas at years two and three were associated with AR at age four. Regression models using specific aeroallergen wheal areas were superior to models summing the wheal areas of all aeroallergens. The risk of AR was greatest among those children with the largest sum of informative specific aeroallergens (timothy and Alternaria at age two; maple, dog, fescue and Penicillium at age three). These novel findings in this high-risk group could improve identification of early childhood AR. Given the association of AR to asthma and in childhood is mostly allergic there is also the potential to improve asthma risk stratification.43
Supplementary Material
Figure E1- Absolute number of aeroallergen sensitivities among allergic rhinitis children at ages one to four.
Table E1: Holm-Sidak adjusted SPT wheal area at age one, two and three associated with allergic rhinitis (compared to all other phenotypes) at age four. In each year there was at least one subject whose parent refused testing to Milk and Egg. Therefore, the denominator for age one is 595; for age two 589 (for milk) and 588 (for egg); for age three 609 (for milk) and 602 (for egg).
Table E2: Unadjusted association of allergen wheal areas at ages 1, 2, and 3 (stratified to those were aeroallergen sensitized but asymptomatic) with AR at age 4.
Table E3: Unadjusted association of allergen wheal areas at ages 1, 2, and 3 (stratified to those were early AR) with AR at age 4.
Table E4: Stratified adjusted odds ratio of allergen wheal area at age two and three. Stratified analyses were performed for aeroallergen sensitivity associated with AR at age four. First analysis was to among children who were sensitized but not symptomatic. At age two, no aeroallergen wheal area was significant in multivariable model. At age three, elm wheal area size was significantly associated with AR at age four, with covariates including β-glucan, duration of breastfeeding, and season of birth. The second analysis was among children who were sensitized and symptomatic (i.e. had early onset of AR). At age two, alternaria wheal area was significantly associated with AR at age four, with covariates including ethnicity, duration of breastfeeding, and number of children in home at age one. At age three, no aeroallergen was significant in the multivariable model.
Acknowledgments
Supported by: National Institute of Environmental Health Services Grant ES 11170 and ES 10957;
National Institute of Allergy and Infectious Disease AI 60515 T32
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Soni A. Survey MEP. Statistical Brief # 204. Agency for Healthcare Research and Quality; May 21, 2008. Allergic Rhinitis: Trends in Use and Expenditures 2000 and 2005. [Google Scholar]
- 2.Rochat MK, Illi S, Ege MJ, et al. Allergic rhinitis as a predictor for wheezing onset in school-aged children. The Journal of Allergy and Clinical Immunology. 2010 Dec;126(6):1170–1175 e1172. doi: 10.1016/j.jaci.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Settipane RJ, Settipane GA. IgE and the allergy-asthma connection in the 23-year follow-up of Brown University students. Allergy Asthma Proc. 2000 Jul-Aug;21(4):221–225. doi: 10.2500/108854100778248890. [DOI] [PubMed] [Google Scholar]
- 4.Diaz-Sanchez D, Proietti L, Polosa R. Diesel fumes and the rising prevalence of atopy: an urban legend? Curr Allergy Asthma Rep. 2003 Mar;3(2):146–152. doi: 10.1007/s11882-003-0027-4. [DOI] [PubMed] [Google Scholar]
- 5.Gruzieva O, Bellander T, Eneroth K, et al. Traffic-related air pollution and development of allergic sensitization in children during the first 8 years of life. J Allergy Clin Immunol. 2012 Jan;129(1):240–246. doi: 10.1016/j.jaci.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Ryan PH, LeMasters G, Biagini J, et al. Is it traffic type, volume, or distance? Wheezing in infants living near truck and bus traffic. J Allergy Clin Immunol. 2005 Aug;116(2):279–284. doi: 10.1016/j.jaci.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 7.Carr TF, Saltoun CA. Chapter 2: Skin testing in allergy. Allergy Asthma Proc. 2012 May-Jun;33(Suppl 1):S6–8. doi: 10.2500/aap.2012.33.3532. [DOI] [PubMed] [Google Scholar]
- 8.Bernstein IL, Li JT, Bernstein DI, et al. Allergy diagnostic testing: an updated practice parameter. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2008 Mar;100(3 Suppl 3):S1–148. doi: 10.1016/s1081-1206(10)60305-5. [DOI] [PubMed] [Google Scholar]
- 9.Sporik R, Hill DJ, Hosking CS. Specificity of allergen skin testing in predicting positive open food challenges to milk, egg and peanut in children. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2000 Nov;30(11):1540–1546. doi: 10.1046/j.1365-2222.2000.00928.x. [DOI] [PubMed] [Google Scholar]
- 10.Hill DJ, Hosking CS, Reyes-Benito LV. Reducing the need for food allergen challenges in young children: a comparison of in vitro with in vivo tests. Clin Exp Allergy. 2001 Jul;31(7):1031–1035. doi: 10.1046/j.1365-2222.2001.01113.x. [DOI] [PubMed] [Google Scholar]
- 11.Bodtger U, Poulsen LK, Malling HJ. Asymptomatic skin sensitization to birch predicts later development of birch pollen allergy in adults: a 3-year follow-up study. J Allergy Clin Immunol. 2003 Jan;111(1):149–154. doi: 10.1067/mai.2003.37. [DOI] [PubMed] [Google Scholar]
- 12.Kellberger J, Dressel H, Vogelberg C, et al. Prediction of the incidence and persistence of allergic rhinitis in adolescence: a prospective cohort study. The Journal of Allergy and Clinical Immunology. 2012 Feb;129(2):397–402. 402 e391–393. doi: 10.1016/j.jaci.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 13.Schafer T, Wolke G, Ring J, Wichmann HE, Heinrich J. Allergic sensitization to cat in childhood as major predictor of incident respiratory allergy in young adults. Allergy. 2007 Nov;62(11):1282–1287. doi: 10.1111/j.1398-9995.2007.01453.x. [DOI] [PubMed] [Google Scholar]
- 14.LeMasters GK, Wilson K, Levin L, et al. High prevalence of aeroallergen sensitization among infants of atopic parents. J Pediatr. 2006 Oct;149(4):505–511. doi: 10.1016/j.jpeds.2006.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asher MI, Keil U, Anderson HR, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. 1995 Mar;8(3):483–491. doi: 10.1183/09031936.95.08030483. [DOI] [PubMed] [Google Scholar]
- 16.Braun-Fahrlander C, Wuthrich B, Gassner M, et al. Validation of a rhinitis symptom questionnaire (ISAAC core questions) in a population of Swiss school children visiting the school health services. SCARPOL-team. Swiss Study on Childhood Allergy and Respiratory Symptom with respect to Air Pollution and Climate. International Study of Asthma and Allergies in Childhood. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 1997 May;8(2):75–82. doi: 10.1111/j.1399-3038.1997.tb00147.x. [DOI] [PubMed] [Google Scholar]
- 17.Martuzevicius D, Grinshpun SA, Reponen T, et al. Spatial and temporal variations of PM(2.5) concentration and composition throughout an urban area with high freeway density-the Greater Cincinnati study. Atmos Environ. 2004 Mar;38(8):1091–1105. [Google Scholar]
- 18.Ryan PH, Lemasters GK, Biswas P, et al. A comparison of proximity and land use regression traffic exposure models and wheezing in infants. Environ Health Perspect. 2007 Feb;115(2):278–284. doi: 10.1289/ehp.9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sahu M, Hu S, Ryan PH, et al. Chemical compositions and source identification of PM. aerosols for estimation of a diesel source surrogate. The Science of the total environment. 2011 Jun 1;409(13):2642–2651. doi: 10.1016/j.scitotenv.2011.03.032. [DOI] [PubMed] [Google Scholar]
- 20.Hu S, McDonald R, Martuzevicius D, et al. UNMIX modeling of ambient PM2.5 near an interstate highway in Cincinnati, OH, USA. Atmospheric Environment. 2006;40(Supplement 2):378–395. doi: 10.1016/j.atmosenv.2006.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryan PH, Lemasters GK, Levin L, et al. A land-use regression model for estimating microenvironmental diesel exposure given multiple addresses from birth through childhood. Sci Total Environ. 2008 Oct 1;404(1):139–147. doi: 10.1016/j.scitotenv.2008.05.051. [DOI] [PubMed] [Google Scholar]
- 22.Cho SH, Reponen T, Bernstein DI, et al. The effect of home characteristics on dust antigen concentrations and loads in homes. Sci Total Environ. 2006 Dec 1;371(1-3):31–43. doi: 10.1016/j.scitotenv.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biagini JM, LeMasters GK, Ryan PH, et al. Environmental risk factors of rhinitis in early infancy. Pediatr Allergy Immunol. 2006 Jun;17(4):278–284. doi: 10.1111/j.1399-3038.2006.00386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biagini Myers JM, Hershey GK, Deka R, et al. Asking the Right Questions to Ascertain Early Childhood Secondhand Smoke Exposures. The Journal of Pediatrics. 2012 doi: 10.1016/j.jpeds.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campo P, Kalra HK, Levin L, et al. Influence of dog ownership and high endotoxin on wheezing and atopy during infancy. J Allergy Clin Immunol. 2006 Dec;118(6):1271–1278. doi: 10.1016/j.jaci.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Codispoti CD, Levin L, LeMasters GK, et al. Breast-feeding, aeroallergen sensitization, and environmental exposures during infancy are determinants of childhood allergic rhinitis. The Journal of allergy and clinical immunology. 2010 May;125(5):1054–1060 e1051. doi: 10.1016/j.jaci.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eliopoulos C, Klein J, Phan MK, et al. Hair concentrations of nicotine and cotinine in women and their newborn infants. Jama. 1994 Feb 23;271(8):621–623. [PubMed] [Google Scholar]
- 28.Sucharew H, Ryan PH, Bernstein D, et al. Exposure to traffic exhaust and night cough during early childhood: the CCAAPS birth cohort. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2010 Mar;21(2 Pt 1):253–259. doi: 10.1111/j.1399-3038.2009.00952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brunst KJ, Ryan PH, Lockey JE, et al. Unraveling the relationship between aeroallergen sensitization, gender, second-hand smoke exposure, and impaired lung function. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2012 Aug;23(5):479–487. doi: 10.1111/j.1399-3038.2012.01292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harrell J, Frank E. Regression modeling strategies: with applincation to linear models, logistic regression, and survival analysis. New York: Springer-Verlag New York Inc.; 2001. [Google Scholar]
- 31.Goodenough AE, Hart AG, Stafford R. Regression with empirical variable selection: description of a new method and application to ecological datasets. PLoS One. 2012;7(3):e34338. doi: 10.1371/journal.pone.0034338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marinho S, Simpson A, Lowe L, Kissen P, Murray C, Custovic A. Rhinoconjunctivitis in 5-year-old children: a population-based birth cohort study. Allergy. 2007 Apr;62(4):385–393. doi: 10.1111/j.1398-9995.2006.01294.x. [DOI] [PubMed] [Google Scholar]
- 33.Marinho S, Simpson A, Soderstrom L, Woodcock A, Ahlstedt S, Custovic A. Quantification of atopy and the probability of rhinitis in preschool children: a population-based birth cohort study. Allergy. 2007 Dec;62(12):1379–1386. doi: 10.1111/j.1398-9995.2007.01502.x. [DOI] [PubMed] [Google Scholar]
- 34.Hoppin JA, Jaramillo R, Salo P, Sandler DP, London SJ, Zeldin DC. Questionnaire predictors of atopy in a US population sample: findings from the National Health and Nutrition Examination Survey, 2005-2006. American journal of epidemiology. 2011 Mar 1;173(5):544–552. doi: 10.1093/aje/kwq392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lodge CJ, Lowe AJ, Gurrin LC, et al. House dust mite sensitization in toddlers predicts current wheeze at age 12 years. The Journal of Allergy and Clinical Immunology. 2011 Oct;128(4):782–788 e789. doi: 10.1016/j.jaci.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 36.Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med. 2006 Jan 15;25(1):127–141. doi: 10.1002/sim.2331. [DOI] [PubMed] [Google Scholar]
- 37.Naggara O, Raymond J, Guilbert F, Roy D, Weill A, Altman DG. Analysis by categorizing or dichotomizing continuous variables is inadvisable: an example from the natural history of unruptured aneurysms. AJNR Am J Neuroradiol. 2011 Mar;32(3):437–440. doi: 10.3174/ajnr.A2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diaz-Sanchez D, Garcia MP, Wang M, Jyrala M, Saxon A. Nasal challenge with diesel exhaust particles can induce sensitization to a neoallergen in the human mucosa. The Journal of Allergy and Clinical Immunology. 1999 Dec;104(6):1183–1188. doi: 10.1016/s0091-6749(99)70011-4. [DOI] [PubMed] [Google Scholar]
- 39.Diaz-Sanchez D, Tsien A, Fleming J, Saxon A. Combined diesel exhaust particulate and ragweed allergen challenge markedly enhances human in vivo nasal ragweed-specific IgE and skews cytokine production to a T helper cell 2-type pattern. J Immunol. 1997 Mar 1;158(5):2406–2413. [PubMed] [Google Scholar]
- 40.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 3rd. Philadelphia, PA: Lippincott Williams and Wilkins; 2008. [Google Scholar]
- 41.Newman NC, Ryan P, Lemasters G, et al. Traffic-related air pollution exposure in the first year of life and behavioral scores at 7 years of age. Environ Health Perspect. 2013 Jun;121(6):731–736. doi: 10.1289/ehp.1205555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beelen R, Raaschou-Nielsen O, Stafoggia M, et al. Effects of long-term exposure to air pollution on natural-cause mortality: an analysis of 22 European cohorts within the multicentre ESCAPE project. Lancet. 2014 Mar 1;383(9919):785–795. doi: 10.1016/S0140-6736(13)62158-3. [DOI] [PubMed] [Google Scholar]
- 43.Jackson DJ, Gangnon RE, Evans MD, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. American journal of respiratory and critical care medicine. 2008 Oct 1;178(7):667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure E1- Absolute number of aeroallergen sensitivities among allergic rhinitis children at ages one to four.
Table E1: Holm-Sidak adjusted SPT wheal area at age one, two and three associated with allergic rhinitis (compared to all other phenotypes) at age four. In each year there was at least one subject whose parent refused testing to Milk and Egg. Therefore, the denominator for age one is 595; for age two 589 (for milk) and 588 (for egg); for age three 609 (for milk) and 602 (for egg).
Table E2: Unadjusted association of allergen wheal areas at ages 1, 2, and 3 (stratified to those were aeroallergen sensitized but asymptomatic) with AR at age 4.
Table E3: Unadjusted association of allergen wheal areas at ages 1, 2, and 3 (stratified to those were early AR) with AR at age 4.
Table E4: Stratified adjusted odds ratio of allergen wheal area at age two and three. Stratified analyses were performed for aeroallergen sensitivity associated with AR at age four. First analysis was to among children who were sensitized but not symptomatic. At age two, no aeroallergen wheal area was significant in multivariable model. At age three, elm wheal area size was significantly associated with AR at age four, with covariates including β-glucan, duration of breastfeeding, and season of birth. The second analysis was among children who were sensitized and symptomatic (i.e. had early onset of AR). At age two, alternaria wheal area was significantly associated with AR at age four, with covariates including ethnicity, duration of breastfeeding, and number of children in home at age one. At age three, no aeroallergen was significant in the multivariable model.