Abstract
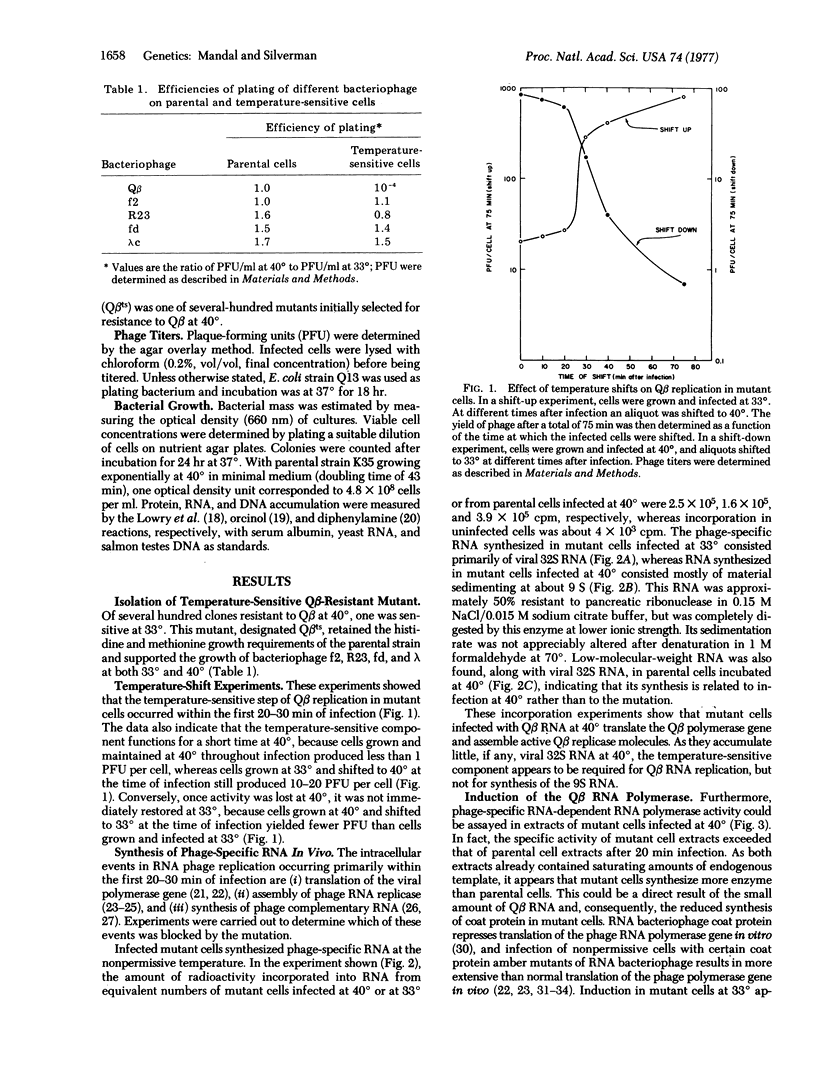
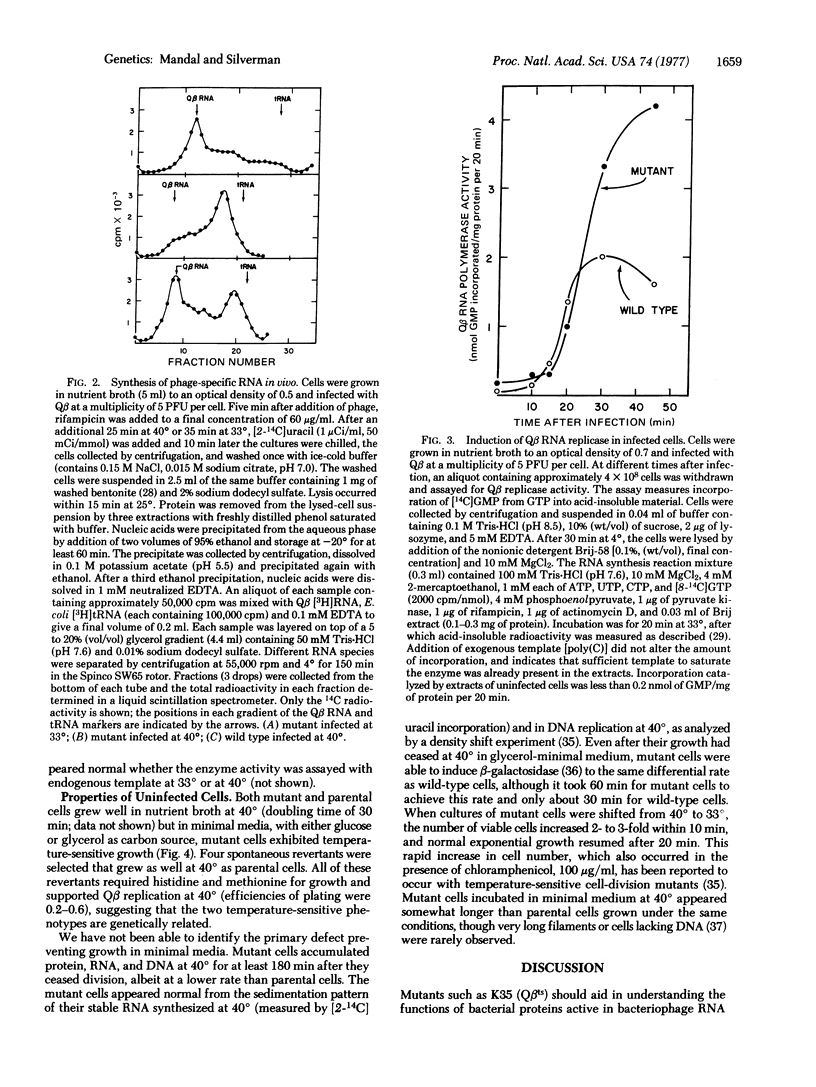
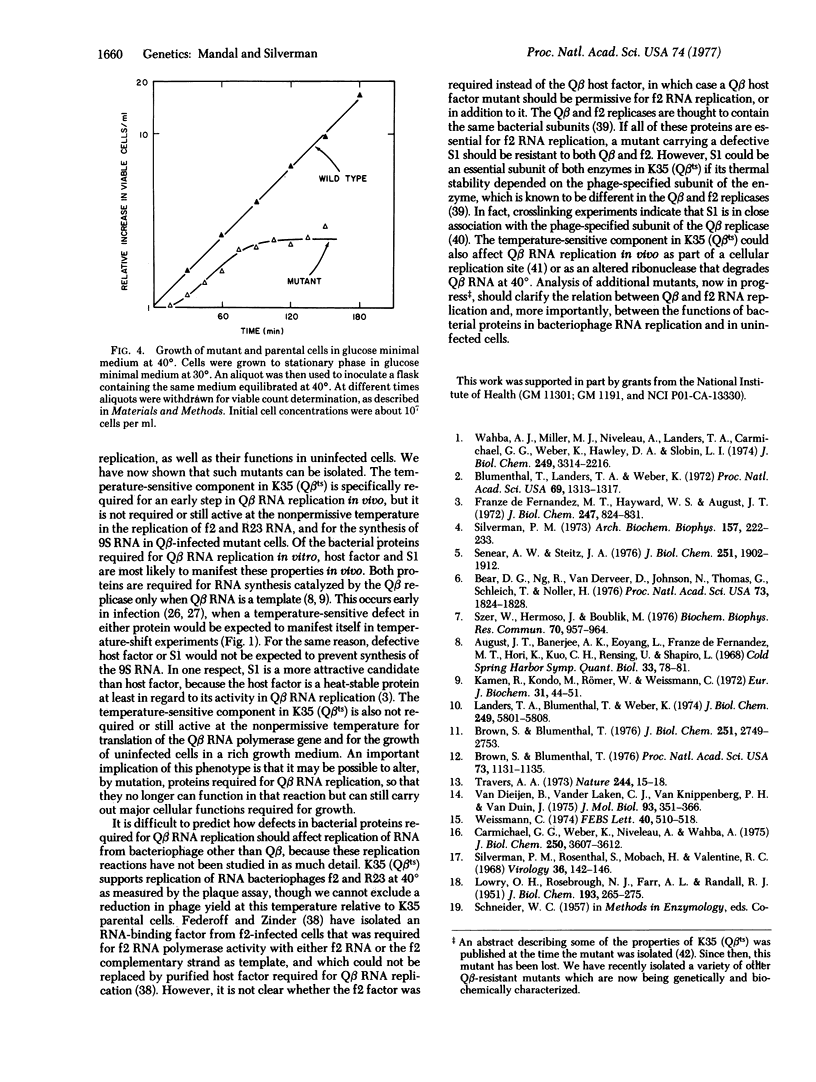
We report the isolation of E. coli mutant capable of supporting replication of bacteriophage Qbeta at 33 degrees, but not at 40 degrees. Coliphages f2, R23, fd, and yamma formed plaques on mutant cells at both temperatures. Temperature-shift experiments showed that bacteriophage Q beta replication was blocked in the mutant within the first 20-30 min of infection. The defect did not prevent translation of the Qbeta polymerase gene or assembly of catalytically active Qbeta replicase molecules. In fact, mutant cells infected at 40 degrees hyperinduced replicase active both in vivo and in vitro. However, zone sedimentation of the in vivo RNA product showed it to consist of partially double-stranded material sedimenting at 9 S, with little or no viral 32S RNA. The 9S RNA was also found, along with a predominant peak of 32S RNA in parental cells infected at 40 degrees, but not in cells infected at 33 degrees. It thus appears that the temperature-sensitive component is required for viral RNA replication, but not for other RNA synthesis catalyzed by the replicase. Uninfected mutant cells grew normally at 40 degrees in nutrient broth, but not in glucose- or glycerol-minimal media. Revertants selected for their abillity to grow in minimal medium at 40 degrees also supported bacteriophage Qbeta replication at 40 degrees.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bear D. G., Ng R., Van Derveer D., Johnson N. P., Thomas G., Schleich T., Noller H. F. Alteration of polynucleotide secondary structure by ribosomal protein S1. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1824–1828. doi: 10.1073/pnas.73.6.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeter M. A., Libonati M., Viñuela E., Weissmann C. Replication of viral ribonucleic acid. X. Turnover of virus-specific double-stranded ribonucleic acid during replication of phage MS2 in Escherichia coli. J Biol Chem. 1966 Oct 25;241(20):4750–4757. [PubMed] [Google Scholar]
- Blumenthal T., Landers T. A., Weber K. Bacteriophage Q replicase contains the protein biosynthesis elongation factors EF Tu and EF Ts. Proc Natl Acad Sci U S A. 1972 May;69(5):1313–1317. doi: 10.1073/pnas.69.5.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S., Blumenthal T. Function and structure in ribonucleic acid phage Qbeta ribonucleic acid replicase. Effect of inhibitors of EF-Tu on ribonucleic acid synthesis and renaturation of active enzyme. J Biol Chem. 1976 May 10;251(9):2749–2753. [PubMed] [Google Scholar]
- Brown S., Blumenthal T. Reconstitution of Qbeta RNA replicase from a covalently bonded elongation factor Tu-Ts complex. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1131–1135. doi: 10.1073/pnas.73.4.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael G. G., Weber K., Niveleau A., Wahba A. J. The host factor required for RNA phage Qbeta RNA replication in vitro. Intracellular location, quantitation, and purification by polyadenylate-cellulose chromatography. J Biol Chem. 1975 May 25;250(10):3607–3612. [PubMed] [Google Scholar]
- Eikhom T. S. Isolation of free minus strands from Qbeta-infected Escherichia coli. J Mol Biol. 1975 Mar 25;93(1):99–109. doi: 10.1016/0022-2836(75)90363-0. [DOI] [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H., SINGER B., TSUGITA A. Purification of viral RNA by means of bentonite. Virology. 1961 May;14:54–58. doi: 10.1016/0042-6822(61)90131-3. [DOI] [PubMed] [Google Scholar]
- Fedoroff N. V., Zinder N. D. Factor requirement of the bacteriophage f2 replicase. Nat New Biol. 1973 Jan 24;241(108):105–108. doi: 10.1038/newbio241105a0. [DOI] [PubMed] [Google Scholar]
- Fedoroff N. V., Zinder N. D. Structure of the poly(G) polymerase component of the bacteriophage f2 replicase. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1838–1843. doi: 10.1073/pnas.68.8.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franze de Fernandez M. T., Hayward W. S., August J. T. Bacterial proteins required for replication of phage Q ribonucleic acid. Pruification and properties of host factor I, a ribonucleic acid-binding protein. J Biol Chem. 1972 Feb 10;247(3):824–831. [PubMed] [Google Scholar]
- Haywood A. M. Two classes of membrane binding of replicative RNA of bacteriophage MS2. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2381–2385. doi: 10.1073/pnas.70.8.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y., Ryter A., Jacob F. Thermosensitive mutants of E. coli affected in the processes of DNA synthesis and cellular division. Cold Spring Harb Symp Quant Biol. 1968;33:677–693. doi: 10.1101/sqb.1968.033.01.077. [DOI] [PubMed] [Google Scholar]
- Kamen R. Characterization of the subunits of Q-beta replicase. Nature. 1970 Nov 7;228(5271):527–533. doi: 10.1038/228527a0. [DOI] [PubMed] [Google Scholar]
- Kamen R., Kondo M., Römer W., Weissmann C. Reconstitution of Q replicase lacking subunit with protein-synthesis-interference factor i. Eur J Biochem. 1972 Nov 21;31(1):44–51. doi: 10.1111/j.1432-1033.1972.tb02498.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Landers T. A., Blumenthal T., Weber K. Function and structure in ribonucleic acid phage Q beta ribonucleic acid replicase. The roles of the different subunits in transcription of synthetic templates. J Biol Chem. 1974 Sep 25;249(18):5801–5808. [PubMed] [Google Scholar]
- Lodish H. F., Zinder N. D. Mutants of the bacteriophage f2. 8. Control mechanisms for phage-specific syntheses. J Mol Biol. 1966 Aug;19(2):333–348. doi: 10.1016/s0022-2836(66)80008-6. [DOI] [PubMed] [Google Scholar]
- MASTER R. W. POSSIBLE SYNTHESIS OF POLYRIBONUCLEOTIDES OF KNOWN BASE-TRIPLET SEQUENCES. Nature. 1965 Apr 3;206:93–93. doi: 10.1038/206093b0. [DOI] [PubMed] [Google Scholar]
- Nathans D., Oeschger M. P., Polmar S. K., Eggen K. Regulation of protein synthesis directed by coliphage MS2 RNA. I. Phage protein and RNA synthesis in cells infected with suppressible mutants. J Mol Biol. 1969 Jan;39(2):279–292. doi: 10.1016/0022-2836(69)90317-9. [DOI] [PubMed] [Google Scholar]
- PIECHAUD M. La coloration sans hydrolyse du noyau des bactéries. Ann Inst Pasteur (Paris) 1954 Jun;86(6):787–793. [PubMed] [Google Scholar]
- Senear A. W., Steitz J. A. Site-specific interaction of Qbeta host factor and ribosomal protein S1 with Qbeta and R17 bacteriophage RNAs. J Biol Chem. 1976 Apr 10;251(7):1902–1912. [PubMed] [Google Scholar]
- Silverman P. M. Replication of RNA viruses: specific binding of the Q RNA polymerase to Q RNA. Arch Biochem Biophys. 1973 Jul;157(1):222–233. doi: 10.1016/0003-9861(73)90408-6. [DOI] [PubMed] [Google Scholar]
- Silverman P. M., Rosenthal S., Mobach H., Valentine R. C. Two new classes of F-pili mutants of Escherichia coli resistant to infection by the male specific bacteriophage F2. Virology. 1968 Sep;36(1):142–146. doi: 10.1016/0042-6822(68)90125-6. [DOI] [PubMed] [Google Scholar]
- Stavis R. L., August J. T. The biochemistry of RNA bacteriophage replication. Annu Rev Biochem. 1970;39:527–560. doi: 10.1146/annurev.bi.39.070170.002523. [DOI] [PubMed] [Google Scholar]
- Szer W., Hermoso J. M., Boublik M. Destabilization of the secondary structure of RNA by ribosomal protein S1 from Escherichia coli. Biochem Biophys Res Commun. 1976 Jun 7;70(3):957–964. doi: 10.1016/0006-291x(76)90685-9. [DOI] [PubMed] [Google Scholar]
- Travers A. Control of ribosomal RNA synthesis in vitro. Nature. 1973 Jul 6;244(5410):15–18. doi: 10.1038/244015a0. [DOI] [PubMed] [Google Scholar]
- Van Dieijen G., Van Der Laken C. J., Van Knippenberg P. H., Van Duin J. Function of Escherichia coli ribosomal protein S1 in translation of natural and synthetic messenger RNA. J Mol Biol. 1975 Apr 15;93(3):351–366. doi: 10.1016/0022-2836(75)90282-x. [DOI] [PubMed] [Google Scholar]
- Viñuela E., Algranati I. D., Feix G., Garwes D., Weissmann C., Ochoa S. Virus-specific proteins in Escherichia coli infected with some amber mutants of phage MS2. Biochim Biophys Acta. 1968 Feb 26;155(2):558–565. doi: 10.1016/0005-2787(68)90199-8. [DOI] [PubMed] [Google Scholar]
- Viñuela E., Algranati I. D., Ochoa S. Synthesis of virus-specific proteins in Escherichia coli infected with the RNA bacteriophage MS2. Eur J Biochem. 1967 Mar;1(1):3–11. doi: 10.1007/978-3-662-25813-2_2. [DOI] [PubMed] [Google Scholar]
- Wahba A. J., Miller M. J., Niveleau A., Landers T. A., Carmichael G. G., Weber K., Hawley D. A., Slobin L. I. Subunit I of G beta replicase and 30 S ribosomal protein S1 of Escherichia coli. Evidence for the identity of the two proteins. J Biol Chem. 1974 May 25;249(10):3314–3316. [PubMed] [Google Scholar]
- Watanabe M., Watanabe H., August J. T. Replication of RNA bacteriophage R23. I. Quantitative aspects of phage RNA and protein synthesis. J Mol Biol. 1968 Apr 14;33(1):1–20. doi: 10.1016/0022-2836(68)90277-5. [DOI] [PubMed] [Google Scholar]
- Young R. A., Blumenthal T. Phage Q-beta ribonucleic acid replicase. Subunit relationships determined by intramolecular cross-linking. J Biol Chem. 1975 Mar 10;250(5):1829–1832. [PubMed] [Google Scholar]


