Abstract
Knowing the extent to which nonhumans and humans share mechanisms for metacognition will advance our understanding of cognitive evolution and will improve selection of model systems for biomedical research. Some nonhuman species avoid difficult cognitive tests, seek information when ignorant, or otherwise behave in ways consistent with metacognition. There is agreement that some nonhuman animals “succeed” in these metacognitive tasks, but little consensus about the cognitive mechanisms underlying performance. In one paradigm, rhesus monkeys visually searched for hidden food when ignorant of the location of the food, but acted immediately when knowledgeable. This result has been interpreted as evidence that monkeys introspectively monitored their memory to adaptively control information seeking. However, convincing alternative hypotheses have been advanced that might also account for the adaptive pattern of visual searching. We evaluated seven hypotheses using a computerized task in which monkeys chose either to take memory tests immediately or to see the answer again before proceeding to the test. We found no evidence to support the hypotheses of behavioral cue association, rote response learning, expectancy violation, response competition, generalized search strategy, or postural mediation. In contrast, we repeatedly found evidence to support the memory monitoring hypothesis. Monkeys chose to see the answer when memory was poor, either from natural variation or experimental manipulation. We found limited evidence that monkeys also monitored the fluency of memory access. Overall, the evidence indicates that rhesus monkeys can use memory strength as a discriminative cue for information seeking, consistent with introspective monitoring of explicit memory.
Keywords: metacognition, information seeking, explicit memory, memory monitoring, tubes
You have likely had the experience of reading something, realizing that you have no memory for the last paragraph, and then having to re-read the passage. This example demonstrates an ability often called metacognition (e.g., Fernandez-Duque, et al., 2000; Koriat, 1997; Kuhn, 2000). Metacognition refers to the ability to monitor one’s cognitive processes, the ability to take action to control those processes, and general knowledge about how one’s cognitive processes function (Flavell, 1979; Nelson, 1996).
One of the most intriguing questions about metacognition is the extent to which species other than humans also monitor and control their cognitive processes. The answer to this question will inform longstanding issues about the evolution of consciousness, self-awareness, theory-of-mind, subjective experience, and about human uniqueness (for example, see Metcalfe & Son, 2012; Smith, et al., 2014 for good discussions of these issues). Such difficult issues may currently be intractable, but comparative psychologists have been remarkably productive in identifying instances of adaptive behavior in tasks designed to test metacognitive ability. Investigations have found that some species reliably “succeed” in these metacognitive tests (e.g., Beran, et al., 2013; Call, 2010; Call & Carpenter, 2001; Foote & Crystal, 2007; Goto & Watanabe, 2012; Hampton, 2001; Hampton, et al., 2004; Kornell, et al., 2007; Shields, et al., 2005; Shields, et al., 1997; Smith, et al., 2006; Smith, et al., 1995; Smith, et al., 1998; Templer & Hampton, 2012), but also evidence that the ability to behave adaptively on these tests may not be uniformly distributed among species (e.g., Basile, et al., 2009; Beran & Smith, 2011; Brauer, et al., 2004; Inman & Shettleworth, 1999; Marsh, 2014; Roberts, et al., 2009; Sole, et al., 2003).
As apparent demonstrations of metacognitive competence in nonhumans have accumulated, particularly in rhesus monkeys (Macaca mulatta), research has begun to shift away from asking whether particular species “have metacognition” and toward attempts to identify the mechanisms underlying purportedly metacognitive performances in nonhumans (Basile & Hampton, 2014; Hampton, 2009b; Jozefowiez, et al., 2009; Kornell, 2013; Le Pelley, 2012; Smith, et al., 2014). Currently, there is disagreement about what these mechanisms might be, and this controversy has engaged researchers in philosophy, mathematical modelling, human cognitive psychology, and comparative psychology (Browne, 2003; Carruthers, 2008; Crystal & Foote, 2011; Hampton, 2009b; Jozefowiez, et al., 2009; Kornell, 2013; Le Pelley, 2012; Metcalfe, 2003; Smith, et al., 2014). Understanding the mechanisms underlying nonhuman metacognition may ultimately help us understand those underlying human metacognition by encouraging forms of experimental design, theoretical approaches, and skepticism that are especially prominent in the animal learning and comparative cognition communities (e.g., Crystal & Foote, 2011; Hampton, 2009b; Jozefowiez, et al., 2009; Kornell, 2013; Le Pelley, 2012, 2014). The ability to test these mechanisms in nonhumans will also allow for investigations of their neural bases using highly-informative neuroscientific methods not commonly used in human subjects (e.g., Middlebrooks & Sommer, 2012). Thus, identifying the mechanisms that support metacognitive behavior in nonhumans will have broad implications.
We argue that researchers will make the most progress in identifying the mechanisms underlying metacognition in nonhumans by adopting a discriminative approach (Basile & Hampton, 2014). Subjects perform a primary cognitive task, such as a delayed match-to-sample memory test, in which cognitive state varies across trials. In a secondary task, subjects are given the opportunity to behave differently depending on their cognitive state in the primary task, for example by choosing to see the answer again or to proceed directly to the memory test. We infer that subjects can monitor their cognitive state in the primary task if they can use it as a discriminative cue in the secondary task, for example, by selectively choosing to see the answer when ignorant. In the above example, potential discriminative cues might include memory strength, the retention interval, prior reinforcement history of specific stimuli, and retrieval fluency. By systematically manipulating these putative discriminative cues, we can determine the extent to which each controls behavior.
Metamemory is a form of metacognition in which the cognitive process subject to monitoring and control is memory (Koriat, 1996; Nelson & Narens, 1990; Pannu & Kaszniak, 2005; Rhodes & Tauber, 2011; Schwartz, 1994). Metamemory paradigms are useful in distinguishing between explicit and implicit memory systems because explicit memories are, by definition, those memories of which subjects are aware and which they can therefore monitor. As indicated by Narens, Graf, and Nelson (1996), “The metacognitive component … of deciding whether a possible answer occurred during study is generally acknowledged … as being an important distinguishing factor between implicit and explicit memory, with its presence indicating explicit memory.” In contrast, “By definition, then, implicit memory is, at least in some cases, memory without metamemory” (Dunlosky & Bjork, 2008). Thus, some studies of metamemory in nonhumans have been motivated by the need to establish methods to distinguish between explicit and implicit memory in nonverbal species (Hampton, 2001, 2003, 2005, 2006, 2009b, 2011; Hampton & Hampstead, 2006; Hampton, et al., 2004; Templer & Hampton, 2012). The distinction between explicit and implicit memory is central to taxonomies of human memory (Squire, et al., 1993; Squire & Wixted, 2011), but behavioral measures for making this distinction in nonverbal species are poorly developed. Because explicit types of memory, such as episodic and semantic memory, are accessible to monitoring but implicit types of memory are not, metamemory experiments provide one behavioral approach to making this critical distinction. Note that we use “explicit memory” to mean only that the memory can be monitored by the subject. Although monitoring reflects a functional parallel to human explicit memory, it does not by itself evince other qualities often associated with explicit memory in humans, such as consciousness, self-awareness, or theory of mind. By taking this approach, we do not mean to deny that implicit cognitive processes influence metacognitive behavior, such as strategy selection (e.g., Cary & Reder, 2002), or that metacognitive decisions can be based on monitorable states that originally arose from implicit processes (e.g., Koriat & Levy-Sadot, 2000).
Comparative research in metamemory suggests that the distinction between explicit and implicit memories may also apply in at least some other species (Beran, et al., 2013; Call, 2010; Call & Carpenter, 2001; Fujita, 2009; Goto & Watanabe, 2012; Hampton, 2001; Hampton, et al., 2004; Kornell, et al., 2007; Smith, et al., 1998; Templer & Hampton, 2012; Washburn, et al., 2010). Humans distinguish between remembering and forgetting using a variety of cues, ranging from direct introspective memory access to inference of memory status based on retention interval, retrieval fluency, or ease of study (Koriat, 1996; Kornell, 2013). One of the major focuses of research on human metamemory has been to identify the extent to which different cues control reports of remembering or forgetting (Besner & Son, 2007; Schwartz, 1994). We take a similar approach here with rhesus monkey subjects by evaluating seven potential controlling cues in one well-established metacognitive task. This approach accommodates the possibility that monkeys’ metacognitive behavior is controlled by multiple cues and provides a baseline for comparing which cues control behavior in different purportedly metacognitive tasks.
A variety of paradigms have yielded converging evidence for metamemory in nonhuman primates. For example, in “decline” paradigms, subjects selectively declined individual memory tests when their memory was weak (e.g., Hampton, 2001; Smith, et al., 1998; Templer & Hampton, 2012; Washburn, et al., 2010). Similarly, in “betting” paradigms, subjects selectively chose a high-risk-high-gain reinforcement contingency following correct answers but a low-risk-low-gain strategy following incorrect answers (e.g., Kornell, et al., 2007).
The paradigm that may most closely approximate how nonhuman animals might use memory monitoring in natural environments is the “information seeking” paradigm (Call & Carpenter, 2001). Researchers hide food in one of several horizontal tubes, such that subjects cannot see the food directly without bending down and carefully peering into the tubes. Researchers manipulate the status of subjects’ memory for the location of the food by allowing the subject to observe where the food is hidden on some trials (Seen trials) and blocking the subject’s view of the baiting process on other trials (Unseen trials). Subjects can either immediately attempt to recover the food, or delay food retrieval while visually searching the possible hiding locations. Choosing incorrectly results in no food and a delay until the next opportunity to get food. Searching requires additional effort and takes time. Subjects that can monitor the status of memory can maximize rewards and minimize effort by selecting a location immediately on Seen trials, when they know the location of the food, and making the extra effort to search before choosing on Unseen trials. When initially ignorant, human children, great apes (Call, 2010; Call & Carpenter, 2001), and rhesus monkeys (Hampton, et al., 2004) reliably look down the tubes and find the food before making a choice. In contrast, they immediately select a tube when knowledgeable. In a related paradigm, great apes selectively searched containers for hidden food when ignorant of the type of food, but requested the hidden food by “naming” it using a lexigram keyboard when knowledgeable (Beran, et al., 2013). Tests of information seeking in other species show much less reliable discrimination between seen and unseen trials. Capuchin monkeys showed equivocal evidence for selective information seeking in both manual and computerized tasks (Basile, et al., 2009; Beran & Smith, 2011), and dogs and pigeons have repeatedly failed to search for more information when ignorant in similar tests (Brauer, et al., 2004; McMahon, et al., 2010; Roberts, et al., 2009). These results suggest species differences in the extent to which nonhumans can use their memory state as a discriminative cue for information seeking.
Selective looking in the standard manual foraging tasks may be explained in ways that do not invoke introspective monitoring of explicit memory states (Hampton, 2009b). In the last few years, researchers have proposed many viable explanations that posit that selective looking in these manual foraging tasks might be controlled by cues or processes that are public or non-mnemonic. It is difficult to evaluate these explanations across studies because different species and different experimental designs might favor different strategies. Finding evidence against a particular account in one species or with one methodology does not invalidate that same account in another species or even the same species when tested with a different methodology. Therefore, it may be most informative to evaluate a large set of hypothetical mechanisms within a single experimental design and species. Here we describe tests evaluating seven competing hypotheses for selective information seeking in rhesus monkeys: memory monitoring, behavioral cue associations, rote response learning, expectancy violation, response competition, generalized search strategy, and postural mediation. Each of these hypotheses will be introduced as they are operationalized in the experiments below. Although most of these hypotheses are mutually exclusive, it is possible that behavior in this information-seeking paradigm is controlled by multiple mechanisms. Thus, the goal of this work was to test each proposed hypothesis and identify the cue or cues that most strongly control behavior.
EXPERIMENT 1
In Experiment 1, we evaluated whether monkeys in our computerized information-seeking task selectively used the information response when initially ignorant, as has been found in manual versions of this task. Our methods were based on the manual tubes task that has been used to find strong evidence for selective visual searching in rhesus monkeys (Hampton, et al., 2004), but equivocal evidence in capuchin monkeys (Basile, et al., 2009). Monkeys earned food by remembering in which of four locations on a touchscreen a red dot had appeared. At test, they chose either to select one of those locations immediately (the take-the-test option), or to see the location of the red dot again (the see-the-answer option). On half of critical trials, the red dot was presented during the study phase (Seen trials) and on the other half, the red dot was omitted during study (Unseen trials). If this computerized task measures the same cognitive capacities as previous manual versions, monkeys should choose to see the answer more often when ignorant on Unseen trials, than when knowledgeable on Seen trials. In addition, choosing to see the answer should change the monkey’s memory state, allowing him to choose the correct option during the memory test, when he would otherwise have guessed.
Subjects
Eleven adult male rhesus macaques (Macaca mulatta; mean age at start of testing = 7.3 years) were tested in their home cages. Each subject was fed full food rations daily and had unrestricted access to water at all times. The majority of subjects were pair-housed except during testing, during which a divider separated the monkeys into two compartments such that they could still access their partner but not their partner’s computer screen. All subjects had prior experience with a touchscreen-based decline-test paradigm similar to that reported by Hampton (2001). In addition, five of the eleven subjects had previously participated in a manual decline-test paradigm (Templer & Hampton, 2012). All subjects also had prior experience with basic touchscreen-based cognitive tasks including psychophysical discriminations, perceptual classifications, and delayed matching of images. Subjects had no experience with information-seeking paradigms similar to that reported here. Except where noted, all experiments included the same subjects.
Apparatus
We tested subjects six days a week using portable testing rigs equipped with a 15” color LCD touch screen (3M, St. Paul, MN; and ELO, Milpitas, CA) stereo speakers, and two automatic food dispensers (Med Associates Inc., St. Albans, VT) which dispensed nutritionally-balanced food pellets into cups below the screen. The testing rigs were attached to the front of the cages for seven hours a day, allowing subjects to test at their discretion. All experiments use the same apparatus.
Stimuli
A red dot (150 × 150 pixels) marked the correct location, four blue squares (225 × 225 pixels) marked the four possible response locations, a blue arrow (200 × 200 pixels) activated the take-the-test response, and a green recycle symbol (200 × 200 pixels) activated the see-the-answer response (Figure 1). Except where noted, all experiments use the same stimuli.
Figure 1.
Trial progression of the computerized information-seeking task as presented during the final test phase. Monkeys initiated each trial by touching a green square (top). At study, monkeys either saw a blank screen (Unseen trials) or a sample in one of four screen locations (Seen trials). After an unfilled memory delay, four dim blue boxes appeared to mark the possible locations, but were not yet responsive to touches. Two symbols also appeared to mark the metacognitive response options. Touching the green recycle symbol extinguished all symbols, caused the red dot to appear as in the sample phase of Seen trials, and then proceeded to the memory test. Touching the blue arrow extinguished the metacognitive symbols and initiated the memory test. At the memory test, four bright blue boxes marked the possible locations. If the monkey touched the box marking the correct location, the box disappeared, revealing the red dot, and touching the red dot produced a food reward and a positive audio reinforcement (“Excellent!”). If the monkey touched one of the three incorrect boxes, that box disappeared, revealing nothing, followed by a negative audio stimulus (“D’oh!”) and an unfilled two-second timeout. Trials were separated by an unfilled 10-second interval. To prevent accidental choices, all responses required two consecutive touches to the same response location. Touches to the correct location during the memory delay aborted the trial.
Procedure
In the final task, we required monkeys to accurately remember the location of the red dot over a short memory interval of one second, and to use the take-the-test and see-the-answer responses (Figure 1). Prior to final testing, monkeys completed a series of training phases to ensure that memory was accurate, and that subjects’ overall use of the see-the-answer and take-the-test responses was away from floor and ceiling levels.
Phase 1: Introduction of the Dot
In Phase 1, we trained monkeys to associate the red dot with a food reward. Monkeys initiated each trial by touching the green start box. Next, the red dot appeared in one of the four target locations. Each location was correct twice in each block of eight randomized trials. Touching the red dot resulted in a food pellet and a positive audio reinforcer. In this phase and all subsequent phases, trials were separated by an unfilled 10-second inter-trial interval during which the screen was black. Each response required two consecutive touches to prevent registration of accidental touches as choices. We trained monkeys for one 40-trial session.
Phase 2: Introduction of the Blue Squares and Delay
In Phase 2, we trained monkeys to remember the location of the red dot over a one-second memory delay. Trials proceeded as in Phase 1 with the following changes. At study, the red dot appeared for 200 ms, after which there was an unfilled memory delay during which the screen was dark gray. Monkeys were not required to touch the red dot during this study phase, and touches to the correct location during the memory delay aborted the trial. At test, the blue squares appeared in the four response locations. If monkeys touched the correct location twice, the selected blue square disappeared, revealing the red dot. Touching the red dot twice resulted in a food reward. If the monkey touched one of the three incorrect blue boxes, that box disappeared, revealing nothing, followed by a negative audio stimulus and two-second timeout during which the screen was black. Each session consisted of 40 trials. The delay between study and test was initially 100ms. For each session in which the monkey was above 80% correct, the delay increased by 100 ms. Monkeys progressed to Phase 3 when they completed a session above 80% correct at a 1000 ms delay.
Phase 3: Introduction to the Metacognitive Responses
In Phase 3, we introduced two response buttons before the final memory test: a blue arrow and a green recycling symbol (hereafter: metacognitive responses, for ease of reference). Selecting the blue arrow initiated the memory test whereas selecting the green recycling symbol re-presented the red dot in its study location (hereafter: the take-the-test and see-the-answer responses, respectively). Trials proceeded in the same fashion as in Phase 2 with the following changes. At test, the four response locations were dimmed, which indicated that they could not be selected, and one of the two metacognitive buttons was presented concurrently with the dimmed response locations. Half of the trials were Forced Answer Trials, on which the see-the-answer response was presented in the lower left corner, in the absence of the take-the-test button (Figure 2). When selected, the see-the-answer response and the dim response locations extinguished, the sample was re-presented for 200 ms, and then the bright blue response locations appeared for the test phase. The other half of the trials were Forced Test Trials, on which the take-the-test button was presented in the lower right corner, in the absence of the see-the-answer button (Figure 2). When selected, the dimmed response locations immediately brightened for the test phase. For both trial types, brightening of the response locations to their normal blue color indicated that they could now be selected, at which point the test phase proceeded as in Phase 2. Each session consisted of 40 trials: 20 Forced Answer, and 20 Forced Test Trials. We trained monkeys until they reached 80% correct on Forced Test Trials for each of four consecutive sessions.
Figure 2.
Diagram of screen displays used to test metamemory in monkeys. Each panel represents an example display as monkeys saw it. The first row represents the study phases of trials, and the second row represents what monkeys saw at the time of metacognitive choice. If monkeys chose to see the answer, they then saw what is depicted in the third row, followed by a test like that depicted in the fourth row. If monkeys chose to proceed to the test, what is depicted in the third row was skipped and they proceeded directly to the test depicted in the fourth row. From left to right, columns represent displays from the concurrent metacognitive judgment of memory for a spatial location in Experiments 1 and 2, the Forced Answer and Forced Test trials as used in the spatial experiments, the missing-location probe trials in Experiment 3, the high-value probe trials in Experiment 4, the prospective metacognitive judgment of memory for a spatial location in Experiment 5, and the prospective metacognitive judgment of memory for an image in Experiment 6. For Forced Answer and Forced Test trials in Experiments 5 and 6, the single available metacognitive option was presented alone on the screen. The progression of trials in all experiments followed the pattern shown in Figure 1.
If monkeys did not meet criterion within 100 sessions, we introduced remedial training. Sessions consisted of 20 Forced Answer Trials and 20 Forced Test Trials, as well as 40 Baseline trials on which the metacognitive buttons were absent, as in Phase 2. Again, we trained monkeys until they reached 80% correct on Forced Test Trials for each of four consecutive sessions.
Testing Phase
In the Testing Phase, we presented the two metacognitive options together for the first time. The main task proceeded as in Phase 3, with the following changes. To test how monkeys used the two metacognitive options as a function of the status of their memory, they now received Seen trials and Unseen trials, on which both metacognitive options were available. On Seen trials, the red dot was presented, as in Phase 3. On Unseen trials the red dot was omitted. Each session consisted of 20 Seen Trials, 20 Unseen Trials, 20 Forced Answer Trials, and 20 Forced Test Trials. Forced Answer and Forced Test trials always occurred with a presented sample. To avoid floor and ceiling effects in the use of the metacognitive buttons, we titrated the number of touches required to select each button after each session. If monkeys selected one of the metacognitive options on over 75% of all Seen and Unseen trials combined, that option was designated as the preferred option and the number of touches required to select that response was increased by two touches. If monkeys selected the previously-preferred metacognitive option on fewer than 25% of trials, the cost of that option decreased by two touches. If monkeys selected the previously-preferred option on fewer than 5% of trials, the cost of that option was reset to two touches, the other option was now designated as the preferred option, and the cost of the newly-preferred option increased by two touches. We analyzed the first two consecutive sessions in which each response was used on between 25–75% of Seen and Unseen trials combined, and on which accuracy on Forced Test trials was above 80%.
Data Analysis
Response time measures are median latencies in ms. Proportions were arcsine transformed prior to statistical analysis to better approximate normality (Aron & Aron, 1999). Performance was compared between conditions using two-tailed paired t-tests, and to chance using one-sample t-tests, with = .05. Effect sizes are presented as partial eta-squared for ANOVA, r-squared for correlations, and Cohen’s d for paired or one-sample t-tests as appropriate (Hurlburt, 2006). For conditions in which not all monkeys contributed data (e.g., if a monkey never sought information in a particular condition), paired tests excluded those monkeys. Except where noted, all experiments use these statistical analyses.
Results and Discussion
Monkeys reached criteria for Phase 1 in an average of 1 session (SD=0), Phase 2 in 40.4 sessions (SD=56.3), and Phase 3 in 143.5 sessions (SD=111.85). Six of the eleven monkeys required the remedial training in Phase 3. In the testing phase, monkeys reached criteria in an average of 31.2 sessions (SD=27.4). All monkeys preferred the see-the-answer response, and consequently that response required an average of 16.9 touches to activate (SD=8.5).
Monkeys chose to see the answer significantly less often on Seen trials than on Unseen trials (Figure 3; t(10) = 6.90, p < .001, d = 2.08). On Unseen trials, monkeys were significantly more accurate on trials on which they chose to see the answer than on trials on which they did not (Figure 4; t(6) = 29.39, p < .001, d = 11.11). Selective use of the see-the-answer response when ignorant replicates the main finding of the manual tubes task (Hampton, et al., 2004), suggesting that this task assesses the same underlying cognitive capacity. In Experiments 2–6, we attempted to specify what that cognitive capacity might be, by evaluating seven hypotheses for selective use of the see-the-answer response.
Figure 3.
Proportion (SEM) of Seen and Unseen trials on which monkeys chose to see the answer in Experiment 1.
Figure 4.
Proportion correct (SEM) on Seen and Unseen trials in Experiment 1. Light bars represent trials on which the monkeys chose to see the answer. Dark bars represent trials on which monkeys chose to take the test. The horizontal dashed line represents accuracy expected by chance.
EXPERIMENT 2: MANIPULATING DELAY TO EVALUATE MEMORY MONITORING
In Experiment 1, monkeys chose to see the answer more frequently on trials during which they had not seen the sample than on trials during which they had seen the sample. This suggests that the presence or absence of memory for the target location was the discriminative cue controlling use of the see-the-answer response. In Experiment 2, we evaluated the memory monitoring hypothesis, which posits that the discriminative cue controlling the see-the-answer response results from introspective monitoring of memory. In Experiment 1 we manipulated memory by either presenting or omitting the to-be-remembered sample. Because monkeys were trained with this manipulation, it is possible they learned some non-mnemonic cue specific to those trial types. If they were using their memory state as the discriminative cue, then choice to see the answer should generalize to novel and unexpected variations of memory. Thus, in Experiment 2, we introduced a different manipulation of memory: variation of memory delay length. We re-established baseline performance at the trained delay of one second and then presented monkeys with a single probe session that contained delays of 0.5, 1, 2, and 4 seconds. Prior to Experiment 2, monkeys had never experienced delays longer than one second in this task. Because memory is expected to decay over time (e.g., Basile & Hampton, 2011; Miles, 1971), if monkeys based their choice to see the answer on the strength of their memory, then increasing the delay should weaken memory and cause them to choose to see the answer more often.
Procedure
Phase 1: Reestablishing baseline
Phase 1 was identical to the Testing Phase from Experiment 1, and monkeys were required to meet the same criteria to progress to the probe session. This ensured that the monkeys were still choosing to see the answer at appropriate levels and that accuracy was still high on seen trials. Except where noted, all subsequent experiments reestablished baseline performance in this same way immediately prior to the probe session.
Probe Session
We ran one probe session consisting of 80 Probe Seen Trials (20 at each of four delays), 80 Unseen Trials, 80 Forced Answer Trials, and 80 Probe Forced Test Trials (20 at each of four delays), for a total of 320 Trials. On probe trials, the memory delay was either 0.5, 1, 2, or 4 seconds. Seen trials and Forced Test trials measured information seeking and accuracy, respectively. Unseen trials and Forced Answer trials were run at the trained delay of 1 second and were included to balance all trial types, as changing the overall proportion of trial types would likely change the monkeys’ bias in selecting between the two metacognitive options. Aside from the different delay lengths, all trials were run as described in the final phase of Experiment 1.
Results and Discussion
During reestablishment of baseline, monkeys replicated the main finding from Experiment 1. Monkeys choose to see the answer significantly more often on Unseen trials than on Seen trials (Unseen = .81, Seen = .24; t(10) = 8.45, p < .001, d = 2.55). On Unseen trials, monkeys were significantly more accurate on trials on which they chose to see the answer than on trials on which they did not (see answer = .98, take test = .22; t(8) = 8.75, p < .001, d = 2.92). All monkeys preferred the see-the-answer response, and consequently that response required an average of 17.6 touches to activate (SD=7.7).
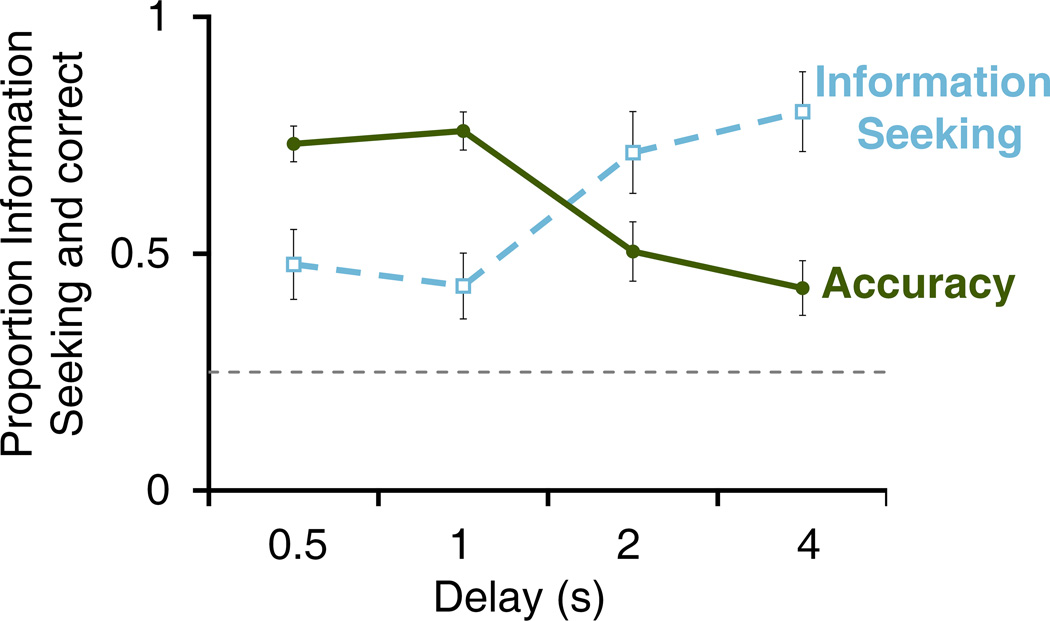
Delay length significantly affected both accuracy and the frequency with which monkeys chose to see the answer before responding (Figure 5; accuracy: F(3,30) = 11.84, p < .001, partial η2 = .542; see-the-answer: F(3,30) = 19.01, p < .001, partial η2 = .655). Accuracy was lower at longer delays (1s vs 4s: t(10) = 3.72, p = .004, d = 1.66) and the probability of choosing to see the answer was higher at longer delays (1s vs 4s: t(10) = 4.92, p = .001, d = 1.48). The shorter half-second delay did not change either accuracy (0.5s vs 1s: t(10) = 0.88, p = .401) or choice to see the answer (0.5s vs 1s: t(10) = 0.94, p = .372). It is unlikely that the monkeys had acquired enough reinforcement history with the long-delay trials for associative learning to produce the observed change in behavior, as each novel delay was only experienced in 20 Seen and 20 Forced Test trials intermixed with 200 normal trials. To better test this hypothesis, we analyzed just the first 10 Seen and first 10 Forced Test trials at each novel delay from the first half of the session, and found the same pattern of results (accuracy: 1s mean = .83, 4s mean = .41, t(10) = 4.70, p = .001, d = 1.95; choice to see the answer: 1s mean = .28, 4s mean = .8, t(10) = 5.15, p < .001, d = 1.55). Thus, delay length likely had an immediate effect on both accuracy and the frequency with which monkeys chose to see the answer. Monkeys likely chose to see the answer more often as a direct result of weaker memory, suggesting that memory strength is the discriminative cue controlling the see-the-answer response.
Figure 5.
Accuracy and choice to see the answer as a function of memory delay in Experiment 2. The solid green line depicts the proportion (SEM) of Forced Test trials at each delay on which the monkey was correct. The dashed blue line depicts the proportion (SEM) of Seen trials at each delay on which the monkey chose to see the answer. The horizontal dashed gray line represents the proportion correct expected by chance.
Individual monkeys varied in the extent to which they were affected by the manipulation of memory delay. If choosing to see the answer was controlled by the memory strength, changes in the proportion of see-the-answer responses should correlate with changes in accuracy. To test this hypothesis, we computed both the change in accuracy and the change in proportion of see-the-answer responses between the trained delay and the longest delay for each monkey. There was a significant negative correlation (Figure 6; r(9) = −.743, p = .009). Monkeys that showed a greater decrease in accuracy due to the increased delay also showed a greater increase in how often they chose to see the answer. The direct relation between an individual monkey’s change in choice to see the answer and that same monkey’s change in accuracy further suggests that choice to see the answer was driven by the effect of delay on that monkey’s memory strength.
Figure 6.
Individual change in accuracy as a function of individual change in choice to see the answer in Experiment 2. Each dot represents one monkey. Individual changes are the values at the longest delay subtracted from the values at the trained one-second delay. For example, the most upper-left point represents a monkey for which proportion correct was 1 at the 1s delay and .25 at the 4s delay, and for which proportion of trials on which he chose to see the answer was .3 at the 1s delay and 1 at the 4s delay.
EXPERIMENT 3: MANIPULATING AVAILABILITY OF TEST OPTIONS TO EVALUATE ROTE RESPONDING
In Experiments 1 and 2, monkeys sought information on Unseen trials, took the test on Seen trials, and choice to see the answer immediately tracked accuracy across novel memory delays. One possible explanation for this pattern of performance is that the monkeys had learned by rote which metacognitive button produced the highest reinforcement rate after having seen, or not seen, the red dot. According to this rote response hypothesis, monkeys had not learned to use the see-the-answer response because they needed information, but had learned by rote over many trials that they could receive the most rewards by pressing the blue arrow after seeing the red dot and the green recycle symbol after seeing a blank screen. To evaluate the rote response hypothesis, we put the response predicted by rote responding in competition with the response predicted by information seeking. On probe trials, the red dot was presented as usual, but the correct location was absent at test. If the monkeys had learned to seek information when necessary, they should choose to see the answer on these trials. However, if the monkeys had learned by rote to select the blue arrow after having seen the red dot, they would continue to do so at normal rates.
Procedure
Probe Phase
In the Probe Phase, the blue box that signified the correct location at test was absent on some trials. On these missing location trials, after the monkey had seen the sample and the memory delay, only the three incorrect blue squares appeared on the screen, along with the two metacognitive options (Figure 2). The red dot was randomly assigned to one of the three remaining locations, as if the red dot had moved during the delay. Thus, the chance rate on these probe trials was 33%. If monkeys chose to see the answer, the red dot appeared in its new location for 200 ms, and selecting that location was correct at test. If subjects elected to take the test, the response location where the dot had appeared during study remained absent and the monkey had to choose from among the remaining three locations.
We ran one session of 40 Probe Seen Trials (20 with all four locations and 20 with the correct location missing), 40 Unseen Trials, 40 Forced Answer Trials, and 40 Probe Forced Test Trials (20 with all four locations and 20 with the correct location missing), for a total of 160 trials. Aside from the missing-location probe trials, trials were as described in the final phase of Experiment 1.
Results and Discussion
During reestablishment of baseline, monkeys replicated the main finding from Experiment 1. Monkeys choose to see the answer significantly more often on Unseen trials when they had not seen the sample, than on Seen trials when they had seen the sample (Unseen = .84, Seen = .19; t(10) = 11.48, p < .001, d = 3.46). On Unseen trials, monkeys were significantly more accurate on trials on which they chose to see the answer than on trials on which they did not (see answer = .99, take test = .16; t(8) = 13.71, p < .001, d = 4.47). All monkeys preferred the see-the-answer response, and consequently that response required an average of 21.6 touches to activate (SD=8.7).
On probe trials, monkeys chose to see the answer significantly more often when the correct location was absent than when it was present (Figure 7; t(10) = 2.57, p = .028, d = 1.51). For comparison, mean proportion choice of the see-the-answer response on the intermixed Unseen trials was .95 (SEM = .02). This manipulation likely affected choice to see the answer without affecting memory. On forced-test trials, accuracy was low when the correct location was absent (mean = .28, SEM = .02; chance = .33), but remained high when the correct location was present (mean = .85, SEM = .03; chance = .25). This indicates that monkeys still remembered the sample’s location, but could not report it because the correct response location was absent. It is unlikely that the monkeys had acquired enough reinforcement history with the missing-location trials for associative learning to produce the observed change in behavior, as they only experienced a single probe session with 20 Seen and 20 Forced Test missing-location trials intermixed with 120 normal trials. To better test this claim, we analyzed the first half of the session separately, which contained only 10 Seen and 10 Forced Test missing-location trials, and found the same pattern of results (choice to see the answer: location present mean = .39, location absent mean = .54, t(10) = 2.33, p = .042, d = 0.70). Thus, monkeys likely responded immediately to the need for additional information and acted appropriately.
Figure 7.
Choice to see the answer as a function of whether the correct location was present at test in Experiment 3. Bars depict the proportion (SEM) of Seen trials on which the monkey chose to see the answer.
Unlike the case with the manipulation of delay length, we did not expect a correlation between change in the proportion of see-the-answer responses and change in accuracy in this experiment. With a longer delay, the need for additional information should vary among individuals because some monkeys will remember the correct location better than others. However, with the correct location missing, the need for additional information should not vary among individuals, as no monkey could know the new correct location any more or less than any other monkey. This was supported by our post-hoc correlational analysis, which found that the change in choice to see the answer did not correlate with the change in accuracy (r(9) = .317, p = .341). Regardless, the fact that monkeys chose to see the answer more often when necessary suggests that they were not responding by rote.
EXPERIMENT 4: MANIPULATING REWARD VALUE AS A TEST OF MEMORY MONITORING AND EXPECTANCY VIOLATION
In Experiments 1–3, monkeys chose to see the sample more often when it had not been presented, when it had been forgotten due to an extended memory delay, and when it had been presented but the correct test option was unavailable. One possible explanation for these findings is that monkeys were using the see-the-answer response whenever their expectations had been violated. The expectancy violation hypothesis posits that monkeys become accustomed to proceeding directly to the test, and that they use the see-the-answer option whenever there is a deviation from the expected course of events, as is the case on most probe trials (Templer & Hampton, 2012). In all previous manipulations in this study, monkeys choose to take the test most often when trials were presented normally, and choose to see the answer when presented with infrequent probe trials in which some aspect of the procedure was unusual. Thus, it is possible that monkeys had learned to select the see-the-answer response whenever an aspect of the trial violated expectations. Because previous probe trials were both unusual and resulted in weak memory, we cannot use those tests to reliably distinguish between control of metacognitive responding by memory strength and control by violation of expectation.
To test the expectancy violation hypothesis, we introduced probe trials with a high-value “hidden” stimulus. Remembering the location of the high-value stimulus was rewarded with four food pellets instead of the single pellet associated with standard trials. High-value stimuli are likely to be remembered better than normal-value stimuli, possibly because they are better encoded due to their high salience. Importantly, memory monitoring and expectancy violation make competing predictions about the direction of change in the see-the-answer response on unusual high-value trials. The memory monitoring hypothesis predicts that stronger memory on the unusual high-value trials will result in fewer choices to see the answer. In contrast, the expectancy violation hypothesis predicts that the unusual nature of the infrequent high-value probes will result in more choices to see the answer (also see the General Discussion where we compare our use of high-value trials with that of Call, 2010). Here, we present data from two runs of the high-reward manipulation: first with the trained delay of 1s, and then with a longer delay of 4s which we predicted would allow us more sensitivity to measure an increase in accuracy and a decrease in use of the see-the-answer response.
Stimuli
In addition to normal stimuli used in previous experiments, a yellow star (150 × 150 pixels) was the to-be-remembered stimulus for trials with increased reward.
Procedure
Phase 1: Stimulus value training
In this phase, we trained the monkeys that the yellow star signaled high-reward trials, and to accurately remember the location of the star. At the beginning and end of each session, we gave monkeys six preference trials in which both the red dot and yellow star stimuli appeared simultaneously on the screen in two of the four randomly-selected test locations. Selecting the red dot yielded a one-pellet reward whereas selecting the yellow start yielded a four-pellet reward. Trials in the rest of these sessions proceeded in the same fashion as Phase 2 of Experiment 1, but on half of the trials, the yellow star was used as the target stimuli instead of the red dot. A correct response in locating the yellow star produced four pellets, instead of one for the locating the red dot. Each session consisted of the 12 preference trials plus 40 memory trials, half with the hidden red dot and half with the hidden yellow star. To progress to Phase 2, monkeys had to accurately remember the location of each type of target on over 80% of memory trials, and had to select the high-reward target on 100% of preference trials, within a single session. The metacognitive responses were not available for either sample type in this phase. Thus, prior to the single probe session, monkeys never experienced the option of using the metacognitive buttons in conjunction with the high-value stimulus. For this experiment, the reestablishment of baseline performance occurred between this value training phase and the single probe session. It was run as described in Experiment 2, with only the normal-value red dot used as the sample.
Probe Phases
In the Probe Phases, the high value yellow star was used as the target stimulus on half of trials (Figure 2). On these high-value trials, correct responses were rewarded with four pellets, instead of one pellet. These two probe sessions were the first time the monkeys received the option to use the metacognitive buttons in response to the high-value stimulus.
We ran one session of 40 Probe Seen Trials (20 with the normal-value red dot target and 20 with the high-value yellow star target), 40 Unseen Trials, 40 Forced Answer Trials, and 40 Probe Forced Test Trials (20 with the normal-value red dot target and 20 with the high-value yellow star target), for a total of 160 trials. Aside from the high-value probe trials, all trials were as described in the final phase of Experiment 1. At the end of the probe session, we ran a six-trial preference test, as described in the Phase 1, to ensure that the monkeys had retained their preference for the high-value target throughout the duration of the session.
We then ran a second probe session that was identical to the first except that that the memory retention interval on all trials was increased from 1s to 4s.
Results and Discussion
Initially, monkeys had no inherent preference for the high-value or low-value targets (one sample t-test, proportion choice of high-value vs 0.5: t(10) = 0.07, p = .946), and all monkeys acquired a 100% preference for the high-value target by the end of Phase 1. During the post-probe session preference test, all monkeys still had a 100% preference for the high-value target.
During reestablishment of baseline, immediately prior to the probe session, monkeys replicated the main finding from Experiment 1. Monkeys chose to see the answer significantly more often when they had not seen the sample, on Unseen trials, than when they had seen the sample, on Seen trials (Unseen = .9, Seen = .15; t(10) = 14.31, p < .001, d = 4.31). On Unseen trials, monkeys were significantly more accurate on trials on which they chose to see the answer than on trials on which they did not (see answer = .99, take test = .15; t(9) = 11.57, p < .001, d = 3.53). All monkeys preferred the see-the-answer response, and consequently that response required an average of 20.2 touches to activate (SD=9.0).
In the 1s delay probe session, the high-value target produced a non-significant trend towards increased accuracy (Figure 8 left; t(10) = 2.11, p = .061) and a significant decrease in choices to see the answer (Figure 8 left; t(10) = 3.85, p = .003, d = 1.38). In the 4s delay probe session, the high-value target significantly increased accuracy (Figure 8 right; t(10) = 4.36, p = .001, d = 1.32) and significantly decreased choices to see the answer (Figure 8 right; t(10) = 4.21, p = .002, d = 1.57). This indicates that monkeys remembered the high-value target better than the normal-value target, and that they consequently choose to see the answer less often. The effect of reward value on choice to see the answer was more pronounced at the 4s memory delay than the 1s memory delay (two-factor repeated-measures ANOVA interaction: F(1,10) = 5.82, p = .037, partial η2 = .368) likely because the longer delay brought baseline accuracy away from ceiling, allowing more sensitivity to measure an increase in accuracy. It is unlikely that the monkeys had acquired enough reinforcement history with using the metacognitive response on high-value trials for associative learning to produce the observed change in behavior, as each probe session only contained 20 Seen and 20 Forced Test high-value trials intermixed with 120 normal trials. To better test this claim, we analyzed the first half of each probe session separately, which contained only 10 Seen and 10 Forced Test high-value trials, and found a similar, though not statistically identical pattern of results (accuracy at 1s delay: low-value mean = .69, high-value mean = .88, t(10) = 3.40, p = .007, d = 1.03; choice to see the answer at 1s delay: low-value mean = .20, high-value mean = .04, t(10) = 3.46, p = .006, d = 1.04; accuracy at 4s delay: low-value mean = .45, high-value mean = .70, t(10) = 2.61, p = .025, d = 0.79; choice to see the answer at 4s delay: low-value mean = .44, high-value mean = .05, t(10) = 3.62, p = .005, d = 1.09). The fact that we found significantly higher accuracy in the first half of the 1s delay trials, whereas this difference only approached significance (p=.061) when the entire session was analyzed, likely reflects an overall effect of reward combined with statistical noise. In any case, finding a significant difference in the first half, but not second half of the probe session would not alter interpretation of these results. Thus, monkeys likely chose to see the answer less often as an immediate response to stronger memory, suggesting that memory strength is the discriminative cue controlling the see-the-answer response.
Figure 8.
Accuracy and choices to see the answer as a function of stimulus value in Experiment 4. The solid green line depicts the proportion (SEM) of Forced Test trials on which the monkey was correct. The dashed blue line depicts the proportion (SEM) of Seen trials on which the monkey chose to see the answer. The horizontal dashed gray line represents the proportion correct expected by chance. The left panel depicts performance at the trained 1s memory delay, whereas the right panel depicts performance at a longer 4s memory delay.
It is unlikely that the monkeys had enough pre-training experience with the high-value stimulus to invalidate its use as a test of expectancy violation. First, monkeys had much more experience with the normal-value stimulus, and their limited experience with the high-value stimulus during pre-training was paralleled by an equal amount of additional experience with the normal-value stimulus. Second, the two probe sessions were the very first time monkeys had the opportunity to use either metacognitive button in response to the high-value stimulus, thus this was a trial progression that the monkeys had not experienced before. Third, expectancy violation could only explain the pattern of results, in which monkeys selected the see-the-answer response more on normal-value trials, if normal-value trials were now more surprising than high-value trials. There is no reason to think normal trials would be surprising at this point in these studies. Thus, the lower proportion of see-the-answer responses during high-value probes is inconsistent with the expectancy violation hypothesis.
If monkeys chose to see the answer less often because their memory was better, we would predict that changes in the two measures in individual subjects would be negatively correlated as they were in Experiment 2. For the 1s delay, a post-hoc correlational analysis of individual responses revealed a significant negative correlation between the change in accuracy and the change in proprotion of choices to see the answer (Figure 9, left; r(9) = −.706, p = .015). Monkeys that showed a greater increase in accuracy for the higher value target also showed a greater decrease in how often they chose to see the answer. For the 4s delay, visual inspection also suggested a negative relationship between the change in proportion of choices to see the answer and the change in accuracy (Figure 9, right), but the post-hoc correlational analysis did not reach statistical significance (r(9) = −.425, p = .195). It is unclear why this was the case, as a significant correlation was found in the same test using a 1s memory delay. It is possible that the low statistical power of this test hid an existing relationship; given eleven subjects and an observed R2 of .181, the observed power of this test was .262. It is also possible that no relationship exists. This cannot be determined with the present data. Regardless, the fact that the high-value probe trials produced better memory and less choice of the see-the-answer response is evidence against the expectancy violation hypothesis and for the memory monitoring hypothesis.
Figure 9.
Individual change in accuracy as a function of the individual change in choice to see the answer in Experiment 4. Each dot represents one monkey. Individual changes are the values with the high-value target subtracted from the values with the normal-value target. The left panel depicts performance at the trained 1s memory delay, whereas the right panel depicts performance at a longer 4s memory delay.
EXPERIMENT 5: USING PROSPECTIVE CHOICE TO EVALUATE RESPONSE COMPETITION AND BEHAVIORAL CUEING
In previous manual versions of this task, it is possible that adaptive choice to see the answer on Unseen trials could have resulted from the fact that the primary memory test and the secondary metacognitive discrimination were presented simultaneously. At test, the sight of the correct answer likely elicits a strong propensity to select that location and retrieve the food. In the absence of this propensity, the rate of all other behaviors increases, including choosing to see the answer. This possibility has been termed response competition. In Experiment 5, we evaluated the response competition hypothesis by requiring the monkeys to make the metacognitive decision prospectively (Hampton, 2001, 2009b). Because response competition posits that the strong propensity to complete the test is produced by the sight of the correct test option, this competition is eliminated if we require monkeys to decide whether to take the test or see the answer before they have seen the test. If monkeys fail to selectively choose to see the answer when they have to decide prospectively, it would support the response competition hypothesis. In contrast, if they still selectively choose to see the answer, it would reduce the viability of the response competition hypothesis.
Experiment 5 also provides a test of the behavioral cue association hypothesis, which posits that subjects may use one of their own publicly observable behaviors elicited by the primary task as the discriminative cue for the see-the-answer response (Hampton, 2009b). We focused on the class of behavior most commonly suggested as a possible cue: vacillation or hesitation (Goto & Watanabe, 2012; Hampton, 2009b; Terrace & Son, 2009). A common observation in comparative cognition research is that correct responses are relatively quick whereas incorrect responses are relatively slow (e.g., Hampton & Hampstead, 2006). This was the case in the primary memory test in Experiment 1, in which median latency to choose one of the four possible target locations during Forced Test trials was significantly longer for incorrect choices than for correct choices (correct = 783 ms; incorrect = 1081 ms; t(10) = 2.73, p = .021, d = 1.03). If the primary memory test and the secondary metacognitive decision are presented simultaneously, subjects could potentially learn to use the see-the-answer response on trials in which the primary memory test elicits vacillation or hesitation. One way to test whether behaviors elicited by the primary memory test are controlling the metacognitive responding to require subjects to make the metacognitive choice before the test is presented. If monkeys continue to selectively choose to see the answer when the primary memory test is not present, then it is unlikely that their choices were controlled by behavioral cues elicited by the primary test. Note that we use this hypothesis as it has been discussed in the past (Hampton, 2009b), to refer to behavioral cues elicited by the primary test. Thus, even if behavior elicited by the primary memory test does not control metacognitive responding, it is possible that behavior elicited by other sources (e.g., the delay of a failed memory search) might control metacognitive responding.
Subjects
One monkey was removed from the laboratory prior to this experiment. Consequently, this experiment and the subsequent experiment include data from 10 subjects.
Procedure
Phase 1: Confirming Accurate Memory under New Conditions
Phase 1 was the identical to Phase 3 from Experiment 1, with the exception that the blue boxes marking the possible test locations were absent when the monkeys had to select one of the metacognitive options. This training phase was conducted to ensure that monkeys could still remember the location of the dot under the new procedures. Monkeys did not have the option to choose between seeking information and taking the test. Each session consisted of 40 Seen trials: 20 Forced Answer, and 20 Forced Test Trials. Monkeys were trained until they reached 80% correct Forced Test Trials for four consecutive sessions.
Testing Phase
The Testing Phase was identical to the Testing Phase from Experiment 1, with the exception that the four blue boxes marking the possible test locations were absent when the monkeys selected one of the metacognitive options (Figure 2).
Results and Discussion
When required to make a metacognitive choice prospectively, monkeys chose to see the answer significantly less often on Seen trials than on Unseen trials (Figure 10; t(9) = 23.00, p < .001, d = 7.27). All monkeys preferred the see-the-answer response, and consequently that response required an average of 12.8 touches to activate (SD=7.6). Unlike in previous experiments, a majority of monkeys (6 of 10) sought information on 100% of Unseen trials, and the remaining monkeys only took the test on an average of 3.25 of the possible 40 Unseen trials. Thus, a statistical test of whether choosing to see the answer improved accuracy on Unseen trials would likely be invalid. Nevertheless, accuracy on Unseen trials following take-the-test response would be expected to be at chance (25%) and accuracy following a choice to see the answer was significantly above chance (mean = 98.8%; t(9) = 28.45, p < .001, d = 19.25). This reproduces the main finding of Experiment 1 under conditions where the monkeys had to choose prospectively, suggesting that the sight of the correct test option did not control choice to see the answer, and reducing the likelihood of the response competition account. Because the test options were not present, the monkeys’ hesitation to select the correct option on the primary memory test could not have served as the discriminative cue, providing evidence against the behavioral cue association hypothesis. However, latency to choose to see the answer was significantly longer than latency to take the test (see answer = 647ms, take test = 425ms; t(9) = 2.86, p = .019, d = 0.93). In the absence of the primary test options, increased latency to choose to see the answer may be due to a failed memory search. This might arise if the discriminative cue controlling behavior were memory status or retrieval fluency. We consider these findings further in the general discussion. Nonetheless, choosing to see the answer selectively under prospective conditions makes the response competition hypothesis and behavioral cue association hypothesis unlikely.
Figure 10.
Proportion (SEM) of Seen and Unseen trials on which monkeys prospectively chose to see the answer in Experiment 5.
EXPERIMENT 6: ASSESSING GENERALIZATION TO IMAGE MEMORY TO EVALUATE POSTURAL MEDIATION
In Experiments 1 – 5, we tested whether monkeys could identify a target location after a delay under the assumption that this paradigm tested memory, and therefore that the secondary task might assess the monitoring of that memory. However, in this and most tests of spatial responding, it is possible that subjects used postural mediation instead of remembering the target location (Hunter, 1913). That is, when presented with a target location, subjects could lean towards or look at that location throughout the retention interval and choose correctly at test by simply selecting the location to which they are already oriented or closest. They could then learn to use the take-the-test response when they are already oriented toward a response location and to use the see-the-answer response when they are not (e.g., if they had become distracted or moved around the cage, or if no sample had been presented to trigger a specific orientation). This could explain performance in most manual tubes tasks (Basile, et al., 2009; Call & Carpenter, 2001; Hampton, et al., 2004) and most of the previous experiments in this paper (see General Discussion). In Experiment 6, we evaluated the postural mediation hypothesis by testing whether selectively choosing to see the answer generalized to a non-spatial test of memory for images. As in Experiment 5, monkeys had to make the metacognitive choice prospectively. If monkeys used memory status as the discriminative cue, they should transfer performance to this new memory task. However, if monkeys used postural mediation as the discriminative cue, they should not transfer performance to this non-spatial task.
Stimuli
Sample stimuli consisted of four visually distinct still-life color photographs (225 × 225 pixels).
Procedure
In the final task, we required monkeys to remember which of four images had most recently been presented, and then to make a prospective metacognitive judgment as in Experiment 5. At study, one of the four images was presented in the center of the screen. Each image served as the sample twice randomly in each block of eight trials. At test, the four images appeared in the locations previously occupied by the blue boxes, in a pseudorandomized manner such that each image appeared equally often in each location. To obtain a reward, monkeys had to select the image they had seen at study. Selecting the see-the-answer response re-presented the sample in the center of the screen (Figure 2). Otherwise, training phases, trial timing, and performance criteria were identical to those in Experiment 1.
Results and Discussion
In the test of image memory, monkeys chose to see the answer significantly less often on Seen trials than on Unseen trials (Figure 11; t(9) = 15.44, p < .001, d = 4.88). Nine of the ten monkeys preferred the see-the-answer response, and consequently that response required an average of 12.4 touches to activate (SD=5.4). The tenth monkey preferred the take-the-test response, and that response required 4 touches to activate. On Unseen trials, monkeys were significantly more accurate on trials on which they chose to see the answer than on trials on which they did not (see answer = .83, take test = .13; t(9) = 9.12, p < .001, d = 2.71). This replicates, with images, the selective choice of the see-the-answer response observed with spatial stimuli in Experiments 1 and 5, indicating that the monkeys did not rely on postural mediation to solve this task. Similar to Experiment 5, monkeys showed a nonsignificant trend towards slower latency when choosing to see the answer than when choosing to take the test (see answer = 500ms, take test = 374ms; t(9) = 2.11, p = .064). Because monkeys made this choice prospectively, this hesitation could not be due to vacillation among possible responses in the primary memory test. Instead, it might indicate a failed memory search, or that choices were controlled by retrieval fluency. We consider these possibilities further in the general discussion.
Figure 11.
Proportion (SEM) of Seen and Unseen trials on which monkeys prospectively chose to see the answer prior to the image-memory test in Experiment 6.
GENERAL DISCUSSION
This evaluation of seven hypotheses advanced to account for apparent metamemory indicates that monkeys introspectively monitored their memory to determine whether or not to view the answer again before proceeding to a memory test. Monkeys chose to see the answer more often when their memory was absent, weak, or indicated an unavailable option. In contrast, they chose to proceed directly to the memory test when their memory was present, strong, and indicated an available response. They used the see-the-answer response selectively and adaptively both concurrently and prospectively, and in tests of both spatial and image memory. In all experiments, when a manipulation affected memory, it immediately affected use of the see-the-answer response. This strongly suggests that some aspect of memory was the cue most strongly controlling behavior in this information-seeking paradigm. If a stimulus, whether internal or external, can serve as a discriminative cue, it follows that the stimulus can be monitored. Because monitorability is generally acknowledged to be a defining characteristic of explicit memory (Dunlosky & Bjork, 2008; Narens, et al., 1996; Squire, et al., 1993; Squire & Wixted, 2011), our results suggest that explicit memory underlies accurate responding in the matching to sample tests used here with monkeys. The presence of explicit memory in monkeys draws a strong parallel with human memory and indicates that explicit memory, and associated monitoring processes, evolved in ancestors shared by many extant primates. How widely distributed these processes are among nonhuman species remains an actively researched question. The present set of hypotheses for apparent metamemory performance, and the procedures described for evaluating them, provide benchmarks for future research in other species and with other tests of memory. Below, we discuss each hypothesis further (Table 1).
Table 1.
Competing hypotheses for selective information-seeking
| Hypothesis | Claim | Method to evaluate |
Experiments | Result |
|---|---|---|---|---|
| Memory monitoring | Use status of memory as a discriminative cue | Manipulate memory | 2 & 4 | + |
| Behavioral cue association | Use own hesitation as a discriminative cue | Prospective choice | 5 & 6 | − |
| Rote response | Inflexibly use most-rewarded response in Seen/Unseen trials | Missing test location | 3 | − |
| Expectancy violation | Choose see-the-answer in unexpected situations | High-value stimulus | 4 | − |
| Response competition | Secondary task directly competes with primary task | Prospective choice | 5 & 6 | − |
| Generalized search strategy | Domain-specific foraging behavior | Abstract computerized test | 1–6 | − |
| Postural mediation | Orient towards to-be-remembered location | Transfer to image memory | 3 & 6 | − |
Evaluation of Potential Discriminative Stimuli for Information Seeking
Our converging set of tests favor the memory monitoring hypothesis. We report both positive and negative evidence supporting this hypothesis. The negative evidence consists of evidence against six competing hypotheses (Table 1). The positive evidence is that the probability of choosing to see the answer correlated with both natural variations and experimental manipulations of memory strength. We manipulated memory in three ways: by making it absent on Unseen trials (Experiments 1–6), by making it weak on trials with longer delay intervals (Experiment 2), and by making it strong on high-value trials (Experiment 4). In each case, monkeys appropriately chose to see the answer more often when necessary than when unnecessary. This shift in behavior was immediate and not the result of new training, as it occurred during single probe sessions that usually only contained 20 Seen probe trials on which monkeys could choose between the two metacognitive responses under each new set of conditions. It also remained when we analyzed only the first half of those probe sessions. Additionally, changes in how often monkeys chose to see the answer correlated with changes in memory across individuals in two experiments (Experiments 2 and 4a), with monkeys that showed greater changes in memory accuracy showing greater changes in the frequency with which they chose to see the answer. Together, these results greatly strengthen the memory monitoring hypothesis, indicating that some aspect of memory was the discriminative cue for information-seeking.
We observed evidence against the behavioral cue association hypothesis. Subjects can emit many behaviors during testing, and it is impossible to exhaustively assess them all for discriminative cue properties. Thus, we assessed the behavior most often suggested as a possible discriminative cue for metacognitive responses: hesitation or vacillation in response to the primary memory task (Goto & Watanabe, 2012; Hampton, 2009b; Terrace & Son, 2009). For hesitation in response to the primary test to function as a discriminative cue, it would have to vary systematically between correct and incorrect memory trials, as was the case in this study. It would also have to be available at the time of metacognitive choice, which was not the case during prospective tests. In Experiments 5 and 6, monkeys made their metacognitive choice prospectively before seeing the memory test, which means that hesitation elicited by the primary memory test could not have controlled behavior. This logic extends to rule out control by all behavioral cues elicited by the primary memory test.
The rote response hypothesis was plausible given that repetitive computerized testing provides ample opportunities for associative learning; however, rote responding could not account for monkeys’ performance on the missing-location trials in Experiment 3. Had the monkeys learned rote responses to Seen and Unseen trials, they should always select the blue arrow, and proceed directly to the test, after seeing the sample. Instead, they appropriately chose to see the answer more often when the correct option was not available at test, even though they had seen the sample. This behavior appears to reflect an additional capacity for flexibility in these tests, in that monkeys chose to see the answer even when they likely did have a memory for the sample. When that memory was incompatible with the available options, the monkeys detected the discrepancy and responded adaptively. Previous information-seeking studies have not directly tested the rote response hypothesis (Basile, et al., 2009; Hampton, et al., 2004). Instead, these studies attempted to limit the possibility that subjects learned a rote response by minimizing the number of training trials. The method presented here represents a step forward and may provide a way for future studies to directly evaluate what subjects have learned by rote.
It is unlikely that monkeys chose to see the answer on probe trials due to expectancy violation. In Experiments 1 through 3, and in most previous studies of metamemory in nonhumans, manipulations of memory have been done through infrequent probe trials designed to decrease memory, such as no-sample probes or long-delay probes. These probe trials are unusual, employing parameters or trial-progressions that differ from those with which the subject was trained. It is possible for subjects to learn that unusual trials are associated with a low likelihood of reward, and thus that the see-the-answer response optimal response on any trial that is unusual. Because these probe trials are both unusual and designed to worsen memory, we cannot usually tell whether increased use of the see-the-answer response is controlled by the probe trials’ effects on memory or the fact that these trials violate the monkeys’ expectations. In Experiment 4, the unusual probe trials increased memory, and subjects consequently choose to see the answer less often, contrary to what we would predict if monkeys had learned to choose the see-the-answer response on unusual trials. Thus, it is unlikely that behavior in this study was controlled by expectancy violation.
Response competition is unlikely to account for performance on this computerized task. In previous information-seeking tasks that used the manual tubes apparatus (Basile, et al., 2009; Call & Carpenter, 2001; Hampton, et al., 2004), choosing to see the answer directly competed with food retrieval. The sight of a baited location increases the propensity to retrieve the food, and this propensity may simply displace other behaviors, including the choice to see the answer. In the absence of this propensity, other behaviors, including choosing to see the answer are correspondingly more likely to occur. Thus, in situations where the primary test and secondary discrimination are presented simultaneously, response competition is a viable explanation for selective responding on the secondary discrimination. In Experiments 5 and 6, monkeys made the decision to see the answer or take the test prior to seeing the test locations in the spatial memory test (Experiment 5) and prior to seeing the test images in the item memory test (Experiment 6). Because the test items for the primary memory test were not present when the monkeys made their choice on the secondary metacognitive test, there was no direct competition between the metacognitive choice and food retrieval. Thus, the probability of choosing to see the answer could not be affected by response competition. This finding can be compared to two reports of information seeking in great apes, which found evidence against the response competition hypothesis by increasing the drive for food retrieval (Call, 2010) or by presenting a hidden desirable food item on every trial (Beran, et al., 2013).
The generalized search strategy hypothesis proposed by other researchers (Kornell, et al., 2007; Marsh & MacDonald, 2012; Marsh, 2014) is unlikely to explain performance in these experiments. The generalized search strategy hypothesis posits that animals appear to selectively seek information in naturalistic foraging tasks because those tasks elicit the same default chain of domain-specific behaviors that most animals use in natural foraging situations: seek food, find food, retrieve food, and eat food. Because this hypothesis posits that the default behaviors are elicited by the similarity of the experimental task to natural foraging, others have suggested that the hypothesis cannot explain performance when those same behaviors are demonstrated in abstract computerized tasks (Kornell, et al., 2007). Because the current computerized experiments do not resemble natural foraging, it is unlikely that they elicited some default chain of behaviors specific to the domain of foraging. Thus, the current findings support a domain-general process of memory monitoring.
Although postural mediation is almost always possible in tests of spatial memory, it is unlikely to explain performance in the current study. The sight of the target at study could potentially cause subjects to lean towards or gaze at the target location, and remain oriented towards that location during the retention interval, eliminating the need to actually remember the location. Subjects might fail to initially orient when the target was not presented, increase orientation with high-value targets, or become distracted and cease orienting during long delays, which would produce response patterns that superficially appear to result from changes in memory but which actually resulted from changes in orientation. If monkeys used postural mediation on the primary memory task, it is possible that orientation towards a location, or lack thereof, was the discriminative cue for the see-the-answer response. However, monkeys showed selective metacognitive responding in the test of image memory in Experiment 6, and postural mediation would not support accurate memory for images. Additionally, using one’s orientation on Seen trials as the cue to select the test-taking response would not produce the results in Experiment 3, as the missing response location probes were all Seen trials and would all have produced the initial orienting response. Together, Experiments 3 and 6 provide evidence against the use of postural mediation.
One intriguing interpretation of the current study is that behavior is controlled in part by retrieval fluency (Benjamin & Bjork, 1996), though the current data do not allow for a full evaluation of this hypothesis. In prospective tests, we observed one instance for which latency was longer before choosing to see the answer than before choosing to take the test (Experiment 5), and another instance for which this difference was a nonsignificant trend (Experiment 6). Previous research using a “decline” paradigm did report that one of two monkeys showed this difference with a prospective test of image memory (Hampton, 2001). It is also worth noting that this latency difference was also numerically present in all concurrent tests, but never reached statistical significance (Experiment 2: see answer = 529ms, take test = 418ms; t(10) = 2.01, p = .073; Experiment 3: see answer = 711ms, take test = 574ms; t(10) = 1.69, p = .121; Experiment 4: see answer = 674ms, take test = 494ms; t(10) = 2.19, p = .053). There are two obvious interpretations for a latency difference. First, behavior may be controlled by memory status and longer latencies prior to selecting the see-the-answer response may represent failed memory searches or increased processing necessary to reject an incorrect memory. When presented with the metacognitive options, monkeys may attempt to access a memory of the correct location or image, choosing to take the test as soon as an accurate memory is accessed. If a memory is not accessed within a certain time window, or the accessed memory is evaluated as incorrect, monkeys choose to see the answer. Second, the latency difference may indicate that metacognitive behavior is under control of retrieval fluency, independent of accuracy. In studies of humans, subjects often infer a high probability of successful performance based on the speed with which a piece of information can be retrieved, independent of whether the retrieved information is correct (Benjamin & Bjork, 1996). Control by retrieval fluency and control by memory status can be dissociated through manipulations such as increasing the semantic familiarity of a possible answer, which can increase retrieval fluency independent of answer correctness. It is also possible that monkeys monitor both their memory state and the retrieval fluency, similar to how humans utilize multiple cues to make metamemory judgments. We put this intriguing possibility forward tentatively pending further studies that evaluate in more detail the degree to which different aspects of memory control metacognitive responding in monkeys.
We evaluated hypotheses sequentially rather than concurrently, which creates the possibility that the mechanisms controlling performance changed over time. While possible, it is unlikely that the individual experiments in this study assessed different underlying mechanisms peculiar to each. We primarily used probe designs in which monkeys performed a steady state behavior that was verified before each probe session, and we administered relatively small numbers of infrequent probe trials intermixed without warning among normal trials. This design minimizes experience with the unusual probe trials and prevents prediction of when probe trials will appear. Most importantly, this approach ensures that monkeys perform consistently through the set of experiments. We found similar results when we analyzed only the first half of each probe session, indicating that monkeys did not learn a new response rule for each probe trial type. Thus, it is very unlikely that monkeys switched strategies during each set of probe trials. Instead, it is most likely that these probe trials assessed the same underlying cognitive mechanisms that controlled behavior in the main task. For the two prospective tests, Experiments 5 and 6, the similarity of those tests with the previous tests makes it likely that they also assessed the same mechanisms. A future study might better test this position by combining probe trials similar to those in Experiments 2 and 4 with the prospective tests in Experiments 5 and 6.
Cautions and Limitations
Although the information-seeking task described here provides evidence for memory monitoring in rhesus monkeys, we should be careful not to generalize these findings uncritically. Different species could produce superficially similar performance on the same task using different strategies. Additionally, differences in training could bias subjects to solve the task in different ways; for example, extended training could produce a rote response strategy. Thus, while our findings inform our understanding of how rhesus monkeys perform similar tasks, and certainly inform our understanding of the characteristics of their memory systems, we should be hesitant to reach the same conclusions about other species without directly testing alternative hypotheses. We hope we have identified methods that will stimulate future research with different species to identify which discriminative cues control behavior in information-seeking tasks.
The findings from our manipulation of reward value are at odds with those from a similar manipulation in apes (Call, 2010), but it is likely that different mechanisms are at work in the two methodologies. In apes, high reward value did not affect accuracy but did increase visual searches for the food. This has been attributed to a “passport effect” whereby apes sought information despite strong memory because they were reluctant to risk losing the high-value food, much as a human traveler might double-check that they have their passport even though they remember packing it (Call & Carpenter, 2001). Increased information seeking with high-value rewards convincingly ruled out the response competition hypothesis in apes because the high-value food was expected to increase the propensity to reach for the food, which did not happen. In monkeys, higher reward value made monkeys choose to see the answer less often, but significantly increased memory accuracy. The high-value stimulus was likely more salient and better encoded. Both patterns of performance are consistent with the hypothesis that subjects monitored their memory. Therefore, manipulations of reward value can only validly evaluate the response competition hypothesis if they do not affect memory. So, while we could not use this manipulation to evaluate the response competition hypothesis in the current study, it did provide support for the memory monitoring hypothesis.
After these reported experiments were complete, we noticed that one of the eleven monkeys had potentially developed a non-mnemonic strategy for completing the spatial memory task. After the sample was presented, he gently touched near the target location in a delicate way that did not register as a touch and did not abort the trial. Anecdotally, it appeared that he often chose the take-the-test response if his hand was already on the screen and the see-the-answer response if his hand was off the screen. Such postural mediation could potentially produce a pattern of results where monkeys selectively choose to see the answer on Unseen trials, long-delay trials, and low-value trials without having to remember the stimulus location. However, it is unlikely that postural mediation explains overall performance for multiple reasons. First, as we described above, it is difficult to account for generalization to image tests with postural mediation. Second, the first two authors selected three additional monkeys and observed each on two separate days, but did not observe them behaving this way. Third, this strategy would not change performance on missing-location trials in Experiment 3, as the sample presentation was identical in both normal and probe trials, and thus would have produced similar orienting responses. Consistent with this, the monkey observed using this strategy was one of only two monkeys that did not choose to see the answer more often on missing-location trials. Nevertheless, the potential use of non-mnemonic strategies highlights an inherent weakness in tests that use spatial memory.
Broader Implications and Conclusions
In recent years, human psychologists, philosophers, mathematical modelers, and comparative psychologists offered new perspectives and disagreed about the psychological mechanisms underlying purported metamemory performance by nonhuman animals (Browne, 2003; Carruthers, 2008; Crystal & Foote, 2011; Hampton, 2009b; Jozefowiez, et al., 2009; Kornell, 2013; Le Pelley, 2012; Metcalfe, 2003; Smith, et al., 2014). Some of the proposed metamemory mechanisms do not involve monitoring of memory, per se, but instead either responding to cues indirectly associated with memory or responding through mechanisms that may not involve a monitoring component at all. The current evidence constrains theorizing about monkey metamemory performance to mechanisms that involve monitoring some aspect of memory. We think there is great promise in using animal models to understand the neural bases of metacognition in humans. Others have pointed out that this promise comes with problems, questioning whether monkey metacognitions are explicit or implicit (Fleming & Frith, 2014), and noting monkeys’ proclivity for “‘cheating’ at metacognitive tasks by using external cues” (Middlebrooks, et al., 2014). While the current findings do not determine whether monkeys’ metamemory judgments are themselves explicit, they do suggest monitoring of explicit memory. Further, the converging tests we report here go a long way toward ensuring that information-seeking in metamemory tasks is unlikely to represent “cheating.” Indeed, the current report lays out a set of behavioral methods for evaluating whether “cheating” is occurring. The primary focus of neuroscientific studies of metacognition using nonhumans has been on judgments of perception rather than memory (Middlebrooks, et al., 2014), and we hope that the current results enable future neuroscientific studies of metamemory using nonhumans.
Nonhuman metacognition is relevant to deep questions about consciousness, self-awareness, theory-of-mind, subjective experience, and human uniqueness (for example, see Metcalfe & Son, 2012; Smith, et al., 2014 for good discussions of these issues). However, such longstanding and difficult questions are unlikely to be answered with any single new study and we wish to be clear that we provide no such answers here. We have instead focused on making some progress in the study of explicit memory in monkeys by adhering to an operational definition of explicit memory that emphasizes monitorability. The discriminative approach (Basile & Hampton, 2014) used in most studies of nonhuman metacognition was designed to determine which cues can and cannot be monitored. Even in cases where the monitored cue is internal, as we argue here for memory, such methods cannot directly assess the subjective experience of remembering. Thus, we recognize that our work is partly motivated by broad questions about nonhuman cognition and its relation to our subjective experience of human cognition. But we adhere to the position that we can make the most progress by using definitions of cognitive processes that can be readily operationalized in nonhumans, such as by defining the explicitness of memory as the extent to which memory can be introspectively monitored (see Basile & Hampton, 2014; Hampton, 2009a for similar arguments).
One of the major controversies in the field of nonhuman metacognition is whether metacognitive performance can be explained using cognitive mechanisms widely recognized to operate in nonhumans, or requires us to invoke mechanisms not generally attributed to nonhumans (Le Pelley, 2012; Smith, et al., 2014). The argument is often phrased in terms of “high-level” versus “low-level” explanations (e.g., Smith, et al., 2014). For example, quantitative associationist models have been developed in an effort to explain apparent metamemory performance using well-accepted principles of conditioning and learning (Jozefowiez, et al., 2009; Le Pelley, 2012), and have elicited a strong critique from proponents of “high-level” accounts (Smith, et al., 2014). We take issue with the basic assumptions of this conflict. In practice, labels such as “high-level” or “low-level” do not impart useful information for understanding cognition or evaluating hypotheses (Basile & Hampton, 2014; Hampton, 2009a) and, unfortunately, some “low-level” explanations appear to be rejected in part because they are considered boring or killjoy (Shettleworth, 2010).
In the case of nonhuman metamemory, we suggest that current associationist accounts are objectively indistinguishable from accounts of introspective memory monitoring, and may actually be in agreement. Le Pelley (2012) argued, “For several reasons, the sample might leave a stronger trace on some trials than others, and a strong trace might generate an internal cue that differs from that generated by a weak trace. Associative principles would then ensure that the animal learned to make the decline the test response on choice trials when the weak-trace internal cue is present.” Here we have argued that some aspect of memory state varied and the monkeys learned to use that variation as a discriminative cue, which indicates that their memory state must be monitorable. Learning to pair strong and weak memory states with two different responses would proceed according to accepted associative principles, but the conclusion is still that those memory states are monitorable. We see little objective behavioral difference between these accounts, and little utility in stratifying them into high- and low-levels. Both of these accounts advance our understanding of the types of information that enter into the cognitive processing underlying metamemory judgments. A variety of events in the environment cause changes in the state of memory, including presentation of memoranda (Experiment 1), delay interval (Experiment 2), and quality of reward (Experiment 4). We argue that monkeys respond adaptively to the variation in memory caused by each of these, and by other factors, by introspectively monitoring changes in the state of memory, rather than by monitoring each individual environmental event according to a separate rule. Such generalized adaptive memory monitoring is what it means to know when you know.
In conclusion, we evaluated seven hypotheses advanced to account for the performance of monkeys in tests of metamemory. We found no reliable evidence that performance was based on behavioral cue associations, rote response learning, expectancy violation, response competition, generalized search strategy, or postural mediation. In contrast, we repeatedly found evidence that choosing to see the answer was controlled by some aspect of memory, possibly a combination of memory state and retrieval fluency. Despite lacking the ability to declare that they remember, monkeys likely monitor some forms of memory, paralleling the distinction between explicit and implicit memory systems in humans. Studies of metacognition in humans have revealed that humans make metamemory judgments based not only on direct memory access, but on a variety of mnemonic cues (Koriat, 1996; Kornell, 2013). Given the robust evidence that monkeys can monitor some aspect of their memory, future studies may make the most progress by attempting to identify which mnemonic cues are present in monkey metacognitive judgments and the degree to which each mnemonic cue controls behavior.
Acknowledgments
We thank Steven L. Sherrin, Tara A. Dove-VanWormer, and Jessica A. Joiner for help running subjects. This work was supported by the National Science Foundation (Grants 0745573; 1146316), the National Center for Research Resources P51RR000165, the Office of Research Infrastructure Programs / OD P51OD011132, the Center for Behavioral Neuroscience under the Science and Technology Center Program of the National Science Foundation (under agreement IBN-9876754), and the National Institute of Mental Health (Grant R01M H082819). The authors declare no competing financial interests.
References
- Aron A, Aron E. Statistics for psychology. Upper Saddle River, NJ: Prentice Hall; 1999. [Google Scholar]
- Basile BM, Hampton RR. Monkeys recall and reproduce simple shapes from memory. Current Biology. 2011;21(9):774–778. doi: 10.1016/j.cub.2011.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile BM, Hampton RR. Metacognition as discrimination: Commentary on Smith et al. (2014) Journal of Comparative Psychology. 2014;128(2):135–137. doi: 10.1037/a0034412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile BM, Hampton RR, Suomi SJ, Murray EA. An assessment of memory awareness in tufted capuchin monkeys (Cebus apella) Animal Cognition. 2009;12(1):169–180. doi: 10.1007/s10071-008-0180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin AS, Bjork RA. Retrieval fluency as a metacognitive index. In: Reder LM, editor. Implicit memory and metacognition. Mahwah, NJ: Lawrence Erlbaum Associates, Inc.; 1996. pp. 309–338. [Google Scholar]
- Beran MJ, Smith JD. Information seeking by rhesus monkeys (Macaca mulatta) and capuchin monkeys (Cebus apella) Cognition. 2011;120(1):90–105. doi: 10.1016/j.cognition.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran MJ, Smith JD, Perdue BM. Language-Trained Chimpanzees (Pan troglodytes) Name What They Have Seen but Look First at What They Have Not Seen. Psychological Science. 2013;24(5):660–666. doi: 10.1177/0956797612458936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besner NR, Son LK. Underlying mechanisms of initial feelings of knowing in children. Scandinavian Journal of Psychology. 2007;48(6):449–457. doi: 10.1111/j.1467-9450.2007.00614.x. [DOI] [PubMed] [Google Scholar]
- Brauer J, Call J, Tomasello M. Visual perspective taking in dogs (Canis familiaris) in the presence of barriers. Applied Animal Behaviour Science. 2004;88(3–4):299–317. [Google Scholar]
- Browne D. Some sceptical thoughts about metacognition. Behavioral And Brain Sciences. 2003;26(3):340–341. doi: 10.1017/S0140525X03220082. [DOI] [PubMed] [Google Scholar]
- Call J. Do apes know that they could be wrong? Animal Cognition. 2010;13(5):689–700. doi: 10.1007/s10071-010-0317-x. [DOI] [PubMed] [Google Scholar]
- Call J, Carpenter M. Do apes and children know what they have seen? Animal Cognition. 2001;4:207–220. [Google Scholar]
- Carruthers P. Meta-cognition in Animals: A Skeptical Look. Mind & Language. 2008;23(1):58–89. [Google Scholar]
- Cary M, Reder L. Metacognition in Strategy Selection. In: Chambres P, Izaute M, Marescaux P-J, editors. Metacognition. Springer US; 2002. pp. 63–77. [Google Scholar]
- Crystal JD, Foote AL. Evaluating information-seeking approaches to metacognition. Current Zoology. 2011;57:531–542. doi: 10.1093/czoolo/57.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlosky J, Bjork RA. The Integrated Nature of Metamemory and Memory. In: Dunlosky J, Bjork RA, editors. Handbook of Metamemory and Memory. New York, NY: Psychology Press; 2008. pp. 11–28. [Google Scholar]
- Fernandez-Duque D, Baird JA, Posner MI. Executive attention and metacognitive regulation. Consciousness and Cognition. 2000;9(2):288–307. doi: 10.1006/ccog.2000.0447. [DOI] [PubMed] [Google Scholar]
- Flavell JH. Metacognition and cognitive monitoring: A new area of cognitive-developmental inquiry. American Psychologist. 1979;34:906–911. [Google Scholar]
- Fleming S, Frith C. Metacognitive Neuroscience: An Introduction. In: Fleming SM, Frith CD, editors. The Cognitive Neuroscience of Metacognition. Springer Berlin Heidelberg; 2014. pp. 1–6. [Google Scholar]
- Foote AL, Crystal JD. Metacognition in the rat. Current Biology. 2007;17(6):551–555. doi: 10.1016/j.cub.2007.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita K. Metamemory in tufted capuchin monkeys (Cebus apella) Anim Cogn. 2009;12(4):575–585. doi: 10.1007/s10071-009-0217-0. [DOI] [PubMed] [Google Scholar]
- Goto K, Watanabe S. Large-billed crows (Corvus macrorhynchos) have retrospective but not prospective metamemory. Animal Cognition. 2012;15(1):27–35. doi: 10.1007/s10071-011-0428-z. [DOI] [PubMed] [Google Scholar]
- Hampton RR. Rhesus monkeys know when they remember. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:5359–5362. doi: 10.1073/pnas.071600998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton RR. Metacognition as evidence for explicit representation in nonhumans. Behavioral and Brain Sciences. 2003;26:346–347. doi: 10.1017/S0140525X03300081. [DOI] [PubMed] [Google Scholar]
- Hampton RR. Can Rhesus monkeys discriminate between remembering and forgetting? In: Terrace HS, Metcalfe J, editors. The missing link in cognition: Origins of self-reflective consciousness. New York: Oxford University Press; 2005. pp. 272–295. [Google Scholar]
- Hampton RR. Memory awareness in rhesus monkeys. In: Fujita K, Itakura S, editors. Diversity of Cognition. Kyoto, Japan: Kyoto University Press; 2006. pp. 282–299. [Google Scholar]
- Hampton RR. Focusing the uncertainty about nonhuman metacognition. Comparative Cognition & Behavior Reviews. 2009a;4:56–57. doi: 10.3819/ccbr.2009.40006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton RR. Multiple demonstrations of metacognition in nonhumans: Converging evidence or multiple mechanisms? Comparative Cognition & Behavior Reviews. 2009b;4:17–28. doi: 10.3819/ccbr.2009.40002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton RR. Status of nonhuman memory monitoring and possible roles in planning and decision making. In: Menzel R, Fischer J, editors. Animal thinking: contemporary issues in comparative cognition. Cambridge, MA: MIT Press; 2011. pp. 105–119. [Google Scholar]
- Hampton RR, Hampstead BM. Spontaneous behavior of a rhesus monkey (Macaca mulatta) during memory tests suggests memory awareness. Behavioural Processes. 2006;72(2):184–189. doi: 10.1016/j.beproc.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Hampton RR, Zivin A, Murray EA. Rhesus monkeys (Macaca mulatta) discriminate between knowing and not knowing and collect information as needed before acting. Animal Cognition. 2004;7:239–254. doi: 10.1007/s10071-004-0215-1. [DOI] [PubMed] [Google Scholar]
- Hunter WS. The delayed reaction in animals and children : studies from the Psychological Laboratory of the University of Chicago. Cambridge, Boston, Mass. ; New York:: H. Holt & co.; 1913. [Google Scholar]
- Hurlburt RT. Comprehending behavioral statistics. 4th ed. Australia ; Belmont, CA: Thomson/Wadsworth; 2006. [Google Scholar]
- Inman A, Shettleworth SJ. Detecting metamemory in nonverbal subjects: A test with pigeons. Journal of Experimental Psychology-Animal Behavior Processes. 1999;25(3):389–395. [Google Scholar]
- Jozefowiez J, Staddon J, Cerutti D. Metacognition in animals: How do we know that they know. Comparative Cognition & Behavior Reviews. 2009;4:29–39. [Google Scholar]
- Koriat A. Metamemory: The feeling of knowing and its vagaries. International Journal of Psychology. 1996;31(3–4):4611–4611. [Google Scholar]
- Koriat A. Monitoring one's own knowledge during study: A cue-utilization approach to judgments of learning. Journal of Experimental Psychology-General. 1997;126(4):349–370. [Google Scholar]
- Koriat A, Levy-Sadot R. Conscious and Unconscious Metacognition: A Rejoinder. Consciousness and Cognition. 2000;9(2):193–202. doi: 10.1006/ccog.2000.0436. doi: http://dx.doi.org/10.1006/ccog.2000.0436. [DOI] [PubMed] [Google Scholar]
- Kornell N. Where Is the "Meta" in Animal Metacognition? Journal of Comparative Psychology. 2013 doi: 10.1037/a0033444. [DOI] [PubMed] [Google Scholar]
- Kornell N, Son LK, Terrace HS. Transfer of metacognitive skills and hint seeking in monkeys. Psychological Science. 2007;18(1):64–71. doi: 10.1111/j.1467-9280.2007.01850.x. [DOI] [PubMed] [Google Scholar]
- Kuhn D. Metacognitive development. Current Directions in Psychological Science. 2000;9(5):178–181. [Google Scholar]
- Le Pelley ME. Metacognitive monkeys or associative animals? Simple reinforcement learning explains uncertainty in nonhuman animals. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2012;38(3):686–708. doi: 10.1037/a0026478. [DOI] [PubMed] [Google Scholar]
- Le Pelley ME. Primate polemic: Commentary on Smith, Couchman, and Beran (2014) Journal of Comparative Psychology. 2014;128(2):132–134. doi: 10.1037/a0034227. [DOI] [PubMed] [Google Scholar]
- Marsh H, MacDonald S. Information seeking by orangutans: a generalized search strategy? Animal Cognition. 2012;15(3):293–304. doi: 10.1007/s10071-011-0453-y. [DOI] [PubMed] [Google Scholar]
- Marsh HL. Metacognitive-like information seeking in lion-tailed macaques: a generalized search response after all? Animal Cognition. 2014:1–16. doi: 10.1007/s10071-014-0767-7. [DOI] [PubMed] [Google Scholar]
- McMahon S, Macpherson K, Roberts WA. Dogs choose a human informant: metacognition in canines. Behavioural Processes. 2010;85(3):293–298. doi: 10.1016/j.beproc.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Metcalfe J. Drawing the line on metacognition. Behavioral and Brain Sciences. 2003;26(3):350–351. doi: 10.1017/S0140525X03340087. [DOI] [PubMed] [Google Scholar]
- Metcalfe J, Son LK. Anoetic, noetic, and autonoetic metacognition. The foundations of metacognition. Oxford University Press, Oxford, UK. 2012:289–301. [Google Scholar]
- Middlebrooks PG, Abzug ZM, Sommer MA. Studying Metacognitive Processes at the Single Neuron Level. In: Fleming SM, Frith CD, editors. The Cognitive Neuroscience of Metacognition. Springer Berlin Heidelberg; 2014. pp. 225–244. [Google Scholar]
- Middlebrooks PG, Sommer MA. Neuronal Correlates of Metacognition in Primate Frontal Cortex. Neuron. 2012;75(3):517–530. doi: 10.1016/j.neuron.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles RC. Species differences in "transmitting" spatial location information. In: Jarrard LE, editor. Cognitive Processes of Nonhuman Primates. New York and London Academic Press; 1971. [Google Scholar]
- Narens L, Graf A, Nelson TO. Metacognitive Aspects of Implicit/Explicit Memory. In: Reder L, editor. Implicit Memory & Metacognition. Mahwah, NJ: Lawrence Erlbaum Associates, Inc.; 1996. pp. 137–170. [Google Scholar]
- Nelson TO. Consciousness and metacognition. American Psychologist. 1996;51(2):102–116. [Google Scholar]
- Nelson TO, Narens L. Metamemory: A theoretical framework and new findings. The psychology of learning and motivation. 1990;26:125–141. [Google Scholar]
- Pannu JK, Kaszniak AW. Metamemory experiments in neurological populations: A review. Neuropsychology Review. 2005;15(3):105–130. doi: 10.1007/s11065-005-7091-6. [DOI] [PubMed] [Google Scholar]
- Rhodes MG, Tauber SK. The Influence of Delaying Judgments of Learning on Metacognitive Accuracy: A Meta-Analytic Review. Psychological Bulletin. 2011;137(1):131–148. doi: 10.1037/a0021705. [DOI] [PubMed] [Google Scholar]
- Roberts WA, Feeney MC, McMillan N, MacPherson K, Musolino E, Petter M. Do pigeons (Columba livia) study for a test? Journal of Experimental Psychology: Animal Behavior Processes. 2009;35(2):129–142. doi: 10.1037/a0013722. [DOI] [PubMed] [Google Scholar]
- Schwartz B. Sources of information in metamemory: Judgments of learning and feelings of knowing. Psychonomic Bulletin & Review. 1994;1(3):357–375. doi: 10.3758/BF03213977. [DOI] [PubMed] [Google Scholar]
- Shettleworth SJ. Clever animals and killjoy explanations in comparative psychology. Trends in Cognitive Science. 2010;14(11):477–481. doi: 10.1016/j.tics.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Shields WE, Smith JD, Guttmannova K, Washburn DA. Confidence judgments by humans and rhesus monkeys. Journal of General Psychology. 2005;132(2):165–186. [PMC free article] [PubMed] [Google Scholar]
- Shields WE, Smith JD, Washburn DA. Uncertain responses by humans and rhesus monkeys (Macaca mulatta) in a psychophysical same-different task. Journal of Experimental Psychology: General. 1997;126(2):147–164. doi: 10.1037//0096-3445.126.2.147. [DOI] [PubMed] [Google Scholar]
- Smith JD, Beran MJ, Redford JS, Washburn DA. Dissociating uncertainty responses and reinforcement signals in the comparative study of uncertainty monitoring. Journal of Experimental Psychology: General. 2006;135(2):282–297. doi: 10.1037/0096-3445.135.2.282. [DOI] [PubMed] [Google Scholar]
- Smith JD, Couchman JJ, Beran MJ. Animal metacognition: A tale of two comparative psychologies. Journal of Comparative Psychology. 2014;128(2):115–131. doi: 10.1037/a0033105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JD, Schull J, Strote J, McGee K, Egnor R, Erb L. The uncertain response in the bottlenosed dolphin (Tursiops truncatus) Journal of Experimental Psychology: General. 1995;124(4):391–408. doi: 10.1037//0096-3445.124.4.391. [DOI] [PubMed] [Google Scholar]
- Smith JD, Shields WE, Washburn DA. Memory monitoring by animals and humans. Journal of Experimental Psychology: General. 1998;127(3):227–250. doi: 10.1037//0096-3445.127.3.227. [DOI] [PubMed] [Google Scholar]
- Sole LM, Shettleworth SJ, Bennett PJ. Uncertainty in pigeons. Psychonomic Bulletin & Review. 2003;10(3):738–745. doi: 10.3758/bf03196540. [DOI] [PubMed] [Google Scholar]
- Squire LR, Knowlton B, Musen G. The Structure and Organization of Memory. Annual Review of Psychology. 1993;44:453–495. doi: 10.1146/annurev.ps.44.020193.002321. [DOI] [PubMed] [Google Scholar]
- Squire LR, Wixted JT. The Cognitive Neuroscience of Human Memory Since HM. In: Hyman SE, Jessell TM, Shatz CJ, Stevens CF, Zoghbi HY, editors. Annual Review of Neuroscience, Vol 34. Vol. 34. Palo Alto: Annual Reviews; 2011. pp. 259–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templer VL, Hampton RR. Rhesus monkeys (Macaca mulatta) show robust evidence for memory awareness across multiple generalization tests. Animal Cognition. 2012;15(3):409–419. doi: 10.1007/s10071-011-0468-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrace HS, Son LK. Comparative metacognition. Current Opinion in Neurobiology. 2009;19(1):67–74. doi: 10.1016/j.conb.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Washburn DA, Gulledge JP, Beran MJ, Smith JD. With his memory magnetically erased, a monkey knows he is uncertain. Biology Letters. 2010;6(2):160–162. doi: 10.1098/rsbl.2009.0737. [DOI] [PMC free article] [PubMed] [Google Scholar]