Abstract
Individuals with Parkinson’s disease (PD) with symptom onset on the left side of the body (LPD) show mild type of left-sided visuospatial neglect, whereas those with right-onset (RPD) generally do not. The functional mechanisms underlying these observations are unknown. Two hypotheses are that the representation of left-space in LPD is either compressed or reduced in salience. We tested these hypotheses psychophysically. Participants were 31 non-demented adults with PD (15 LPD, 16 RPD) and 17 normal control adults (NC). The spatial compression hypothesis was tested by showing two sinusoidal gratings, side by side. One grating’s spatial frequency (SF) was varied across trials, following a staircase procedure, whereas the comparison grating was held at a constant SF. While fixating on a central target, participants estimated the point at which they perceived the two gratings to be equal in SF. The reduced salience hypothesis was tested in a similar way, but by manipulating the contrast of the test grating rather than its SF. There were no significant differences between groups in the degree of bias across hemifields for SF discrimination or for contrast discrimination. Results did not support either the spatial compression hypothesis or the reduced salience hypothesis. Instead, they suggest that at this perceptual level, LPD do not have a systematically biased way of representing space in the left hemifield that differs from healthy individuals, nor do they perceive stimuli on the left as less salient than stimuli on the right. Neglect-like syndrome in LPD instead presumably arises from dysfunction of higher-order attention.
Keywords: hemiparkinsonism, parkinsonism, LPD, visuospatial neglect, hemineglect, perception
1. Introduction
Recently an emphasis has been placed on exploring the non-motor aspects of Parkinson’s disease (PD) such as cognitive and perceptual disturbances, which substantially impact quality of life beyond the disease’s classical motor symptoms (Cronin-Golomb, 2013). PD is usually asymmetrical in its onset, and individuals whose motor symptoms start on the left side of their body (LPD) have shown particular perceptual abnormalities that are suggestive of a mild form of visuospatial neglect. First, those with LPD have been shown to bisect lines in a way that is milder but similar to that shown by individuals with neglect syndrome, perceiving the middle of the line to be shifted rightward from its physical location (Lee, Harris, Atkinson, & Fowler, 2001). Second, they more frequently begin exploring a stimulus by first gazing to its right side than its left side, which is opposite to the pattern seen in healthy control adults and in PD with right side onset (RPD) (Ebersbach et al., 1996). Third, LPD view objects on the left as smaller than they really are, as compared to objects on the right side of space (Harris, Atkinson, Lee, Nithi, & Fowler, 2003). These perceptual disturbances may have negative effects on daily life: LPD more frequently report bumping into the left side of doorways (Davidsdottir, Cronin-Golomb, & Lee, 2005), and it takes little imagination to generate additional sequelae in walking, navigation, and especially in regard to driving.
Despite the clinical importance of this phenomenon in LPD, the functional mechanisms underlying this neglect-like pattern of performance in LPD remain unknown. At a neurophysiological level, the differential deterioration of the right hemisphere, which accounts for the fact that motor symptoms begin on the left side of the body, also presumably accounts for perceptual disturbance in LPD (Cronin-Golomb, 2010). At a functional level, the mechanisms underlying LPD’s neglect-like performance are less certain. One explanation that has been offered is that in LPD, the representation of the left side of space is compressed (Davidsdottir, Wagenaar, Young, & Cronin-Golomb, 2008; Harris et al., 2003). If this were the case, it might explain why some LPD bisect lines rightward of their true center, because the left portion of the line would be compressed and therefore appear smaller—leading to a shift of perceived center.
Another possibility is that visual signals (such as salience or contrast) in the left hemifield are generally weakened in LPD. Several studies have found reduced contrast sensitivity in PD (e.g., Kupersmith, Shakin, Siegel, & Lieberman 1982; Pieri, Diederich, Raman, & Goetz, 2000; Amick et al., 2003), with some suggesting a generalized loss of contrast sensitivity across spatial frequencies (Price, Feldman, Adelberg, & Kayne 1992), and others indicating a shift in the contrast sensitivity function resulting from changes at specific spatial frequencies (Bodis-Wollner et al., 1987). With respect to LPD-specific biases, Davidsdottir and colleagues (2008) found no evidence for such. Whether LPD may view objects in the left hemifield to be lower in contrast than those in the right hemifield, using some sort of contrast-matching procedure (Georgeson & Sullivan, 1975) is as yet far unknown. If visual signals were weakened in the left hemifield relative to in the right hemifield in LPD, it might affect perception of stimulus length, which would subsequently affect line bisection performance. Such a disparity in signal strength would also seem to be a potential explanation for patterns of exploratory eye movements seen in LPD, who tend to begin exploring the right side of a stimulus rather than the left on a visual search task (Ebersbach et al., 1996), since the salience of a physical stimulus (largely determined by visual signals such as contrast or motion) is an important factor in determining where eye movements will be directed (Hart, Schmidt, Klein-Harmeyer, & Einhauser, 2013).
In the present study, we tested both of these hypotheses using psychophysical methods. To avoid the potential confound of biased eye movements, we employed a brief presentation time in both tasks (<100 msec) and used eye tracking to ensure fixation in the center of the screen. The spatial compression hypothesis was assessed using a task in which the spatial frequency of an object on the left was compared with the spatial frequency of an object on the right. Healthy adults show mild spatial compression of the left hemifield on spatial frequency discrimination tasks (Edgar & Smith, 1990). For the hypothesis to be supported, LPD (relative to the control group) would have to overestimate the spatial frequency of objects in the left hemifield as compared to those in the right hemifield. The reduced salience hypothesis was tested in a similar way, but using contrast as the physical metric of comparison rather than spatial frequency. For the reduced salience hypothesis to be supported, LPD would have to underestimate the contrast of stimuli in the left hemifield as compared to those in the right.
2. Experiment 1
2.1 Methods
2.1.1 Participants
Thirty-one non-demented individuals with Parkinson’s disease (15 LPD and 16 RPD) and 16 normal control adults (NC) participated in the study. Demographic and other participant information is shown in Table 1. The groups were matched on age, education, male:female ratio, and premorbid intelligence as measured by the vocabulary section of the Wide Range Achievement Test (Wilkinson, 1993). Potential participants were excluded from the study on the basis of having neurological conditions other than PD, coexisting serious chronic medical illnesses including psychiatric illness, use of psychoactive medication besides antidepressants and anxiolytics in the PD group, history of intracranial surgery (e.g., deep brain stimulation or other invasive PD treatments), traumatic brain injury, current alcohol dependence or substance abuse. All participants except two RPD, one LPD, and two NC received a detailed neuro-ophthalmological examination to rule out visual disorders including significant glaucoma, cataracts, or macular degeneration. All participants were screened for dementia using the Columbia Modified Mini-Mental State Examination (MMSE) (Stern, Sano, Paulson, & Mayeux, 1987). The minimum score for inclusion in the study was 27. The LPD and RPD groups each had a median Hoehn and Yahr score of 2, with most being at a mild to moderate motor stage. The range of scores for LPDs was between 1 and 4 (single individual for the latter) and the range of scores for RPDs was between 1 and 3. LPD and RPD did not differ significantly on their Hoehn and Yahr scores (Kolmogorov-Smirnov, Z = .97, p = .31) nor on motor severity as measured by the Unified Parkinson’s Disease Rating Scale (UPDRS) (Movement Disorders Society Task Force on Rating Scales for Parkinson’s Disease, 2003). The Beck Depression Inventory II and Beck Anxiety Inventory were administered to ensure that the groups were matched on mood (Beck & Steer, 1993; Beck, Steer, & Brown, 1996).
Table 1.
Participant Characteristics
| Measure | LPD (n = 15) | RPD (n = 16) | NC (n = 16) | Significance |
|---|---|---|---|---|
| Age (years) | 64.0 (7.4) | 64.5 (6.7) | 66.4 (8.1) | NS |
| Education (years) | 17.5 (1.9) | 16.9 (1.5) | 17.4 (2.1) | NS |
| Gender (M/F) | 8/7 | 7/9 | 5/11 | NS |
| UPDRS Motor Score | 19.3 (7.4) | 19.4 (9.8) | -- | NS |
| H & Y Stage (median) | 2.0 | 2.0 | -- | NS |
| LED (mg/day) | 488 (261) | 460 (335) | -- | NS |
| Acuity (Log MAR) | 0.11 (0.14) | 0.09 (0.12) | 0.04 (0.11) | NS |
| BDI-II | 5.2 (3.6) | 6.7 (5.7) | 2.5 (3.8) | NS |
| BAI | 5.7 (3.7) | 7.2 (5.5) | 3.1 (4.6) | NS |
Note. LPD = left-onset Parkinson’s disease; RPD = right-onset Parkinson’s disease; NC = normal control participants. UPDRS = Unified Parkinson’s Disease Rating Scale; H & Y = Hoehn & Yahr staging criteria; LED = Levodopa equivalent dosage; BDI-II = Beck Depression Inventory – II; BAI = Beck Anxiety Inventory. Values presented are means (standard deviations), unless otherwise indicated.
2.1.2 Stimulus and procedures
Data were obtained in compliance with regulations of the Institutional Review Board of Boston University, in accordance with the Declaration of Helsinki. All participants provided informed consent.
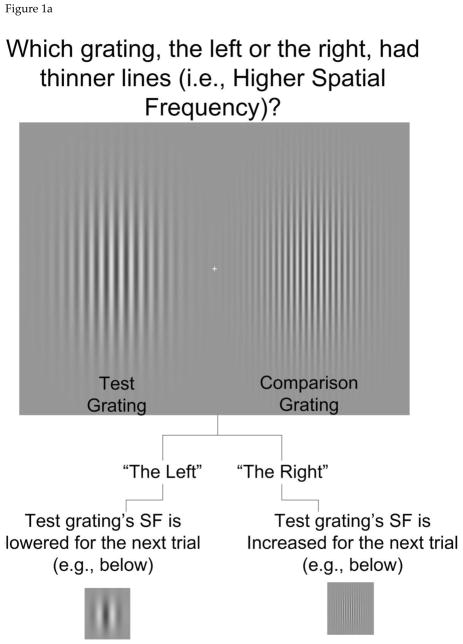
The stimulus was a pair of static Gabor patches, presented side by side as shown in Figure 1a. One was designated as the “test”, meaning its spatial frequency (SF) varied from trial to trial, and the other was designated as the comparison, meaning it was held constant throughout the testing block. In each trial, the task was to determine which grating, the test or the comparison, had the higher SF (i.e., thinner bands of light and dark), while fixating on the center cross. Eye tracking was used to ensure fixation, as detailed below. The test grating’s SF was adjusted over 20 trials in response to the participant’s responses, according to a QUEST procedure (quantile method) (Watson & Pelli, 1983). The test grating’s SF was adjusted broadly at the start of the procedure, and became more fine-tuned as it progressed (Figure 1b), approaching the participant’s point of subjective equality (PSE) regarding the two gratings’ SF. The PSE was quantified as the average of test SF at each of the points at which the staircase changed direction (e.g., from increasing to decreasing SF), excluding the first 5 trials, in which SF varied quite widely.
Figure 1.
a. Stimulus and task for Experiment 1, spatial frequency discrimination. Stimulus duration was 50 msec, but the fixation cross was presented 600 msec prior to the Gabor grating presentation, to ensure participants had time to fixate. b. Illustration of QUEST staircase procedure for determining the point of subjective equality (PSE).
A PSE was derived for each test condition (when it was on the left versus the right), and converted to a percent of spatial compression. The contrast of the comparison grating was set at 31% Michelson contrast. The contrast of the test was randomly jittered by up to 1.2 log unit in either direction (above or below the comparison’s contrast), but was centered on 0.3 Michelson contrast also. This was done in order to remove the potential confound that the perceived contrast of a visual object is affected by its spatial frequency (Robson & Campbell, 1997), and presumably vice versa. Jittering the contrast of the test ensured that participants could not use the perceived contrast of the Gabor patches (linked to SF) as a cue to help them do the task. Stimulus duration was 50 msec. Stimuli were programmed using Psychophysics Toolbox and MatLab (Brainard, 1997) and were presented on a 21″ CRT monitor (Hewlitt Packard FP2141SB) running at 120 Hz.
The procedure was done separately with the test in the left hemifield and in the right hemifield, and a separate PSE was attained for each. This meta-procedure was then repeated at four baseline SFs: 0.5, 1.0, 2.0 and 4.0 cpd. For example, with the Gabor patch on the left (the comparison) set to the baseline SF of 1 cpd, the Gabor patch on the right (the test) might be initially chosen to have a higher SF than that of the test (e.g., 1.8 cpd). The participant would report that the SF of the grating on the right was higher than that of the grating on the left; this response would cause the SF of the grating on the right to be reduced on the next trial. This procedure was repeated for 20 trials over which the SF of the adjusted grating approached the point at which the participants thought that the two gratings had equal SFs (as shown in Figure 1b).
Half of the participants in each group reported which gratings had a higher SF and half reported which grating had a lower SF in order to control for any effect of higher-order cognition or bias in saying the word “right” or saying the word “left” that might exist, particularly in individuals with PD who often have a “good side” or “bad side”. The test was explained in the following way: “Tell me which blob, the one on the left or the right, has thinner (or thicker) lines.” A demonstration of the test was used to introduce the task to the participants and ensure their ability to perform it. The experimenter observed the stimuli along with the participant and ensured that the participant answered with at least 75% accuracy on the demo trials where the spatial frequency difference was very clear to the experimenter. Usually, only one round of demonstration was required to meet this criterion, but it was repeated if necessary..
2.1.3 Eye tracking
An ASL Eye Trac Six camera was used to ensure maintenance of fixation during the task (for details see Laudate, Neargarder, & Cronin-Golomb, 2013). Eye gaze was recorded during each 50 msec trial at 120 Hz, resulting in 6 samples per trial. According to the manufacturer (Applied Science Laboratories), the system accuracy was within 1 degree of visual angle, and precision was approximately 0.25 deg. The gaze position across those six frames was averaged to produce a single point of gaze for each 50 msec trial. Three metrics were calculated for each participant across all trials in each condition. First, the average horizontal and vertical components of gaze position were taken by averaging the x and y coordinates of the individual trials. Second, the standard deviation was taken across all trials and conditions. Third, the proportion of trials was recorded for which participants maintained fixation within 3 degrees of visual angle of the center of the screen.
2.1.4 Statistical Analysis
PSE scores were analyzed in the following way. For the condition in which the test stimulus was on the left, the PSE was converted to a percentage of the baseline SF. For example, where the SF was 0.5 cpd, if a participant’s PSE for the test grating was 0.51, then the PSE score would be considered 2% higher than the test. If the test were on the right, this would represent 2% spatial compression at 0.5 cpd baseline SF. If the test were adjusted to be .49 cpd, then it would represent −2% spatial compression at the same baseline. If the test were on the left, the sign of these percentages would be reversed. Ultimately, the negative of the test-left condition was averaged with the test-right condition to produce a spatial compression score, the units of which are percentage of spatial compression of the left hemifield. The hypothesis that spatial compression occurs in LPD was tested by performing a mixed model analysis of variance (ANOVA) on the averaged spatial compression scores at each SF, with group as the between subjects variable, and SF as the within subjects repeated measure. The spatial compression hypothesis would be supported if a main effect for group or an interaction between group and SF emerged, driven by LPD’s increased spatial compression index at one or more SF’s.
2.2 Results
Results are shown in Figure 2. There were no group differences in the main ANOVA comparing LPD, RPD and NC across the four baseline spatial frequencies in their degree of spatial compression, F (2, 44) = 1.1, p = .34, η2= .05. There was a significant effect for SF, F(2.6, 116.3) = 5.5, p = .002,,, η2 = .11, characterized by each group’s spatial compression bias decreasing as SF of the comparison increased. There was no interaction between group and SF, F(5.3, 116.3) = .21, p = .96,, η2 = .01. To summarize, there were no group differences, and LPD showed (non-significantly) less spatial compression than NC; the opposite result that would be expected by the spatial compression hypothesis.
Figure 2.
Results of Experiment 1. While there were no statistically significant group differences, individuals with left onset Parkinson’s disease (LPD) showed a bias pattern that was different from than that of the age-matched normal control group (NC), in the direction that was opposite to that predicted by the spatial compression hypothesis.
One way ANOVAs were performed across groups for horizontal and vertical gaze position and standard deviation, as well as proportion of trials fixated. Groups did not differ in any of the eye tracking metrics computed: horizontal gaze position, 2,45) = .14, p = .87, η2 = .01, vertical gaze position, F(2,45) =2.99, p = .06, η2 = .13, horizontal spread, F(2,45) = 1.91, p = .16, η2 = .08, vertical spread, F(2,45) = .71, p = .50, η2 = .03, and proportion of trials successfully fixated F(2,45) = .57, p =.57, η2 = .03.
3. Experiment 2
3.1 Methods
3.1.1 Participants
Participants were the same as in Experiment 1 except for one fewer LPD, one fewer RPD, and two fewer NC, who were unavailable for repeat testing. The groups continued to be matched for all variables as described in Experiment 1.
3.1.2 Stimulus and Procedures
The stimulus was a pair of sinusoidal Gabor patches, one on the left and one on the right of a central fixation cross (Figure 3a). The task was to compare the contrast levels of the two Gabors (one designated as the test, the other the comparison) while fixating on the center cross. Eye tracking was used to ensure maintenance of fixation, in the same manner as Experiment 1. The comparison patch was held constant at 31% Michelson contrast throughout the testing block, and the test patch was set at one of 8 predetermined levels: 0.5, 0.667, 0.9, 0.95, 1.05, 1.1, 1.2, and 1.4 times the contrast of the comparison. There were 8 repetitions per condition, 8 test contrasts, and 2 test/comparison positions (left/right or right/left), for a total of 128 trials. The SF of both test and comparison Gabors was set at 1 cpd. Again, trials where the test was on the left were interspersed with trials where the test was on the right. The proportion of trials on which the participant reported, “right has higher contrast” (or one minus the proportion of trials reported as “right has lower contrast” for participants reporting in this way) was calculated at each test contrast. Contrast bias across hemifields was considered to be the difference between this proportion when the test was on the right and the same proportion when the test was on the left at each test contrast level (Figure 3b).
Figure 3.
a. Stimulus and task for Experiment 2. b. Derivation of the contrast discrimination bias score. The difference between the two functions shown (test on left and test on right) represents the bias. For participants reporting which Gabor patch was fainter (as opposed to the participant shown, who was reporting which patch was more vivid), the scores were converted to proportion of trials in which the test was more vivid by taking one minus the proportion of trials in which the test was reported as more faint.
Similar in procedure to Experiment 1, half of the participants reported which of the two patches had the higher contrast, and the others reported which had lower contrast. The task was explained in this way: “Tell me which blob has a higher contrast, or appears more vivid to you. It will have brighter brights and darker darks than the other.” If the participant was assigned to report the lower contrast patch, the task was explained in the following way: “Tell me which of the two blobs, left or right, appears fainter than the other.” The purpose of splitting the form of reporting percepts (high versus low contrast) was to avoid the confound of any non-perceptually-driven biases to say left or right. A demonstration was done for this task to ensure that the participants understood the instructions.
3.1.3 Eye tracking
Eye tracking procedures were the same as in Experiment 1.
31.4 Data Analysis
The main analysis was a repeated measures ANOVA on bias scores with group as the between subjects variable (3 levels, LPD, NC, RPD), and test-contrast as the within subjects repeated measure (8 levels corresponding to the 8 contrasts used for the test grating).
3.2 Results
3.2.1 Performance
Results are shown in Figure 4. LPD did not show a bias in either hemifield, nor in the overall bias between the two hemifields. That is, LPD did not view gratings on the left as less salient than those on the right. A mixed model, two way ANOVA across groups and contrast level of the test grating showed no effect for group F(2,40) = .25, p = .78, η^2 = .01, or contrast level of the test grating F(3.3, 132.8) = 1.1, p = .36, η^2 = .03. There was also no interaction between the two variables, F(6.6, 132.8) = .40, p = .90, η^2 = .02.
Figure 4.
Results of Experiment 2. There were no significant group differences.
3.2.2 Eye Tracking
As in Experiment 1, groups did not differ in any of the eye tracking metrics computed. One way ANOVAs showed no group differences for horizontal gaze position, F(2,42) = .25, p = .78, η2 = .01vertical gaze position, F(2,42) = 2.4, p = .11,, η2 = .11, horizontal spread, F(2,42) = 1.4 p = .26,, η2 = .07, vertical spread, F(2,42) = .49, p = .61,, η2 = .03, and proportion of trials successfully fixated F(2,42) = 2.1, p =.14,, η2 = .10.
4. Discussion
We found that individuals with LPD did not show any perceptual biases that differed from neurologically healthy control participants. The results do not support the hypothesis, advanced by results of other studies, that LPD perceive the left side of space as compressed. Accordingly there is no support for the idea that spatial compression is a mechanism for neglect-like performance in LPD.
There were also no group differences in contrast discrimination biases across hemifields. The results from this task did not support the novel hypothesis that in general, visual signals are weakened in the left hemifield in LPD. If the strength of visual signals were reduced overall in the left hemifield in LPD, one would expect that objects in the right hemifield would appear to be at higher contrast than objects of equal contrast on the left. This did not occur in any of the participant groups in this study, who all performed similarly. Reduced signal strength in the left hemifield in LPD therefore does not appear to be the mechanism underlying neglect-like performance in LPD.
Two potential confounding variables present themselves in a study of perceptual bias preferences of this type, but both were well controlled for in our study. First, it is possible that participants may have a bias to report “left” or “right” more frequently that is independent of stimulus characteristics. The design of the task ensured freedom from the left/right reporting bias because the test stimulus was in both hemifields, and half of the participants reported which SF was higher, and half reported which SF was lower. Further, the biases that did exist in both LPD and NC were seen only on tests of lower SFs (0.5 and 1 cpd), suggesting that they were linked to low-level perceptual processing; higher-order cognitive biases would be expected to affect all SFs similarly. Second, the fact that PD is primarily a motor disorder, and that eye movements are affected in the disease, would raise the possibility that abnormal eye movements could be a factor in neglect-like performance. In the present study, however, the stimulus was brief enough to prevent any strategic eye movements during its presentation, and eye tracking was used to ensure fixation. Further, if motor deficits affecting eye movements were a primary factor in neglect-like performance, we would expect to see a symmetrical deficit in RPD, perhaps in the opposite direction, since their eye movements should also be affected similarly, but RPD performed similarly to healthy adults on the tasks listed above in which LPD show neglect. It is therefore likely that the results are accurate reflections of the perceptual processes involved, rather than the result of confounding variables such as eye movement biases or higher-order cognitive biases. In light of other studies showing perceptual biases in LPD (Davidsdottir et al., 2008; Harris et al., 2003; Lee, Harris, Atkinson, & Fowler, 2001; Villardita, Smirni, & Zappala, 1983), the lack of abnormal results in LPD in this study suggest that such higher-order processes as attentional biases may in fact drive the observed perceptual bias.
Our sample of LPD, RPD, and NC was larger than those in previous studies that have documented neglect-like biases in LPD (Harris et al., 2003; Lee et al., 2001). Like other studies, it was restricted to individuals with only mild to moderate PD. The lack of perceptual biases in the LPD group in the present study may be reflective of a true lack of bias in these perceptual processes in individuals with LPD at any disease stage, or it could be that biases may arise only with advanced disease.
The perceptual tasks used in our study presumably rely on low-level visual processing centers, such as in the primary visual cortex (Boynton et al., 1999). The absence of reduced saliency or spatial compression in LPD implicates higher-order attentional difficulties in explaining the neglect-like syndrome in LPD. Neurobiologically, these processes rely on visual attention centers such as in the parietal cortex, which have also been shown to be structurally affected in PD (Pereira et al., 2009). While speculative, this notion accords with a recent review by Diederich et al. (2014), which suggests that the perceptual deficits in PD arise from deficient processing in non-conscious visual pathways (the retino-colliculo-thalamo-amygdala and retino-geniculo-extrastriate pathways), whereas the primary visual pathway connecting retina to V1 (occipital cortex) is relatively intact.
The task for future research exploring the nature of potential neglect syndrome in LPD is clear. First, the original studies on size and length perception in the left and right hemifields should be replicated, as some were done with sample sizes under 10 per group (e.g. Lee et al., 2001). Also, assessments should be expanded to include cancellation and other tasks that are related to line bisection performance and are typically used for diagnosing neglect syndrome (Albert, 1973; Guariglia, Matano, & Piccardi, 2014). It is possible that perceptual biases as measured in the present study are altered in some individuals with PD (who may in fact experience something like traditional hemineglect), but that these individuals are not systematically selected by dividing PD samples by side of motor symptom onset. It may be that other PD subgroups need to be considered when determining the crucial factors for vulnerability to neglect-like performance in PD. For example, individuals with non-tremor dominant PD have been reported to be more deficient than those with tremor dominance with respect to self-reported visual difficulties (Seichepine et al., 2011) and clock drawings (Seichepine et al., 2014). In the present study, our sample was too small for such a breakdown by tremor/non-tremor subgroup. It is unclear at this time how such subgroup differences relate to the primary subgroup breakdown explored in the present study: that of LPD versus RPD.
We recently found that LPD as a group exhibited rightward bias on line bisection when measured psychophysically, but also we observed that not all individuals with LPD showed this bias, and that there were individuals with RPD who did show the bias. The group difference did not, however, emerge on a traditional paper and pencil version of the test (Norton, Munro, & Cronin-Golomb, submitted). Future studies should compare discrimination biases with performance on tasks on which individuals actually demonstrate neglect-like biases: for example, visual stimulus exploration, cancellation, and line bisection using long line stimuli. Results from such additional studies could strengthen the claim that behavioral neglect as assessed by line bisection tasks is unrelated to perceptual discrimination biases.
In summary, our findings do not support either of the functional hypotheses offered to explain hemineglect-like performance in LPD: spatial compression or weakened salience of the left hemifield. The perceptual distortions associated with PD are complex. Neglect-like performance by individuals with LPD (or RPD) on line bisection tasks and visual exploration tasks presumably reflect altered attentional processing rather than the relatively low-level visual processes examined in this study.
Highlights.
Left-onset Parkinson’s patients (LPD) perform consistently with left-hemineglect.
In LPD, left-space may be compressed or signals there may be weakened.
Left-space is not compressed in LPD, nor are contrast signals weakened.
Neglect in LPD, if present, may stem from higher order mechanisms such as attention.
Acknowledgments
This research was supported by grants from the National Institute of Neurological Disorders and Stroke, including a Ruth L. Kirschstein National Research Service Award (F31 NS07682) to DJN and RO1 NS067128 to ACG, and by a MOBINT-GRANT from the AGAUR Catalan Agency of University Grants and Research to XGP. We would like to thank all of the individuals who participated in this study. Our recruitment efforts were supported, with our gratitude, by Marie-Saint Hilaire, M.D., and Cathi Thomas, R.N., M.S.N., of Boston Medical Center Neurology Associates, and by Boston area Parkinson’s disease support groups and the Michael J. Fox Trial Finder. We thank Victoria Nguyen, Catherine Munro, Chelsea Toner, Laura Pistorino, Matthew DiBiase, and Sandy Neargarder, Ph.D., for their assistance on this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albert ML. A simple test of visual neglect. Neurology. 1973;23:658–664. doi: 10.1212/WNL.23.6.658. [DOI] [PubMed] [Google Scholar]
- Amick MM, Cronin-Golomb A, Gilmore GC. Visual processing of rapidly presented stimuli is normalized in Parkinson’s disease when proximal stimulus strength is enhanced. Vision Research. 2003;43:2827–35. doi: 10.1016/s0042-6989(03)00476-0. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Beck Anxiety Inventory Manual. San Antonio, TX: Psychological Corporation; 1993. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spatial Vision. 1997;10:433–436. doi: 10.1163/156856897X00357. [DOI] [PubMed] [Google Scholar]
- Bodis-Wollner I, Marx MS, Mitra S, Bobak P, Mylin L, Yahr M. Visual dysfunction in Parkinson’s disease: Loss in spatiotemporal contrast sensitivity. Brain. 1987;110:1675–1698. doi: 10.1093/brain/110.6.1675. [DOI] [PubMed] [Google Scholar]
- Boynton GM, Demb JB, Glover GH, Heeger DJ. Neuronal basis of contrast discrimination. Vision Research. 1999;39:257–69. doi: 10.1016/s0042-6989(98)00113-8. [DOI] [PubMed] [Google Scholar]
- Cronin-Golomb A. Parkinson’s disease as a disconnection syndrome. Neuropsychology Review. 2010;20:191–208. doi: 10.1007/s11065-010-9128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin-Golomb A. Emergence of nonmotor symptoms as the focus of research and treatment of Parkinson’s disease: introduction to the special section on nonmotor dysfunctions in Parkinson’s disease. Behavioral Neuroscience. 2013;127:135–138. doi: 10.1037/a0032142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidsdottir S, Wagenaar R, Young D, Cronin-Golomb A. Impact of optic flow perception and egocentric coordinates on veering in Parkinson’s disease. Brain. 2008;131:2882–2893. doi: 10.1093/brain/awn237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebersbach G, Trottenberg T, Hättig H, Schelosky L, Schrag A, Poewe W. Directional bias of initial visual exploration. A symptom of neglect in Parkinson’s disease. Brain. 1996;119:79–87. doi: 10.1093/brain/119.1.79. [DOI] [PubMed] [Google Scholar]
- Edgar GK, Smith AT. Hemifield differences in perceived spatial frequency. Perception. 1990;19:759–766. doi: 10.1068/p190759. [DOI] [PubMed] [Google Scholar]
- Georgeson MA, Sullivan GD. Contrast constancy: Deblurring in human vision by spatial frequency channels. The Journal of Physiology. 1975;252:627–656. doi: 10.1113/jphysiol.1975.sp011162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guariglia P, Matano A, Piccardi L. Bisecting or not bisecting: this is the neglect question. Line bisection performance in the diagnosis of neglect in right brain-damaged patients. PLoS One. 2014;9:e99700. doi: 10.1371/journal.pone.0099700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JP, Atkinson EA, Lee AC, Nithi K, Fowler MS. Hemispace differences in the visual perception of size in left hemiParkinson’s disease. Neuropsychologia. 2003;41:795–807. doi: 10.1016/S0028-3932(02)00285-3. [DOI] [PubMed] [Google Scholar]
- Hart BM, Schmidt HC, Klein-Harmeyer I, Einhauser W. Attention in natural scenes: contrast affects rapid visual processing and fixations alike. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2013;368:20130067. doi: 10.1098/rstb.2013.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupersmith MJ, Shakin E, Siegel IM, Lieberman A. Visual system abnormalities in patients with Parkinson’s disease. Archives of Neurology. 1982;39:284–286. doi: 10.1001/archneur.1982.00510170026007. [DOI] [PubMed] [Google Scholar]
- Laudate TM, Neargarder S, Cronin-Golomb A. Line bisection in Parkinson’s disease: investigation of contributions of visual field, retinal vision, and scanning patterns to visuospatial function. Behavioral Neuroscience. 2013;127:151–163. doi: 10.1037/a0031618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AC, Harris JP, Atkinson EA, Fowler MS. Evidence from a line bisection task for visuospatial neglect in left hemiparkinson’s disease. Vision Research. 2001;41(20):2677–2686. doi: 10.1016/S0042-6989(01)00129-8. [DOI] [PubMed] [Google Scholar]
- Norton DJ, Munro CE, Cronin-Golomb A. Horizontal and vertical line bisection biases in left-onset Parkinson’s disease are independent of saccadic abnormalities. (submitted) [Google Scholar]
- Movement Disorders Society Task Force on Rating Scales for Parkinson’s Disease. The Unified Parkinson’s Disease Rating Scale (UPDRS): Status and recommendations. Movement Disorders. 2003;18:738– 750. doi: 10.1002/mds.10473. [DOI] [PubMed] [Google Scholar]
- Pereira JB, Junqué C, Martí MJ, Ramirez-Ruiz B, Bargalló N, Tolosa E. Neuroanatomical substrate of visuospatial and visuoperceptual impairment in Parkinson’s disease. Movement Disorders. 2009;24:1193–9. doi: 10.1002/mds.22560. [DOI] [PubMed] [Google Scholar]
- Pieri V, Diederich NJ, Raman R, Goetz CG. Decreased color discrimination and contrast sensitivity in Parkinson’s disease. Journal of the Neurological Sciences. 2000;172:7–11. doi: 10.1016/S0022-510X(99)00204-X. [DOI] [PubMed] [Google Scholar]
- Price MJ, Feldman RG, Adelberg D, Kayne H. Abnormalities in color vision and contrast sensitivity in Parkinson’s disease. Neurology. 1992;42:887–890. doi: 10.1212/WNL.42.4.887. [DOI] [PubMed] [Google Scholar]
- Robson J, Campbell F, editors. A quick demonstration of your own contrast sensitivity function. New York: New York Arts Magazine; 1997. [Google Scholar]
- Seichepine DR, Neargarder S, Davidsdottir S, Reynolds GO, Cronin-Golomb A. Side and type of initial motor symptom influences visuospatial functioning in Parkinson’s disease. Journal of Parkinson’s Disease. doi: 10.3233/JPD-140365. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seichepine DR, Neargarder S, Miller IN, Riedel TM, Gilmore GC, Cronin-Golomb A. Relation of Parkinson’s disease subtypes to visual activities of daily living. Journal of the International Neuropsychological Society. 2011;1:841–52. doi: 10.1017/S1355617711000853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Sano M, Paulson J, Mayeux R. Modified Mini-Mental State Examination: Validity and reliability. Neurology. 1987;37(Suppl 1):S179. [Google Scholar]
- Villardita C, Smirni P, Zappala G. Visual neglect in Parkinson’s disease. Archives of Neurology. 1983;40(12):737–739. doi: 10.1001/archneur.1983.04050110055008. [DOI] [PubMed] [Google Scholar]
- Watson AB, Pelli DG. QUEST: A Bayesian adaptive psychometric method. Perception & Psychophysics. 1983;33(2):113–120. doi: 10.3758/BF03202828. [DOI] [PubMed] [Google Scholar]
- Wilkinson G. Wide Range. Wilmington; DE: 1993. WRAT-3: Wide Range Achievement Test administration manual. [Google Scholar]