Abstract
Background
Seven male Labrador Retriever puppies from 3 different litters, born to clinically normal dams and sires, were evaluated for progressive weakness and muscle atrophy. Muscle biopsies identified a congenital myopathy with pathologic features consistent with myotubular myopathy. Further investigations identified a pathogenic mutation in the myotubularin gene, confirming that these puppies had X‐linked myotubular myopathy (XLMTM).
Objective
To review the clinical phenotype, electrodiagnostic and laboratory features of XLMTM in this cohort of Labrador Retrievers.
Results
Male puppies with XLMTM were small and thin compared with their normal littermates. Generalized weakness and muscle atrophy were present by 7 weeks of age in some puppies and evident to most owners by 14 weeks of age. Affected puppies stood with an arched spine and low head carriage, and walked with a short, choppy stride. Muscle atrophy was severe and progressive. Patellar reflexes were absent. Laryngeal and esophageal dysfunction, and weakness of the masticatory muscles occurred in puppies surviving beyond 4 months of age. Serum creatine kinase activity was normal or only mildly increased. EMG findings were nonspecific and included positive sharp waves and fibrillation potentials. Clinical signs progressed rapidly, with most affected puppies unable to walk within 3–4 weeks after clinical signs were first noticed.
Conclusions and Clinical Importance
Although initial clinical signs of XLMTM are similar to the phenotypically milder centronuclear myopathy in Labrador Retrievers, XLMTM is a rapidly progressive and fatal myopathy. Clinicians should be aware of these 2 distinct myopathies with similar clinical presentations in the Labrador retriever breed.
Keywords: Canine, Congenital myopathy, Myotubularin
Abbreviations
- CNM
centronuclear myopathy
- CSF
cerebrospinal fluid
- CXLMD
canine X‐linked muscular dystrophy
- CXLMTM
canine X‐linked myotubular myopathy
- EMG
electromyography
- MTM1
myotubularin
Congenital myopathies including dystrophin‐deficient muscular dystrophy,1 autosomal recessive centronuclear myopathy (CNM),2 and sarcolemmal‐specific collagen VI deficiency3 have been reported in Labrador Retriever puppies. Recently, myotubular myopathy, an X‐linked subclassification of centronuclear myopathy,4 was described affecting young male Labrador Retriever puppies (XLMTM) and a mutation in the myotubularin gene (MTM1) was identified.5 In people, XLMTM is caused by a myriad of mutations in the MTM1 gene coding for the protein myotubularin.6 Myotubularin belongs to a large family of lipid phosphatases that are broadly expressed in many tissues, including skeletal muscle. In nonmuscle tissue, myotubularin plays a role in signaling pathways specifically involved in intracellular vesicle trafficking and autophagy.7, 8 In myofibers, myotubularin localizes to the terminal cisternae of the sarcoplasmic reticulum, where it plays an important role in promoting proper membrane curvature and triad morphology leading to proper excitation contraction coupling.9
Materials and Methods
Five young related male Labrador Retrievers with a rapidly progressive disorder causing muscle atrophy and weakness were evaluated at the Veterinary Teaching Hospital at the Western College of Veterinary Medicine (VTH‐WCVM), University of Saskatchewan between August 2006 and March 2009. All clinical examinations, diagnostic procedures and testing performed on these puppies were in accordance with guidelines established by the University of Saskatchewan's Animal Care Committee. Clinical and historical information regarding these puppies and 2 additional related puppies were obtained by medical record review and consultation with owners and referring veterinarians. All affected puppies were tested for the mutation in the PTPLA gene causing autosomal recessive CNM in Labrador Retrievers2 , 1 and 1 was tested for the mutation causing dystrophin‐deficient muscular dystrophy (DMD) in Golden Retrievers.2 Complete blood counts and routine serum chemistry profiles were performed on all dogs and Toxoplasma gondii and Neospora caninum serology were performed on the first two dogs evaluated. Histologic, histochemical, and immunohistochemical studies of muscle and peripheral nerve biopsy specimens were performed at the Comparative Neuromuscular Laboratory, University of California – San Diego (La Jolla, California, USA).
Results
Familial History for Affected Male Puppies from 3 Different Litters
Litter 1. Seven male and 4 female puppies were born to a clinically normal 4‐year‐old female chocolate Labrador Retriever bred to a clinically normal male chocolate Labrador Retriever (Fig. 1). As reported previously,5, 10 5 of the male puppies developed signs of muscle weakness and atrophy between 12 and 17 weeks of age. Four were euthanized without further evaluation; 1 pup (pup 1) was referred to the VTH‐WCVM. Muscles and peripheral nerves were collected from this pup for evaluation and a congenital myopathy was diagnosed. DNA tests were submitted for the CNM mutation and for the Golden Retriever DMD mutation, which were not found. According to the owner, the dam of this litter had 2 previous litters, sired by different males, each producing multiple male pups with early onset progressive muscle atrophy and weakness. All female puppies were normal. This dam was presented to the VTH‐WCVM for euthanasia because of behavioral problems a few months after evaluation of pup 1. Physical and neurologic examinations at that time were normal.
Figure 1.
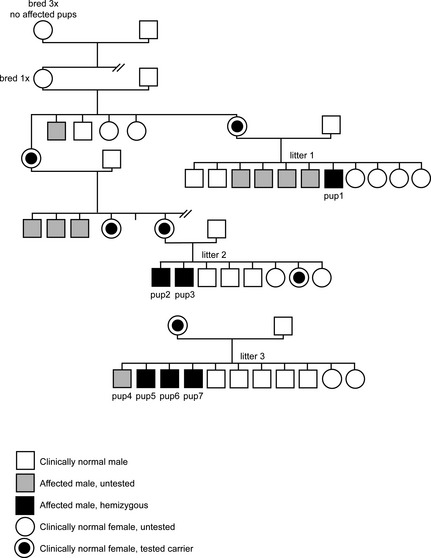
Pedigrees showing 3 litters of Labrador Retrievers with X‐linked myotubular myopathy.
Litter 2. A litter of 5 male and 3 female chocolate Labrador Retriever puppies was born to a clinically normal 3‐year‐old female chocolate Labrador Retriever bred to a clinically normal male chocolate Labrador Retriever.5 Two male pups from this litter developed signs of progressive muscular weakness and atrophy and were presented for veterinary evaluation between 3 and 4 months of age. Muscle specimens and cheek swabs for DNA analysis were collected from 1 of the affected male puppies (pup 2) immediately after humane euthanasia by the general veterinarian. DNA was tested for the PTPLA gene mutation, which was not found. Examination of the muscle biopsy supported a diagnosis of a congenital myopathy. The second affected puppy (pup 3) was referred to the VTH‐WCVM. When questioned, the breeder indicated that 3 of the dam's male littermates had been euthanized at a young age for progressive muscle weakness (Fig. 1).
This dam and 1 female puppy from the litter subsequently were examined at the WCVM and determined to be clinically and neurologically normal. Cheek swabs were submitted for CNM testing and both were clear. Muscle biopsies from the female littermate were normal.
Litter 3. A litter of 9 male (8 black and 1 chocolate) and 2 female (1 black and 1 chocolate) puppies was born to a clinically normal 5‐year‐old female black Labrador Retriever bred to a clinically normal 3.5‐year‐old male black Labrador Retriever.5 In this litter, 4 black male puppies showed signs of muscle weakness and atrophy at 7 weeks of age (Fig. 1). The affected puppies seemed to improve, but then were re‐evaluated for progressive muscle weakness and atrophy at 13–18 weeks of age. Limited clinical information was available from 1 of the affected male puppies (pup 4). The remaining 3 affected male puppies were evaluated at the VTH‐WCVM (pups 5, 6, and 7). One additional male puppy from this litter reportedly died shortly after birth from accidental trauma, and it was uncertain whether that puppy was affected. This dam had 1 previous litter that was the result of an accidental breeding to a nonpurebred male dog, and the breeder reported no problems with any of the puppies.
Clinical Findings in Affected Male Puppies
In total, 5 affected male puppies from 3 separate litters, ranging from 14 to 26 weeks of age were evaluated over a 3‐year period at the VTH‐WCVM. Clinical information, tissues, or both also were available from 2 affected puppies not evaluated at the WCVM (pup 2 from litter 2 and pup 4 from litter 3; Fig. 1).
All affected puppies were considered small for their age and all were thin to emaciated with body condition scores (BS) 0.5–1.5/5 when they were evaluated at the VTH‐WCVM. Age of onset of clinical signs varied. Although most owners and breeders did not notice clinical abnormalities until the puppies were approximately 14 weeks of age (range, 8–18 weeks; median, 14 weeks), the veterinarian who examined litter 3 at 7 weeks of age reported that the affected male puppies were abnormal at that time. Those 4 puppies (pups 4–7) were small for their age (2.0–2.7 kg) and thin (BCS 2), and they all had pelvic limb weakness, generalized muscle atrophy, and masticatory muscle weakness leading to a dropped jaw. Deworming, hand feeding, and physiotherapy reportedly resulted in improvement and the puppies were sent to their new owners. All of these pups were re‐presented to a veterinarian between 13 and 18 weeks of age because of progressive weakness and muscle atrophy.
Clinical abnormalities in all affected puppies progressed rapidly once they were severe enough to be noted by owners. By the age of 15–23 weeks, most puppies were unable to stand and walk. The owners of pup 6 were convinced that muscular weakness became much more pronounced during cold weather when the ambient temperature remained below −30°C for several consecutive days. Pups 1 and 2 had been treated with corticosteroids (prednisone 1.5–2.0 mg/kg BW q24h) for presumed juvenile cellulitis early in the course of their weakness and pup 7 had been treated with corticosteroids after becoming nonambulatory. Pups 1 and 6 had been treated with nonsteroidal anti‐inflammatory drugs (meloxicam 0.1 mg/kg BW PO q24 h). There was no noticeable improvement or slowing of disease progression with either of these treatments.
Body temperature, pulse, and respiratory rate were normal in all dogs evaluated at the VTH‐WCVM with the exception of the oldest and most severely affected puppy (pup 7; 26 weeks of age) that exhibited rapid, shallow, abdominal respirations (60/min), suggesting intercostal muscle weakness. A dry hair coat with excessive scaling was noted in most of the puppies. Complete ophthalmologic examination including fundoscopic evaluation was normal in the 2 dogs in which this was evaluated. Orthopedic examinations were unremarkable in all dogs; no bone, joint, or spinal pain was identified. Pup 2 did show evidence of mild discomfort when limb muscles were palpated.
Only 3 of the puppies (pups 1, 3, and 6) could still walk with assistance when they were evaluated at the VTH‐WCVM. They stood with an arched spine and were unable to lift their heads, resulting in low head carriage (Fig. 2). When they walked, they exhibited severe weakness, with a short, choppy stride, and collapsing after only a few steps. Within 1 week of presentation, these pups were unable to walk, even with assistance.
Figure 2.
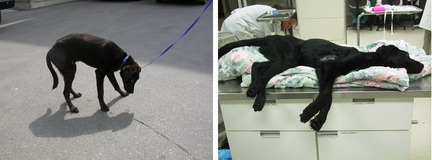
Image of pup 1 (left) showing an arched spine and typical low head carriage resulting from neck muscle weakness. Severe muscle wasting is shown in the image of pup 7 (right).
Proprioception, as assessed by response to knuckling and hopping, was normal in all affected pups that could be evaluated. Pup 7 did not have voluntary movement in any limbs, and postural reactions could not be assessed. Generalized muscle atrophy was pronounced and muscle tone was decreased in all affected pups, with flaccid muscles reported in the most severely affected pups. Patellar reflexes were consistently absent in all pups. Withdrawal reflexes were normal or diminished in most pups but absent in pup 7. Although paravertebral skin pinch elicited vocalization in pup 7, there was no detectable twitch of the cutaneous trunci muscle. Skin sensation, tail function, anal tone, and the perineal reflex were normal in all affected pups.
Mentation was assessed to be normal in all pups and the palpebral reflex was present and was not fatigable. The most severely affected pups (pups 5, 6, and 7) had dropped jaws attributed to severe atrophy and weakness of the muscles of mastication. Tongue strength and gag response were subjectively decreased in pup 7. Laryngeal, pharyngeal, and esophageal dysfunction were suspected or confirmed in all 5 affected dogs evaluated at the VTH‐WCVM. Laryngeal dysfunction was suspected based on a hoarse bark and confirmed by visual observation of failure of the arytenoid cartilages to abduct during inspiration in unsedated pups. Fluoroscopic swallowing and esophagram studies in 2 pups (pups 5 and 6) confirmed pharyngeal and esophageal dysfunction.
Clinical Pathology
Laboratory results were available from the 5 affected pups evaluated at the VTH‐WCVM and from 1 other affected pup from litter 3 (pup 4). None of the CBC findings were remarkable; 1 affected pup (pup 1) had a mild neutropenia (2.295 × 109/L; reference range, 3.0–10 × 109/L) and a few pups had reactive lymphocytes thought to be secondary to recent vaccination. Serum biochemical abnormalities included mild increases in alkaline phosphatase activity (103–172 U/L; reference range, 9–90 U/L) in 3 pups and mild increases in alanine aminotransferase activity (136–211 U/L; reference range, 19–59 U/L) in all pups that had been treated previously with glucocorticoids. Mild hyperphosphatemia (range, 2.25–2.72 mmol/L; reference range, 0.63–2.41 mmol/L) was evident in 4 of the affected pups consistent with age‐related bone turnover. Serum creatinine concentration was slightly decreased in the affected pups (range, 15–41 μmol/L; reference range, 61–97 μmol/L) with the decrease most marked in the oldest and most severely affected pup (pup 7) suggesting that decreased muscle mass was the cause. Serum creatine kinase (CK) activity was either normal (n = 3) or only slightly increased (n = 3) in all puppies tested (range, 196–972 U/L; reference range, 51–418 U/L). Urine specific gravity ranged from 1.018 to 1.044.
Diagnostic Imaging
Thoracic radiographs were evaluated in 2 pups (pups 1 and 7) and were normal. After pain was identified during deep palpation of the limbs of pup 2, radiographs of all limbs were performed and findings were normal. Fluoroscopic swallowing studies and esophagrams were performed in 2 pups (pups 5 and 6) using liquid barium and then multiple consistencies of barium coated food. Pup 5 was unable to form a bolus with liquid barium, and showed impaired bolus‐forming ability with canned food and dry kibble until a large volume of food had accumulated in the oropharynx, but then esophageal transit was normal. Pup 6 exhibited normal swallowing and esophageal transit with canned food, but slightly delayed bolus formation and decreased primary and secondary esophageal contractions with liquid and with dry kibble. Echocardiography was performed in pup 7, the oldest and most severely affected dog, with normal cardiac dimensions and blood flow identified with slightly decreased fractional shortening (29.9%), suggesting mild myocardial dysfunction.
Electromyography
Electromyographic studies (EMGs) performed under general anesthesia showed nonspecific abnormalities suggesting destabilization of the sarcolemmal membrane in the 3 pups tested (pups 2, 5, and 6). Positive sharp waves were identified in the neck and shoulder girdle muscles of pup 2. Moderate to severe fibrillation potentials were recorded in the left digastricus, masseter, and temporalis muscles of pup 5 and positive sharp waves were found in the left cranial tibial muscle. Positive sharp waves were recorded in the cervical, temporalis, lingual, gastrocnemius, lumbar muscles, and extensor carpi radialis muscles in pup 6 (Fig. 3).
Figure 3.
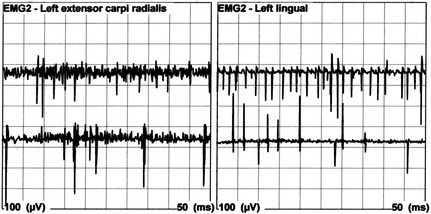
Electromyographic tracings from pup 6 showing positive sharp waves recorded from the left extensor carpi radialis and lingual muscles.
Miscellaneous Diagnostic Testing
Edrophonium chloride3 (0.2 mg/kg BW IV) was administered to pups 1 and 5 with neither showing any improvement in muscle strength. Circulating serum antibodies against acetylcholine receptors were measured and were normal (0.21 nmol/L; reference range, <0.6 nmol/L) in pup 1. Serological testing for antibodies to Toxoplasma gondii and Neospora caninum were negative in the 2 pups tested (pups 1 and 2) and cerebrospinal fluid (CSF) cytology and protein content were normal in these 2 pups as well. DNA testing for the PTPLA exon 2 insertion associated with autosomal recessive CNM of Labrador Retrievers was negative for affected and carrier states in all puppies. The dam of pup 1 was tested for CNM and also for the DMD gene mutation causing X‐linked dystrophin‐deficient muscular dystrophy in Golden Retrievers and was negative for both.
Postmortem Findings
Complete postmortem examinations were performed on all affected puppies except pup 4. The only clinically relevant findings were severe generalized muscle atrophy and emaciation. Microscopic abnormalities were confined to the skeletal muscles.
Muscle and Peripheral Nerve Biopsies
Comprehensive descriptions of histopathology, histochemistry, immunohistochemistry, and ultrastructural analysis of muscle biopsies from these affected pups and controls have been reported previously.5 Pathologic changes were diagnostic of myotubular myopathy. Typical pathologic changes included excessive variability in myofiber size, type 1 fiber predominance, centrally placed nuclei in most myofibers, and subsarcolemmal ringed and central dense areas highlighted with mitochondrial specific reactions typical of “necklace fibers”.11 By immunohistochemical and ultrastructural analysis, centrally located muscle nuclei were confirmed, and muscle triads were disorganized and exhibited an abnormal orientation of T tubules. None of these changes were evident in similarly processed muscle biopsy specimens obtained from the dam of litter 1 and a normal female puppy from litter 2. Peroneal nerve biopsies from pups 1, 5, and 7 were evaluated in frozen and resin sections. The nerve biopsies were normal without evidence of axonal degeneration, demyelination, or abnormalities of supporting structures.
Pedigree Analysis
As previously published, inspection of the pedigrees confirmed that the myopathy in these dogs was inherited as an X‐linked recessive trait (Fig. 1).5 All affected dogs evaluated were male and each had multiple affected male siblings and all female siblings were normal. All affected puppies resulted from the mating of a clinically normal dam and sire. Breeding a carrier female to multiple normal sires resulted in affected puppies, typical of X‐linked inheritance.
Subsequent genetic analysis confirmed that each of the affected pups described above carried a pathogenic MTM1 gene mutation that was considered responsible for their severe skeletal muscle myopathy.5
Discussion
Here, we provide a complete clinical description of a cohort of puppies with X‐linked myotubular myopathy. The initial clinical presentation of these puppies was similar to that expected with CNM, with 3‐ to 4‐month‐old puppies exhibiting weakness, muscle atrophy, absent patellar reflexes, and relatively normal serum CK activities.12, 13, 14, 15, 16, 17 There were, however, several differences between these XLMTM puppies and puppies with CNM, which prompted further evaluation. All affected puppies were male, and pedigree analysis supported an X‐linked mode of inheritance rather than the autosomal recessive inheritance typical of CNM in Labrador Retrievers.12 In contrast to dogs with PTPLA mutations, clinical abnormalities in the XLMTM puppies were more severe and rapidly progressive, uniformly resulting in death or euthanasia by 15–26 weeks of age. Autosomal recessive CNM usually is a less severe, more slowly progressive disorder, with many dogs surviving into adulthood.12, 13, 14, 15, 16, 17 All of these puppies were negative for the PTPLA mutation responsible for CNM in Labrador Retrievers.
Muscular dystrophy has been recognized in many breeds of dogs and initially was considered in the differential diagnosis for these puppies.12 The most common form of muscular dystrophy in dogs, canine X‐linked muscular dystrophy (CXLMD), is associated with a mutation in the gene encoding the sarcolemmal protein dystrophin.14, 15 This disorder usually results in an absence of dystrophin and severe muscular degeneration, but rarely a mutation can lead to a structurally altered and partially functional dystrophin protein resulting in a less severe phenotype.14, 15, 18 Dystrophin‐deficient CXLMD has been reported in 1 male Labrador Retriever puppy1 and has been extensively investigated in Golden Retrievers.19 Most puppies with dystrophin‐deficient CXLMD show muscular weakness as soon as they begin to ambulate. Unlike puppies with CNM and XLMTM, patellar reflexes are maintained until muscle fibrosis and contractures occur.1, 14, 20 A reliable feature of dystrophin‐deficient CXLMD is markedly increased serum CK activity as early as 7 days of age and peaking at 6–8 weeks of age at 100 times normal.12, 14, 20 Despite the observation that only males were affected, our clinical findings of absent patellar reflexes and normal CK activities, and normal immunohistochemical staining and immunoblotting for dystrophin and associated proteins, eliminated CXLMD as a diagnosis in the puppies described here.
Based on the histopathologic diagnosis of myotubular myopathy in these puppies, the X‐linked nature of the pedigree analysis, and the absence of myotubularin in the muscles on immunohistochemistry and immunoblotting, a candidate gene approach was used to test for a mutation in the myotubulin gene MTM1.5 All 15 exons of the canine MTM1 gene located on the X chromosome were sequenced. A C‐to‐A transversion in exon 7 was present in all 7 affected males described here.5 Affected puppies were hemizygous for the mutation, whereas known carrier females were heterozygous. The mutation was not detected in unaffected male dogs from the 3 litters, in a panel of control DNA samples obtained from 237 unrelated and unaffected Labrador Retrievers from throughout North America, Europe, and Australia, or in 59 additional control dogs from 25 breeds, supporting it as the pathogenic causative mutation. Although litters 1 and 2 initially were thought to be unrelated, further investigation revealed the relationship illustrated in Figure 1. Insufficient pedigree details are available for the third litter to prove or disprove a relationship, however, genetic haplotype has shown that a spontaneous mutation occurred in a local bitch and was transmitted to affected puppies in all 3 litters through a founder effect.5
Similar to the clinical course in these puppies, XLMTM in humans is a severe disease that, in the absence of intensive medical support, often follows a fatal course over the first few years of life. Affected male infants are clinically abnormal at or even before birth, exhibiting decreased muscle tone, decreased movement, and areflexia.5, 21, 22 Tube feeding usually is required because of impaired ability to suck and swallow. Respiratory muscle weakness is severe, often requiring mechanical ventilation and complications related to ventilation cause death in many patients during infancy or early childhood.5, 21, 22 Diagnosis is based on typical histopathologic findings on muscle biopsy in combination with suggestive clinical features. Biopsies disclose uniformly small muscle fibers with centrally placed nuclei resembling fetal myotubes.23
Mutations in the myotubularin (MTM1) gene on human chromosome Xq28 are responsible for XLMTM. The incidence is estimated at 1/50000 male births.5, 22 Over 200 different spontaneous deletion, insertion, nonsense, missense, and splice mutations have been found in the MTM1 gene, with the majority occurring in exons 12, 4, 11, 8, and 9.5 The mutation identified in these puppies was a unique exon 7 missense mutation that has not been reported in a human patient.4 Identification of the mutation in the affected puppies allowed us to not only identify carrier females and counsel owners regarding future breedings but also allowed us to recruit carrier females to establish a breeding colony. Establishment of a breeding colony has contributed to ongoing investigation of the pathophysiology of muscle deterioration and weakness in individuals with XLMTM,24 and to evaluation of potential treatments.25
Spontaneous mutation in the MTM1 gene resulting in XLMTM in male offspring is well described in human medicine but only recently reported in dogs.4, 5 Given the frequency with which these mutations occur in humans, it is likely that similar mutations occur occasionally in dogs, but that the resulting myopathies are incorrectly diagnosed as some other congenital myopathy. Evaluation of affected Labrador Retriever puppies in this report highlights the clinical differences between XLMTM and the other reported congenital myopathies in this breed, and may help veterinarians recognize similar clinical presentations in other breeds.
Acknowledgments
Work was performed at the University of Saskatchewan and University of California San Diego. This study was partially supported by grants R01 AR044345 and R01 HL115001 (AHB) from the National Institutes of Health, U.S.A. This work was presented as an abstract at the 2009 ACVIM Forum & Canadian Veterinary Medical Association Convention, Montreal, Quebec, Canada.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Footnotes
CNM Project; Alfort School of Veterinary Medicine, Alfort, France
HealthGene, Saskatoon, SK, Canada
Enlon; Baxter, Toronto, ON, Canada
References
- 1. Bergman RL, Inzana KD, Monroe WE, et al. Dystrophin‐deficient muscular dystrophy in a Labrador Retriever. J Am Anim Hosp Assoc 2002;38:255–261. [DOI] [PubMed] [Google Scholar]
- 2. Pele M, Tiret L, Kessler J‐L, et al. SINE exonic insertion in the PTPLA gene leads to multiple splicing defects and segregates with the autosomal recessive centronuclear myopathy in dogs. Hum Mol Genet 2005;14:1417–1427. [DOI] [PubMed] [Google Scholar]
- 3. Marioni‐Henry K, Haworth P, Scott H, et al. Sarcolemmal specific collagen VI deficient myopathy in a Labrador Retriever. J Vet Intern Med 2014;28:243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pierson CR, Tomczak K, Agrawal P, et al. X linked myotubular and centronuclear myopathies. J Neuropathol Exper Neurol 2005;64:555–564. [DOI] [PubMed] [Google Scholar]
- 5. Beggs AH, Bohm J, Snead E, et al. MTM1 mutation associated with X‐linked myotubular myopathy in Labrador Retrievers. Proc Nat Acad Sci USA 2010;107:14697–14702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jungbluth H, Wallgren‐Pettersson C, Laporte J. Centronuclear (myotubular) myopathy. Orphanet J Rare Dis 2008;3:26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Robinson FL, Dixon JE. Myotubularin phosphatases: Policing 3‐phosphoinosides. Trends Cell Biol 2006;16:403–412. [DOI] [PubMed] [Google Scholar]
- 8. Nicot AS, Laporte J. Endosomal phosphoiositides and human diseases. Traffic 2008;9:1240–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Amoasii L, Hnia K, Chicanne G, et al. Myotubularin and Ptdlns3P remodel the sarcoplasmic reticulum in muscle in vivo . J Cell Sci 2013;126(Pt 8):1806–1819. [DOI] [PubMed] [Google Scholar]
- 10. Cosford L, Taylor SM, Thompson L, Shelton GD. Possible new inherited myopathy in a young Labrador retriever. Can Vet J 2008;49:393–397. [PMC free article] [PubMed] [Google Scholar]
- 11. Bevilacqua JA, Bitoun M, Biancalana V, et al. “Necklace fibers”, a new histologic marker of late‐onset MTM1‐related centronuclear myopathy. Acta Neuropathol 2009;117:283–291. [DOI] [PubMed] [Google Scholar]
- 12. Cosford K, Taylor SM. Exercise intolerance in retrievers. Vet Med 2010;105:64–74. [Google Scholar]
- 13. McKerrell RE, Braund KG. Hereditary myopathy in Labrador retrievers: Clinical variations. J Small Anim Pract 1987;28:479–489. [Google Scholar]
- 14. Shelton GD, Engvall E. Muscular dystrophies and other inherited myopathies. Vet Clin N Am: Sm Anim Pract 2002;32:103–124. [DOI] [PubMed] [Google Scholar]
- 15. Shelton GD, Engvall E. Canine and feline models of human inherited muscle diseases. Neuromuscul Disord 2005;15:127–138. [DOI] [PubMed] [Google Scholar]
- 16. Gortel K, Houston DM, Kuiken T, et al. Inherited myopathy in a litter of Labrador retrievers. Can Vet J 1996;37:108–110. [PMC free article] [PubMed] [Google Scholar]
- 17. Maurer M, Mary J, Guillaud L, et al. Centronuclear myopathy in Labrador retrievers: A recent founder mutation in the PTPLA gene has rapidly disseminated worldwide. PLoS ONE 2012;7:e46408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baroncelli AB, Abellonio F, Pagano TB, et al. Muscular dystrophy in a dog resembling human Becker muscular dystrophy. J Comp Path 2014;150:429–433. [DOI] [PubMed] [Google Scholar]
- 19. Kornegay JN, Bogan JR, Bogan DJ, et al. Canine models of Duchenne muscular dystrophy and their use in therapeutic strategies. Mamm Genome 2012;23:85–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Valentine BA, Cooper BJ, de Lahunta A, et al. Canine X‐linked muscular dystrophy. An animal model of Duchenne muscular dystophy: Clinical studies. J Neurol Sci 1988;88:69–81. [DOI] [PubMed] [Google Scholar]
- 21. Young‐Mi H, Kyoung‐Ah K, Yun‐Jin L, et al. X‐linked recessive myotubular myopathy with MTM1 mutations. Korean J Pediatr 2013;56:139–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eun HL, Mi‐Sun Y, Seong JP, et al. Two cases of X‐linked myotubular myopathy with novel MTM1 mutations. J Clin Neurol 2013;9:57–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. North KN. Congenital myopathies In: Engel AG, Franzini‐Armstrong C, eds. Myology, vol. 2, 3rd ed New York, NY: McGraw‐Hill; 2004:1473–1533. [Google Scholar]
- 24. Grange RW, Doering J, Mitchell E, et al. Muscle function in a canine model of X‐linked myotubular myopathy. Muscle Nerve 2012;46:588–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Childers MK, Joubert R, Poulard K, et al. Gene therapy prolongs survival and restores function in murine and canine models of myotubular myopathy. Sci Transl Med 2014;6:1–15 (220ra10). [DOI] [PMC free article] [PubMed] [Google Scholar]


