Abstract
Objectives
We sought to elucidate the risks for access site-related complications (ASC) following percutaneous lower extremity revascularization and to evaluate benefit of routine ultrasound-guided access (RUS) in decreasing ASC.
Methods
We reviewed all consecutive percutaneous revascularizations (PTA or stent) performed for lower extremity atherosclerosis at our institution from 2002–2012. RUS began September 2007. Primary outcome was any ASC (bleeding, groin or retroperitoneal [RP] hematoma, vessel rupture, or thrombosis). Multivariable logistic regression was used to determine predictors of ASC.
Results
A total of 1,371 punctures were performed on 877 patients (43% women, median age 69 [IQR 60–78] yrs for claudication (29%), critical limb ischemia (59%), or bypass graft stenosis (12%) using 4–8Fr sheaths. There were 72 ASCs (5%): 52 instances of bleeding or groin hematoma, 9 pseudoaneurysms, 8 retroperitoneal hematomas, 2 artery lacerations, and 1 thrombosis. ASC were less frequent when RUS was used (4% vs. 7%, P=.02). Multivariable predictors of ASC were age >75 years (OR 2.0, 95% CI 1.1–3.7, P=.03), CHF (OR 1.9, 95% CI 1.1–1.3, P=.02), preoperative warfarin use (OR 2.0, 95% CI 1.1–3.5, P=.02), & RUS (OR 0.4, 95% CI 0.2–0.7, P<.01). Vascular closure devices (VCDs) were not associated with lower rates of ASCs (OR 1.1, 95% CI 0.2–0.7, P<.01). RUS lowered ASCs in those >75 years (5% vs. 12%, P<.01) but not those on warfarin preoperatively (10% vs. 13%, P=.47). RUS did not decrease VCD failure (6% vs. 4%, P=.79).
Conclusions
We were able to decrease the rate of ASCs during lower extremity revascularization with the implementation of RUS. VCDs did not impact ASCs. Particular care should be taken on patients >75 years old, those with CHF, and those on warfarin.
INTRODUCTION
Vascular access site-related complications (ASCs) are a major cause of perioperative morbidity and mortality among patients undergoing percutaneous endovascular intervention. Occurring at a rate of 1–9% in contemporary series of coronary angiography/intervention1,2, the effects of ASCs include not only prolonged hospital stay, patient discomfort, and higher healthcare costs, but increased mortality rates even one year post-procedure2–4.
Although well described in the interventional cardiology literature, the data on the incidence and risk factors for ASCs among patients undergoing lower extremity revascularization is relatively sparse. Relative to patients with coronary artery disease, patients with peripheral vascular disease may be more likely to have atherosclerosis affecting the common femoral artery. Vascular surgeons in our division routinely use ultrasound for all percutaneous procedures to improve our ability to access the intended artery in the best location. Thus, the objectives of this study were to describe the prevalence and predictors of access site related complications in patients undergoing percutaneous lower extremity revascularization and to evaluate the potential benefit of routine ultrasound-guided access (RUS) in decreasing the rate of ASCs and potentially improving the technical success of vascular closure devices (VCDs).
METHODS
Subjects and settings
The Beth Israel Deaconess Medical Center institutional review board approved this study. Patient consent was not required since this was a retrospective chart review that did not involve using any patient identifiers. We performed a retrospective chart review on all consecutive patients undergoing lower extremity percutaneous revascularization procedures from August 2002 through October 2012. Patients were identified using Current Procedural Terminology (CPT) codes for angioplasty and/or atherectomy, ± stenting of the lower extremities, including the iliac arteries. Indications for intervention included intermittent claudication, critical limb ischemia (rest pain or tissue loss), and stenosis/impending graft failure of an existing bypass graft as documented on surveillance duplex. Procedures performed for acute limb ischemia or for reasons other than peripheral arterial occlusive disease (e.g. trauma, aneurysms) were excluded. Only cases utilizing common femoral artery (CFA) access were included. Procedures were performed through 4–8Fr sheaths preferentially through retrograde contralateral and occasionally anterograde ipsilateral access. Routine ultrasound guided access (RUS), defined as universal usage in all consecutive patients, was instituted by the vascular surgeons at our institution in September 2007. Prior to this period, ultrasound guided access was used infrequently and only selectively. Arterial closure was either by direct manual compression or through the use of a vascular closure device (we use most frequently the Perclose Proglide, Abbott Laboratories, Redwood City, CA). Unless contraindicated, patients who were on warfarin preoperatively were instructed to discontinue it 5 days prior to their procedure for elective procedures. Preoperative International Normalized Ratio (INR) was not routinely measured and therefore not reported here. Intraoperatively, patients were heparinized with 80–100 units per kilogram and activated clotting times were typically maintained > 250 seconds for iliofemoral and >300 seconds for tibial interventions. Postoperatively, patients were typically loaded with clopidogrel 300mg and then maintained on clopidogrel 75mg/day for 30 days and aspirin 325mg/day indefinitely thereafter. Clopidogrel was not typically loaded preoperatively as the patients who are not intervened upon percutaneously often undergo surgical bypass shortly thereafter.
Measurements and Outcomes
We identified the following types of access site-related complications (ASCs): bleeding or groin hematomas that required transfusion, led to hemodynamic instability, or caused an increased length of stay; pseudoaneurysm (diagnosed by ultrasound); retroperitoneal hematoma (diagnosed by computer tomography); artery laceration or rupture (diagnosed intraoperatively); and thrombosis. Procedural success was defined as residual stenosis <30% as assessed on single-view completion angiography. Procedures were defined as multilevel if lesions in more than one vascular bed (aortoiliac, femoro-popliteal, tibial, etc.) were intervened upon. Vascular closure device (VCD) failure was defined as inadequate hemostasis, failure of the device to deploy, or occlusion of the common femoral artery after deployment. Academic year was divided into two halves: the first half from July through December and the second from January through June. Information on post-discharge mortality was obtained using the Social Security Death Index.
Ultrasound Technique
At our institution, nearly all punctures are performed by the vascular surgery fellows (and occasionally general surgery residents under supervision of the fellow). Each July, the junior fellow is taught the following technique by his or her senior fellow: After sterile draping, the ultrasound probe is used to visualize the common femoral artery. The femoral bifurcation is first identified with a transverse view. Keeping the bifurcation in focus, the view is switched to longitudinal and the femoral bifurcation and femoral head are identified giving a clear view of the common femoral artery. The probe is maintained in a position parallel with the table, which requires substantial pressure in an obese groin. This avoids the misperception of an external iliac artery appearing to be a flat common femoral artery over the femoral head. Keeping the bifurcation at the inferior edge of the screen also aids in avoiding a high puncture. Care is taken to identify a segment of common femoral artery with a minimum of plaque and calcification not only on the anterior wall but the posterior wall as well which could interfere with the function of the Proglide closure device. A nick in the skin is made with an 11 blade and the subcutaneous tissue along the course of the wire is spread with a straight snap under ultrasound surveillance. This creates a tract for the suture mediated closure device to freely pass down to the artery at completion of the procedure. A micropuncture needle is then inserted under ultrasound guidance taking care to see the entire length of the needle as well as the length of the common femoral artery filling the ultrasound screen with the long view. (Note: during the earlier years of the study (2002–2004), 19 gauge needles were often used rather than micropuncture needles). This maneuver has a learning curve but assures an anterior wall puncture. After micropuncture needle access and 0.018 inch wire passage the ultrasound image demonstrating this is stored and fluoroscopy is used to confirm puncture over the femoral head. We feel that ultrasound alone cannot exclude a high puncture. If fluoroscopy demonstrates puncture above the femoral head, the needle is removed and re-inserted lower taking care to assure that the femoral bifurcation is seen together with the needle.
Statistical Analysis
All analyses were performed on a per-puncture and intention-to-treat basis. Thus, if an attempt at closure was made with a VCD, the puncture was categorized as such, even if the VCD failed and hemostasis was ultimately achieved via manual compression. Patient demographics and outcomes were reported as absolute numbers and percentages. Pearson chi-square and Fisher’s exact test were used for comparisons of categorical variables. Means of continuous variables were compared using the Student t-test assuming equal variances whereas medians of continuous variables were compared using the Wilcoxon rank sum test. Multivariable logistic regression modeling was used to identify independent predictors of access site complications. Statistical significance was defined as a P-value <.05. All statistical analyses were performed using STATA 12.0 (StataCorp, College Station, Tex).
RESULTS
Patient Characteristics
During the study period there were a total of 925 patients who underwent 1,356 procedures, resulting in 1,419 punctures. Forty-eight punctures were excluded due to intentional non-CFA access; these included 36 brachial, 9 bypass graft, 2 superficial femoral artery, and 1 axillary artery access procedures. The study population thus included 1,371 punctures (43% women, mean age 69 ± 12 years). Indications for the procedure were claudication in 29%, critical limb ischemia in 59%, and bypass graft stenosis/failure in the remaining 12%. Routine ultrasound-guided access was used for 924 (67%) punctures. The proportion of punctures obtained under ultrasound guidance increased over time (Table I). Access closure was obtained via manual compression in 49% punctures and the use of vascular closure device in 47%. The method of closure was undocumented in the remaining 4% of punctures. However, of note, >50% of these were performed in the first four years of the study, suggesting closure was more likely to have been via manual compression. In univariate analysis, patients who underwent RUS were less likely to be obese and have CHF and more likely to have hyperlipidemia and diabetes, have a past smoking history, and be dialysis-dependent (see Table II).
TABLE I.
Number and proportion of punctures obtained under ultrasound guidance over time.
| Year | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total number of punctures | 10 | 30 | 109 | 180 | 64 | 59 | 186 | 189 | 183 | 232 | 129 |
| Number in which ultrasound was used | 0 | 2 | 1 | 2 | 3 | 33 | 164 | 180 | 182 | 229 | 128 |
| Percent in which ultrasound was used | 0.0 | 6.7 | 0.9 | 1.1 | 4.7 | 55.9 | 88.2 | 95.2 | 99.5 | 98.7 | 99.2 |
TABLE II.
Comparison of demographics, baseline comorbidities, and preoperative anticoagulant use between patients who underwent routine ultrasound guided access vs. those who did not.
| Non-RUS N=447 (33%) |
RUS N=924 (67%) |
P | |
|---|---|---|---|
| No. (%) | No. (%) | ||
| Age | |||
| <65 years | 164 (34) | 321 (35) | |
| 65–74 years | 107 (24) | 261 (28) | 0.24 |
| >75 years | 176 (39) | 342 (37) | |
| Female | 190 (42) | 405 (44) | 0.68 |
| BMI | |||
| Underweight (BMI <18.5) | 26 (6) | 36 (4) | |
| Normal weight (BMI 18.5–24.9) | 124 (28) | 262 (28) | |
| Overweight (BMI 25–29.5) | 107 (24) | 301 (33) | |
| Obese (BMI 30+) | 190 (42) | 325 (35) | |
| HTN | 346 (80) | 723 (78) | 0.57 |
| HPL | 229 (53) | 594 (64) | <.01 |
| DM | 236 (54) | 590 (64) | <.01 |
| CAD | 212 (49) | 406 (44) | 0.09 |
| Hx of MI | 86 (20) | 164 (18) | 0.37 |
| Afib | 59 (14) | 130 (14) | 0.87 |
| Hx TIA | 19 (4) | 34 (4) | 0.55 |
| Hx CVA | 59 (14) | 98 (11) | 0.12 |
| CRI | 60 (14) | 164 (18) | 0.07 |
| ESRD on HD | 31 (7) | 100 (11) | 0.04 |
| CHF | 96 (22) | 160 (17) | 0.04 |
| COPD | 47 (11) | 93 (10) | 0.70 |
| Current smoker | 85 (21) | 162 (18) | 0.25 |
| Prior smoker | 139 (38) | 390 (45) | 0.02 |
| Preoperative Anticoagulants | |||
| Aspirin | 225 (65) | 644 (70) | 0.06 |
| Clopidogrel | 128 (37) | 345 (38) | 0.80 |
| Warfarin | 61 (17) | 145 (16) | 0.67 |
Afib, atrial fibrillation; BMI, body mass index; CAD, coronary artery disease; CRI, chronic renal insufficiency; COPD, chronic obstructive pulmonary disorder; CVA, cerebrovascular accident; DM, diabetes mellitus; ESRD, end stage renal disease; HD, hemodialysis; HPL, hyperlipidemia; HTN, hypertension; Hx, history; MI, myocardial infarction; RUS, routine ultrasound guided access; TIA, transient ischemic attack
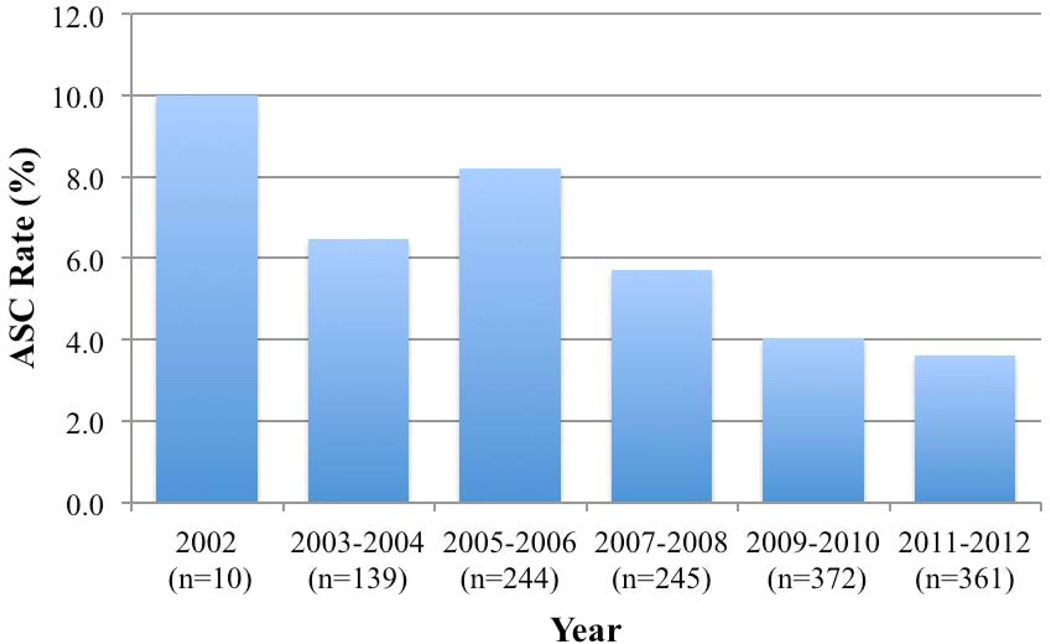
Access site-related complications (ASCs) occurred in 72 patients (5%): 52 incidences of bleeding/groin hematoma, 9 pseudoaneurysms, 8 retroperitoneal hematomas, 2 artery lacerations, and 1 thrombotic complication. Additional procedures were performed for 22 complications: 2 complications were recognized intraoperatively and immediately addressed while 11 patients returned to the operating room after their complications were identified postoperatively. All 9 patients who developed pseudoaneurysms were treated with ultrasound-guided thrombin injection. Figure 1 shows the rate of ASCs over time.
FIGURE 1.
ASC rates by year of study. The total number of procedures per period is indicated in parentheses. Routine ultrasound-guided access was initiated in September of 2007.
In general, demographics and baseline comorbidities were similar between patients who developed ASCs and those who did not (Table III). However, patients who had ASCs were significantly older (median age 73 vs. 69 years, P<.01), more likely to have congestive heart failure (35% vs. 18%, P<.01), and were more likely to be on warfarin preoperatively (31% vs. 15%, P<.01). Notable, factors that did not have a significant association with access-related complications were gender, body mass index (BMI), tobacco use, and the preoperative use of aspirin and clopidogrel.
TABLE III.
Comparison of demographics, baseline comorbidities, and preoperative anticoagulant use between patients who developed access site complications and those who did not.
| Access Site Complication N=72 (5%) |
No Access Site Complication N=1299 (95%) |
P | |
|---|---|---|---|
| No. (%) | No. (%) | ||
| Age | |||
| <65 years | 15 (21) | 470 (36) | |
| 65–74 years | 20 (28) | 348 (27) | 0.01 |
| ≥ 75 years | 37 (51) | 481 (37) | |
| Female | 36 (50) | 559 (43) | 0.27 |
| BMI | |||
| Underweight (BMI <18.5) | 26 (6) | 58 (5) | |
| Normal weight (BMI 18.5–24.9) | 29 (28) | 368 (31) | 0.77 |
| Overweight (BMI 25–29.5) | 17 (37) | 384 (32) | |
| Obese (BMI ≥30) | 39 (29) | 382 (32) | |
| HTN | 56 (78) | 1013 (79) | 0.88 |
| HPL | 37 (51) | 786 (61) | 0.11 |
| DM | 39 (34) | 787 (61) | 0.26 |
| CAD | 38 (53) | 580 (45) | 0.23 |
| Hx of MI | 15 (21) | 235 (18) | 0.64 |
| Afib | 13 (18) | 176 (14) | 0.30 |
| Hx TIA | 3 (4) | 50 (4) | 0.76 |
| Hx CVA | 7 (10) | 150 (12) | 0.71 |
| CRI | 8 (11) | 216 (17) | 0.25 |
| ESRD on HD | 6 (8) | 125 (10) | 0.84 |
| CHF | 25 (35) | 231 (18) | <.01 |
| COPD | 4 (6) | 136 (11) | 0.23 |
| Current smoker | 16 (23) | 231 (19) | 0.43 |
| Prior smoker | 24 (37) | 505 (44) | 0.31 |
| Preoperative Anticoagulants | |||
| Aspirin | 53 (77) | 816 (68) | 0.18 |
| Clopidogrel | 28 (41) | 445 (37) | 0.61 |
| Warfarin | 22 (31) | 184 (15) | <.01 |
Afib, atrial fibrillation; BMI, body mass index; CAD, coronary artery disease; CRI, chronic renal insufficiency; COPD, chronic obstructive pulmonary disorder; CVA, cerebrovascular accident; DM, diabetes mellitus; ESRD, end stage renal disease; HD, hemodialysis; HPL, hyperlipidemia; HTN, hypertension; Hx, history; MI, myocardial infarction; TIA, transient ischemic attack
Procedural Characteristics
On univariate analysis, there was no difference in ASC rates based on various procedural characteristics such retrograde vs. anterograde access, procedural success, multilevel intervention, or protamine reversal (Table IV). Operator experience did not appear to be associated with an excess of ASCs, as rates were similar between the first and second halves of the academic year (6% vs. 5%). Procedure-related factors that were associated with lower rates of ASCs were the use of ultrasound-guided access (4% vs. 7%) and the use of vascular closure devices (4% vs. 6%). Failure of the VCD occurred in 40 (6%) cases of which 6 (15%) developed an ASC.
TABLE IV.
Comparison of access site-related complication rates for various procedure-related characteristics.
| No. (%) | P | |
|---|---|---|
| Indication | ||
| Claudication | 15 (4) | 0.16 |
| Critical Limb Ischemia | 45 (6) | |
| Bypass graft failure/Restenosis of native vessel | 12 (7) | |
| Ultrasound-guided access | ||
| Used | 33 (4) | 0.02 |
| Not used | 39 (7) | |
| Access direction | ||
| Retrograde | 59 (5) | 0.13 |
| Antegrade | 13 (8) | |
| Sheath Size | ||
| <5 Fr | 3 (6) | 0.06 |
| 5 Fr | 26 (4) | |
| 6 Fr | 39 (7) | |
| >6 Fr | 4 (5) | |
| Technical Success | ||
| Achieved | 71 (4) | 1.00 |
| Failed | 1 (5) | |
| Level | ||
| Single level | 44 (5) | 0.24 |
| Multilevel | 28 (6) | |
| Closure | ||
| Manual compression | 38 (6) | 0.24 |
| Vascular closure device | 29 (4) | |
| Unknown | 5 (9) | |
| Reversal with protamine | ||
| Used | 5 (6) | 0.80 |
| Not used | 67 (5) | |
| Academic Period | ||
| July – December | 38 (6) | 0.40 |
| January – June | 34 (5) |
Results of our multivariable logistic regression model indicated that independent predictors of access site-related complications were age >75 years (OR 2.0, 95% CI 1.1–3.7), CHF (OR 1.9, 95% CI 1.1–3.5), and preoperative warfarin use (OR 2.0, 95% CI 1.1–3.5) (Table V). Routine ultrasound surveillance significantly decreased the odds of having an access complication (OR 0.4, 95% CI 0.2–0.7) whereas vascular closure devices did not (OR 1.1, 95% CI 0.6–1.9).
TABLE V.
Multivariable predictors of access site-related complications.
| OR | 95% CI | P | |
|---|---|---|---|
| Age | |||
| <65 years | - | - | - |
| 65–74 years | 1.4 | 0.7–3.0 | 0.32 |
| ≥ 75 years | 2.0 | 1.1–3.7 | 0.03 |
| CHF | 1.9 | 1.1–3.3 | 0.02 |
| Preoperative warfarin use | 2.0 | 1.1–3.5 | 0.02 |
| VCD vs. MC | 1.1 | 0.6–1.9 | 0.79 |
| RUS | 0.4 | 0.2–0.7 | <.01 |
CHF, congestive heart failure; CI, confidence interval; MC, manual compression; OR, odds ratio; RUS, routine ultrasound-guided access; VCD, vascular closure device
Routine Ultrasound-Guided Access
Differences in ASC rates between patients in whom routine ultrasound-guided access was used and those in whom it was not were analyzed among particularly high-risk groups (Table VI) who had been identified on univariate analysis. RUS appeared to be protective against ASCs for elderly patients (5% vs. 12%, P<.01) but not for those with CHF and those on warfarin preoperatively. Additionally, RUS did not appear to improve VCD failure rates (6% vs. 4%, P=.79).
TABLE VI.
Comparison of access site-related complication (ASC) rates among high risk groups in whom routine ultrasound-guided access (RUS) was used vs. those in whom it was not
| No RUS (%) |
RUS (%) |
P | |
|---|---|---|---|
| Overall ASC rate | 7 | 4 | 0.02 |
| ASC rate in elderly (≥ 75yrs) patients | 12 | 5 | <.01 |
| ASC rate in patients with CHF | 13 | 7 | 0.13 |
| ASC rate in patients on warfarin preoperatively | 13 | 10 | 0.47 |
| VCD failure rate | 4 | 6 | 0.79 |
ASC, access site-related complication; CHF, congestive heart failure; RUS, routine ultrasound-guided access; VCD, vascular closure device
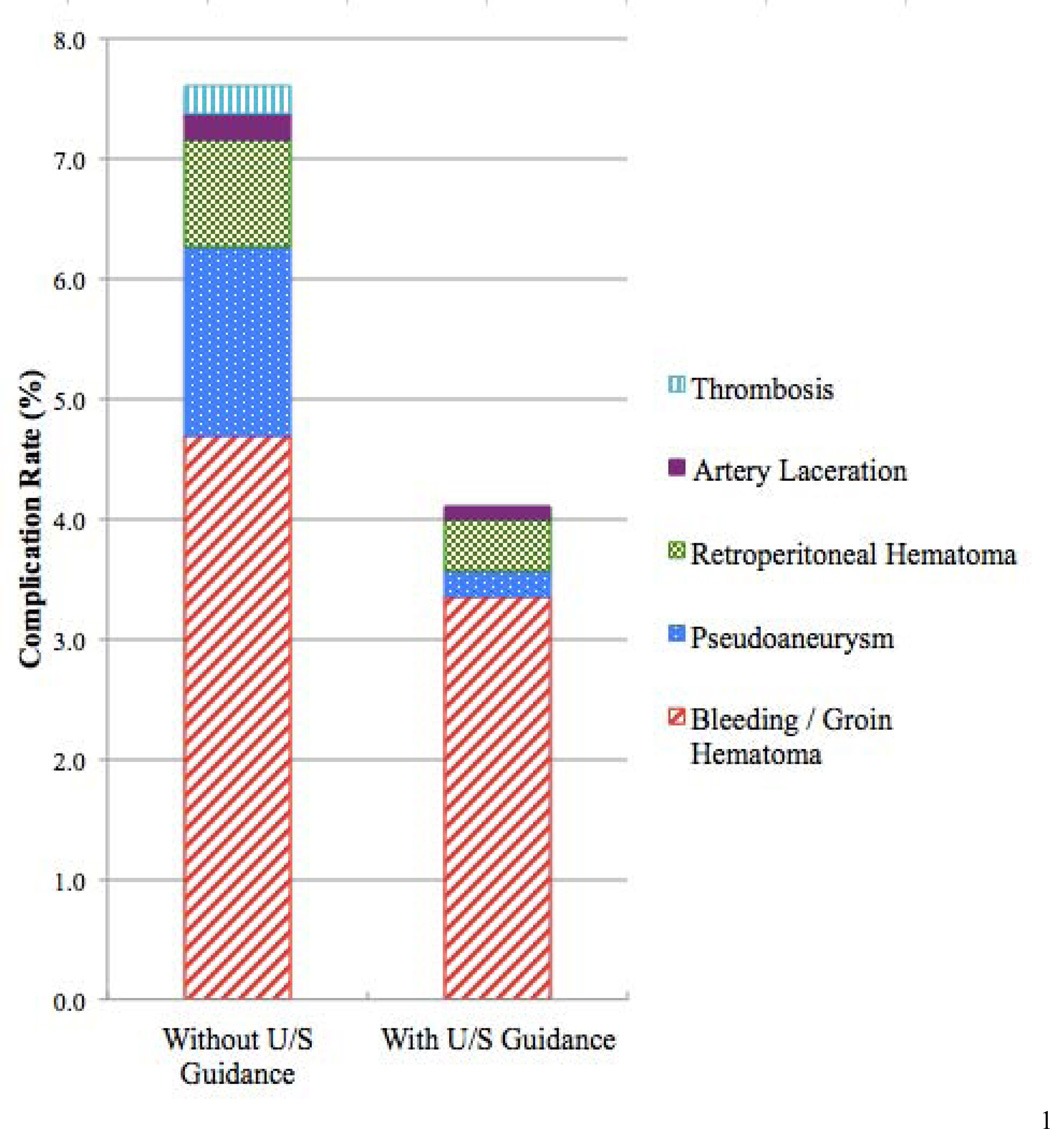
When the specific types of ASCs were analyzed (Figure 2), we found that the complication that had the greatest reduction in incidence after RUS was pseudoaneurysm development. Pseudoaneurysm occurrence was 86% lower in patients in whom RUS was used. Retroperitoneal hematomas and artery lacerations were both reduced by 52% and bleeding/groin hematomas by 29%. There were no thrombotic complications when RUS was used.
FIGURE 2.
Break down of types of access site-related complications with and without routine ultrasound guided access.
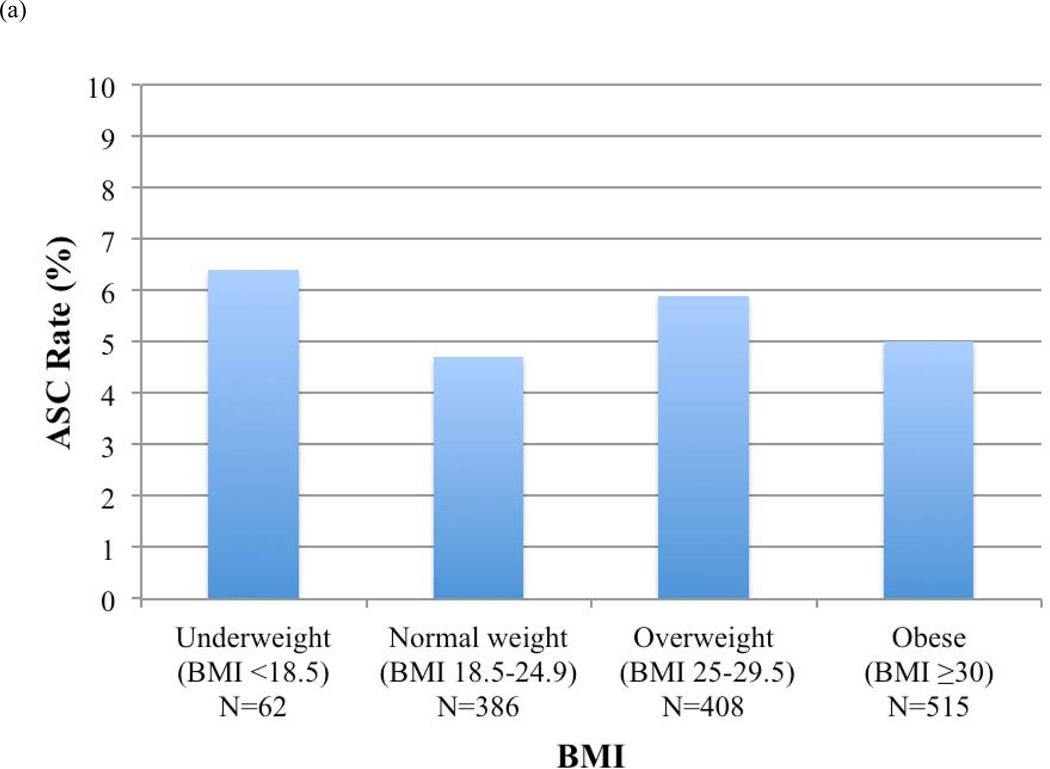
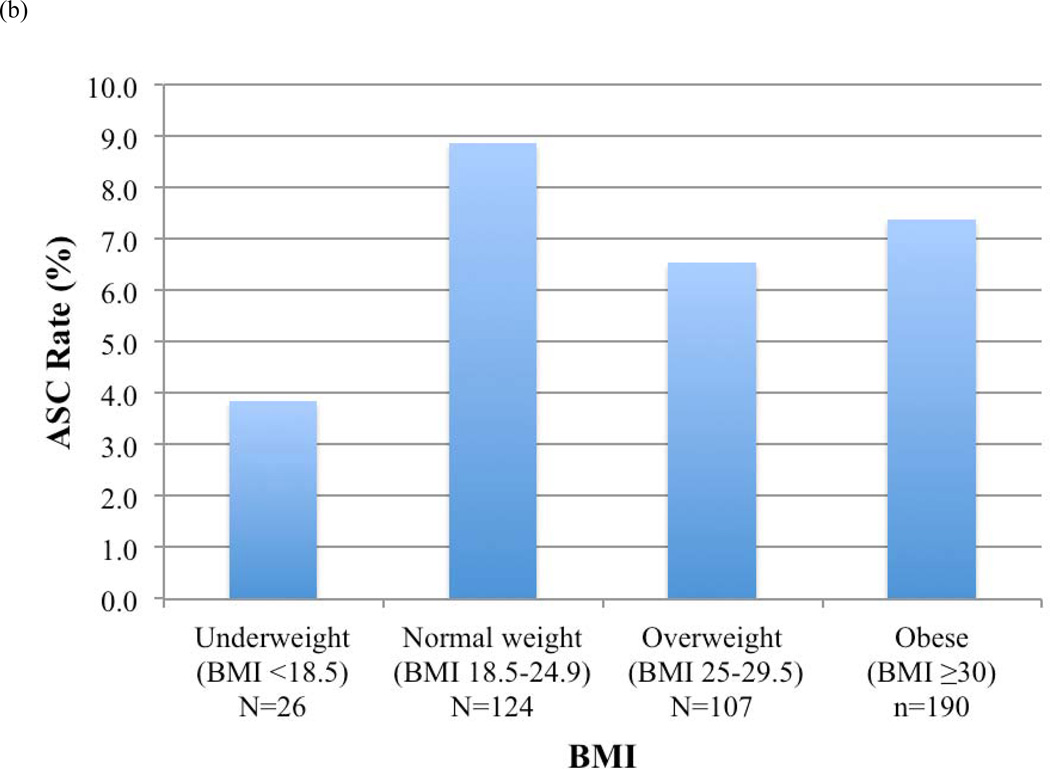
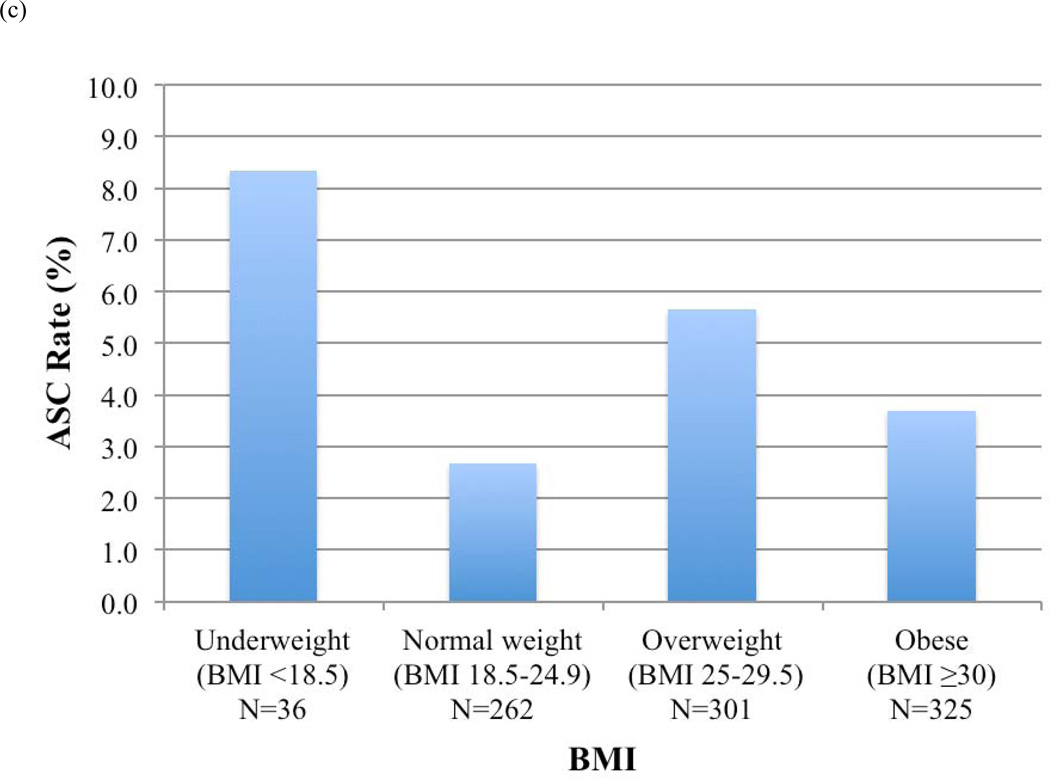
ASC rates before and after RUS for each BMI category were calculated (Figure 3). Overall, patients who were underweight (BMI <18.5) had the highest ASC rates. Rates of ASCs decreased for normal weight (9% vs. 3%, P=.01), overweight (7% vs. 6%, P=.81), and obese patients (7% vs. 4%, P=.09) in whom RUS was used, but not for underweight patients.
FIGURE 3.
Access site-related complications (ASCs) by body mass index (BMI) category (a) in all patients, (b) in patients in whom routine ultrasound-guided access was not used, and (c) in patients in whom routine ultrasound-guided access was used
DISCUSSION
In our series of femoral punctures for lower extremity peripheral vascular intervention, access site-related complications occurred at a rate of 5%. Independent predictors of ASCs were age, CHF, and preoperative warfarin use. RUS more than halved the odds of developing an ASC but VCDs were not protective. Among high-risk groups, RUS was able to decrease ASC rates for elderly patients but not those on warfarin or which CHF. Finally, RUS did not improve VCD failure rates.
Advanced age (≥ 75 years), CHF, and preoperative warfarin use are risk factors that have been previously described by other authors1. Age and comorbidities such as CHF are likely surrogate markers for more advanced atherosclerotic disease and vessel calcification. Although patients in our study were instructed to stop warfarin preoperatively, it has been shown that the INR in up to 7% of patients may not normalize even when warfarin is held 6 days in advance of surgery5. Furthermore, even when the INR is confirmed to have normalized, patients on chronic warfarin therapy still demonstrate increased intraoperative blood loss and requirement for postoperative transfusion6.
Procedural factors previously found to predict higher rates of ASCs are antegrade vs. retrograde approach7, interventional rather than diagnostic procedures8–10, and procedural indication11. Diagnostic angiograms were not included in our study so we are unable to comment on whether a differential association exists. However, we did notice a general trend towards higher ASC rates among patients with more advanced or complex disease. For example, though the differences were non-significant, there were higher complication rates among patients with critical limb ischemia vs. those with claudication, among procedures performed via an antegrade vs. retrograde approach, among patients who had multilevel vs. single level disease, and among procedures that were technically unsuccessful. All these variables appear to be proxies for a heavier burden of disease.
Micropuncture needles were admittedly used more frequently in the latter half of the study period. However, the needle gauge would only be expected to influence complications that arose from accidental posterior wall arteriotomies, since the anterior wall arteriotomy is dilated to the size of the eventual sheath used. Additionaly, given that no differences in ASC rates were identified among sheaths sized 4–8Fr (a range between1.3mm to 2.7mm), increasing the gauge of the puncture needle from 19 gauge to 21 gauge (a 0.2mm difference) would not be expected to significantly affect ASC rates either.
After controlling for other relevant variables, we did not find VCDs to be associated with lower ASC rates. Despite their widespread use and increasing popularity12, VCDs have in fact never been conclusively shown to decrease ASC rates relative to manual compression13–16. While they do reduce the time to hemostasis and patient mobility, VCDs may in fact increase the rate of vascular complications by 5-fold when they fail17, 18. This certainly was the case in our study, in which VCD failure was associated with a 15% ASC rate. RUS did not appear to decrease VCD failure rates, but this is perhaps because failure of suture-mediated devices is often due to device mishandling or defect (suture breakage or sutures pulling through the arterial wall) in addition to vessel calcification19–21. In fact, in their randomized controlled trial comparing the use of suture-mediated closure devices vs. manual compression for endovascular interventions performed for peripheral vascular disease, Starnes et al. reported no association of calcified plaque to vascular access site or device complications. Interestingly, neither of the two patients who developed major access site complications in their study had evidence of calcified plaque in the femoral vessels.
Given that patient factors are rarely modifiable and VCDs, despite their other benefits, have not made a demonstrable difference in vascular complication rates, arguably the best target for reducing ASC rates is improving femoral puncture technique. Indeed, data from our institution show that the most significant multivariable predictor of developing a vascular access-related complication was whether routine ultrasound guidance was used for puncture. When using external anatomic or fluoroscopic landmarks to guide access, the optimal location of femoral puncture is missed in approximately 13% of patients undergoing femoral access22.
Considering the association of retroperitoneal hematomas with high punctures23, 24 and pseudoaneurysms with low punctures25, it naturally follows that the benefit of using ultrasound guidance to identify the optimal location for femoral arteriotomy would be most evident in these two complications. Looking at individual categories of ASCs, we confirmed this to be the case, as we found that RUS had the greatest impact in decreasing the development of retroperitoneal hematomas and pseudoaneurysms.
With regard to BMI, RUS reduced ASC rates in patients of all BMI categories except those who were underweight, who in fact had the highest ASC rates overall. Although not intuitive, this phenomenon has in fact been previously described by multiple authors studying the association between BMI and outcomes after percutaneous coronary interventions1, 26–29. This so-called “obesity paradox”, in which the distribution of postoperative complications assumes a U-shape, with the highest rates of complications at the extremes of BMIs and the lowest rates of complications occurring in obese patients, may be explained by several potential factors. Patients with lower BMI may have less intravascular reserve and therefore be more prone to manifesting hemodynamic changes and requiring blood transfusion. Similarly, patients with lower BMIs may have commensurately lower creatinine clearance, which can lead to higher relative concentrations of anti-platelet and other anticoagulant medications (e.g. clopidogrel) that are not dosed based on weight.
Ultrasound-guided access requires a certain amount of tissue between the ultrasound probe and the target vessel to best visualize the trajectory of the needle. Thus, thinner patients may not have sufficient space to derive benefit from ultrasound guidance. Perhaps for patients with low BMI, our technique should be modified to simply localize the femoral bifurcation with ultrasound and then perform femoral puncture using the standard Seldinger technique with fluoroscopic confirmation.
Finally, underweight patients are more likely to have smaller femoral vessels more prone to injury secondary to arteriotomy and instrumentation. Indeed, women, who have been shown to have common femoral arteries of smaller diameter and shorter length than men30, have also been consistently shown to have higher rates of ASCs1, 2, 31. Additionally, BMI appears to be a more important predictor of ASCs in women than in men29. Of note, we did not find female gender to be associated with higher ASC rates, but perhaps our study was underpowered to detect a significant difference.
Two multicenter randomized controlled trials have attempted to elucidate the benefit of ultrasound-guided femoral puncture. In the Femoral Arterial Access with Ultrasound Trial (FAUST), Seto et al. compared outcomes of 501 patients randomized to fluoroscopic guided puncture vs. 503 patients randomized to ultrasound guided puncture32. Procedures included coronary (91%) or peripheral (9%) diagnostic angiograms and percutaneous interventions. Ultrasound guidance was associated with fewer numbers of attempts and reduced time for access, higher first-pass rates, and a lower rate of accidental venipuncture. Additionally, ultrasound guidance reduced the risk of vascular access complications by 59% (1.4% vs. 3.4%, p=.041) compared to fluoroscopic guidance.
In a more recent trial, interventional radiologists from two institutions randomized 208 patients undergoing retrograde puncture of the common femoral artery to either ultrasound-guided or palpation-guided puncture33. As with the FAUST trial, higher first pass success rates, fewer total number of attempts required for access, and reduced times to sheath insertion were seen in the group in whom ultrasound-guided puncture was used. Access site complication rates were also lower in the ultrasound group but this difference did not quite reach statistical significance (4% vs. 0%, p=.052). Unfortunately, the procedures performed in this study were done for a wide range of indications, including peripheral artery disease, carotid artery stenosis, subarachnoid hemorrhage, renal artery stenosis, and others, thereby limiting the generalizability of the results. Our study has several limitations. First, this was a single-institution retrospective review. Thus, our results are subject to selection and information bias. Our division currently uses the Perclose Proglide but in the earlier years of this study, several types of VCDs were used. Additionally, unlike ultrasound guidance, which was used routinely after 2007, VCDs were not used in all patients and were in fact typically avoided in patients with extensive common femoral artery calcification depending on surgeon judgment and preference. Thus, the VCD failure rate would likely have been higher without RUS. It is possible that operator experience over time may be responsible for some improvements associated with the institution of RUS. However, fellows (and some residents under supervision of the fellow) perform nearly all punctures and the senior fellow trains the junior fellow in July, which makes this less likely. The switch to micropuncture needles in 2004 may have also contributed to some degree, and we certainly use them because we do believe they may help prevent ASCs, particularly in situations where multiple attempts/punctures are made. However, there was no appreciable decrease in ASC rates that correlated with the adoption of micropuncture needles and only a minor proportion of punctures were performed with 19-gauge needles. Finally, our study was not inclusive of diagnostic angiograms, which are historically associated with fewer vascular complications than interventional procedures given the typically smaller profile and lack of anticoagulation. It would be interesting to see if RUS would similarly demonstrate a reduction in ASC rates in purely diagnostic procedures. This would likely require a much larger cohort. Regardless, we use RUS for diagnostic procedures as well.
CONCLUSION
Among patients undergoing percutaneous lower extremity revascularization, those at highest risk of developing access site-related complications include patients of advanced age (≥ 75 years), patients with CHF, and those on warfarin preoperatively. Routine ultrasound guided puncture of the femoral artery decreases the rate of vascular access complications by optimizing the site of arteriotomy. In contrast, vascular closure devices are not associated with fewer vascular complications and in fact can lead to higher rates of hemorrhage and hematoma when they fail.
Acknowledgments
This work was supported by the NIH T32 Harvard-Longwood Research Training in Vascular Surgery grant HL007734.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosures: Author Disclosures: RC Lo, none; MTM Fokkema, none; T Curran, none; J Darling, none; AD Hamdan, Endologix Consultant; M Wyers, Endologix Consultant, Boston Scientific Consultant; EL Chaikof, none; ML Schermerhorn, Endologix Consultant, Medtronic Consultant.
Presented at the 2013 New England Society for Vascular Surgery Annual Meeting, Stowe, VT
REFERENCES
- 1.Piper WD, Malenka DJ, Ryan TJ, Jr, Shubrooks SJ, O’Connor GT, Jr, Robb JF, et al. Predicting vascular complications in percutaneous coronary interventions. Am Heart J. 2003;145(6):1022–1029. doi: 10.1016/S0002-8703(03)00079-6. [DOI] [PubMed] [Google Scholar]
- 2.Yatskar L, Selzer F, Feit F, Cohen HA, Jacobs AK, Williams DO, et al. Access site hematoma requiring blood transfusion predicts mortality in patients undergoing percutaneous coronary intervention: data from the National Heart, Lung, and Blood Institute Dynamic Registry. Catheter Cardiovasc Interv. 2007;69(7):961–966. doi: 10.1002/ccd.21087. [DOI] [PubMed] [Google Scholar]
- 3.Kinnaird TD, Stabile E, Mintz GS, Lee CW, Canos DA, Gevorkian N, et al. Incidence, predictors, and prognostic implications of bleeding and blood transfusion following percutaneous coronary interventions. Am J Cardiol. 2003;92(8):930–935. doi: 10.1016/s0002-9149(03)00972-x. [DOI] [PubMed] [Google Scholar]
- 4.Romaguera R, Wakabayashi K, Laynez-Carnicero A, Sardi G, Maluenda G, Ben-Dor I, et al. Association between bleeding severity and long-term mortality in patients experiencing vascular complications after percutaneous coronary intervention. Am J Cardiol. 2012;109(1):75–81. doi: 10.1016/j.amjcard.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Schulman S, Elbazi R, Zondag M, O'Donnell M. Clinical factors influencing normalization of prothrombin time after stopping warfarin: a retrospective cohort study. Thromb J. 2008;6:15. doi: 10.1186/1477-9560-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young EY, Ahmadinia K, Bajwa N, Ahn NU. Does chronic warfarin cause increased blood loss and transfusion during lumbar spinal surgery? Spine J. 2013;13(10):1253–1258. doi: 10.1016/j.spinee.2013.05.052. [DOI] [PubMed] [Google Scholar]
- 7.Wheatley BJ, Mansour MA, Grossman PM, Munir K, Cali RF, Gorsuch JM, et al. Complication rates for percutaneous lower extremity arterial antegrade access. Arch Surg. 2011;146(4):432–435. doi: 10.1001/archsurg.2011.47. [DOI] [PubMed] [Google Scholar]
- 8.Muller DW, Shamir KJ, Ellis SG, Topol EJ. Peripheral vascular complications after conventional and complex percutaneous coronary interventional procedures. Am J Cardiol. 1992;69(1):63–68. doi: 10.1016/0002-9149(92)90677-q. [DOI] [PubMed] [Google Scholar]
- 9.Fruhwirth J, Pascher O, Hauser H, Amann W. [Local vascular complications after iatrogenic femoral artery puncture] Wien Klin Wochenschr. 1996;108(7):196–200. [PubMed] [Google Scholar]
- 10.Applegate RJ, Sacrinty MT, Kutcher MA, Kahl FR, Gandhi SK, Santos RM, et al. Trends in vascular complications after diagnostic cardiac catheterization and percutaneous coronary intervention via the femoral artery, 1998 to 2007. JACC Cardiovasc Interv. 2008;1(3):317–326. doi: 10.1016/j.jcin.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 11.Stegemann E, Hoffmann R, Marso S, Stegemann B, Marx N, Lauer T. The frequency of vascular complications associated with the use of vascular closure devices varies by indication for cardiac catheterization. Clin Res Cardiol. 2011;100(9):789–795. doi: 10.1007/s00392-011-0313-4. [DOI] [PubMed] [Google Scholar]
- 12.Subherwal S, Peterson ED, Dai D, Thomas L, Messenger JC, Xian Y, et al. Temporal trends in and factors associated with bleeding complications among patients undergoing percutaneous coronary intervention: a report from the National Cardiovascular Data CathPCI Registry. J Am Coll Cardiol. 2012;59(21):1861–1869. doi: 10.1016/j.jacc.2011.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koreny M, Riedmuller E, Nikfardjam M, Siostrzonek P, Mullner M. Arterial puncture closing devices compared with standard manual compression after cardiac catheterization: systematic review and meta-analysis. Jama. 2004;291(3):350–357. doi: 10.1001/jama.291.3.350. [DOI] [PubMed] [Google Scholar]
- 14.Nikolsky E, Mehran R, Halkin A, Aymong ED, Mintz GS, Lasic Z, et al. Vascular complications associated with arteriotomy closure devices in patients undergoing percutaneous coronary procedures: a meta-analysis. J Am Coll Cardiol. 2004;44(6):1200–1209. doi: 10.1016/j.jacc.2004.06.048. [DOI] [PubMed] [Google Scholar]
- 15.Biancari F, D'Andrea V, Di Marco C, Savino G, Tiozzo V, Catania A. Meta-analysis of randomized trials on the efficacy of vascular closure devices after diagnostic angiography and angioplasty. Am Heart J. 2010;159(4):518–531. doi: 10.1016/j.ahj.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 16.Das R, Ahmed K, Athanasiou T, Morgan RA, Belli AM. Arterial closure devices versus manual compression for femoral haemostasis in interventional radiological procedures: a systematic review and meta-analysis. Cardiovasc Intervent Radiol. 2011;34(4):723–738. doi: 10.1007/s00270-010-9981-0. [DOI] [PubMed] [Google Scholar]
- 17.Bangalore S, Arora NF, Resnic S. Vascular closure device failure: frequency and implications: a propensity-matched analysis. Circ Cardiovasc Interv. 2009;2(6):549–556. doi: 10.1161/CIRCINTERVENTIONS.109.877407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vidi VD, Matheny ME, Govindarajulu US, Normand SL, Robbins SL, Agarwal VV, et al. Vascular closure device failure in contemporary practice. JACC Cardiovasc Interv. 2012;5(8):837–844. doi: 10.1016/j.jcin.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou ZJ, Cui K, Cao SP, Huang Z, Guo ZG, Xiu JC, et al. [Evaluation of two arterial closure devices, Angioseal and Perclose, in coronary catheter interventions] Nan Fang Yi Ke Da Xue Xue Bao. 2011;31(10):1767–1770. [PubMed] [Google Scholar]
- 20.Starnes BW, O'Donnell SD, Gillespie DL, Goff JM, Rosa P, Parker MV, et al. Percutaneous arterial closure in peripheral vascular disease: a prospective randomized evaluation of the Perclose device. Journal of vascular surgery. 2003;38(2):263–271. doi: 10.1016/s0741-5214(03)00291-x. [DOI] [PubMed] [Google Scholar]
- 21.Etezadi V, Katzen BT, Naiem A, Johar A, Wong S, Fuller J, et al. Percutaneous suture-mediated closure versus surgical arteriotomy in endovascular aortic aneurysm repair. J Vasc Interv Radiol. 2011;22(2):142–147. doi: 10.1016/j.jvir.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Pitta SR, Prasad A, Kumar G, Lennon R, Rihal CS, Holmes DR. Location of femoral artery access and correlation with vascular complications. Catheter Cardiovasc Interv. 2011;78(2):294–299. doi: 10.1002/ccd.22827. [DOI] [PubMed] [Google Scholar]
- 23.Ellis SG, Bhatt D, Kapadia S, Lee D, Yen M, Whitlow PL. Correlates and outcomes of retroperitoneal hemorrhage complicating percutaneous coronary intervention. Catheter Cardiovasc Interv. 2006;67(4):541–545. doi: 10.1002/ccd.20671. [DOI] [PubMed] [Google Scholar]
- 24.Tiroch KA, Arora N, Matheny ME, Liu C, Lee TC, Resnic FS. Risk predictors of retroperitoneal hemorrhage following percutaneous coronary intervention. Am J Cardiol. 2008;102(11):1473–1476. doi: 10.1016/j.amjcard.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 25.Kim D, Orron DE, Skillman JJ, Kent KC, Porter DH, Schlam BW, et al. Role of superficial femoral artery puncture in the development of pseudoaneurysm and arteriovenous fistula complicating percutaneous transfemoral cardiac catheterization. Cathet Cardiovasc Diagn. 1992;25(2):91–97. doi: 10.1002/ccd.1810250203. [DOI] [PubMed] [Google Scholar]
- 26.Gurm HS, Whitlow PL, Kip KE. The impact of body mass index on short- and long-term outcomes inpatients undergoing coronary revascularization Insights from the bypass angioplasty revascularization investigation (BARI) J Am Coll Cardiol. 2002;39(5):834–840. doi: 10.1016/s0735-1097(02)01687-x. [DOI] [PubMed] [Google Scholar]
- 27.Gurm HS, Brennan DM, Booth J, Tcheng JE, Lincoff AM, Topol EJ. Impact of body mass index on outcome after percutaneous coronary intervention (the obesity paradox) Am J Cardiol. 2002;90(1):42–45. doi: 10.1016/s0002-9149(02)02384-6. [DOI] [PubMed] [Google Scholar]
- 28.Cox N, Resnic FS, Popma JJ, Simon DI, Eisenhauer AC, Rogers C. Comparison of the risk of vascular complications associated with femoral and radial access coronary catheterization procedures in obese versus nonobese patients. Am J Cardiol. 2004;94(9):1174–1177. doi: 10.1016/j.amjcard.2004.07.088. [DOI] [PubMed] [Google Scholar]
- 29.Ahmed B, Lischke S, De Sarno M, Holterman LA, Straight F, Dauerman HL. Gender related differences in predictors of vascular complications: role of vessel size and BMI. J Thromb Thrombolysis. 2013;36(1):84–90. doi: 10.1007/s11239-012-0847-y. [DOI] [PubMed] [Google Scholar]
- 30.Schnyder G, Sawhney N, Whisenant B, Tsimikas S, Turi ZG. Common femoral artery anatomy is influenced by demographics and comorbidity: implications for cardiac and peripheral invasive studies. Catheter Cardiovasc Interv. 2001;53(3):289–295. doi: 10.1002/ccd.1169. [DOI] [PubMed] [Google Scholar]
- 31.Vogel TR, Dombrovskiy VY, Haser PB, Graham AM. Evaluating preventable adverse safety events after elective lower extremity procedures. Journal of vascular surgery. 2011;54(3):706–713. doi: 10.1016/j.jvs.2011.03.230. [DOI] [PubMed] [Google Scholar]
- 32.Seto AH, Abu-Fadel MS, Sparling JM, Zacharias SJ, Daly TS, Harrison AT, et al. Real-time ultrasound guidance facilitates femoral arterial access and reduces vascular complications: FAUST (Femoral Arterial Access With Ultrasound Trial) JACC Cardiovasc Interv. 2010;3(7):751–758. doi: 10.1016/j.jcin.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 33.Gedikoglu M, Oguzkurt L, Gur S, Andic C, Sariturk U, Ozkan C. Comparison of ultrasound guidance with the traditional palpation and fluoroscopy method for the common femoral artery puncture. Catheter Cardiovasc Interv. 2013;82(7):1187–1192. doi: 10.1002/ccd.24955. [DOI] [PubMed] [Google Scholar]