Abstract
A hallmark of negative symptoms in schizophrenia is reduced motivation and goal directed behavior. While preclinical models suggest that blunted striatal dopamine levels can result in reduced motivation and goal-directed behavior, this mechanism is inconsistent with evidence for enhanced striatal dopamine levels in schizophrenia. In seeking to reconcile this discrepancy, one possibility is that negative symptoms reflect a failure of striatal motivational systems to mobilize appropriately in response to reward–related information. In the present study, we used a laboratory effort-based decision-making task in a sample of patients with schizophrenia and healthy controls. We found that patients and controls did not differ in the overall amount of effort expenditure, but patients made significantly less optimal choices in terms of maximizing rewards. These results provide further evidence for a selective deficit in the ability of schizophrenia patients to utilize environmental cues to guide reward-seeking behavior.
INTRODUCTION
A growing body of evidence suggests that reduced motivation and goal-directed behavior may occur in schizophrenia without concomitant alterations of hedonic responsivity (Barch and Dowd, 2010; Folley and Park, 2010; Gard et al., 2007; Gold et al., 2008). In preclinical models, effort-based decision-making paradigms that assess the willingness to invest greater effort in order to obtain larger or preferred rewards have repeatedly implicated disruption of corticostriatal dopamine (DA) as a possible substrate for motivational impairments (Salamone and Correa, 2012; Treadway and Zald, 2013); indeed, potentiation or attenuation of DA signaling can increase or decrease effort expenditure for rewards in both rodents (Bardgett et al., 2009; Floresco et al., 2008; Salamone et al., 2007) and humans (Venugopalan et al., 2011; Wardle et al., 2011).
In the context of schizophrenia, however, it appears unlikely that negative symptoms are mediated by a global reduction in striatal DA given robust evidence for striatal DA elevations as a mechanism underlying symptoms of psychosis (Fusar-Poli and Meyer-Lindenberg, 2012; Howes et al., 2013). An alternative explanation is that both positive and negative symptoms results from irregular (as opposed to simply enhanced or reduced) striatal DA release that may fail to appropriately respond to meaningful reward incentives. Consistent with this model, several recent studies of effort-based decision-making in patients with schizophrenia have found no evidence for a global reduction of effort expenditure, but rather an apparent failure to mobilize effort in response to maximally rewarding cues (Barch et al., 2014; Fervaha et al., 2013; Gold et al., 2013). In the present study, we adopted a similar methodology in an attempt to replicate these prior findings and extend them with a more direct investigation into the utilization of reward magnitude and probability information in guiding effort-based choice in schizophrenia patients.
METHODS
Participants and Procedure
All subjects provided written informed consent, and all study procedures were approved by the Vanderbilt Institutional Review Board. 13 outpatients with schizophrenia (SZ) participated in the study, and data from 15 healthy control subjects (HC) were drawn from a prior published study (Treadway et al., 2012). All patients were recruited from private-care facilities in Nashville, TN, and completed the Structured Clinical Interview for DSM-IV (First et al., 2005) to confirm diagnosis. Exclusion criteria for the SZ were: IQ lower than 85, a prior history of head trauma/neurological disorder, or a history of drug use in the previous year. The severity of symptoms was evaluated with SANS and SAPS interviews (Andreasen, 1984a, b; Zigler and Levine, 1981). Subjects were then instructed to complete the Effort-Expenditure for Rewards Task (EEfRT). One patient failed to comply with task instructions, and was excluded from the study. A summary of demographics and symptom scores are reported in Table 1. The two groups differed in years of education, which was included as a covariate in subsequent group analyses. However, note that SZ had intact IQ (mean =100; S.D. = 10.4).
Table 1.
Demographic characteristics
| SZ (n=12) | HC (n=15) | p-value | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
|
|
|
|
|
||
| Sex | 5/12 female | 9/15 female | 0.34 | ||
| Age | 38.5 | 10.1 | 39.7 | 8.2 | 0.72 |
| Years of Education | 13.7 | 1.9 | 16.3 | 1.8 | 0.008 |
| SAPS (Andreasen, 1984a) | 19.2 | 11.2 | - | - | |
| SANS (Andreasen, 1984b) | 25.8 | 12.7 | - | - | |
| Chlorpromazine Equivalent Dose (CPZ) (Woods, 2003) | 428.5 | 362.3 | - | - | |
Effort Expenditure for Rewards Task (EEfRT) is a computerized effort-based decision-making task (Treadway et al., 2009) (Fig 1). On each trial the participant must chose between, a high effort option and a low effort option, which require varying amounts of speeded manual button pressing. For low-effort choices, participants are eligible to win $1.00, while eligible amounts for selecting the high effort option ranged between $1.24 – $4.30 (“reward magnitude”). Additionally, probability of reward receipt (regardless of choice) varied across trials. Participants were provided with accurate probability cues at the beginning of each trial indicating that they had a “high” (88%), “medium” (50%) or “low” (12%) probability of receiving money for the choice made on that trial. The inclusion of probability and reward magnitude manipulations in the EEfRT facilitates the evaluation of two constructs: overall willingness to expend effort for rewards (since the high effort option is always associated with a greater expected value), and the utilization of reinforcement parameters in the allocation of effort resources (i.e. is effort allocated when reward values and probabilities are most favorable).
Figure 1.
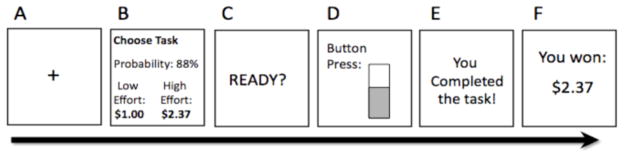
Schematic diagram of a single trial of the Effort-Expenditure for Rewards Task. A. 1s fixation cue. B. 5s choice period where subjects are presented with information regarding the reward magnitude of the High Effort option, and the probability of receiving a reward. C. A 1s “ready” screen. D. Subjects must make rapid button presses to complete the chosen task while a virtual “bar” fills up to indicate their progress. E. Subjects receive feedback on task completion. F. Subjects receive feedback about any money received for that trial.
Statistical Analyses
Statistical analyses were consistent with prior studies using the EEfRT in samples with schizophrenia patients (Barch et al., 2014). Participant choice data was entered into a 2 (group) x 3 (probability level) x 4 (reward value) Repeated-measures ANOVA, with reward magnitude values for the high effort option binned into four groups: <$1.96; $1.96–$2.77; $2.77–$3.58; ≥$3.58. A Huynh-Feldt correction was applied in cases where the assumption of sphericity was not met.
To further evaluate group differences in the utilization for both probability and reward value information when making effort allocations, individual logistic regression analyses were performed for each subject with expected value (defined as per-trial reward value * probability) as a single regressor predicting effort choice.
RESULTS
Main Effects of the EEFRT
Consistent with prior studies using the EEfRT, there were significant main effects of probability (Huynh-Feldt F(2, 39.7) = 9.69, p = 0.001) and reward value (Huynh-Feldt F(3, 64.9) = 15.31, p < 0.00001), such that all subjects were more likely to choose the high effort option when the reward value and probability of receiving reward were higher. There were no group differences in average reaction time between HC (M = 1.32, SD = 0.44) and SZ (M = 1.10 SD = 0.71) (t25 = 1.02, p = 0.319), and no differences in successful completion of high and low effort options (t25 = −0.75, p = 0.458), with both groups completing over 95% of trials on average.
Group Effects
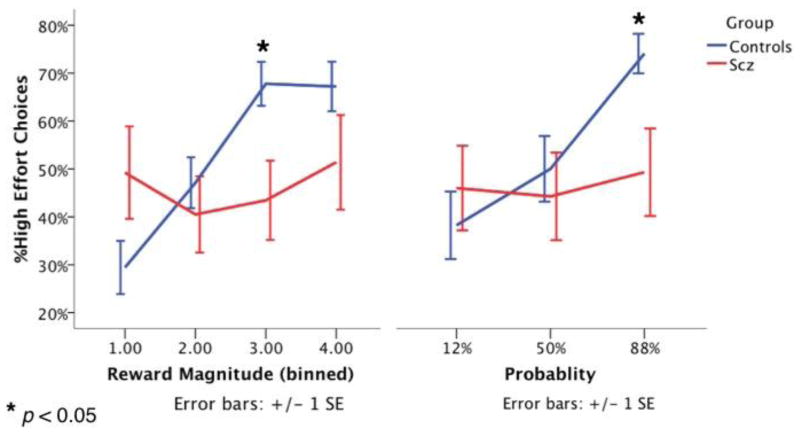
There was no main effect of group in terms of the total number of high-effort options made (F(1, 25) = 0.62, p = 0.440). This was true even when controlling for years of education. However, there were significant interactions of group-by-probability (Huynh-Feldt F(2, 39.8) = 5.42, p = 0.012) (Cohen s d = 0.90) and group-by-reward value (Huynh-Feldt F(3, 62.2) = 7.50, p < 0.0004) (Cohen s d = 1.06), such that HC were significantly more likely to choose the high effort option when the probability and reward magnitudes were higher, while choice behavior among SZ remained relatively constant across different levels of probability and reward (Fig 2). There was no three-way interaction (Huynh-Feldt F(6, 5.9) = 1.35, p = 0.24). These interaction effects remained significant even when controlling for chlorpromazine equivalent medication doses (CPZ), and when 2 patients who chose no high effort options were excluded. There were no interactions between education level and either probability or reward magnitude, though there was a trend-level main effect of years of education such that higher education levels predicted a larger number of high effort choices.
Figure 2.
High effort choices made by controls and patients with schizophrenia across differing levels of probability and reward magnitude. We observed significant interactions for both group-by-probability and group-by-reward value such that control participants increased the proportion of high effort choices made during trials with high probability and reward magnitude to a much a greater extent than did schizophrenia patients.
Sensitivity to Reinforcement Parameters
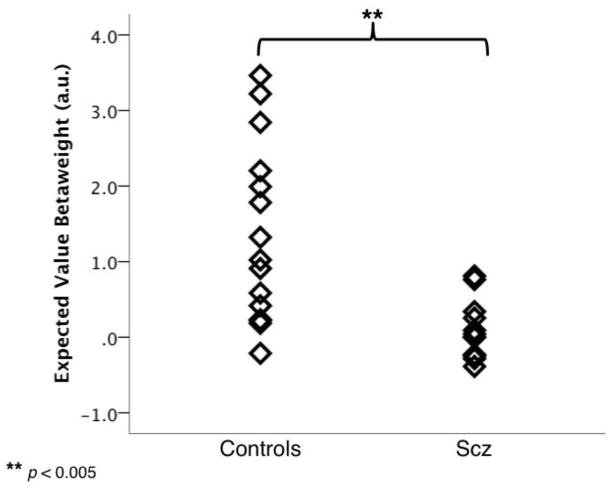
The pattern of interactions suggested that HC relied much more on the probability and reward magnitude information to guide their allocation of effort than did SZ. To further test this hypothesis, per-subject expected value (EV) beta weights were compared across groups; higher beta weights indicate EV was a stronger predictor of choice behavior. EV beta weights were significantly larger for HC than SZ (t25 = 3.59, p = 0.002) and follow-up one-sample t-tests confirmed that EV beta weights were significantly greater than 0 for HC (t14 = 4.40, p = 0.001) but not for SZ (t11 = 0.86, p = 0.410), suggesting that HC, but not SZ were relying on EV information to guide effort decisions (Fig 3).
Figure 3.
Utilization of expected value (EV) when making effort-allocation choices. Single-subject logistic regression analyses revealed that betaweights for EV were significantly higher for controls than patients with schizophrenia, indicating that controls utilized EV information when making effort-allocation decisions, while schizophrenia patients did not. au = arbitrary units.
Associations with Negative Symptoms
Given the theoretical link between effort-based decision-making and negative symptoms, we tested for an association between EV beta weights and negative symptom severity assessed by the SANS. Using a regression analysis with CPZ as a nuisance covariate, we observed a trend-level association between SANS and EV beta weights (b = −0.60, p = 0.051), such that individuals with higher negative symptoms had more difficulty incorporating reward and probability information when making effort-related decisions.
DISCUSSION
In this study, we replicated prior findings suggesting that SZ patients show significant impairment in the ability to allocate physical effort resources in pursuit of rewards. This is in keeping with three prior independent effort-based decision-making studies, all of which found that SZ did not make fewer high effort choices than HC on average, but generally failed to select the high effort option for the most rewarded trials (Barch et al., 2014; Fervaha et al., 2013; Gold et al., 2013).
Our study extended this finding through an examination of the use of expected value information in predicting choice behavior; whereas controls appeared to rely heavily on expected value to guide allocation of effort, this information had little effect on choices made by SZ, with EV being a significant predictor of choice behavior for only two patients. Moreover, we observed a trend-level association between negative symptoms and magnitude of EV betaweights. The inability to translate reward-relevant information into goal-directed behavior has emerged as a key deficit in daily functioning of individuals with schizophrenia, with recent ecological momentary assessment data highlighting the difficulty that patients experience in completing effortful actions, even when they expect to enjoy them (Gard et al., 2014). These deficits may reflect a parallel to the aberrant salience model of schizophrenia (Winton-Brown et al., 2014), although in this case the problem arises in discriminating between different incentives rather than attentional cues.
This study has several caveats. First, our sample size was small, though we note that prior studies with patient samples ranging from 16 to 59 all found significant group-by-probability and group-by-value interactions with effect sizes ranging from d = 0.31 to 1.06 and d = 0.37 to 1.35, consistent with the current report. A second limitation is that all patients were receiving antipsychotic drugs, which can produce side effects of apathy and lethargy. That said, there were no group differences in completion rates, reaction times, or overall number of high effort options, and our main effects were unchanged by controlling for the medication dose. Lastly, given the persistent working memory deficit in schizophrenia, it is possible that failure to maintain the task goal or the mental representation of the reward value may have played a role in EEfRT performance. Future studies should examine the impact of working memory deficits in optimal allocation of effort.
In sum, this paper replicates and extends prior work suggesting that schizophrenia is associated with selective deficits in reward-maximizing allocation of effort during goal-directed activity.
Acknowledgments
This study was supported by the National Institute of Mental Health (NIMH) grants F31MH087015, K99MH102355 (MTT), and T32 MH018921-21A1 (JSP), and a Brain & Behavior Research Foundation NARSAD grant (SP). The authors wish to thank Lindsey McIntosh and Heath Nichols for help with recruitment and symptom assessment.
Footnotes
CONTRIBUTORS
MTT, DHZ and SP designed the study, MTT and JSP collected and analyzed the data, MTT, JSP, DHZ, and SP wrote the paper.
CONFLICT OF INTEREST
The authors report no conflict of interest related to this work, financial or otherwise.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreasen NC. The scale for the asessment of positive symptoms (SAPS) University of Iowa; Iowa City: 1984a. [Google Scholar]
- Andreasen NC. Scale for the assessment of negative symptoms. University of Iowa; Iowa City: 1984b. [Google Scholar]
- Barch DM, Dowd EC. Goal representations and motivational drive in schizophrenia: the role of prefrontal-striatal interactions. Schizophr Bull. 2010;36(5):919–934. doi: 10.1093/schbul/sbq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Treadway MT, Schoen N. Effort, anhedonia, and function in schizophrenia: Reduced effort allocation predicts amotivation and functional impairment. J Abnorm Psychol. 2014;123(2):387. doi: 10.1037/a0036299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardgett ME, Depenbrock M, Downs N, Points M, Green L. Dopamine modulates effort-based decision making in rats. Behav Neurosci. 2009;123(2):242–251. doi: 10.1037/a0014625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fervaha G, Graff-Guerrero A, Zakzanis KK, Foussias G, Agid O, Remington G. Incentive motivation deficits in schizophrenia reflect effort computation impairments during cost-benefit decision-making. J Psychiatr Res. 2013;47(11):1590–1596. doi: 10.1016/j.jpsychires.2013.08.003. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structred Clinical Interview for DSM-IV-TR Axis I Disorders - Patient Edition (With Psychotic Screen) Biometrics Research Department; 2005. [Google Scholar]
- Floresco SB, Tse MT, Ghods-Sharifi S. Dopaminergic and glutamatergic regulation of effort- and delay-based decision making. Neuropsychopharmacology. 2008;33(8):1966–1979. doi: 10.1038/sj.npp.1301565. [DOI] [PubMed] [Google Scholar]
- Folley BS, Park S. Relative food preference and hedonic judgments in schizophrenia. Psychiatry Res. 2010;175(1):33–37. doi: 10.1016/j.psychres.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Meyer-Lindenberg A. Striatal presynaptic dopamine in schizophrenia, Part II: meta-analysis of [18F/11C]-DOPA PET studies. Schizophr Bull. 2012:sbr180. doi: 10.1093/schbul/sbr180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DE, Kring AM, Gard MG, Horan WP, Green MF. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophr Res. 2007;93(1–3):253–260. doi: 10.1016/j.schres.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DE, Sanchez AH, Cooper K, Fisher M, Garrett C, Vinogradov S. Do People With Schizophrenia Have Difficulty Anticipating Pleasure, Engaging in Effortful Behavior, or Both? 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Strauss GP, Waltz JA, Robinson BM, Brown JK, Frank MJ. Negative symptoms of schizophrenia are associated with abnormal effort-cost computations. Biol Psychiatry. 2013;74(2):130–136. doi: 10.1016/j.biopsych.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Waltz JA, Prentice KJ, Morris SE, Heerey EA. Reward processing in schizophrenia: a deficit in the representation of value. Schizophr Bull. 2008;34(5):835–847. doi: 10.1093/schbul/sbn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Shotbolt P, Bloomfield M, Daalman K, Demjaha A, Diederen KMJ, Ibrahim K, Kim E, McGuire P, Kahn RS. Dopaminergic function in the psychosis spectrum: an [18F]-DOPA imaging study in healthy individuals with auditory hallucinations. Schizophr Bull. 2013;39(4):807–814. doi: 10.1093/schbul/sbr195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M. The mysterious motivational functions of mesolimbic dopamine. Neuron. 2012;76(3):470–485. doi: 10.1016/j.neuron.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology (Berl) 2007;191(3):461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Bossaller NA, Shelton RC, Zald DH. Effort-based decision-making in major depressive disorder: a translational model of motivational anhedonia. J Abnorm Psychol. 2012;121(3):553. doi: 10.1037/a0028813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Schwartzman AN, Lambert WE, Zald DH. Worth the 'EEfRT'? The effort expenditure for rewards task as an objective measure of motivation and anhedonia. PLoS ONE. 2009;4(8):e6598. doi: 10.1371/journal.pone.0006598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Zald DH. Parsing Anhedonia Translational Models of Reward-Processing Deficits in Psychopathology. Current Directions in Psychological Science. 2013;22(3):244–249. doi: 10.1177/0963721412474460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopalan VV, Casey KF, O'Hara C, O'Loughlin J, Benkelfat C, Fellows LK, Leyton M. Acute Phenylalanine/Tyrosine Depletion Reduces Motivation to Smoke Cigarettes Across Stages of Addiction. Neuropsychopharmacology. 2011 doi: 10.1038/npp.2011.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle MC, Treadway MT, Mayo LM, Zald DH, de Wit H. Amping Up Effort: Effects of d-Amphetamine on Human Effort-Based Decision-Making. J Neurosci. 2011;31(46):16597–16602. doi: 10.1523/JNEUROSCI.4387-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winton-Brown TT, Fusar-Poli P, Ungless MA, Howes OD. Dopaminergic basis of salience dysregulation in psychosis. Trends Neurosci. 2014;37(2):85–94. doi: 10.1016/j.tins.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64(6):663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- Zigler E, Levine J. Premorbid competence in schizophrenia: what is being measured? J Consult Clin Psychol. 1981;49(1):96. doi: 10.1037//0022-006x.49.1.96. [DOI] [PubMed] [Google Scholar]