Abstract
Adult Mesenchymal Stem Cells (MSCs) were described previously as multipotent cells that could differentiate into bone, cartilage, muscle and other mesenchymal tissues. New information suggests that MSCs can be found in every tissue of the body because they function as perivascular cells, pericytes, found outside of all blood vessels. When those vessels break or are inflamed the pericyte is detached and forms MSCs that is activated by its local microenvironment of injury. Such MSCs function to secrete powerful immune-modulatory and regenerative agents; over 426+ clinical trials are now in play covering a huge spectrum of clinical conditions. How such activated MSCs affect the menstrual cycle, menopause or osteotrophic cancers has only recently been studied. This manuscript outlines these issues and challenges the scientific and medical community to use this new-found knowledge to uncover new clinical logics and medial solutions for women.
Keywords: Mesenchymal Stem Cells, tissue regeneration, pericytes, menstrual cycle, cell proliferation
Introduction
Every tissue in the human body houses adult Mesenchymal Stem Cells (MSCs). What are they? What do they do? And how can they be managed to enhance Women's Healthcare? Some of the answers to these questions are now emerging and as a component of my invitation to deliver the 2014 NAMS/Pfizer-Wulf H. Utian Endowed Lecture, I offer this manuscript as a treatise on the latest information about MSCs and as a challenge to the scientific/medical community to use the MSC technology to improve healthcare delivery to women.
MSC
The Old
In the late 1980's, I formulated as a cell-based therapy working hypothesis the outline of which is represented in Figure 1 (1-3). Indeed, in cell culture, we and others have shown that marrow-or adipose-derived MSCs can be induced into differentiation lineage pathways that sustain the formation of bone, cartilage, muscle, marrow stroma (hematopoietic lineage support), tendon-ligament, fat, etc. (4-10). This multipotency and a specific set of cell-surface antigens (C73, CD90, CD105, etc.) have been used as a definition for MSCs (11). The hypothesis, based on cell culture experiments, was that the MSCs in situ provide replacement units for injured or expiring differentiated cells in various mesenchymal tissues. In addition, vigorous and extensive use of MSCs in tissue engineered tissues has been and continues to be aggressively explored (12-15). I can say from the efforts of my lab and others that there are no MSC-derived engineered tissues that have been successfully differentiated and implanted to provide therapeutic benefit. Moreover, we now understand that this tissue-specific differentiation will require a different spectrum of multiple growth factors in sequential exposure to generate, for example, ear, nose, throat, sternal, hip, knee and ankle cartilage (16,17) which are all quite different in the identity and relative quantities of molecular constituents and mechanical functionality. The management of multipotent progenitors generated by a number of newer technologies is now being actively pursued using this sequential exposure logic (18-20), but is out of context of this treatise.
Figure 1. The Mesengenic Process.
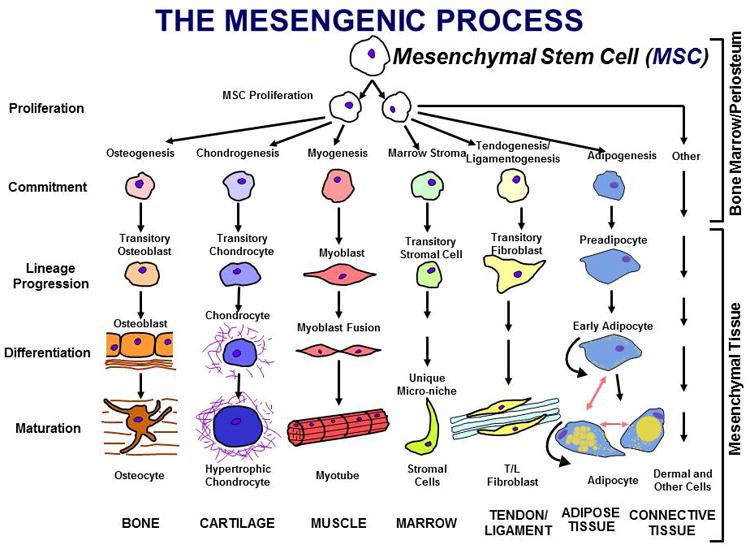
It was hypothesized that marrow contained an MSC capable of being induced into multiple, separate lineage pathways resulting in cells which fabricated mesenchymal tissues such as bone, cartilage, etc.
The New
Information is now available which firmly establishes that all MSCs are derived from perivascular cells, pericytes (21-23). Thus, every vascularized tissue in the body has MSCs by virtue of the fact that all blood vessels are covered with pericytes. These pericytes are released from their anchorage in the extracellular matrix (ECM) surrounding each blood vessel or sinusoid to generate local MSCs upon injury or inflammation. These newly released MSCs are activated by the local micro-environment and react by secreting two sets of bioactive molecules: from the front of the MSCs, curtains of molecules cause the inhibition of interrogating immune cells from entering the injury field (the first line of defense against the establishment of autoimmune reactions) (24-28). From the back of the locally activated MSCs, another complex set of factors are secreted which help establish a regenerative microenvironment. These trophic factors (27) function to: A. Inhibit ischemia-caused apoptosis. B. Inhibit scar formation; C. Stimulate angiogenesis and, D. Stimulate the mitosis of tissue-specific progenitors (28). Given these functional parameters, I now propose to redefine the MSC as a Medicinal Signaling Cell that functions as a drug store at sites of tissue injury or inflammation (28).
Given the above, how do these newly described properties relate to women's health?
Menstrual Cycle and MSCs
Several reports clearly document that large quantities of MSCs can be isolated from the menstrual blood (29-31). These MSCs are released from the uterine wall vessels as the uterine tissue replaces itself and regenerates its endometrial lining. Whether the number and timing of the released MSCs affects the severity and duration of the menstrual flow has not been investigated (a suggestion!). Indeed, I would further suggest that perhaps the delivery of a bolus of exogenous MSCs at the right time into the uterine lining might speed healing and repair/regeneration of the uterine wall and potentially decrease the severity of menstruation. The premise for this suggestion is that exogenously supplied MSCs might provide paracrine factors which decrease the pain and enhance re-establishment of functional and unbroken blood vessels in the regenerating muscular tissue of the uterus.
In the cases of urinary and fecal incontinence which have prevalence among women, preliminary data from animal models and humans indicates that exogenously provided MSCs has a profound enhancement of regeneration of the afflicted tissues to provide normal function and a cure for these maladies (32-36). I stress that any tissue condition that can benefit from immune-modulation, trophic activity or a regenerative micro-environment is a candidate for MSC-based therapy. Indeed, by searching the website “clinicaltrials.gov” for mesenchymal stem cell-based trials, over 425 such trials is listed with an amazing array of clinical symptoms which all have an immune- and regenerative component. Surprisingly, very few of these focus on issues of women's healthcare.
Therapeutic Uses of MSCs
As an example of the therapeutic use of either autologous or allogeneic MSCs, consider acute or chronic cardiac issues. For acute myocardial infarcts (AMI), culture expanded marrow-derived allogeneic MSCs have been administered within 48 hours of the first symptoms of AMI. The results of Phase II clinical trial has been published and indicate that considerable protection of heart tissue occurs from the infusion of allogeneic MSCs (37, Osiris Therapeutics, Inc.). Likewise, very positive research has been reported by Cytori, Inc. and others (38) using autologous MSC preparation from adipose tissue. In this context, positive outcomes of injecting MSCs into osteoarthritic joints or into the spinal discs (Osiris Therapeutics, Inc. and 39). Thus, in cardiac and orthopaedic sites, exogenous MSCs appear to have profound therapeutic outcomes although no Phase III trial has yet to be completed.
Menopause
There are no studies that relate MSC-function to the hormonal status of the patient. Specifically, how does the loss of circulating estrogen affect pericyte titers and MSC-function? As a first step in this regard, we have conducted one set of experiments to determine if MSCs from male and female donors are different. As a first step, we compared the proliferative capacity of the marrow MSCs from male and female donors. MSCs from females divide faster and undergo more population doubling compared to male MSCs (unpublished observations, D. Lennon, et al). Figure 2 shows the schematic protocol for these experiments. The most stringent test in such a comparison is to start the cultures with equal number of MSCs derived from both male and female donors. The data seen in Figure 2, documents that by the end of one week in culture, the female MSCs have almost completely taken over the cultures. The simplest explanation for this is the fact that this MSC-culture expansion has been optimized (40) using selected batches of fetal calf serum (FCS). Such serum, of course, has huge levels of estrogen provided by the pregnant mother. This estrogen in FCS preferentially supports the expansion of MSCs from females.
Figure 2. Competitive Proliferation of Female versus Male MSCs.
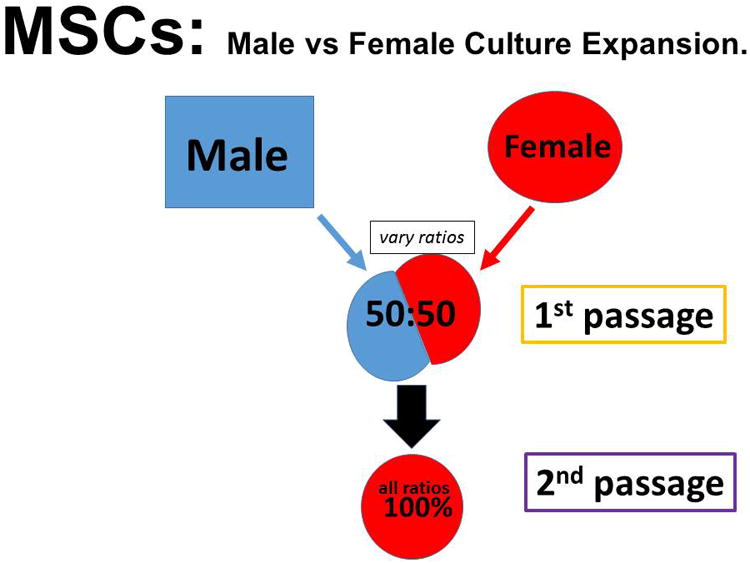
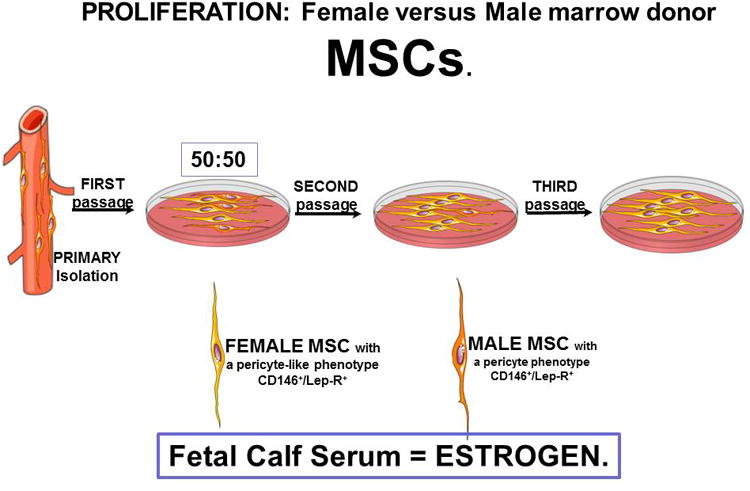
MSCs were purified from a female and from a male donor. Figure A is the general experimental scheme. At first passage, an equal mixture of each was placed into culture in expansion medium that contains 10% of a selected batch of Fetal Calf Serum (40). At second and third passages FISH analysis was performed. At second passage 90-99% of the cells were female while at third passage all of the cells were female. Figure B provides an oversimplified view of the data.
Whether MSCs from males are intrinsically different from MSCs from females has not been determined? This comparison would be extremely difficult to assess since we know that the expressional capacity (genome-controlled) of every donor MSC is quantitatively different (41). Moreover, the FCS-supplemented growth medium for the cell culture-produced MSCs is quite influential over the observed secretory and differentiation properties of the MSCs (40, 42). In the majority of clinical trials now in-play testing allogeneic MSCs for safety, potency and efficacy, the starting marrow has been obtained (mostly) from 20-30 year old males (43). These young donor MSCs have been culture expanded in selected lots of FCS as we originally suggested was optimal for MSC expansion with retention of multipotential. An exception is the isolation and expansion of MSC-like cells derived from full-term, discarded placentas (44-45). Will these placental MSCs prove to be superior to the marrow-derived male MSCs?
Osteotrophic Cancers
Breast cancer and melanoma are not only frequent amongst women, but these solid tumors are known to metastasize to bone (46-47). If every blood vessels in the body is covered with pericytes, how do the metastasizing cancer cells get through the basement membrane ECM that surrounds all blood vessels and then past the covering and ECM-embedded pericyte? Figure 3 provides our current research hypothesis in which we can document that PDGF-BB anchors the pericyte in the basement membrane perivascular ECM (48). As the melanoma cell bridges the endothelial layer, platelets are broken releasing their contents including large amounts of PDGF-BB. The melanoma cell is attracted to the pericyte by its secretion of SDF-1 (49) and, importantly, the melanoma binds to the pericyte/MSC via a CD146 homodimer (50) coupling on both cells' outer membrane. This bound couple is now incorporated into the stroma of bone where the melanoma corrupts the secretory capacity of the MSCs to provide both angiogenic and proliferative stimulants. The end result is the establishment and expansion of a metastasis of melanoma in bone resulting in osteolytic events, bone loss and fracture and pain. This detailed chemistry now provides a platform for looking for inhibitory molecules for osteotrophic metastatic melanoma and breast cancers with the MSC as the central mediator (42).
Figure 3. Mechanistic Candidates.
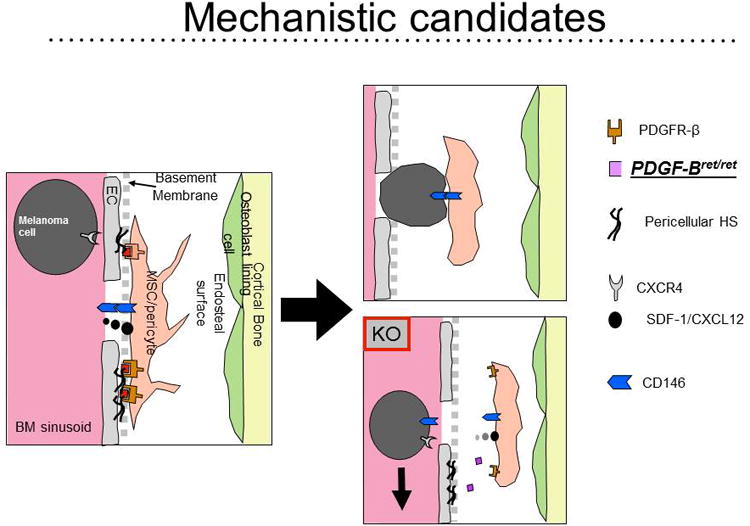
Our working hypothesis for how melanoma cells cross the endothelial/basement barrier to actively establish a metastasis in bone. We have evidence to suggest that the MSC/pericyte secretes SDF-1/CXCL12 causing the melanoma cells to slide into range by virtue of their CXCR4. The release of PDFG-BB by platelets that surround the circulating melanoma cells detaches the pericyte from its anchorage controlled by the PDGFR-β, pericellular heparin sulfate (HS) binding of PDGF-BB to the pericytes PDGFR-β. Because both the melanoma cell and the pericyte/MSC have CD146 on their outer membranes, a homodimer of CD146 is formed and the melanoma/MSC is brought into the bone stroma s a coupled pair (50, 42).
MSCs and Women's Health
The basic and clinical science of MSCs is a rapidly emerging field. Insufficient energy and resources have been placed into applying cell-based therapies to prevent and cure maladies central to women's health. As a speculative example, if MSCs are anti-scarring, can they be used to inhibit the expansion or, indeed, shrink uterine fibroids? Small facial scars have disappeared in women receiving MSC-fat injections for cosmesis (wrinkle reduction) (52). This suggestion must first be approached in suitable pre-clinical models prior to proper FDA-approved clinical trials (investigator or commercial initiated). The future for cell-based therapies in women's health is an open door waiting for creative, reasonable and testable hypotheses followed by new clinical logics and medical solutions for women.
Conclusion
The new science and clinical use of both endogenously and exogenously supplied MSCs will have a huge impact on the practice of medicine. Numerous problems associated with women's health should be addressed using this cell-based therapeutic approach. Although women have used MSC-based technologies for cosmetic indications for the last 10-15 years, it would seem reasonable to suggest that more serious clinical symptoms could now be addressed using MSC-based therapies.
Acknowledgments
Financial Support/Funding: The work reported here was supported by NIH (NCI, R01-CA163562) and the L. David and E. Virginia Baldwin Fund.
Footnotes
The content of this article was presented by Dr. Arnold I. Caplan as the NAMS/Pfizer – Wulf H. Utian Endowed Lecture on October 17, 2014, in Washington, DC at the Annual Meeting of The North American Menopause Society (NAMS).
An endowment to NAMS from Pfizer established this annual lectureship, with faculty selected by The North American Menopause Society Scientific Program Committee.
Conflict of Interest:/Financial Disclosure: CWRU receives royalties from Osiris Therapeutics, which they share with the author, Arnold I. Caplan.
References
- 1.Caplan AI. Cell delivery and tissue regeneration. J Contr Release. 1989;11:157–165. [Google Scholar]
- 2.Caplan AI. Mesenchymal stem cells. J Ortho Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 3.Caplan AI. The mesengenic process. Clin Plast Surg. 1994;21:429–435. [PubMed] [Google Scholar]
- 4.Nakahara H, Goldberg VM, Caplan AI. Culture-expanded human periosteal-derived cells exhibit osteochondral potential in vivo. J Ortho Res. 1991;9:465–476. doi: 10.1002/jor.1100090402. [DOI] [PubMed] [Google Scholar]
- 5.Yoo JU, Barthel TS, Nishimura K, et al. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J Bone and Joint Surg. 1998;80:1745–1757. doi: 10.2106/00004623-199812000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Saito T, Dennis JE, Lennon DP, et al. Myogenic expression of mesenchymal stem cells within myotubes of mdx mice in vitro and in vivo. Tissue Eng. 1996;1:327–344. doi: 10.1089/ten.1995.1.327. [DOI] [PubMed] [Google Scholar]
- 7.Kemp KC, Hows J, Donaldson C. Bone marrow-derived mesenchymal stem cells. Leukemia Lymphoma. 2005;46(11):1531–1544. doi: 10.1080/10428190500215076. [DOI] [PubMed] [Google Scholar]
- 8.Young RG, Butler DL, Weber W, et al. Use of mesenchymal stem cells in achilles tendon repair. J Orthop Res. 1998;16:406–413. doi: 10.1002/jor.1100160403. [DOI] [PubMed] [Google Scholar]
- 9.Tseng C, Kolonin MG. Adipose Tissue-derived progenitor cells and cancer. In: Cao Yihai., editor. Angiogenesis in Adipose Tissue. 2013. pp. 321–337. [Google Scholar]
- 10.Chan TM, Chen JY, Ho LI, et al. ADSC therapy in neurodegenerative disorders. Cell Transpl. 2014;23:549–557. doi: 10.3727/096368914X678445. [DOI] [PubMed] [Google Scholar]
- 11.Cominici M, LeBlanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cythotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 12.Park H, Temenoff JS, Tabata Y, et al. Injectable biodegradable hydrogel composites for rabbit marrow mesenchymal stem cell and growth factor delivery for cartilage tissue engineering. Biomaterials. 2007;28:3217–3227. doi: 10.1016/j.biomaterials.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rowland CR, Lennon DP, Caplan AI, et al. The Effects of crosslinking of scaffolds engineered from cartilage ECM on the chondrogenic differentiation of MSCs. Biomaterials. 2013;34(12):5802–581. doi: 10.1016/j.biomaterials.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caplan AI. Tissue Engineering Designs for the Future: New Logics, Old Molecules. Tiss Eng. 2000;6:1–8. doi: 10.1089/107632700320838. [DOI] [PubMed] [Google Scholar]
- 15.Caplan AI. Tissue engineering strategies for mesenchymal or skeletal tissues. In: Ikada Y, Shimizu Y, editors. Tissue Eng for Therapeutic Use 4: Proceedings of the 4th International Symposium on Tissue Engineering. Elsevier Science, BV; Kyoto, Japan: 1999. pp. 67–72. [Google Scholar]
- 16.Naumann A, Dennis JE, Awadallah A, et al. Immunochemical and mechanical characterization of cartilage subtypes in rabbit. J Histochem Cytochem. 2002;50(8):1049–1058. doi: 10.1177/002215540205000807. [DOI] [PubMed] [Google Scholar]
- 17.Naumann A, Dennis JE, Staudenmaier R, et al. Mesenchymal stem cells – a new pathway for tissue engineering in reconstructive surgery. Laryngo Rhino Otol. 2003;81:521–527. doi: 10.1055/s-2002-33287. [DOI] [PubMed] [Google Scholar]
- 18.Niibe K, Kawamura Y, Araki D, et al. Purified mesenchymal stem cells are an efficient source for iPS cell induction. PLoS ONE. 2011;6(3):e17610. doi: 10.1371/journal.pone.0017610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trivedi P, Hematti P. Derivation and immunological characterization of mesenchymal stromal cells from human embryonic stem cells. Exp Hematol. 2008;36:350–359. doi: 10.1016/j.exphem.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pearson S, Sroczynska P, Lacaud G, et al. The stepwise specification of embryonic stem cells to hematopoietic fate is driven by sequential exposure to Bmp4, activin A, bFGF and VEGF. Development. 2008;135:1525–1535. doi: 10.1242/dev.011767. [DOI] [PubMed] [Google Scholar]
- 21.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Caplan AI. All MSCs are pericytes? Cell Stem Cell. 2008;3:229–330. doi: 10.1016/j.stem.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Murray IR, West CC, Hardy WR, et al. Natural history of mesenchymal stem cells, from vessel walls to culture vessels. Cell Mol Life Sci. 2014;71:1353–1374. doi: 10.1007/s00018-013-1462-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Da Silva Meirelles L, Fontes AM, Covas DT, et al. Mechanisms involved in the therapeutic properties of Mesenchymal Stem Cells. Cytokine Growth F R. 2009;20:419–427. doi: 10.1016/j.cytogfr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213:341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 26.Caplan AI. MSCs as therapeutics. In: Hematti P, Keating A, editors. Stem Cell Biology and Regenerative Medicine Mesenchymal Stromal Cells: Biology and Clinical Applications, Stem Cell Biology and Regenerative Medicine. Springer Science+Business Media; New York: 2012. pp. 79–90. [Google Scholar]
- 27.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 28.Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: Environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45:e54. doi: 10.1038/emm.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ding DC, Shyu WC, Lin SZ. Mesenchymal stem cells. Cell Transpl. 2011;20:5–14. doi: 10.3727/096368910X. [DOI] [PubMed] [Google Scholar]
- 30.Allickson J, Xiang C. Human adult stem cells from menstrual blood and endometrial tissue. J Zhejiang Univ Sci B. 2012;13(5):419–420. doi: 10.1631/jzus.B1200062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Musina RA, Belyavski AV, Tarusova OV, et al. Endometrial mesenchymal stem cells isolated from the menstrual blood. Bull Exp Biol Med. 2008;145(4):539–543. doi: 10.1007/s10517-008-0136-0. [DOI] [PubMed] [Google Scholar]
- 32.Kuismanen K, Sartoneva R, Haimi S, et al. Autologous adipose stem cells in treatment of female stress urinary incontinence: Results of a pilot study. Stem Cells Trans Med. 2014;3:936–941. doi: 10.5966/sctm.2013-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nikolavsky D, Chancellor MB. Stem cell therapy for stress urinary incontinence. Neurology and Urodynamics. 2010;29(S1):S36–S41. doi: 10.1002/nau.20833. [DOI] [PubMed] [Google Scholar]
- 34.Wang HJ, Chuang YC, Chancellor MB. Development of cellular therapy for the treatment of stress urinary incontinence. Int Urogynecol J. 2011;22:1075–1083. doi: 10.1007/s00192-011-1432-1. [DOI] [PubMed] [Google Scholar]
- 35.Cruz M, Dissaranan C, Cotleur A, et al. Pelvic organ distribution of mesenchymal stem cells injected intravenously after simulated childbirth injury in female rats. Obstet Gynecol Int. 2012;2012:612946. doi: 10.1155/2012/612946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salcedo L, Mayorga M, Damaser M, et al. Mesenchymal stem cells can improve anal pressures after anal sphincter injury. Stem Cell Res. 2013;10:95–102. doi: 10.1016/j.scr.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amado LC, Saliaris AP, Schuleri KH, et al. Cardiac repair with intramyocardinal injection of allogeneic mesenchymal stem cells after myocardial infarction. PNAS. 2005;102(32):11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quevedo HC, Hatzistergos KE, Oskouei BN, et al. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. PNAS. 2009;106(33):14022–14027. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghosh P, Moore R, Vernon-Roberts B, et al. Immunoselected STRO-3+ mesenchymal precursor cells and restoration of extracellular matrix of degenerate intervertebral discs. J Neurosurg Spine. 2012;16:479–488. doi: 10.3171/2012.1.SPINE11852. [DOI] [PubMed] [Google Scholar]
- 40.Lennon DP, Haynesworth SE, Bruder SP, et al. Human and animal mesenchymal progenitor cells from bone marrow: Identification of serum for optimal selection and proliferation. Vitro Cell Develop Bio. 1996;32:602–611. [Google Scholar]
- 41.Haynesworth SE, Baber MA, Caplan AI. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: Effects of dexamethasone and IL-1α. J Cell Physiol. 1996;166:585–592. doi: 10.1002/(SICI)1097-4652(199603)166:3<585::AID-JCP13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 42.Correa D, Greenberg SA, Somoza RA, et al. Regulation of MSCs ultimate phenotype: Repercussions for cartilage tissue engineering. Invited review article, eCM Journal. 2014 Submitted. [Google Scholar]
- 43.See Osiris Therapeutics, Inc. and Mesoblast websites (http://www.osiris.com/index.php, http://www.mesoblast.com/).
- 44.Hariri RJ. Tissue matrices comprising placental stem cells, and methods of making same. US Patent Publication number US7311904 B2. 2002 Filing date February 13, 2001.
- 45.Li X, Ling W, Pennisi A, et al. Human placenta-derived adherent cells prevent bone loss, stimulate bone formation, and suppress growth of multiple myeloma in bone. Stem Cells. 2011;29:263–273. doi: 10.1002/stem.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mohammad KS, Javelaud D, Fournier PGJ, et al. TGF-β-RI kinase inhibitor SD-208 reduces the development and progression of melanoma bone metastases. Cancer Res. 2011;71:175–184. doi: 10.1158/0008-5472.CAN-10-2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kang Y, Siegel PM, Shu W, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 48.Guise TA, Mundy GR. Breast cancer and bone. Curr Opin Endocrinol. 1995;2:548–555. [Google Scholar]
- 49.Xian X, Hakansson J, Stahlberg A, et al. Pericytes limit tumor cell metastasis. J Clin Invest. 2006;116(3):642–651. doi: 10.1172/JCI25705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng C, Qiu Y, Zeng Q, et al. Endothelial CD146 is required for in vitro tumor-induced angiogenesis: the role of a disulfide bond in signaling and dimerization. Intl J Biochem & Cell Biol. 2009;41:2163–2172. doi: 10.1016/j.biocel.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 51.Caplan AI, West MD. Progressive approval: A proposal for a new regulatory pathway for regenerative medicine. Stem Cells Trans Med. 2014;3(5):560–563. doi: 10.5966/sctm.2013-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mazzola RF, Cantarella G, Torretta S, et al. Autologous fat injection to face and neck: from soft tissue augmentation to regenerative medicine. Acta Otorhinolaryngol Ital. 2011;31(2):59–69. [PMC free article] [PubMed] [Google Scholar]


