Summary
Tunicates are invertebrate members of the chordate phylum, and are considered to be the sister group of vertebrates. Tunicates are composed of ascidians, thaliaceans, and appendicularians. With the advent of inexpensive high-throughput sequencing, the number of sequenced tunicate genomes is expected to rise sharply within the coming years. To facilitate comparative genomics within the tunicates, and between tunicates and vertebrates, standardized rules for the nomenclature of tunicate genetic elements need to be established. Here we propose a set of nomenclature rules, consensual within the community, for predicted genes, pseudogenes, transcripts, operons, transcriptional cis-regulatory regions, transposable elements, and transgenic constructs. In addition, the document proposes guidelines for naming transgenic and mutant lines.
Keywords: tunicates, genome annotation, gene, transposable element, cis-regulatory sequences
INTRODUCTION
Affordable high-throughput sequencing is leading to a paradigm shift in evolutionary developmental biology (aka, Evo-Devo) as an increasing number of near-complete genome sequences will soon be available for most taxa, including the tunicates. The genomes of two solitary species of the genus Ciona, Ciona intestinalis (Dehal et al., 2002) and Ciona savignyi (Small et al., 2007), were sequenced in the early 2000s, followed by the genome of the appendicularian Oikopleura dioica (Denoeud et al., 2010). The first genome of a colonial ascidian, Botryllus schlosseri, was released in 2013 (Voskoboynik et al., 2013), and the genomes of at least seven more species belonging to the genera Phallusia, Halocynthia, and Molgula (Stolfi et al., 2014) are currently being sequenced and annotated. Comparison of these genomes to each other, and to the lancelet (Amphioxus) and vertebrate genomes, will shed light on the last common ancestor of tunicates and vertebrates, and help explain how ascidians could retain a particularly conserved embryonic development in spite of rapid genome divergence (Lemaire, 2011; Lemaire et al., 2008).
The definition of precise naming rules for genetic elements, which would greatly facilitate tunicate comparative genomics, has so far not been attempted. This article proposes uniform guidelines for ascidians, thaliaceans, and appendicularian genetic elements. A central concern when designing these rules is that orthologous features across the subphylum receive the same name, and that this name is chosen, when possible, to reflect the orthology to features in the human genome, the most completely sequenced and annotated chordate genome.
Computational analyses are taking center stage in biology and a second concern was to make sure that the nomenclature rules would be compatible with the efficient parsing of large files, while remaining understandable to the bench biologist. This involved the avoidance of symbols and identifiers of characters that could be interpreted as separators in tabulated files. We also made sure that alphabetical or numerical symbols and identifiers have a constant syntax and number of characters.
The following sections will first define species abbreviations, then rules for coding and non-coding genetic features, before closing on the nomenclature for transgenic elements and lines of transgenic or mutant animals.
SPECIES SYMBOLS
Comparative analyses frequently necessitate distinguishing between orthologous elements in different species. In such cases, an abbreviated species symbol is used as a prefix to the feature name.
A species is identified by a binomial name composed of a generic epithet and a specific epithet, written in italics (International Code for Zoological nomenclature). The first epithet describes the genus, and its first letter is capitalized (e.g., Ciona). The second epithet describes the particular species within the genus and is written in lower case italics (e.g., intestinalis). A third epithet, usually written in lower case italics, can be added to describe subspecies, when applicable.
Such binomial species names can be abbreviated and used as prefix or suffix in the names of genetic elements. The following abbreviation rules are proposed, which were tested on the 3,018 Tunicate species listed in the World Registry of Marine Species (WoRMS) database at the time of writing. Current validated abbreviations are listed in the Supporting Information Table 1 and deposited with the WoRMS. Novel abbreviations should be registered with WoRMS.
A species symbol is a binomial abbreviation consisting of six letters. In most cases, the syntax is “2G4S.” The first two letters are an abbreviation of the generic epithet (G), and the final four letters are an abbreviation of the specific epithet (S). In some cases, explained below, three letters are used to abbreviate generic epithets, in which case the binomial abbreviation is 3G3S.
Abbreviations of generic epithets should not be ambiguous. When possible, two-letter abbreviations should be used, built from the first two letters of the generic name (e.g., Py for Pyura). In case of ambiguity, (e.g., Pycnoclavella vs. Pyura), one of the conflicting genera receives an unambiguous abbreviation built from the first letter of the generic name, followed by a discriminative letter from the generic name (Pv for Pycnoclavella). In rare cases, there are too many genera starting with the same first letter to define unambiguous two-letter abbreviations for all genera (e.g., the names of 42 genera start with a P). In such case, some genera receive a three-letter abbreviation (e.g., Prm for Protomolgula). There should be no ambiguities between two-letter and three-letter abbreviations. While there can be Prm for Protomolgula and Prh for Protoholozoa, there is no genus whose two-letter abbreviation is simply Pr, as this would cause confusion as to whether a binomial abbreviation follows the 2G4S or 3G3S formula.
Abbreviations of specific epithets are usually four letters long (2G4S rule), except if they belong to a genus abbreviated with three letters, in which case their abbreviations are also three letters long (3G3S rule). Specific epithet abbreviations are usually built from the first three or four letters of the specific name (e.g., inte for intestinalis), except when this results in ambiguities within a genus (e.g., Pyura squamata vs. Pyura squamulosa). In these cases, a unique species abbreviation can incorporate discriminative letters from the specific epithet, which maximize intuitive reading (e.g., sqma for squamata vs. sqml for squamulosa). In cases of 2G4S abbreviations, when the specific epithet only has three letters, a letter from the generic epithet is added before the species abbreviation (e.g., Didemnum ahu = Didahu). Some prefixes are frequently used in specific epithet, and can be systematically abbreviated in a standard way. These prefixes are currently used: poly- (abbr: py-); psam- (psm-); pseudo- (ps-); longi- (1-); multi-(m-); trans- (tr-). Examples: pseudogrisiatum is abbreviated as psgr, translucidum becomes trlu; and polyducta becomes pydu. These abbreviations of frequently used prefixes do not apply to generic epithets.
Identical or very similar specific epithets in different genera can share a common abbreviation (or at least the first three letters of the abbreviation, for species following the 3G3S rule), as long as this does not result in identical, conflicting specific abbreviations within a genus. In this case, one of the conflicting species abbreviations should be modified accordingly.
GENES
A gene is defined in Sequence Ontology (Eilbeck et al., 2005) as “a region (or regions) that includes all of the sequence elements necessary to encode a functional transcript. A gene may include regulatory regions, transcribed regions, and/or other functional sequence regions” (Sequence Ontology term SO:0000704, Table 1). A gene can encode one or more proteins, or one or more non-coding RNAs.
Table 1.
Classes of Genes Defined in Sequence Ontology and Corresponding Class Descriptors in Unique Gene Identifiers
| Class of gene | Sequence ontology ID |
Sequence ontology definition | Class descriptor |
|---|---|---|---|
| Protein-coding gene | SO:0001217 | A gene that codes for a protein | CG |
| Ribosomal RNA gene | SO:0001637 | A gene that encodes a ribosomal RNA | rRNA |
| Transfer RNA gene | SO:0001272 | A gene that encodes a transfer RNA | tRNA |
| Non-coding RNA gene | ncRNA | ||
| Long non-coding RNA gene | SO:0001877 | A non-coding RNA over 200 nucleotides in length. | IncRNA |
| Long intergenic non-coding RNA gene | SO:0001463 | A multiexonic non-coding RNA transcribed by RNA polymerase II. | lincRNA |
| Small nuclear RNA gene | SO:0001268 | A gene that encodes a small nuclear RNA | snRNA |
| Small nucleolar RNA gene | SO:0001267 | A gene that encodes a small nucleolar RNA | snoRNA |
| Micro RNA gene | SO:0001265 | A gene that encodes a microRNA | mi RNA |
| piwi-associated RNA gene | SO:0001035 | A small non coding RNA, part of a silencing system that prevents the spreading of selfish genetic elements. | piRNA |
| Enhancer RNA | SO:0001870 | A short ncRNA that is transcribed from an enhancer. May have a regulatory function. | eRNA |
| Mitochondrial gene | SO:0000088 | A gene located in mitochondrial sequence | mt |
| Pseudogene | SO:0000336 | A sequence that closely resembles a known functional gene, at another locus within a genome, that is non-functional as a consequence of (usually several) mutations that prevent either its transcription or translation (or both). In general, pseudogenes result from either reverse transcription of a transcript of their “normal” paralog (SO:0000043) (in which case the pseudogene typically lacks introns and includes a poly(A) tail) or from recombination (SO:0000044) (in which case the pseudogene is typically a tandem duplication of its “normal” paralog). | ps |
A precise gene nomenclature system should be able to:
Unambiguously and stably identify a gene, across the successive releases of genome assemblies and gene builds for a given species.
Track the history of successive gene models.
Identify the gene as the ortholog, or a close relative, of a Human gene in order to facilitate a connection to available information on gene function, gathered from the larger corpus of biomedical research.
Reflect the belonging to a structural gene family or the phenotype obtained following perturbation of the gene activity.
Finally the evolution of a gene name should be traceable, when additional knowledge builds up on the function of the gene in tunicate or other species.
To achieve these goals, a tunicate gene is defined by a combination of a unique gene locus identifier, a gene model identifier, a primary gene name and primary symbol, and synonymic gene names and symbols.
Unique Gene Locus Identifier
This is a stable identifier, which is guaranteed to follow the gene throughout any changes that may be made to its structure. Locus identifiers are composed of a species prefix and an eight-digit number. The unique identifier does not provide information on the type of the gene (coding, non-coding, etc.).
Example:
Cisavi.00009682 defines the Ciona savignyi gene number 00009682, which, in this case, is a coding gene.
As these identifiers are generated automatically and independently for each species, there is no reason that the genes Ciinte.00004567 and Phmamm.00004567 should be orthologous.
Genes are initially identified on the basis of ab initio prediction and experimental evidence. As new evidence accumulates, the structure of a predicted gene can evolve with time, leading to the fusion of two predicted genes, to the split of a gene into two or more genes, or to the disappearance of the gene. Genes resulting from fusions or splits receive new unique gene identifiers, and a tracking system will link the new genes to their precursors. In such cases, a suffix is added to the unique gene identifier, indicating a change of status. The suffix _suppr[year] indicates that the gene has been suppressed from the species gene list in the indicated year. The suffix _split[year] indicates that the gene has been split into two or more genes in the indicated year. The suffix _fused [year] indicates that the gene has been fused with one or more other genes in the indicated year. In case of complex patterns of splits and fusions, fusions are considered dominant.
Examples:
Ciinte.00000657_suppr2013 means that this Ciona intestinalis gene has ceased to be considered a gene in 2013.
Cisavi.00008765_split2011 indicates that this Ciona savignyi gene has been split into two or more genes in 2011.
Boschl.00012998_fused20l4 indicates that this Botryllus schlosseri gene has been fused with one or more other genes in 2014.
Gene Model Identifier
This identifier characterizes a particular instance (model) of a gene in a given assembly. It is composed of a species prefix, a descriptor of the class of gene considered (CG for a protein-coding gene, tRNA, miRNA, eRNA. Table 1 lists accepted Sequence Ontology gene class descriptors), a reference of the gene build and assembly considered, and a unique identifier that specifies on which contig/scaffold/chromosome the gene is located. This identifier can change with time as novel information/analyses become available. Different species can initially adopt different syntaxes for the gene build and assembly identifiers as exemplified below, although an effort should be made to unite these syntaxes in genomes that are considered stable.
Examples:
Ciinte.CGKH2012.C1.841 defines the Ciona intestinalis coding gene of KH assembly, gene build 2012, “ranked” 841 on Chromosome 1. Note that in C intestinalis, genes on a given chromosome/scaffold are “ranked” in arbitrary order, and genes with successive ranking numbers are not necessarily neighbors on the chromosome.
Cisavi.miRNA.ENS75.R16.3924783–3924863 defines the Ciona savignyi miRNA gene of built ENSEMBL release 75 located on Reftig (scaffold) 16 between coordinates 3,924,783 and 3,924,863. Note that in C savignyi, genes are identified by coordinates on a scaffold.
Boschl.CG.Botznik2013.botctg020918.g2519 defines the Botryllus schlosseri protein coding gene reference g2519, located on the contig 020918 of assembly Botznik2013. In B. schlosseri, genes are identified by their rank in the global gene list.
Primary Coding Gene Name and Symbol
The name of a gene is a word or short phrase describing the structure of a gene (e.g., Cytochrome b5 domain containing 1), its orthology group (Orthodenticle homeobox), its function (Mitogen-activated protein kinase kinase 1/2), or its expression pattern (Posterior end mark). The first letter of the first word is capitalized and names are italicized. The 1-to-1 tunicate orthologs should share a common gene name and symbol.
The primary symbol of a gene is a short-form representation/abbreviation of the primary gene name, unique within the species. Usually 3–5 characters long, no more than 10 (e.g., Cyb5d1 is the gene symbol for Cytochrome b5 domain containing 1). The first letter of the symbol should be capitalized, and the other letters should be in lower case. The use of punctuation, such as period and hyphens, within gene symbols is discouraged, except under specific circumstances described below. Gene symbols are italicized.
Tunicate gene symbols should generally not be preceded by a mention of the species symbol (e.g., Ciinte, Harore.), except when the distinction between orthologous genes in different tunicates needs to be made. In such cases, the species abbreviation (e.g., Ciinte), is added in front of the symbol and separated from it by a dot (“.”).
To facilitate comparison to vertebrates, tunicate gene names and symbols are preferentially named after their mouse or human orthologs, as defined by the HGNC project (Gray et al., 2013), with a preference for the human ortholog(s) when they exist.
In the case of one-to-many orthology relationships between tunicate and human orthologs, the tunicate gene name/symbol reflects all human (or mouse) paralogs.
Example:
Fibroblast growth factor 9/16/20—symbol Fgf9/ 16/20—is the single tunicate ortholog of the three human genes FGF9, FGF16, and FGF20.
To avoid excessively long names or symbols, the numbers are omitted if a single tunicate gene is orthologous to all human members of a subfamily.
Example:
Pitx is the single tunicate ortholog of Human PITX1, 2, and 3.
For simplicity, when the human (or mouse) paralogs have very differing names, a single “class” or widely accepted “family” name is used for the tunicate gene. The Ciona intestinalis choice for this name determines the names in subsequently sequenced tunicate genomes.
Examples:
Ciona intestinalis cAMP response element-binding protein 1 (Creb1) is orthologous to the three human genes cAMP response element-binding protein 1 (CREB1), Activating transcription factor 1 (ATF1), and cAMP Response Element Modulator (CREM).
In Ciona intestinalis, Dan domain family member (Dand) is the single ortholog of Human Cerberus (CER1), Gremlin (GREM1), and Dan domain 5 (DAND5) genes.
Ciona intestinalis Otx is the single ortholog of Human OTX1 OTX2, and CRX genes.
Ciona intestinalis Myogenic regulatory factor (Mrf) is orthologous to the human Myogenic regulatory factor family members Myogenic differentiation 1 (MYOD1, Myogenin (MYOG), Myogenic factor 5 (MYF5), and Myogenic factor 6 (MYF6).
In rare cases, tunicate gene names and symbols can deviate from those officially used for the orthologous human genes, and reflect more closely the terms used to refer to the encoded protein in humans and/or orthologous genes in other vertebrates.
Example:
The human gene named “T, brachyury homolog (Mouse)” encodes a protein whose recommended name is “Brachyury,” according to the UniProt database Jain et al., 2009). The orthologous tunicate gene name is thus Brachyury, and its symbol is Bra.
In case of many-to-one or many-to-many orthology relationships, the tunicate paralogs are distinguished by “.{a–z}” suffixes. In some cases, the tunicate duplication will have taken place at the root of either the tunicate phylogeny or a specific sublineage (ascidians, salps, stolido-branchs, etc.) and the same suffices should be used for all orthologs within this branch. Because of the rapid evolution of tunicate genomes, precise orthology relationships are sometimes difficult to draw. Hence, while we suggest that orthologous tunicate genes receive the same suffix when possible, non-orthologous genes may receive the same suffix, especially in distant species. This rule may be subject to revision when a higher coverage of the taxon with sequenced genomes is achieved, and we have a better vision of the diversity and complexity of orthology relationships within families, orders, and genera.
Examples:
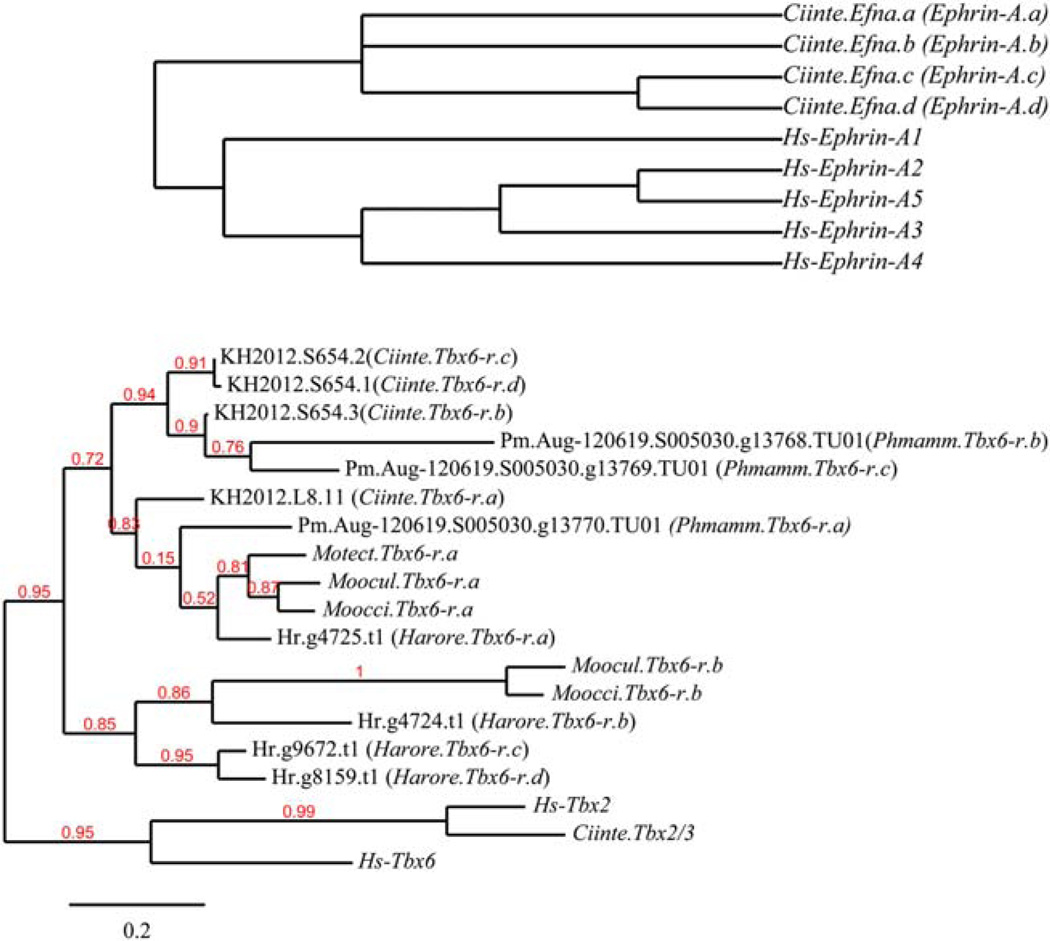
The Ciona intestinalis Ephrin-A genes have undergone independent duplications in the vertebrate and ascidian lineages (Fig. 1A), giving rise to Ciona intestinalis Ephrin-A a to .d, (symbols: Efna.a to .d) which are all orthologous to human Ephrin-A1to 5 (EFNA1to 5, Fig. 1A).
Hedgehog.a and .b are the two paralogous Ciona intestinalis orthologs of human sonic, Indian, and desert hedgehog genes.
FIG. 1.
(A) Phylogenetic tree for the Ciona intestinalis and Human Ephrin A gene family. (B) Phylogenetic tree for the tunicate Tbx6-related class of genes in Ciona intestinalis, Halocynthia roretzi, Phallusia mammillata, and Molgula spp., and their human (Hs-) relatives.
When a tunicate gene shows preferential similarity to a mammalian gene without robust orthology association, it inherits the vertebrate name followed by the suffixes -related (-r in the symbol). We discourage the use of the suffix “-like” in this case because it occurs in some human gene names (e.g., Fer3-like BHLH transcription factor). Should several tunicate genes be preferentially similar to the same mammalian gene, they are distinguished by adding a “.{a–z}” suffix to their names and symbols.
Examples:
Ciona savignyi prophet of Pit-1-related (Prop-r) is similar to human prophet of Pit-1 (PROP) without being a confident ortholog.
In Ciona intestinalis several gene duplications have given rise to four paralogous T-box six-related (Tbx6-r) genes, all related to the single human TBX6 gene. These genes are named Tbx6-r.a, .b, .c, .d. There are also four Halocynthia roretzi Tbx6-r genes, which however do not have clear 1-to-1 Ciona orthologs. These genes are also named Tbx6-r.a, .b, ,c, ,d, but there is no strong inference that Harore.Tbx6-r.x is orthologous to Ciinte.Tbx6-r.x. (Fig. 1B).
Ciona intestinalis, Ciona savignyi, and Halocynthia have two groups of Zic-related genes, which are called Macho-1 and ZicL (or ZicR). All of these species have one copy of the gene initially called Macho-1, and this gene is named Zic-r.a (Macho-1 remains as a secondary synonym, see below). Ciona intestinalis has five copies of ZicL, and they are named Zic-r.b to Zic-r.f. Similarly, Halocynthia has a single copy of ZicR, named Zic-r.b. Note that Ciin-te.Zic-r.a and Harore.Zic-r.a are orthologous, but Ciinte.Zic-r.b and Harore.Zic-r.b might not be orthologous in a strict sense.
Genes that belong to a protein family, but show only limited similarity to non-tunicate genes are considered “tunicate-specific” members of this family. Their name is “Tunicate,” followed by the protein family name, and by a numerical identifier. Numerical identifiers are unique within a protein family. A tunicate-specific gene may be present in some but not all tunicate genomes, in which case its numerical identifier remains unused in species that do not have this gene. Use of the term “orphan” in gene names and symbol is discouraged for genes without non-tunicate orthologs. The symbols of “tunicate-specific” genes are of the form [Family] tun[#].
Example:
Tunicate bhlh 2 (Bhlhtun2) is the tunicate bHLH gene number 2 with no characterized ortholog out-side of tunicates. This gene is present in Ciona intestinalis, but not Halocynthia roretzi, and in this species tunicate bhlh identifiers skip 2 and jump from Bhlhtun 1 to Bhlhtun3.
Genes without significant protein sequence similarity (BlastP E-value > 1e-5), or conserved domain, with any non-tunicate species do not receive a descriptive name, unless they have been functionally characterized in tunicates (e.g., Posterior end mark). They are named using their stable gene identifier.
Pseudogenes inherit the name of the functional gene from which they are derived, followed by the suffix -ps and a serial number if there are multiple pseudogenes. If only one pseudogene copy of a particular gene exists, it is given the suffix -ps1.
Gene Synonyms
A gene can have several “secondary” synonymous names or symbols, which differ from those that have been applied to the gene at various times. Such names, which often reflect the specialized function of a gene in tunicates, can be used in publications, provided the primary symbol is also mentioned. Secondary synonyms are important to ensure that databases will trace deprecated names to their current primary name and symbol. The current primary gene name and symbol should always be mentioned in new work. Primary gene names and symbols can evolve in time, for instance to reflect more accurate orthology relationships. In such cases, the modified primary names and symbols become synonyms to the new primary name and symbols.
Examples:
Macho-1 is a secondary synonym for Zic-r.a.
Hepatocyte nuclear factor 6 (Hnf6) is a secondary synonym for One cut homeobox (Onecut).
Overlapping, Antisense, and Opposite Strand Genes
In general, genes whose transcripts partially overlap on opposite strands (for instance overlapping 3′ ends) should be given distinct names and symbols. This rule also applies to genes encoded at the same locus, in the same orientation, but using different reading frames.
A gene of unknown function running on the opposite strand to a coding gene, and included within this gene locus, should receive the name and symbol of the coding gene with the suffix “-os” for opposite strand.
Example:
In Ciona intestinalis the Bra-os gene (KH2012:KH.S1404.3) is running on the opposite strand, and included within the Ciona intestinalis Brachyury locus (KH2012:KH.S1404.1).
A gene running on the opposite strand of a coding gene and known to regulate the function of this gene receives the name of the affected gene, with a suffix “-as” for antisense.
Non-Coding Gene Primary Names and Symbols
In addition to coding genes, many genes encode functional short and long non-coding RNAs, including tRNAs, snoRNAs, rRNAs, snRNAs, miRNAs, piRNAs… In human, these genes have recently been the focus of efforts to design a unique nomenclature (Wright and Bruford, 2011), from which the tunicate nomenclature is adapted. Primary names and symbols can be accompanied by synonyms, to relate the gene to more ancient nomenclatures. Our current knowledge on tunicate non-coding genes is partial, and some of the following rules will need refinement as this knowledge increases. Genes with clear 1-to-1 orthologs share the same name and symbol, one-to-many and many-to-many orthology rules are the same as for coding genes.
Tunicate nuclear tRNA gene names are of the form [Species] transfer RNA [amino acid] [#] (anticodon), where [Species] is the symbol of the species, usually omitted if the context is unambiguous, [amino acid] is the name of the amino acid, [#] is the serial number for the transfer RNA, and (anticodon) is the sequence of the anticodon. The corresponding symbol is of the form trna[aa][#], where [aa] is the single letter abbreviation for the amino acid.
Example:
trnas1 is the symbol for the transfer RNA serine 1 (UGA) gene.
Mitochondrial tRNA gene are named according to the same logic, with the prefix “mt-.”
Example:
mttrnag is the symbol for the mitochondrial transfer RNA Glycine (UCU) gene.
Tunicate small nucleolar RNA genes follow the nomenclature of snoRNABase (Xie et al., 2007) (www-snorna.biotoul.fr) and are split into small nucleolar RNA, C/D Box (SNORD), small nucleolar RNA, H/ACA Box (SNORA), and small Cajal body-specific (SCARNA). In each species, their symbol follows the syntax: {snord, snora, scarna} [#] {a–z}, where [#] is a serial number describing the snoRNA family. SnoRNA genes are frequently duplicated and the different members of each family in a tunicate genome are distinguished by the. {a–z} suffix.
Example:
Ciinte.snord18.a is the symbol of one of the five Ciona intestinalis small nucleolar RNA, C/D Box 18 gene, orthologous to all human SNORD 18 (many-to-many orthology relationship). The others are Ciin-te.snord 18.b, ,c, ,d, and ,e.
Tunicate ribosomal RNA gene names have the syntax [Species] (mitochondrial) ribosomal RNA {5, 5.8, 12, 16, 18, 26}S [#], where [#] is a serial number that reflects the presence of many copies of each ribosomal RNA gene. The corresponding symbol is (mt-)rn{5, 5.8, 12, 16, 18,26}s[#].
Examples:
Ciinte.rn 182 is the symbol of the second nuclear ribosomal 18S RNA gene in Ciona intestinalis.
Harore.mt-rn16s is the symbol of the Halocynthia roretzi mitochondrial ribosomal 16S RNA gene (synonym used in previous work: mt-lrRNA for large mitochondrial rRNA gene).
Small nuclear RNAs (snRNA or U-RNAs) are Uridine-rich small RNAs found in the nucleus of eukaryotic cells. The syntax of the symbol of spliceosomal snRNAs (U1, 2, 3, 4, 4atac, 5, 6, 6atac, 11, and 12) and of the U7 snRNA is rnu{1, 2, 3, 4, 4atac, 5, 6, 6atac, 7, 11, 12}-[#], where [#] is a numerical identifier.
Example:
rnu1–1 is the symbol of the U1 snRNA 1.
Other small nuclear RNAs include vault RNAs (symbol vtrna[#]), 7SK RNA (rn7sk) 7SL RNAs (rn7sl[#]),Y RNAs that form part of the Ro RNP (rny[#]), and telo-merase RNA component (Terc).
Piwi-interacting RNA form the largest family of expressed ncRNAs. piRNAs are designed by symbols with the syntax pirna[#], where [#] is a serial number. piRNAs are often grouped in large clusters, which are themselves identified through symbols with the syntax: pirc[#] for PiRNA cluster [#], where [#] is a serial number.
Finally, tunicate miRNA genes are named according to accepted international standards used by MiRBase (Kozomara and Griffiths-Jones, 2014) and first published in 2003 (Ambros et al., 2003). Briefly, full names are of the form Species miRNA[#]-[#], where [#] is the accepted MiRBase number for this class of miRNA. Symbols of miRNA genes are of the form mir[#]. Mature miRNAs are distinguished from the gene they originate from with a capital R (miR[#]). miRNAs that encode homologous mature transcripts share the same mir number, with differing suffixes. If the mature miRNAs differ by only one or two nucleotides, they are allocated letter suffixes (e.g., mir10A and mir10B), whereas if the mature miRNAs are identical, the genes are given hyphenated numerical suffixes (e.g., mir1–1 and mir1-2) (Wright and Bruford, 2011).
Examples:
In Phallusia mammillata, Phmamm.mir121 is the symbol of the miRNA 121 gene, orthologous to the Human miRNA 121 gene.
miR121 is the mature miRNA produced by the gene mir 121 in a tunicate species.
TRANSCRIPTS
A transcript is “an RNA synthesized on a DNA or RNA template by an RNA polymerase. Several transcripts with alternative structures can be produced by a single gene though the process of alternative splicing and/or alternative promoter usage” (SO:0000673). Transcripts are defined by a transcript model identifier, a transcript name, and a symbol.
Transcript Model Identifier
The transcript model identifier is generally composed of its gene model identifier, a suffix that uniquely identifies each transcript model variant, and the “.t” suffix. The syntax of unique transcript suffixes may differ between species.
Examples:
Ciinte.CG.KH2012.C4.84.v1.A.SL5-1.t is the transcript model variant v1 A.SL5–1 of Ciona intestinalis coding gene Ciinte.CG.KH2012.C4.84.
Cisavi.CG.ENS75.R90.454100–458898.16640.t is the transcript model variant 16,640 of Ciona savignyi coding gene Cisavi.CG.ENS75.R90.454l00–458898.
Transcript Model Name and Symbol
The transcript model inherits its gene name and symbols, followed by a suffix that distinguishes the transcript from the gene. No specific rules are established for the syntax of transcript suffixes in names and symbols, which can thus differ between species.
PROTEINS
Protein Model Identifiers
Proteins receive the same identifiers as the transcript they are produced from, not italicized, except that the “.t” suffix is replaced by “.p.”
Example:
Ciinte.CG.KH2012.C4.84.v1.A.nonSL3–1.p is the protein produced by the transcript variant Ciinte.CG.KH2012.C4.84.v1.A.nonSL3–1.t of the Ciona intestinalis Otx gene.
Protein Names and Symbols
When a single protein is produced by a gene, this protein inherits the name and symbol of the corresponding gene, without italics.
Example:
T-box 6-related.d (Tbx6-r.d) is the sole protein produced from by the gene T-box 6-related.d (Tbx6-r.d).
As a simplified convention to refer to the different protein isoforms translated from different transcripts of the same gene, one may use the gene symbol followed by a suffix of syntax -i{#}, where i stands for isoform and # is an integer.
Example:
In both Ciona intestinalis and Halocynthia roretzi, the Lhx3/4 gene produces two protein isoforms (Christiaen et al., 2009; Kobayashi et al., 2010), which can be named Lhx3/4-i1 and -i2.
TRANSCRIPTIONAL C/S-REGULATORY REGIONS
A transcriptional cis-regulatory region is a segment of DNA, usually non-coding, that modulates the level of activity of one or more genes (SO:0001055). It is defined by a unique identifier, by its coordinates in a given genome assembly, by its type of activity and by optional target gene or genes, when known. Note that only experimentally tested cis-regulatory elements are identified and named, and that no inference is made about the precise boundaries of the functional cis-regulatory region in the genomic context.
Cis-Regulatory Region Unique Identifier
This is a stable identifier, which is guaranteed to follow the region in successive genome assemblies. It is composed of the species symbol, followed by REG and an eight-digit unique number (e.g., Ciinte.REG00000034). Regulatory regions with the same unique number in different species are not inferred to be orthologous.
Cis-Regulatory Region Identifier Within an Assembly
As regulatory sequences can regulate multiple genes, or the target genes may not be known, target gene name is not necessarily included to define a cis-regulatory region within an assembly. A cis-regulatory region identifier is of the form: [Species].REG.[assembly].[start-end](| [target gene symbol1] | [Target gene symbol2]|.), where [Species] is the species abbreviation, “.REG” indicates the class of element, [assembly] identifies the assembly within the species, [start-end] gives the coordinate of the region, and (| [target gene symbol1] |.) is optional and list experimentally determined targets of the cis-regulatory region by alphabetical order.
Examples:
Ciinte.REG.KH2012.C1.289567–289760 is a regulatory sequence located on the Chromosome C1 of KH assembly of Ciona intestinalis, between positions 289,567 and 289,760. Note that the coordinates do not include commas (,) to avoid difficulties when parsing .csv formatted files.
Ciinte.REG.KH2012.C4.4313996–4315697|Otx is a Ciona intestinalis regulatory sequence for the Otx gene, located on Chromosome C4 between positions 4,313,996 and 4,315,697.
Ciinte.REG.KH2012.C2.1981987–1982339 |Admp | Pinhead is a Ciona intestinalis regulatory sequence controlling the expression of both Admp and Pin-head (Imai et al., 2012).
Note that cis-regulatory element names found in scientific articles are usually shorter and more “biologically relevant” than the proposed nomenclature, but short names are difficult to generalize and are often legitimately biased to illustrate the main message of the article. The following guidelines can help authors build such short names in their manuscripts. Naming cis-regulatory sequences with respect to a gene transcription start site is discouraged unless the transcription start site has been precisely mapped in the tissue in which the cis-regulatory region is active. Naming regulatory sequences with respect to the start of the transcript extending furthest 5′ of the gene can be used in articles, provided the identity of the gene build is mentioned. In all cases, the materials and methods of the article should provide the cis-regulatory region identifier defined above.
Example:
The name Otx[−1541/−1417] can be used in the main text of a scientific article dealing with Ciona intestinalis, but the full identifier of this sequence, Ciin-te.REG.KH2012.C4.4315574–4315697 | Otx should be mentioned in the materials and methods.
Often a “minimal” or “basal” promoter of one gene is used in combination with distal cis-regulatory elements from the same or a different locus. The minimal promoter is often defined as the sequence immediately flanking the site of initiation of transcription by RNA polymerase and is necessary, but not sufficient, for transcription. In scientific articles, minimal promoters may be indicated by the prefix “pr” attached to a gene symbol (e.g., prOtx or prCiinte.Otx), but the standard rules for naming of cis-regulatory region identifiers still apply to them.
OPERONS
An operon (or polycistronic gene) is defined as “A group of contiguous genes transcribed as a single (polycistronic) mRNA from a single regulatory region” (SO:0000178). As this may be difficult to assess, a relaxed definition can be used: two or more genes transcribed in the same orientation with no or very short intergenic sequences and evidence for trans-splicing of the downstream gene(s) (Satou et al., 2008). Each Operon receives a unique Operon identifier.
Unique Operon Identifier
This identifier is stable across successive genome assemblies and gene builds. It is composed of a species identifier, followed by “-OP” and an eight-digit number.
Example:
Ciinte.OP00000807. There is no assumption of orthology between operons with the same numerical identifier in different species.
TRANSPOSABLE ELEMENTS (TE)
A TE is “an element that can insert in a variety of DNA sequences. A transposon may contain the genes necessary for its transposition. For example, gag, int, env, and pol are the TE genes of the TY element in yeast” (SO:0000101).
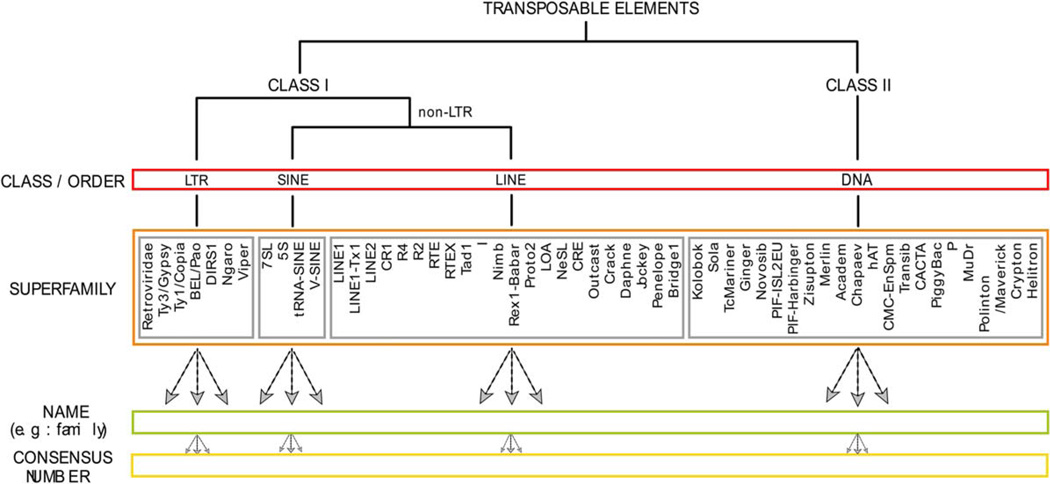
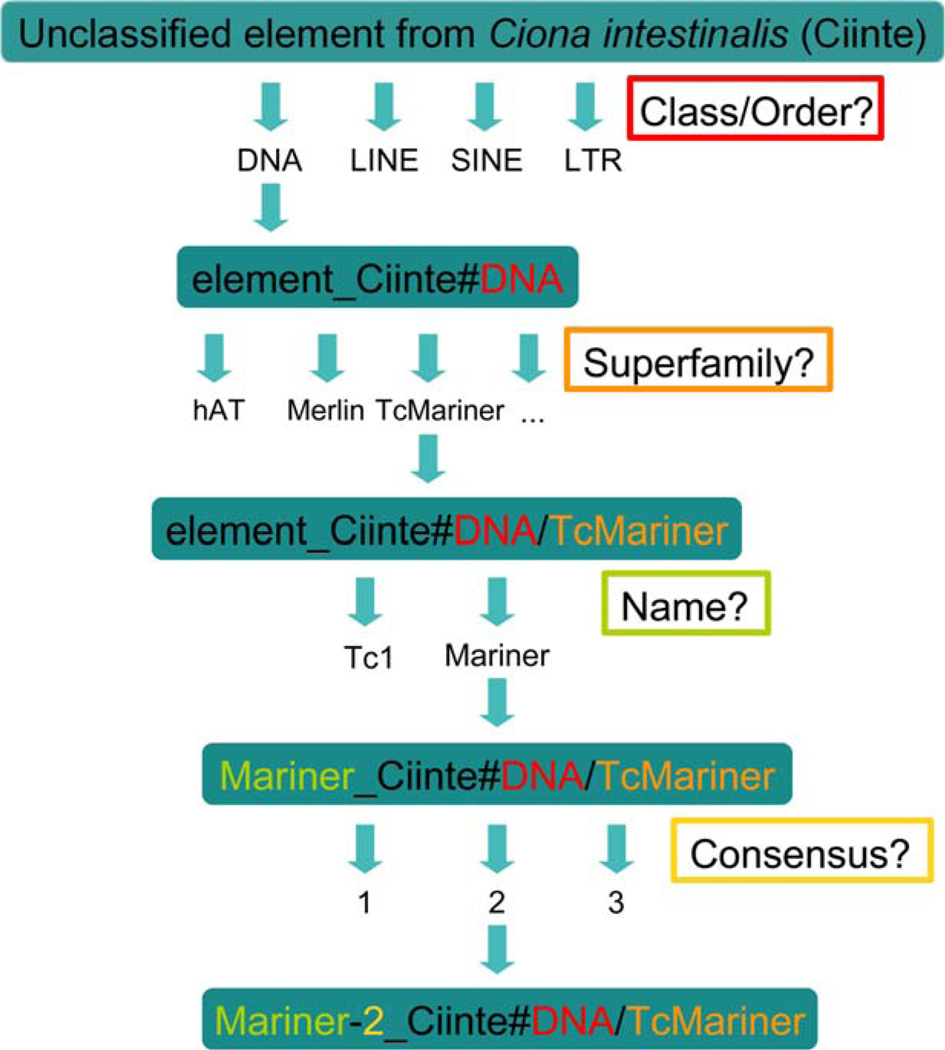
The nomenclature of tunicate TEs follows the international nomenclature (Kapitonov and Jurka, 2008; Wicker et al., 2007), allowing TE researchers to share data and use the common Repbase repeat database (Jurka et al., 2005). This nomenclature is also used by classical repeat-detection software such as Repeat-Masker (http://www.repeatmasker.org/). Figure 2 represents the different levels of TE classification and Figure 3 represents a flowchart for their nomenclature in Ciona intestinalis.
FIG. 2.
Classification of tunicate transposable elements.
FIG. 3.
Flowchart for the classification process for Ciona intestinalis transposable elements.
At the higher level (class), TEs are classified based on their transposition mechanism. Class I elements (retro-transposons) transpose via the reverse transcription of an RNA intermediate through a mechanism commonly called “copy-and-paste.” Class II elements (DNA trans-posons) directly transpose from DNA to DNA sequences using a “cut-and-paste” mechanism. Within both classes, there are several levels of classification (subclasses, orders, superfamilies, and families) that include elements sharing common structural features and transposition mechanisms, and forming phylogenetically distinct groups of sequences.
Retrotransposons (class I elements) are subdivided in two subclasses, depending on the presence or absence of Long Terminal Repeat (LTR) flanking the element: LTR retrotransposons include the LTR and DIRS orders, while non-LTR retrotransposons comprises the Penelope, LINE and SINE orders. Within orders, elements are classified into superfamilies. For instance, the LTR order contains four superfamilies: Ty3/Gypsy, BEL/Pao, Ty1/ Copia, and retroviridae.
DNA transposons (class II elements) are subdivided into two subclasses, depending on the number of DNA strands that are cut at the TE donor site for transposition. Subclass I (single strand cut) includes two orders (TIRs for “Terminal Inverted Repeats” and Crypton). Subclass II (double strand cut) includes two orders, Helitron and Polinton.
TE subfamilies are named according to (Kapitonov and Jurka, 2008): NAME#(SUB)CLASS/SUPERFAMILY,
Where the NAME contains Prefix-Infix 1 [-Infix2]_Suffix
Prefix is the name of the element, in general the family or superfamily name.
Infix 1 is the subfamily identifier, in general a number.
Infix 2 defines particular structural features such as LTR, or I for Internal part (region of the element comprised between the two LTR or TIR).
Suffix is the species identifier (4–5 letters).
Where the (SUB)CLASS can be (strict choice)
LTR for the following orders: LTR, DIRS. LINE for the non-LTR retrotransposons and Penelope orders.
SINE for the non-LTR non-autonomous SINE order. DNA for all DNA subclasses.
Where SUPERFAMILY corresponds to a superfamily, when identified, belonging to one of the classes or subclasses if the superfamily has been identified.
Examples:
Tc1-4_Phmamm#DNA/TcMariner is the consensus sequence of a Tc1 element in Phallusia mammillata (Phmamm).
Gypsy-3-LTR_Ciinte#LTR/Gypsy is the consensus sequence of a Solo-LTR of a Gypsy retrotransposon in Ciona intestinalis (Ciinte).
LTR-10_Harore#LTR/Unknown or LTR-10_Haror-e#LTR is the consensus sequence of a LTR retrotransposon but unclear superfamily in Halocynthia roretzi (Harore).
LINE1_Moocci#LINE/L1 is the consensus sequence of a LINE1 non-LTR retrotransposon in Molgula occidentalis (Moocci).
TRANSGENIC LINES AND TRANSGENIC CONSTRUCTS
A transgene is “a gene that has been transferred naturally or by any of a number of genetic engineering techniques from one organism to another” (SO:0000902). Transgenes are obtained by the introduction into an organism of a transgenic construct, an engineered plas-mid or other recombinant DNA molecule that carries various features including, but not limited to, cis- regulatory sequences, reporter genes, etc. Transgenic lines are named according to the construct(s) used to generate them.
Transgenic Constructs
Most transgenic constructs are defined by a cis-regulatory sequence, and a protein-coding cDNA sequence (or sequence that is transcribed as a non-coding RNA). The general syntax of transgenic constructs is: p{Mi., SB., SBT2., (blank)}-{driver}>{functional RNA- or protein-coding gene}.
The “p” indicates that the construct has a plasmid background, the first bracket indicates the type of trans-poson used, if any: Mi for Minos transposon, SB for sleeping beauty transposon, and SBT2 for the T2 variant of SB used in Ciona intestinalis. This field can be left in blank if no transposon system is used to facilitate trans-gene integration.
The driver is of the form: {Species}.{cis-reg}. When the driver consists of the fusion of an enhancer and a promoter, these two elements are separated by a colon (“:”). cis-regulatory and functional RNA or protein names are separated by the symbol “>.”
Transgenes can be transcribed into protein-coding or non-coding RNAs. The symbol of the sequence to be transcribed can adopt various syntaxes, according to their source. It can code for a non-tunicate protein, in which case the symbol used should be the one frequently used in the literature (e.g., EGFP). If it encodes a tunicate protein, the gene symbol conventions established in these guidelines should be used (e.g., Creb1). If the distinction between different tunicate species needs to be made, then the convention is {species abbreviation}.{gene symbol} (e.g., Ciinte.Creb1).
Additionally, the transcribed sequence can also encode a fusion between two different proteins or parts thereof. A double colon (“::”) indicates an in-frame fusion between two protein-coding sequences.
Example:
EGFP::Ciinte.Creb 1 corresponds to a fusion between the full length EGFP placed N-terminally in-frame with the Creb1 protein from Ciona intestinalis.
The names of both cis-regulatory sequence and coding or non-coding RNAs should be brief and need not provide exhaustive details. When the name of a construct is too long to be used in the main text of a manuscript, an abbreviated form can be used, provided the full name is mentioned in the materials and methods of the article.
Examples:
pCiinte.REG000005>NLSLacZ, is a plasmid construct that drives a nuclear form of β-Galactosidase (encoded by the NLSLacZ gene) under control of the cis-regulatory sequence 000005 from Ciona intestinalis.
pMi-Ciinte.REG.KH2012.C4.4315574-4315697|Otx:prCiinte.Bra>Ciinte.Syt1/2::EGFP is a plasmid construct made in a Minos transposon vector that drives a fusion between Ciona intestinalis syn-aptotagmin 1/2 (symbol Syt1/2) and enhanced GFP (placed in C-terminal position), under control of a composite Ciona intestinalis cis-regulatory sequence made of an Otx enhancer and the Ciona intestinalis Brachyury minimal (Corbo et al. 1997). This Otx regulatory sequence is often referred to as the a-element in the literature, and the abbreviated form of this construct, if the species is not ambiguous, could be pMi-Otx-a-elt:prBra>Syt1/2::EGFP, or even pMi-Otx>Syt::EGFP if there is no ambiguity about which Otx element and Syt gene is being used.
pPhmamm.KEG000056>tdTomato::Ciinte.H2b is a plasmid construct that drives a fusion of the Ciona intestinalis histone 2b and the tdTomato tandem fluorescent protein (placed in N-terminal position), under control of the Phallusia mammillata cis-regulatory sequence 000056.
In some cases (e.g., Ga14/UAS system), a single construct can harbor several cassettes, each containing a regulatory and a coding or functional sequence. In this case, multiple cassettes are distinguished by a semicolon inside parentheses, appended by “p” to denote the fact that both cassettes are on the same plasmid.
Example:
p(Ciinte.Isl>GAL4;6xUAS:prCiinte.Bra>GFP): this construct drives Gal4 under the control of a Ciona intestinalis Islet (Isl) cis-regulatory region. It also harbors a second cassette, with a 6-mer of the UAS sequence, placed in front of the prBra minimal promoter, and driving GFP. Note that short construct names should be completed in materials and methods by the full name including coordinates, in a specified assembly, of Isl and prBra.
Transgenic Line
A transgenic line is a line of organisms derived from a common parent that has been modified by heritable transgenic insertion (SO:0000781): An insertion that derives from another organism, via the use of recombinant DNA technology. A transgenic line is defined by a species, one or more transgenic constructs and, when known, the insertion locus of the construct in the species genome. The general syntax of a line is Species.Tg[-construct]{n}.Insertion_site, where [construct] is built from the above rules and where the insertion descriptor is omitted if the insertion site is unknown. The number at the {n} part is used to distinguish different transgenic lines harboring the same transgenic construct. This number does not always coincide with the number of available transgenic lines produced with the construct, because some of the initial transgenic lines may not have been kept.
Examples:
Ciinte.Tg[pMi-Ciinte.REG.KH2012.L41.267342–27094 9|Zip:prFoxa.a>Kaede]1 (note that prFoxa.a should be formally Ciinte.REG.KH2012.C11.4404828–44047 10), is the first transgenic line of Ciona intestinalis obtained by Minos transposon-mediated transgenesis with a vector that drives the fluorescent protein Kaede under control of a Ciona intestinalis Zip enhancer placed in front of the minimal promoter of Foxa.a (Di Gregorio et al. 2001). This name could be abbreviated as Ciinte. Tg[pMi-Zip>Kaede]1. Transgenic lines can have synonymous names to reflect previous names. For instance, this construct was initially named Tg[MiCiZipCifkhK]1 in the first report (Nakazawa et al, 2013).
Ciinte.Tg[pSB-Ciinte.REG.KH2012.C10.4438567–44 40059| MsiprTpo>NLSDsRed;Ciinte.REG.KH2012.C 14.1414843–1413822|Nut>MiTP]1 is a Ciona intestinalis transgenic line generated using a vector including a sleeping beauty transposon element. The vector contains two expression cassettes (separated by a semi colon). One drives a nuclear form of DsRed under control of the cis element of an enhancer from Musashi (Msi) and a promoter of a gene encoding thyroid peroxidase (Tpo) of Ciona intestinalis. The other cassette drives Minos transposase (MiTP) under control of a cis element from Nut gene of Ciona intestinalis. This transgenic line was initially named Ju[SBFr3dTPORCiNutMiTP]1 (Hozumi et al., 2010).
Enhancer trap lines should be identified by the capital letter affix E instead of Tg, because expression pattern of transgenes cannot be deduced from the transgenic line name. Gene trap and promoter trap lines could be identified by the capital letter affixes G and P, respectively.
Example:
Ciinte.E[pMi-TSA-Ciinte.REG.KH2012.L3.178445–177583| Tpo>NLSEGFP]124.KH2012.L171.188604 is an enhancer trap line of Ciona intestinalis transformed by the Minos-mediated transgenesis that drives nuclear-localized EGFP under control of the cis element of Tpo of Ciona intestinalis. In this line, the insertion site of the vector has been identified, and the information follows the line name. “TSA” indicates the splicing acceptor and transcription termination sequence cassette used in the construct. This transgenic line was initially named EJ[MiTSAdT-POG]124 (Sasakura et al, 2012).
MUTANT LINES
A mutant line is a line of organisms showing a deviation from the wild-type phenotype and derived from a single genetically modified parent, through classical mutagenesis or transgenic insertion. Mutant lines are defined by their genetic alteration, their genetic background and their phenotype. Nomenclatures differ for mutants isolated by forward and reverse genetic means.
Mutants obtained by forward genetic means are defined by their phenotypes, and their name is left to the imagination of the creator of the mutant, who should also provide an abbreviated symbol, which will subsequently be used. Mutant allele names of causative genes are specified by gene names followed by a superscription of abbreviated mutant names.
Example:
swimming juvenile (sj) (Sasakura et al., 2005) is a Ciona intestinalis mutant in which metamorphic events occur in an irregular order. The causative gene of this mutant is the Cellulose synthase A gene (Cesa), and the allele name of mutated Cesa in this mutant line is Cesasj.
The name of mutants or mutant alleles obtained by reverse genetic means, the mutant lines can be specified by the target gene and the method used to produce mutants. The general syntax is: {gene symbol}{TAL, CAS, INS}, where TAL stands for TALEN, CAS for CRISPR/Cas9, INS for insertion, of a transgene for instance. If more than two different mutant alleles are established by the same technology, they can be distinguished by numbers, like BraTAL2 and BraTAL3.
Example:
The BraTAL mutant designates a mutant for the Brachyury gene created with the help of TALEN technology.
The animal specified by the name BraTAL2/Bra CAS is a heterozygous animal that harbors one Brachyury mutant allele created by TALEN (the second such allele to be reported) and one Brachyury mutant allele created by CRISPR/Cas9.
CONCLUDING REMARKS: INSTRUCTIONS FOR AUTHORS AND FOR BIOINFORMATICIANS
The rules listed in this article were designed to balance the occasionally antagonistic wishes of experimental and computational biologists. Experimentalists want compact names that make biological sense to them. The precise formatting of these names is frequently seen as a hurdle that muddles biological messages. By contrast, bioinformaticians’ priorities are reproducible standardized and traceable names, with a syntax facilitating the efficient parsing of large files. As a result, some of the consensus rules listed here will probably be problematic for either categories of users. This final paragraph lists some difficulties for experimental and computational biologists and suggests ways to offset them.
Tunicate biologists may sometimes find the proposed names too long, and too constraining. Human gene names and symbols for some favorite developmental regulators may also not speak to them (e.g., Human RBPJ1 is usually called Suppressor of Hairless, which is the name of its ortholog in Drosophila). Also, tunicate developmental biologists may prefer naming some genes on the basis of their ascidian loss-of-function phe-notype (e.g., Macho-1, Notrlc) than by reference to Human genes (Zic-r.a; Hand-related). In such cases, the name that makes most sense could be used in a publication, provided the primary human-derived gene name or symbol is also mentioned in the main text, for instance the first time the gene is discussed. As primary names of genetic elements may sometimes change, traceability requires the inclusion in the materials and methods of the unique identifier of each gene transcript or protein studied, and the precise coordinates of any new cis-regulatory element/construct described in the work in a well identified genome assembly. We suggest that this is done in a specific section called “Unique identifiers and coordinates of listed genetic elements.” This section will considerably facilitate the work of biocurators.
Although we strongly reduced the number of punctuation marks in names, avoided characters that can be interpreted as delimiters in formatted text files (space, comma, tab) and imposed a fixed number of digits to numerical identifiers, computational biologists may be confronted to some residual issues when using certain strategies to parse the names of genetic elements. The presence of characters (“/”,”>”,”[“,”|”) that serve as operators in some programming languages will need to be taken into account in parsing scripts.
Supplementary Material
Acknowledgments
Contract grant sponsor: National Science Foundation Postdoctoral Research Fellowship in Biology (AS), Contract grant number: NSF-1161835; Contract grant sponsor: National Bioresource Project (Japan) (YS); Contract grant sponsor: NIGMS/NIH (LC), Contract grant number: R01GM096032; Contract grant sponsor: NHLBI/NIH (LC), Contract grant numbers: R01HL108643; R01GM100315 and 1R01AG037968 (AV); Contract grant sponsor: PIME grant from CNRS (PL); Contract grant sponsor: National Science Foundation Cooperative Agreement, Contract grant number: No. DBI-0939454 (BEACON) (BJS)
Footnotes
Additional Supporting Information may be found in the online version of this article.
The latest version of these guidelines can be downloaded from the Tunicate Portal (http://www.tunicate-portal.org/). To better identify the latest version, the file name for the guidelines should follow the following syntax: Genetic_Guidelines_Tunicate_[year]_[month]_[day] (example, Genetic_Guidelines_Tunicate_2014_05_01).
International Code for Zoological nomenclature. http://iczn.org/code
LITERATURE CITED
- Ambros V, Bartel B, Bartel DP, Burge CB, Carrington JC, Chen X, Dreyfuss G, Eddy SR, Griffiths-Jones S, Marshall M, Matzke M, Ruvkun G, Tuschl T. A uniform system for microRNA annotation. RNA. 2003;9:277–279. doi: 10.1261/rna.2183803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiaen L, Stolfi A, Davidson B, Levine M. Spatio-temporal intersection of Lhx3 and Tbx6 defines the cardiac field through synergistic activation of Mesp. Dev Biol. 2009;328:552–560. doi: 10.1016/j.ydbio.2009.01.033. [DOI] [PubMed] [Google Scholar]
- Corbo JC, Levine M, Zeller RW. Characterization of a notochord-specific enhancer from the Bra-chyury promoter region of the ascidian, Ciona intes-tinalis. Development. 1997;124:589–602. doi: 10.1242/dev.124.3.589. [DOI] [PubMed] [Google Scholar]
- Dehal P, Satou Y, Campbell RK, Chapman J, Degnan B, De Tomaso A, Davidson B, Di Gregorio A, Gelpke M, Goodstein DM, Harafuji N, Hastings KEM, Ho I, Hotta K, Huang W, Kawashima T, Lemaire P, Martinez D, Meinertzhagen IA, Necula S, Nonaka M, Putnam N, Rash S, Saiga H, Satake M, Terry A, Yamada L, Wang H, Awazu S, Azumi K, Boore J, Branno M, Chin-bow S, DeSantis R, Doyle S, Francino P, Keys DN, Haga S, Hayashi H, Hino K, Imai KS, Inaba K, Kano S, Kobayashi K, Kobayashi M, Lee B, Makabe KW, Manohar C, Matassi G, Medina M, Mochizuki Y, Mount S, Morishita T, Miura S, Nakayama A, Nishizaka S, Nomoto H, Ohta F, Oishi K, Rigoutsos I, Sano M, Sasaki A, Sasakura Y, Shoguchi E, Shin-i T, Spagnuolo A, Stainier D, Suzuki MM, Tassy O, Takatori N, Tokuoka M, Yagi K, Yoshizaki F, Wada S, Zhang C, Hyatt PD, Larimer F, Detter C, Doggett N, Glavina T, Hawkins T, Richardson P, Lucas S, Kohara Y, Levine M, Satoh N, Rokhsar DS. The draft genome of Ciona intes-tinalis: Insights into chordate and vertebrate origins. Science. 2002;298:2157–2167. doi: 10.1126/science.1080049. [DOI] [PubMed] [Google Scholar]
- Denoeud F, Henriet S, Mungpakdee J-M, Aury S, Da Silva C, Brinkmann H, Mikhaleva J, Olsen LC, Jubin C, Cañestro C, Bouquet JM, Danks G, Poulain J, Campsteijn C, Adamski M, Cross I, Yadetie F, Muffato M, Louis A, Butcher S, Tsagkogeorga G, Konrad A, Singh S, Jensen MF, Cong EH, Eikeseth-Otteraa H, Noel B, Anthouard V, Porcel BM, Kachouri-Lafond R, Nishino A, Ugolini M, Chourrout P, Nishida H, Aasland R, Huzurbazar S, Westhof E, Delsuc F, Lehrach H, Reinhardt R, Weissenbach J, Roy SW, Artiguenave F, Postiethwait JH, Manak JR, Thompson EM, Jaillon O, Du Pasquier L, Boudinot P, Liberies DA, Volff JN, Philippe H, Lenhard B, Roest Crollius H, Wincker P, Chourrout D. Plasticity of animal genome architecture unmasked by rapid evolution of a pelagic tunicate. Science. 2010;330:1381–1385. doi: 10.1126/science.1194167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Gregorio A, Corbo JC, Levine M. The regulation of forkhead/HNF-3 β expression in the Ciona embryo. Dev Biol. 2001;229(1):31–43. doi: 10.1006/dbio.2000.9964. [DOI] [PubMed] [Google Scholar]
- Eilbeck K, Lewis S, Mungall C, Yandell M, Stein L, Durbin R, Ashburner M. The sequence ontology: A tool for the unification of genome annotations. Genome Biol. 2005;6:R44. doi: 10.1186/gb-2005-6-5-r44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray KA, Daugherty LC, Gordon SM, Seal RL, Wright MW, Bruford EA. Genenames.org: The HGNC resources in 2013. Nucleic Acids Res. 2013;41:D545–D552. doi: 10.1093/nar/gks1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hozumi A, Kawai N, Yoshida R, Ogura Y, Ohta N, Satake H, Satoh N, Sasakura Y. Efficient transposition of a single Minos transposon copy in the genome of the ascidian Ciona intestinalis with a transgenic line expressing transposase in eggs. Dev Dyn. 2010;239:1076–1088. doi: 10.1002/dvdy.22254. [DOI] [PubMed] [Google Scholar]
- Imai KS, Daido Y, Kusakabe TG, Satou Y. Cis-acting transcriptional repression establishes a sharp boundary in chordate embryos. Science. 2012;337:964–967. doi: 10.1126/science.1222488. [DOI] [PubMed] [Google Scholar]
- Jain E, Bairoch A, Duvaud S, Phan I, Redaschi N, Suzek BE, Martin MJ, McGarvey P, Gasteiger E. Infrastructure for the life sciences: Design and implementation of the UniProt website. BMC Bioinf. 2009;10:136. doi: 10.1186/1471-2105-10-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurka J, Kapitonov VV, Pavlicek A, Klonowski P, Kohany O, Walichiewicz J. Repbase update, a database of eukaryotic repetitive elements. Cytogenet Genome Res. 2005;110:462–467. doi: 10.1159/000084979. [DOI] [PubMed] [Google Scholar]
- Kapitonov VV, Jurka J. A universal classification of eukaryotic transposable elements implemented in Repbase. Nat Rev Genet. 2008;9:411–412. doi: 10.1038/nrg2165-c1. author reply 414. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Takatori N, Nakajima Y, Kumano G, Nishida H, Saiga H. Spatial and temporal expression of two transcriptional isoforms of Lhx3, a LIM class homeobox gene, during embryogenesis of two phylogenetically remote ascidians, Halocyn-thia roretzi and Ciona intestinalis. Gene Express Patterns. 2010;10(2):98–104. doi: 10.1016/j.gep.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Kozomara A, Griffiths-Jones S. miRBase: Annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014;42:D68–D73. doi: 10.1093/nar/gkt1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire P. Evolutionary crossroads in developmental biology: The tunicates. Development. 2011;138:2143–2152. doi: 10.1242/dev.048975. [DOI] [PubMed] [Google Scholar]
- Lemaire P, Smith WC, Nishida H. Ascidians and the plasticity of the chordate developmental program. Curr Biol. 2008;18:R620–R631. doi: 10.1016/j.cub.2008.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, Yamazawa T, Moriyama Y, Ogura Y, Kawai N, Sasakura Y, Saiga H. Formation of the digestive tract in Ciona intestinalis includes two distinct morphogenic processes between its anterior and posterior parts. Dev Dyn. 2013;242:1172–1183. doi: 10.1002/dvdy.24009. [DOI] [PubMed] [Google Scholar]
- Sasakura Y, Nakashima K, Awazu S, Matsuoka T, Nakayama A, Azuma J, Satoh N. Transposon-mediated insertional mutagenesis revealed the functions of animal cellulose synthase in the ascidian Ciona intestinalis. Proc Natl Acad Sci U S A. 2005;102:15134–15139. doi: 10.1073/pnas.0503640102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasakura Y, Kanda M, Ikeda T, Horie T, Kawai N, Ogura Y, Yoshida R, Hozumi A, Satoh N, Fujiwara S. Retinoic acid-driven Hox1 is required in the epidermis for forming the otic/atrial placodes during ascidian metamorphosis. Development. 2012;139:2156–2160. doi: 10.1242/dev.080234. [DOI] [PubMed] [Google Scholar]
- Satou Y, Mineta K, Ogasawara M, Sasakura Y, Shoguchi E, Ueno K, Yamada L, Matsumoto J, Wasserscheid J, Dewar K, Wiley GB, Macmil SL, Roe BA, Zeller RW, Hastings KEM, Lemaire P, Lindquist E, Endo T, Hotta K, Inaba K. Improved genome assembly and evidence-based global gene model set for the chordate Ciona intestinalis: New insight into intron and operon populations. Genome Biol. 2008;9:R152. doi: 10.1186/gb-2008-9-10-r152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small KS, Brudno M, Hill MM, Sidow A. A hap-lome alignment and reference sequence of the highly polymorphic Ciona savignyi genome. Genome Biol. 2007;8:R41. doi: 10.1186/gb-2007-8-3-r41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolfi A, Lowe EK, Racioppi C, Ristoratore F, Brown CT, Swalla BS, Christiaen L. Divergent mechanisms regulate conserved cardiopharyngeal development and gene expression in distantly related ascidians. eLife. 2014 doi: 10.7554/eLife.03728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voskoboynik A, Neff NF, Sahoo D, Newman AM, Pushkarev D, Koh W, Passarelli B, Fan HC, Mantalas GL, Palmeri KJ, Ishizuka KJ, Gissi C, Griggio F, Ben-Shlomo R, Corey DM, Penland L, White RA, Weissman IL, Quake SR. The genome sequence of the colonial chordate, Botryllus schlosseri. eLife. 2013;2:e00569. doi: 10.7554/eLife.00569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker T, Sabot F, Hua-Van A, Bennetzen JL, Capy P, Chalhoub B, Flavell A, Leroy P, Morgante M, Panaud O, Paux E, SanMiguel P, Schulman AH. A unified classification system for eukaryotic transposable elements. Nat Rev Genet. 2007;8:973–982. doi: 10.1038/nrg2165. [DOI] [PubMed] [Google Scholar]
- Wright MW, Bruford EA. Naming “junk”: Human non-protein coding RNA (ncRNA) gene nomenclature. Hum Genom. 2011;5:90–98. doi: 10.1186/1479-7364-5-2-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Zhang M, Zhou T, Hua X, Tang L, Wu W. Sno/scaRNAbase: A curated database for small nucleolar RNAs and cajal body-specific RNAs. Nucleic Acids Res. 2007;35:D183–D187. doi: 10.1093/nar/gkl873. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.