Abstract
Objective
To assess associations between adiposity and hippocampal-dependent and hippocampal-independent memory forms among prepubertal children.
Study design
Prepubertal children (7–9-year-olds, n = 126), classified as non-overweight (<85th %tile BMI-for-age [n = 73]) or overweight/obese (≥85th %tile BMI-for-age [n = 53]), completed relational (hippocampal-dependent) and item (hippocampal-independent) memory tasks, and performance was assessed with both direct (behavioral accuracy) and indirect (preferential disproportionate viewing [PDV]) measures. Adiposity (%whole body fat mass, subcutaneous abdominal adipose tissue, visceral adipose tissue, and total abdominal adipose tissue) was assessed using DXA. Backward regressions identified significant (P <0.05) predictive models of memory performance. Covariates included age, sex, pubertal timing, socioeconomic status, IQ, oxygen consumption (VO2max), and body mass index (BMI) z-score.
Results
Among overweight/obese children, total abdominal adipose tissue was a significant negative predictor of relational memory behavioral accuracy, and pubertal timing together with socioeconomic status jointly predicted the PDV measure of relational memory. In contrast, among non-overweight children, male sex predicted item memory behavioral accuracy, and a model consisting of socioeconomic status and BMI z-score jointly predicted the PDV measure of relational memory.
Conclusions
Regional, and not whole body, fat deposition was selectively and negatively associated with hippocampal-dependent relational memory among overweight/obese prepubertal children.
Keywords: Cognition, associative memory, subcutaneous fat, visceral fat, DXA
Converging evidence now suggests that poor cognitive function may be yet another complication of obesity1. Obesity is an independent risk factor for developing dementia and Alzheimer disease later in life2. In addition, the complications of obesity are becoming evident in obese children.3 However, there has been only limited research connecting obesity to cognitive function in childhood. Central adiposity, in particular, is implicated in the progression of insulin resistance4. Yet fat around the abdominal viscera in mesentery and omentum, known as visceral fat, is functionally different from fat in the abdominal subcutaneous areas (subcutaneous fat). However, the implications of these fat depositions or their sum (total abdominal adiposity) on pediatric cognitive function remain unknown5. Furthermore, as in adults, different fat compartments in children may have differential functional effects based on weight status6.
Increased waist-to-hip ratio is negatively correlated with memory and hippocampal volume among adults7,8. The hippocampus is critical for relational (associative) memory, which supports representations of the relations between items, such as the relations among the constituent elements of events and their subsequent flexible expression9. It is likely that acquisition of relational knowledge provides a foundation for scholastic achievement, and its flexible expression enables success in handling novel challenges. Therefore, changes in the development of this system in childhood would have wide-ranging impact. In contrast, memory for individual items (item memory) relies on perirhinal cortex, the anterior region of the parahippocampal gyrus9. The dependence of these two memory processes on distinct neural substrates provides an opportunity to study their differential sensitivity to adiposity.
Consequently, this study examined whether relational and/or item memory were related to whole body and central adiposity in prepubertal children and whether these associations varied by weight status. We hypothesized that central adiposity would be selectively and negatively associated with relational memory and this association would vary based on weight status.
Methods
Prepubertal children between 7 and 9 years (n = 126) provided written assent and legal guardians provided written informed consent in accordance with the regulations of the University of Illinois Institutional Review Board. Children were screened for neurological disorders, physical disabilities, psychoactive medication status, and normal or corrected-to-normal vision. Data were also collected on 1) IQ, using the Kaufman Brief Intelligence Test10 or the Woodcock-Johnson Tests of Cognitive Abilities11, 2) socioeconomic status (SES) as estimated based on household income, participation in a school meal-assistance program, maternal and paternal education levels, and how many parents work full-time, and 3) pubertal status12.
Anthropometrics and Body Composition
Height and weight were measured using a stadiometer (Seca; model 240) and a Tanita WB-300 Plus digital scale, respectively. The Centers for Disease Control (CDC, 2000) growth charts were used to determine body mass index (BMI)-for-age percentile and BMI z-scores13. Non-overweight or overweight/obese categorization was based on the 85th percentile BMI-forage cutoff13.
Adiposity was assessed by dual energy x-ray absorptiometry (DXA) using a Hologic QDR 4500A bone densitometer (software version 13.4.2, Bedford, MA). Percent whole body fat mass (%WBFM) was expressed using the standard software measure. The estimation of central adiposity variables has been previously described14. In summary, the abdominal region of interest was a 5 cm wide section placed across the entire abdomen just above the iliac crest at a level that approximately coincides with the 4th lumbar vertebrae on the whole body DXA scan. Total abdominal adiposity (TAAT) was defined as the total adipose tissue area within this region. Subcutaneous abdominal adipose tissue (SAAT) was determined using an algorithm that composites the adipose tissue on the sides of the abdominal cavity and the estimate of subcutaneous fat overlying the abdominal cavity. This estimated SAAT was subtracted from the TAAT to determine visceral adipose tissue (VAT). This estimated VAT has been shown in previous work to correlate (r = 0.92, P <.01) with computed tomography (CT) values of VAT14.
Cardiorespiratory Fitness Assessment
VO2max was measured using a modified Balke treadmill protocol15. Oxygen consumption was measured using a computerized indirect calorimetry system (ParvoMedics True Max 2400, Sandy, UT) with averages for VO2 and respiratory exchange ratio assessed every 20s. VO2max was based upon maximal effort as evidenced by (1) a peak heart rate ≥185 bpm15 and a heart rate plateau16; (2) respiratory exchange ratio >1.017; (3) a score on the children’s OMNI ratings of perceived exertion scale >818; and/or (4) a plateau in oxygen consumption corresponding to an increase of less than 2 ml/kg/min despite an increase in workload15. The absolute VO2max was then adjusted for fat-free mass (derived by DXA) to calculate the measure of fat-free VO2max19.
Memory Tasks
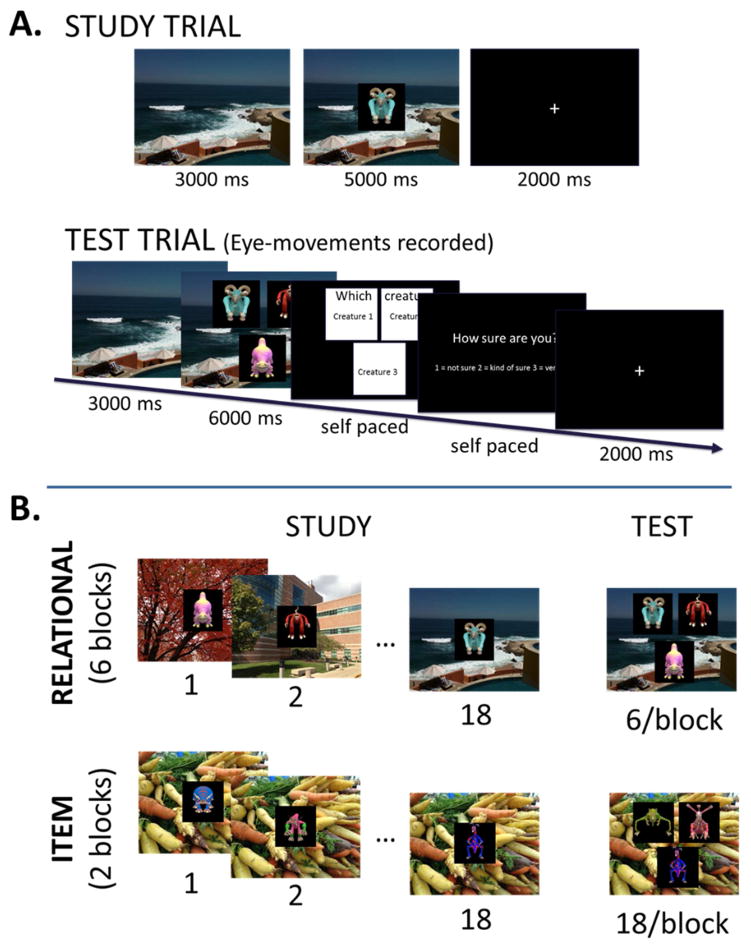
Children completed a task adapted from Monti et al20, however the current version used child-friendly creatures (Electronic Arts Inc., California) rather than faces (Figure 1; available at www.jpeds.com)21. In separate study-test blocks, children studied individual creatures (item condition) or uniquely paired associations between creatures and backgrounds (“habitats”; relational condition). At test, participants were instructed to find the creature originally studied with that scene from an array of three creatures. One of the creatures had been studied with that scene (target) and two had been studied with other scenes (foils). Familiarity across the three creatures was thus matched, necessitating the employment of hippocampal-dependent relational memory22. In the item condition, the background scene was the same for all creature-scene pairings within each block. At test, participants were instructed to find the previously viewed creature. In each test display two creatures were novel and one studied, allowing the discrimination to be made on the basis of familiarity, an ability that is independent of the hippocampus23. Lists of stimuli were counterbalanced across conditions between participants, and target location on test trials was counterbalanced within participant such that the target was equally likely to appear in any of the three possible locations. Order of study-test blocks was counterbalanced across participants such that half the participants began with the relational condition and half with the item condition.
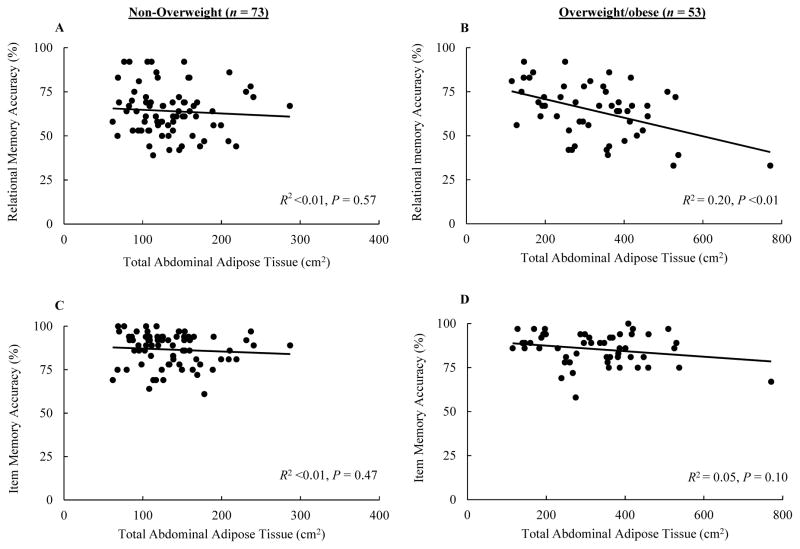
Figure 1.
Scatterplots illustrating the correlation between total abdominal adipose tissue and relational and item memory accuracy.
An Eyelink 1000 eye-tracker (SR Research, Ontario, Canada) was used remotely to record eye-movements at 500 Hz. Time courses were quantified using preferential disproportionate viewing (PDV) – defined as the difference in proportion of time spent viewing correctly-selected matching creatures relative to proportion of time spent viewing incorrectly-selected creatures – prior to behavioral response. This measure corrects for the fact that individuals look more at the stimulus that they will behaviorally select, independent of previous experience. In this way, the magnitude of PDV provides a measure of memory for previous experience, with greater PDV indicating a greater degree of memory accuracy, an effect that has been shown to manifest even prior to the viewer’s behavioral awareness24.
Statistical Analyses
Differences between non-overweight and overweight/obese in memory measures were assessed using an independent samples t-test. Initial Pearson’s correlations assessed bivariate relationships between adiposity and memory measures among all participants. Subsequently, bivariate correlations within weight category were performed. Finally, step-wise multiple regression, within each weight status group, was applied using the backward method of predictor entry (demographics, fitness, and adiposity) to determine predictors of memory performance. This method first includes all variables followed by a step-by-step elimination until no omitted variable would have contributed to the model. Each predictor’s contribution was assessed by studying its significance (α-level at .05). Statistics were performed using SPSS 19 (IBM, Somers, NY).
Results
A total of 126 (71 females) 7–9-year-olds participated in this study. SES categorization of the participants was 35% (low), 35% (medium), and 30% (high). According to the Tanner pubertal staging questionnaire, 85% were stage 1 and 15% belonged to stage 2. BMI-for-age categorization revealed that 5% of the participants were underweight, 53% were normal weight, 14% were overweight, and 28% were obese. Comparisons between non-overweight and overweight/obese groups are presented in Table I. As expected, overweight/obese participants had greater (all Ps <.01) %WBFM (10.9%; 95% confidence interval [CI], 9.1 to 12.6), TAAT (190.3cm2; 95% CI, 153.5 to 227.2), SAAT (156.9cm2; 95% CI, 123.8 to 190.0), and VAT (33.5cm2; 95% CI, 27.3 to 39.7). Although overweight/obese children had lower VO2max relative to total body weight (−7.3kg/ml/min; 95% CI, −9.4 to −5.2, P <.01), the groups did not differ in VO2max relative to fat-free mass (−1.13kg/ml/min; 95% CI, −3.7 to 1.5, P = .41).
Table 1.
Body composition memory performance of participants by weight status
| Non-Overweight (n = 73) | Overweight/obese (n = 53) | |
|---|---|---|
|
| ||
| Intelligence Quotient | 111.1 (14.1) | 108.7 (11.8) |
| BMI z-score* | −0.07 (0.81) | 1.82 (0.54) |
| VO2max (kg/ml/min)* | 44.0 (6.1) | 36.7 (5.8) |
| Fat-Free VO2max (kg/ml/min) | 60.7 (7.4) | 59.6 (7.3) |
| %Whole Body Fat Mass* | 28.5 (4.7) | 39.4 (5.1) |
| TAAT (cm2)* | 135.4 (45.3) | 325.7 (128.5) |
| SAAT (cm2)* | 109.4 (47.2) | 266.2 (113.8) |
| VAT (cm2)* | 26.0 (11.2) | 59.5 (20.6) |
| Relational Memory Accuracy (%) | 64.1 (13.6) | 64.1 (15.1) |
| Item Memory Accuracy (%) | 86.6 (9.5) | 85.5 (8.9) |
| Relational Memory PDV (%) | 8.4 (8.9) | 8.4 (9.9) |
| a Item Memory PDV (%) | 9.6 (13.9) | 11.2 (12.3) |
Data presented as mean ± SD
Data only available for 114 participants (48 overweight/obese)
Significant difference between groups (P <0.05)
BMI, body mass index; VO2max, maximal oxygen consumption; TAAT, total abdominal adipose tissue; SAAT, subcutaneous adipose tissue; VAT, visceral adipose tissue; PDV, preferential disproportionate viewing
There were no differences in accuracy or PDV for either form of memory between non-overweight and overweight/obese participants (Table II). However, relational memory accuracy was positively correlated with age (r = 0.15, P = .04) and being male (r = 0.23, P <.01), but negatively correlated with SAAT (r = −0.28, P = <.01), and TAAT (r = −0.21, P = .01). Item memory was positively correlated with being male (r = 0.21, P <.01) and IQ (r = 0.16, P = .04), but negatively correlated with SAAT (r = −0.18, P = .02) and TAAT (r = −0.15, P = .04). No significant correlates of relational or item memory PDV were identified.
Table 2.
Correlations between memory indices and participant characteristics by weight status
| Non-Overweight (n = 73) | Overweight/obese (n = 53) | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Relational Memory | Item Memory | Relational Memory | Item Memory | |||||
|
|
|
|||||||
| Accuracy | PDV | Accuracy | PDV | Accuracy | PDV | Accuracy | a PDV | |
|
|
|
|||||||
| Age | 0.16 | 0.03 | 0.06 | −0.05 | 0.14 | 0.05 | 0.10 | 0.13 |
| b Sex | 0.18 | 0.18 | 0.30** | 0.19 | 0.29* | 0.10 | 0.09 | −0.07 |
| Pubertal Timing | −0.12 | 0.07 | −0.01 | 0.02 | −0.20 | −.251* | −0.10 | −0.08 |
| SES | −0.05 | 0.23* | 0.03 | 0.06 | 0.22 | −0.19 | 0.25* | 0.01 |
| IQ | 0.08 | 0.20* | 0.13 | 0.12 | 0.14 | −0.15 | 0.21 | 0.14 |
| BMI z-score | −0.05 | −0.18 | 0.09 | 0.13 | −0.19 | −0.10 | −0.14 | 0.03 |
| Fat-free VO2max | 0.20* | −0.05 | −0.06 | −0.03 | 0.05 | −0.12 | 0.15 | 0.15 |
| %WBFM | −0.05 | −0.12 | −0.08 | 0.06 | −0.36** | −0.07 | −0.20 | 0.15 |
| SAAT | −0.10 | −0.11 | −0.16 | −0.01 | −0.46** | −0.15 | −0.24* | 0.07 |
| VAT | 0.15 | 0.10 | 0.30** | 0.17 | −0.23* | −0.03 | −0.11 | 0.12 |
| TAAT | −0.07 | −0.09 | −0.09 | 0.03 | −0.45** | −0.14 | −0.23 | 0.08 |
Data only available for 116 participants (67 non-overweight, 49 overweight/obese)
Females coded as 0 and males coded as 1
Correlation is significant at the 0.01 level (1-tailed).
Correlation is significant at the 0.05 level (1-tailed).
PDV, preferential disproportionate viewing; SES, socioeconomic status; IQ, intelligence quotient; SAAT, subcutaneous adipose tissue; VAT, visceral adipose tissue; TAAT, total fat in abdominal region of interest
Results of the bivariate correlations separated by weight status category are presented in Table II. Among non-overweight children, no adiposity measure was related to relational memory. However, relational memory accuracy was positively correlated with fat-free VO2max (r = 0.20, P = .04). Item memory accuracy was positively related to male sex (r = 0.30, P = .01) and VAT (r = 0.30, P = .01). However, stratifying the correlations by sex revealed that VAT was not correlated with item memory performance for males (r = 0.10, P = .29) or females (r = 0.16, P = .29). Relational memory PDV was positively correlated with SES (r = 0.23, P = .02) and IQ (r = 0.13, P = .14), and no significant correlations were observed for PDV in the item memory condition.
Among overweight/obese children, relational memory accuracy was positively associated with male sex (r = 0.29, P = .02) and negatively related to all DXA measures of adiposity (all Ps <.05). Item memory accuracy was positively correlated with SES (r = 0.25, P = .03) and negatively correlated with SAAT (r = −0.24, P = .04). Pubertal timing (r = −0.25, P = .04) was negatively correlated with relational memory PDV among overweight/obese children.
According to the backward regression analyses, there were no significant predictors of relational memory accuracy and item memory PDV among non-overweight children. However, a model consisting of SES (β = 0.25, P = .03) and BMI z-score (β = −0.20, P = .09) achieved significance (P = .03) while explaining 9% of the variance in relational memory PDV. Although sex alone significantly predicted item memory accuracy (β = 0.30, R2 = 0.09, P = .01), being male was not an independent predictor because sex was not a significant predictor of item memory accuracy following the inclusion of VAT and IQ in the model. Among overweight/obese participants, no independent predictors or significant predictive models of item memory accuracy or PDV were observed. A model consisting of pubertal timing (β = −0.30, P = .03) and SES (β = −0.25, P = .08) achieved significance (P = .04) and explained 12% of the variance in relational memory PDV. However, TAAT was identified as an independent negative predictor of relational memory accuracy (β = −0.45, P <.01) and explained 20% of the variance in relational memory accuracy scores. Figure 2 illustrates the correlation between TAAT and accuracy in both memory forms among non-overweight and overweight/obese children.
Figure 2.
A, Single trial progression in the study (top) and test (bottom) phases. Durations of each trial component are specified in milliseconds. B, Example study and test trials from the Relational condition (top) and Item condition (bottom).
Discussion
The hippocampus is critical for learning and memory throughout the lifespan. In this report, we document an inverse relationship between central adiposity and hippocampal-dependent relational memory among overweight/obese prepubertal children. Following regression analyses, this relationship did not extend to item memory – which relies on the perirhinal cortex – demonstrating a selective association of central adiposity with hippocampal-dependent memory processes.
However, there were no differences in memory performance between groups, when considered solely according to weight. That is, non-overweight and overweight/obese children did not differ in accuracy or PDV in either condition. Therefore, weight status alone may not be the key determinant of memory function among prepubertal children and it is possible that differences in memory performance across the BMI-for-age cutoff for overweight emerge later in development. Separating subsequent analyses by weight status allowed us to examine whether the degree of central adiposity differentially correlates with distinct types of memory among normal and overweight/obese children. The discovery that TAAT, independently and selectively, correlated with relational memory among overweight/obese children raises important questions for future research. Previous studies have demonstrated differences in several lifestyle factors (e.g., diet, physical activity, sleep patterns)25–27 as well as metabolic outcomes (e.g., lipid profiles and inflammatory markers)28,29 between normal and overweight children. It is plausible that these factors may account for the differential associations observed across groups in the current study.
Centralized fat deposition, rather than whole body adiposity or BMI, appears to play a greater role in metabolic diseases30. VAT, relative to SAAT, has been shown to be particularly pathogenic due to its higher lipolytic activity and closer proximity to hepatic portal vasculature31. Therefore, the finding that VAT did not predict memory performance following adjustment for covariates was surprising. Among non-overweight participants, VAT was positively related to item memory accuracy in an uncorrected bivariate correlation, however, this association was not significant following inclusion of sex in the regression models. Hence, the initial correlation observed between VAT and item memory may be a function of males – higher in VAT – outperforming females on the item memory task. Further, VAT was not related to item memory among neither non-overweight males nor females. In contrast, the influence of TAAT on relational memory accuracy appeared to be mediated by SAAT which has also been shown to correlate with hyperinsulinemia among prepubertal children32. Nevertheless, the current study provides evidence that, among prepubertal children, cumulative abdominal adipose tissue is a more significant correlate of hippocampal function than its individual components.
Support for the detrimental effects of fat mass on memory can be found in rodent studies showing that obesity – induced by diet or leptin receptor deficiency – is related to compromised hippocampal function33 and impaired long-term potentiation of neurons in the hippocampus34. Compared with their lean counterparts, obese rats – classified based on their greater weight gain and larger epididymal fat pads – took significantly more time to find a hidden platform during a Morris water maze task designed to access spatial learning and memory, processes known to depend on the hippocampus33. In addition, Gerges et al (33) observed that, relative to lean rats, the CA1 region of the hippocampus of obese Zucker rats exhibited impairment in long-term potentiation. However, whether obesity has a selectively negative effect on the hippocampus or a generalized effect on a wider brain network warrants further study. To our knowledge, no previous study has directly investigated the relationship between measures of adiposity and relational memory among humans. Structural magnetic resonance imaging (MRI) studies indicate that both SAAT and VAT are negatively related to total brain volume, independent of cardiovascular risk factors35. Specific to central adiposity, a 1-SD increase in waist-hip ratio is related to a 0.2-SD decrease in hippocampal volume8. However, these aforementioned studies did not assess cognitive function and thus future studies are needed to assess the effects of the obesity on hippocampal memory. Nevertheless, our results add to the evidence from rodent models and neuroimaging results in adult humans indicating adiposity-related perturbations in hippocampal function and structure.
Several possible mechanisms may underlie the link between central adiposity and impaired hippocampal function36. First, central adiposity-induced changes in glucose homeostasis may affect hippocampal-dependent memory processes. Among rodents, delivering insulin to the hippocampus strengthens spatial memory, while blocking insulin signaling impairs memory function37. Secondly, abdominal adipose tissue secretes proinflammatory markers known to be neurotoxic38,39. Systemic inflammation is known to increase central inflammation, predicting cognitive decline40. Finally, prolonged cortisol release caused by chronic stimulation of the hypothalamic-pituitary-adrenal (HPA) axis may preferentially affect central fat mass due to increased number of glucocorticoid receptors in abdominal fat mass. Abdominal fat has been shown to release cytokines that stimulate the HPA axis to release even more glucocorticoids41. Activation of this cycle results in increased central adiposity, insulin resistance and, critically, hippocampal atrophy42.
Alternatively, central adiposity could have served as a surrogate marker of physical inactivity and our results perhaps reveal the adverse effects of a sedentary lifestyle on hippocampal function. The hippocampus is known to undergo neurogenesis43 into adulthood and to exhibit susceptibility to the negative effects of aging, and positive effects of environmental enrichment and exercise44–46. Environmental enrichment or the “combination of inanimate and social stimulation” induces experience-dependent neuroplasticity in the rodent brain47,48. However, activity is a key component of environmental enrichment and voluntary exercise in a running wheel enhances survival of newborn neurons in the dentate gyrus49. In addition to neurogenesis, exercise has broader effects on the brain including enhanced gliogenesis, synaptogenesis, and angiogenesis as well as increases in growth factors including brain-derived neurotrophic factor (BDNF)50. Low levels of physical activity play an important role in the development of excess of central adiposity in children and adolescents51. Given that overweight children are less likely be physically active, compared with their lean counterparts51, the current results may reflect the negative effects of physical inactivity on hippocampal memory.
The current study has several limitations. DXA generated values for VAT and SAAT are estimated by modeling and are not equivalent to those generated by direct imaging using computed tomography (CT) or MRI. However, DXA has been shown recently to provide accurate estimates of abdominal fat compartmentalization while using only a fraction of the radiation of CT, making DXA a suitable alternative for use in pediatric populations. In addition, although our analyses accounted for key demographic and fitness covariates, future studies should assess biomedical markers of metabolic health and inflammation, such as those noted above.
In conclusion, we provide evidence in children connecting central adiposity, a clinically significant fat deposition in the human body, to the hippocampus and relational memory. That obesity-related impairment is already manifest during the school years has considerable implications for pediatric cognitive health. In light of the currently elevated prevalence of childhood obesity, these findings raise troubling public health concerns by contributing yet another piece of evidence on the negative influence of adiposity even early in life.
Acknowledgments
Funded by the National Institutes of Health (HD055352 and HD069381) and USDA NIFA (2011-67001-30101).
We would like to thank Bonnie Hemrick, Inge Karosevica, Ari Pence, Sebastian Wraight, Patrick Watson, Nicole Boniquit, and Sarah Kinsella.
Abbreviations
- VAT
visceral adipose tissue
- SAAT
subcutaneous abdominal adipose tissue
- TAAT
total abdominal adipose tissue
- IQ
intelligence quotient
- DXA
dual energy x-ray absorptiometry
- %WBFM
percent fat mass
- PDV
preferential disproportionate viewing
- BDNF
brain-derived neurotrophic factor
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Prickett C, Brennan L, Stolwyk R. Examining the relationship between obesity and cognitive function: A systematic literature review. Obesity Research & Clinical Practice. 2014 doi: 10.1016/j.orcp.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Fitzpatrick AL, Kuller LH, Lopez OL, et al. Midlife and late-life obesity and the risk of dementia: Cardiovascular health study. Arch Neurol. 2009;66:336–342. doi: 10.1001/archneurol.2008.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abrams P, Levitt Katz LE. Metabolic effects of obesity causing disease in childhood. Curr Opin Endocrinol Diabetes Obes. 2011;18:23. doi: 10.1097/MED.0b013e3283424b37. [DOI] [PubMed] [Google Scholar]
- 4.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. The Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 5.Ibrahim MM. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obesity reviews. 2010;11:11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 6.Cnop M, Landchild MJ, Vidal J, et al. The concurrent accumulation of intra-abdominal and subcutaneous fat explains the association between insulin resistance and plasma leptin concentrations : Distinct metabolic effects of two fat compartments. Diabetes. 2002;51:1005–1015. doi: 10.2337/diabetes.51.4.1005. [DOI] [PubMed] [Google Scholar]
- 7.Dore GA, Elias MF, Robbins MA, Budge MM, Elias PK. Relation between central adiposity and cognitive function in the Maine–Syracuse study: Attenuation by physical activity. Annals of Behavioral Medicine. 2008;35:341–350. doi: 10.1007/s12160-008-9038-7. [DOI] [PubMed] [Google Scholar]
- 8.Jagust W, Harvey D, Mungas D, Haan M. Central obesity and the aging brain. Arch Neurol. 2005;62:1545. doi: 10.1001/archneur.62.10.1545. [DOI] [PubMed] [Google Scholar]
- 9.Konkel A, Cohen NJ. Relational memory and the hippocampus: Representations and methods. Frontiers in neuroscience. 2009;3:166. doi: 10.3389/neuro.01.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaufman AS. K-BIT: Kaufman brief intelligence test. American Guidance Service Circle; Pines, MN: 1990. [Google Scholar]
- 11.Woodcock RW, McGrew K, Mather N. Woodcock-johnson tests of achievement. Itasca, IL: Riverside Publishing; 2001. [Google Scholar]
- 12.Tanner JM. Growth at adolescence. Oxford: Blackwell Scientific Publications; 1962. [Google Scholar]
- 13.Kuczmarski RJ, Ogden CL, Grummer Strawn LM, et al. Advance data from Vital health statistics of the National Center for Health Statistics. 2000. CDC growth charts: United states; pp. 1–27. [PubMed] [Google Scholar]
- 14.Micklesfield L, Micklesfield J, Goedecke M, Punyanitya K, Wilson T, Kelly Dual-energy X-ray performs as well as clinical computed tomography for the measurement of visceral fat. Obesity. 2012;20:1109–1114. doi: 10.1038/oby.2011.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armstrong L. ACSM’s guidelines for exercise testing and prescription/american college of sports medicine. 7. Philadelphia: Lippincott Williams & Wilkins, Philadelphia; 2006. [Google Scholar]
- 16.Freedson PS, Goodman TL, Rowland TW, editors. Measurement of oxygen consumption. Champaign, IL: Human Kinetics; 1993. Pediatric Laboratory Exercise Testing: Clinical Guidelines. [Google Scholar]
- 17.Bar-Or O. Pediatric sports medicine for the practitioner: From physiologic principles to clinical applications. Springer-Verlag; New York: 1983. [Google Scholar]
- 18.Utter AC, Roberson RJ, Nieman DC, Kang J. Children’s OMNI scale of perceived exertion: Walking/running evaluation. Med Sci Sports Exerc. 2002;34:139–144. doi: 10.1097/00005768-200201000-00021. [DOI] [PubMed] [Google Scholar]
- 19.Goran M, Fields D, Hunter G, Herd S, Weinsier R. Total body fat does not influence maximal aerobic capacity. Int J Obes. 2000;24:841–848. doi: 10.1038/sj.ijo.0801241. [DOI] [PubMed] [Google Scholar]
- 20.Monti JM, Hillman CH, Cohen NJ. Aerobic fitness enhances relational memory in preadolescent children: The FITKids randomized control trial. Hippocampus. 2012;22:1876–1882. doi: 10.1002/hipo.22023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baym CL, Khan NA, Monti JM, et al. Dietary lipids are differentially associated with hippocampal-dependent relational memory in prepubescent children. Am J Clin Nutr. 2014;99:1–8. doi: 10.3945/ajcn.113.079624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hannula DE, Ryan JD, Tranel D, Cohen NJ. Rapid onset relational memory effects are evident in eye movement behavior, but not in hippocampal amnesia. J Cogn Neurosci. 2007;19:1690–1705. doi: 10.1162/jocn.2007.19.10.1690. [DOI] [PubMed] [Google Scholar]
- 23.Davachi L, Wagner AD. Hippocampal contributions to episodic encoding: Insights from relational and item-based learning. J Neurophysiol. 2002;88:982–990. doi: 10.1152/jn.2002.88.2.982. [DOI] [PubMed] [Google Scholar]
- 24.Hannula DE, Althoff RR, Warren DE, Riggs L, Cohen NJ, Ryan JD. Worth a glance: Using eye movements to investigate the cognitive neuroscience of memory. Frontiers in human neuroscience. 2010:4. doi: 10.3389/fnhum.2010.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skinner AC, Steiner MJ, Perrin EM. Self-reported energy intake by age in overweight and healthy-weight children in NHANES, 2001–2008. Pediatrics. 2012;130:e936–42. doi: 10.1542/peds.2012-0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jimenez-Pavon D, Kelly J, Reilly JJ. Associations between objectively measured habitual physical activity and adiposity in children and adolescents: Systematic review. International Journal of Pediatric Obesity. 2010;5:3–18. doi: 10.3109/17477160903067601. [DOI] [PubMed] [Google Scholar]
- 27.Spruyt K, Molfese DL, Gozal D. Sleep duration, sleep regularity, body weight, and metabolic homeostasis in school-aged children. Pediatrics. 2011;127:e345–52. doi: 10.1542/peds.2010-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bennett B, Larson-Meyer DE, Ravussin E, et al. Impaired insulin sensitivity and elevated ectopic fat in healthy obese vs. nonobese prepubertal children. Obesity. 2012;20:371–375. doi: 10.1038/oby.2011.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skinner AC, Steiner MJ, Henderson FW, Perrin EM. Multiple markers of inflammation and weight status: Cross-sectional analyses throughout childhood. Pediatrics. 2010;125:e801–9. doi: 10.1542/peds.2009-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Després J, Lemieux I, Bergeron J, et al. Abdominal obesity and the metabolic syndrome: Contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol. 2008;28:1039–1049. doi: 10.1161/ATVBAHA.107.159228. [DOI] [PubMed] [Google Scholar]
- 31.Després J, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 32.Yanovski JA, Yanovski SZ, Filmer KM, et al. Differences in body composition of black and white girls. Am J Clin Nutr. 1996;64:833–839. doi: 10.1093/ajcn/64.6.833. [DOI] [PubMed] [Google Scholar]
- 33.Jurdak N, Lichtenstein AH, Kanarek RB. Diet-induced obesity and spatial cognition in young male rats. Nutr Neurosci. 2008;11:48–54. doi: 10.1179/147683008X301333. [DOI] [PubMed] [Google Scholar]
- 34.Gerges N, Aleisa A, Alkadhi K. Impaired long-term potentiation in obese zucker rats: Possible involvement of presynaptic mechanism. Neuroscience. 2003;120:535–539. doi: 10.1016/s0306-4522(03)00297-5. [DOI] [PubMed] [Google Scholar]
- 35.Debette S, Beiser A, Hoffmann U, et al. Visceral fat is associated with lower brain volume in healthy middle-aged adults. Ann Neurol. 2010;68:136–144. doi: 10.1002/ana.22062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han JC, Lawlor DA, Kimm S. Childhood obesity. The Lancet. 2010;375:1737–1748. doi: 10.1016/S0140-6736(10)60171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McNay EC, Ong CT, McCrimmon RJ, Cresswell J, Bogan JS, Sherwin RS. Hippocampal memory processes are modulated by insulin and high-fat-induced insulin resistance. Neurobiol Learn Mem. 2010;93:546–553. doi: 10.1016/j.nlm.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56:1010–1013. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- 39.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yaffe K, Kanaya A, Lindquist K, et al. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA: the journal of the American Medical Association. 2004;292:2237. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- 41.Drapeau V, Therrien F, Richard D, Tremblay A. Is visceral obesity a physiological adaptation to stress? Panminerva Med. 2003;45:189–195. [PubMed] [Google Scholar]
- 42.Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57:925. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- 43.Eriksson PS, Perfilieva E, Björk-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 44.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 45.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. The Journal of neuroscience. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proceedings of the National Academy of Sciences. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenzweig MR, Bennett EL, Hebert M, Morimoto H. Social grouping cannot account for cerebral effects of enriched environments. Brain Res. 1978;153:563–576. doi: 10.1016/0006-8993(78)90340-2. [DOI] [PubMed] [Google Scholar]
- 48.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 49.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 50.Van Praag H, Kempermann G, Gage FH. Neural consequences of enviromental enrichment. Nature Reviews Neuroscience. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- 51.Ortega FB, Ruiz JR, Sjöström M. Physical activity, overweight and central adiposity in swedish children and adolescents: The european youth heart study. International Journal of Behavioral Nutrition and Physical Activity. 2007;4:61. doi: 10.1186/1479-5868-4-61. [DOI] [PMC free article] [PubMed] [Google Scholar]