Abstract
Background
Craniofacial reconstructive surgery for craniosynostosis is associated with large blood loss and intraoperative transfusion. This blood loss may continue through the initial postoperative period, potentially resulting in transfusion postoperatively. The purpose of this study is to determine if there is an association between any modifiable intraoperative factors and postoperative blood transfusion in this patient population.
Methods
A cohort of 55 pediatric patients who underwent primary craniofacial reconstruction at Vanderbilt Children’s Hospital from January 1, 2013 to April 31, 2014 was analyzed. The authors analyzed 20 different demographic and perioperative variables for statistical associations with postoperative PRBC transfusion using multiple logistic regression with optimal models being selected by Bayesian Model Averaging.
Results
The optimal regression model only included initial PACU Hct as a predictor and showed a significant association between this variable and postoperative PRBC transfusion (odds ratio 0.69, 95%CI 0.55–0.87, P = 0.0016). Based on the average decrease in postoperative hematocrit (Hct) and the postoperative transfusion trigger, an initial PACU Hct threshold of 30 was calculated. In our patient sample, an initial PACU Hct above 30 was associated with a 50% decrease in the absolute risk of receiving a PRBC transfusion postoperatively.
Conclusions
Based on this retrospective analysis, it may be justifiable to transfuse residual volume from previously exposed intraoperative PRBCs to a Hct above 30 to decrease the likelihood of subsequent blood transfusions from different donors in the postoperative period.
Keywords: Craniosynostosis, Craniofacial Reconstruction, Transfusion, Coagulopathy, Blood Loss, Immunomodulation
Background
Craniofacial reconstruction surgery is often associated with large blood loss, coagulopathy, and perioperative blood transfusion (1). Allogeneic blood transfusion is associated with numerous risks and different approaches have been utilized to decrease transfusion during the intraoperative period such as acceptance of a lower hematocrit (Hct), antifibrinolytics, erythropoetin, and cell saver (2–6). Significant bleeding tends to continue in the postoperative period due to the osteotomies, resulting in frequent blood transfusions. Unfortunately, there has not been as much emphasis placed on reducing blood transfusion in the postoperative period in this patient population. Stricker et al. have published two manuscripts looking at postoperative blood transfusion. In the first, they associated certain intraoperative variables with transfusion of hemostatic blood products in the postoperative period (7). In the second manuscript, they were able to show a decrease in postoperative blood transfusion by implementing a transfusion protocol in the intensive care unit (8). However, is there anything that can be done intraoperatively to decrease blood administration postoperatively for these patients especially PRBCs? The purpose of this study is to determine if there are any controllable intraoperative factors that are associated with blood transfusion in the postoperative period as a means of optimizing intraoperative care.
Methods
Data Acquisition
After approval by the Vanderbilt Institutional Review Board, we identified patients who underwent primary craniofacial reconstruction for craniosynostosis from January 1, 2013 to April 31, 2014 by querying our previous anesthetic records. Demographics, including age, gender, weight, synostosis type, and ASA class, were obtained for each of the patients. In addition, preoperative laboratory values were recorded. Intraoperative variables regarding anesthetic regimen, volume of fluid and blood products administration, and surgical technique were then obtained. Blood loss was estimated based on methods described in Kearney et al (9). Postoperative data recorded included blood products transfused, laboratory values, drain output, and intensive care unit and total hospital lengths of stay.
Anesthetic Management
For this cohort, one neurosurgeon performed the initial exposure and craniotomy and one craniofacial plastic surgeon completed the craniofacial reconstruction. Patients were induced using nitrous oxide and sevoflurane. Large bore IV access and an arterial line were then obtained, an endotracheal tube was inserted, and the patients were maintained on sevoflurane. Crystalloid or 5% albumin was utilized for fluid deficit management and hourly fluid requirements. Aminocaproic acid was initially bolused at 100 mg/kg over 30 minutes and maintained at 33 mg/kg/hr for the remainder of the case. A standard transfusion protocol was utilized for all patients in this cohort that detailed timing of laboratory draws and transfusion triggers. Administration of packed red blood cells (PRBCs) occurred at a hematocrit (Hct) of < 27 or during periods of hemodynamic instability with active bleeding. Fresh frozen plasma (FFP) was administered when R time on Haemonetics® thromboelastography was >10 min, platelets were administered when the platelet count was <100,000/microliter, and cryoprecipitate was given when fibrinogen was <100 mg/dL. Following the procedure, the patient was extubated and brought to the post anesthetic care unit (PACU). After meeting PACU discharge criteria, patients were transported to the pediatric intensive care unit (PICU) where routine care was provided by the surgical and critical care teams. Patients were transfused PRBCs and fresh frozen plasma (FFP) postoperatively for a Hct of ≤ 24 or an INR of ≥ 1.5, respectively.
Statistical Analysis
All statistical analyses were performed using the R software package (version 2.15.1). As a means of determining whether various intraoperative and postoperative variables were associated with postoperative packed red blood cell (PRBC) transfusion, logistic regressions were performed with PRBC administration as the dependent variable. To account for model uncertainty due to the large number of possible variables, Bayesian Model Averaging (BMA) was utilized for variable selection with the Bayesian Information Criterion (BIC) and the posterior probability of the regression model being employed as metrics for model selection (10, 11). The BMA package in the R statistical software was used for this analysis (12). The ‘bic.glm’ function was utilized within this program with all variables set to default including Occam’s Window (OR) being fixed at 20. Model fit was assessed through the use of the Hosmer-Lemeshow test and marginal model plots. A two-tailed P < 0.05 was considered statistically significant.
Results
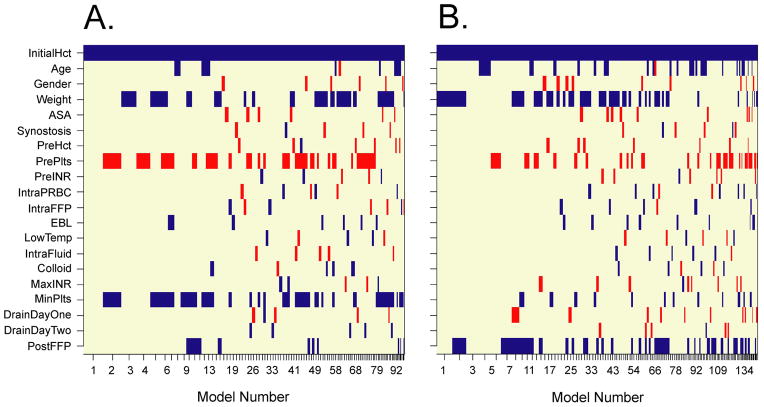
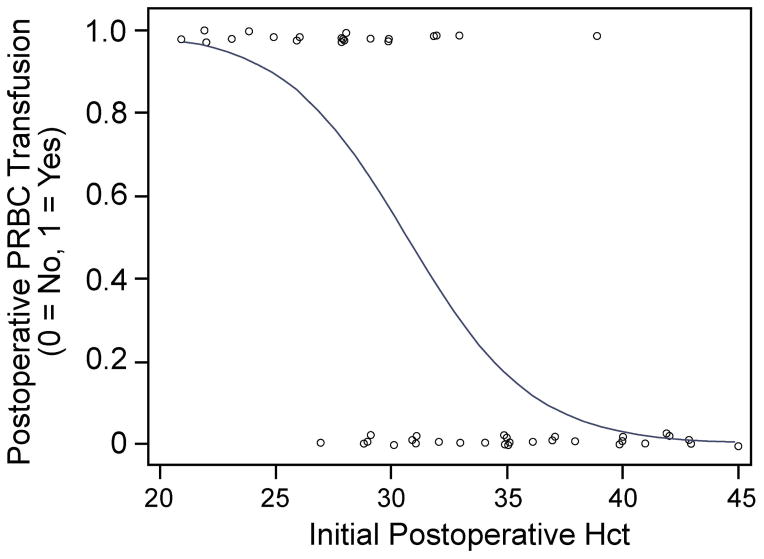
A search of our anesthetic records yielded 55 patients that underwent primary craniofacial reconstruction over this 16 month period. Patient demographics and perioperative variables are represented in Table 1. Since only four patients received fresh frozen plasma (FFP) postoperatively, we only analyzed factors that were associated with postoperative PRBC administration. Variables that were included in our regression analyses were patient age, gender, weight, ASA classification, synostosis type, preoperative Hct, preoperative INR, preoperative platelet count, intraoperative PRBC administration, intraoperative FFP administration, intraoperative fluid administration, colloid exposure, lowest intraoperative body temperature, estimated intraoperative blood loss, initial postoperative (PACU) Hct, Jackson-Pratt (JP) drain output on postoperative day one (from first midnight to 23:59) and two (from second midnight to 23:59), maximum postoperative INR, minimum postoperative platelet count, and postoperative FFP administration. Using BMA for variable selection, multiple logistic regression models were calculated. Based on posterior probability, the optimal regression model only included initial PACU Hct as a predictor. Of note, initial PACU Hct was included as a variable in all of the calculated models (100%) while each of the other variables occurred less than 50% of the time (Figure 1A). There was a significant association between initial PACU Hct and postoperative PRBC transfusion (odds ratio 0.69, 95%CI 0.55–0.87, P = 0.0016, Figure 2) according to this optimal logistic regression model.
Table 1.
Demographic and Perioperative Characteristics*
| Age (months) | 8.7 ± 2.5 |
| Weight (kg) | 8.4 ± 2.1 |
| Male/Female Proportion | 38/17 (69.1%/30.9) |
| ASA class (3/2/1) | 8/43/4 (14.5%/78.2/7.3) |
| Synostosis Type (Multiple Synostoses/Sagittal/Coronal/Metopic) | 7/19/5/24 (12.7%/34.5/9.1/43.6) |
| Preoperative Hct (%) | 35.2 ± 3.8 |
| Preoperative INR | 1.0 ± 0.2 |
| Preoperative Platelet Count per mcL | 415,000 ± 120,000 |
| Intraoperative PRBC Transfusion (yes/no) | 44/11 (80.0%/20.0) |
| Intraoperative FFP Transfusion (yes/no) | 3/52 (5.5%/94.5) |
| Intraoperative Fluid Administration (ml/kg) | 58.0 ± 22.5 |
| Lowest Intraoperative Temperature (°C) | 36.0 ± 0.5 |
| Estimated Blood Loss (ml/kg) | 15.6 ± 9.0 |
| Initial Postoperative Hct (%) | 32.4 ± 6.0 |
| Postoperative PRBC Transfusion (yes/no) | 23/32 (41.8%/58.2) |
| Postoperative FFP Transfusion (yes/no) | 4/51 (7.3%/92.7) |
| Maximum Postoperative INR | 1.4 ± 0.2 |
| Minimum Postoperative Platelet Count | 238,000 ± 66,000 |
| Postoperative Day One JP Drainage (ml) | 118.7 ± 60.5 |
| Postoperative Day Two JP Drainage (ml) | 46.9 ± 30.0 |
Population statistics for non-proportion data expressed as mean ± standard deviation.
Figure 1.
Variables selected for each individual logistic regression model as calculated by Bayesian Model Averaging and represented by bars. Models are numbered sequentially by posterior probability and Bayesian Information Criteria with model 1 being the most optimal model for each analysis. (A) Initial PACU Hct is represented as a continuous variable and appears in all calculated models. (B) Initial PACU Hct represented as a binary variable (≥ 31 vs. <31) and also appears in all calculated models.
Figure 2.
Plot of postoperative PRBC transfusion versus initial PACU hematocrit with logistic fit added. Each circle represents an individual patient. Based on this model, there was a significant association between initial PACU Hct and postoperative PRBC transfusion (odds ratio 0.69, 95%CI 0.55–0.87, P = 0.0016). This model adequately fits the data (Hosmer-Lemeshow test: P = 0.2824, df = 6).
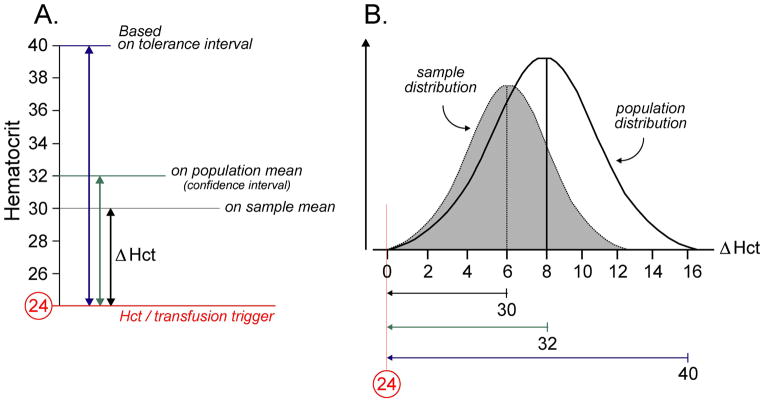
In order to establish an ideal threshold for initial PACU Hct, the average change in postoperative Hct (Δ Hct) was calculated for the patient sample. This variable was defined as the difference in initial PACU Hct and the lowest recorded postoperative Hct (postoperative Hct nadir) during each patient’s entire hospital stay (Figure 3A). For the whole patient sample, the average (± standard deviation) Δ Hct was 6.2 (± 3.8). However, this factor includes patients that received a postoperative PRBC transfusion and the Δ Hct in this subpopulation is actually right censored since their Hct may have decreased further without the transfusion. For example, if a patient’s initial PACU Hct was 28 and they later received a PRBC transfusion for a Hct of 24, their blood count may have decreased further (e.g. 22) without the transfusion and their Δ Hct may be falsely truncated (4 instead of the true value 6). Thus, a more adequate representation of the average Δ Hct would only include patients that were not administered PRBCs postoperatively. Using data from the 32 patients that did not receive a postoperative PRBC transfusion, the average (± standard deviation) Δ Hct was 6.7 (± 3.7). Since this data set was normally distributed (Shapiro-Wilk test with P = 0.6804), a 95% confidence interval of the mean and a (0.95, 95%) tolerance interval were calculated to be 5.4 to 8.0 and −2.6 to 16.0, respectively.
Figure 3.
(A) Average change in postoperative Hct (Δ Hct) as defined as the difference in initial PACU Hct and the postoperative Hct nadir during the entire hospital stay. Since the postoperative trigger for PRBC administration in this patient population at our institution is 24 and Δ Hct in our sample is 6, the threshold for initial PACU Hct was set at 30 for analysis. (B) Schematic of Δ Hct sample and population distributions. If the initial PACU Hct threshold is based on the approximate Δ Hct population mean (derived from the upper limit of the 95% confidence interval), roughly half of the possible Δ Hct values will not be accounted for and this may lead to a number of patients receiving postoperative PRBC transfusions. This transfusion risk may be lessened by accounting for almost the entire Δ Hct population distribution (approximately 97.5%) through the use the upper limit of the (0.95, 95%) tolerance interval derived from the patient sample.
Since the Hct trigger for postoperative PRBC transfusion at our institution is 24 for this patient population and the average Δ Hct was determined to be 6.2, we established an initial PACU Hct threshold of 30 for the purposes of this analysis. To account for the effect of raising the initial PACU Hct to ≥ 31 on postoperative PRBC transfusion while controlling for possible confounders, multiple logistic regression models were calculated similar to our previous analysis with initial PACU Hct set as a binary variable (≥ 31 vs. <31). The optimal regression model for this investigation included both initial PACU Hct and weight as predictors (Table 2). As with the previous analysis, initial PACU Hct as a binary variable was included in all of the calculated models (100%) while each of the other variables occurred less than 50% of the time (Figure 1B). After controlling for patient weight, increasing initial PACU Hct to ≥ 31 decreased the likelihood of postoperative PRBC transfusion (adjusted odds ratio 0.04, 95%CI 0.01–0.20, P < 0.0001). This corresponds to a number needed to treat (NNT) of 2.
Table 2.
Predictors of Postoperative PRBC transfusion*
| Characteristic | OR | 95% CI | P-value |
|---|---|---|---|
| Weight (per kg) | 0.67 | 0.45 to 0.99 | 0.0451 |
| Initial Postoperative Hct | |||
| < 30% | 1.00 | (referent) | |
| ≥ 31% | 0.04 | 0.01 to 0.20 | <0.0001 |
OR = odds ratio, CI = confidence interval.
This logistic regression model was generated using Bayesian Model Averaged based on BIC. Hosmer-Lemeshow test with 8 degrees of freedom yielded P-value of 0.9857, signifying excellent model fit. Weight was modeled as a continuous variable and initial postoperative Hct was modeled as a categorical variable. P-value < 0.05 was considered statistically significant.
Discussion
In this retrospective study, the authors examined numerous demographic and perioperative variables for possible associations with postoperative PRBC transfusion. Our analysis suggests that the only modifiable intraoperative factor is initial PACU Hct. An elevated Hct may be beneficial during the initial postoperative period since a significant amount of bleeding may continue during the early postoperative period.
The majority of patients that undergo primary craniofacial reconstruction for craniosynostosis are transfused PRBCs intraoperatively despite medical attempts to decrease blood loss (3, 4, 13). At our institution, the average volume of a unit of PRBCs is 350 mL and splitting of these units is not performed for the operating rooms. Since this patient population typically weights approximately 8 kg, a 15ml/kg bolus of PRBCs is less than half of the total volume of PRBCs in one unit. The remaining red cells are often discarded since their shelf life is only 4 hours after the initiation of the transfusion. This study suggests that if there is residual volume from a previously exposed unit of PRBCs, there may be a benefit in administering more of the remainder of the product to a target HCT of ≥ 31 if there are no signs of volume overload to prevent subsequent transfusion from a different donor in the postoperative period. In this sample, all patients that received a postoperative PRBC transfusion had residual PRBC volume intraoperatively that was ultimately discarded which they could have received.
After establishing statistically that initial PACU Hct is inversely related to the likelihood of postoperative PRBC transfusion (Figure 2), the question of specific Hct threshold needed to be addressed. In our patient sample, since the average overall Δ Hct was known, we decided to use a threshold derived from adding this quantity to the ICU transfusion trigger (Figure 3B). Utilizing the Hct threshold of 30 resulted in a 50% decrease in the absolute risk of receiving a PRBC transfusion postoperatively and a NNT of 2. To generalize these results to future patients, we will use the upper bound of the 95% confidence interval of the average overall Δ Hct (8.0) to create our Hct threshold. This value is an appropriate approximation of the average Δ Hct for the entire patient population. Utilizing this value will increase the current threshold from 30 to 32 (Figure 3B). However, since the confidence interval only encompasses the mean population Δ Hct and not all possible Δ Hct values in the patient population, basing the threshold on this statistic will most likely result in a number of patients still receiving postoperative PRBC transfusions (Figure 3B). A more liberal strategy would be to incorporate the upper bound of the (0.95, 95%) tolerance interval (16.0) since this method will account for most of the likely Δ Hct values in the patient population (Figure 3B). This practice would increase our threshold to 40. Thus, if previously exposed PRBCs remain at the end of the procedure, it may be beneficial to use this residual volume to increase the Hct to > 8 (or, more liberally, > 16) above the postoperative transfusion trigger.
This practice may seem precarious due to possible associated risks. As mentioned earlier, blood transfusion in itself is fraught with risks that may increase both morbidity and mortality. However, once exposed to a specific unit without an adverse reaction, the likelihood of a reaction occurring with subsequent exposure to the same unit would most likely be minimal. In addition, although increasing Hct also elevates blood viscosity, symptoms of hyperviscosity due to polycythemia rarely occurs at a Hct less than 60 (14). This practice may also increase the chance of transfusion associated cardiovascular collapse (TACO), but this risk may be diminished by anticipating the transfusion and limiting crystalloid and colloid administration. In addition, TACO is more common in adults than children. Lavoie looked at the risks of blood transfusion and noted no reported cases of mortality from TACO in pediatric patients in the serious hazards of transfusion (SHOT) database (15). Furthermore, no patients in this study had issues with respiratory distress postoperatively. In contrast to these potential hazards, this practice may actually mitigate some of the risks such as infectious diseases, surgical site infections, immunomodulatory and alloimmunization effects associated with blood transfusion if it ultimately decreases exposure to multiple donor units (16–21).
Since this study is a retrospective analysis, there are inherent limitations due to the study design. Variables that were unknowingly omitted from our regression analyses may confound our current results. Also there is a possibility that some variables may have been underpowered to show a difference with this limited sample size. However, since the association between initial PACU Hct and postoperative PRBC transfusion was statistically apparent within this small sample, the effect size of this association would most likely be larger than other associations that occur in subsequent analyses with higher power. Lastly, another possible limitation is that these results may not be generalizable beyond the surgical techniques that were employed at this institution. Intraoperative and postoperative blood loss can vary widely amongst institutions, which may be surgeon dependent. One way around this shortcoming would be to calculate the average decrease in postoperative Hct at that particular institution. An intraoperative Hct threshold could then be computed by combining statistics derived from this value with the postoperative transfusion trigger.
In summary, our study suggests that the only modifiable intraoperative factor to reduce the incidence of postoperative transfusion is to increase the immediate postoperative (PACU) Hct. If a patient has already been exposed to a unit of PRBCs and there is remaining volume from the same donor, it may be justifiable to transfuse this volume in order to increase the red cell mass and possibly prevent a subsequent blood transfusion from another donor postoperatively. Transfusion thresholds should be dictated by average postoperative Hct drop and postoperative transfusion triggers. Future studies should focus on delineating the cost reduction and possible decreased morbidity from decreased donor exposures.
What is already known: Previous studies have shown that intraoperative blood product transfusion for patients undergoing craniofacial reconstruction can be minimized through various techniques.
What this article adds: This analysis demonstrated that initial PACU Hct was the only identified modifiable intraoperative variable that is associated with postoperative PRBC administration.
Implications for translation: Transfusion of residual previously-exposed intraoperative PRBCs may decrease postoperative red cell transfusion and the associated complications of multiple donor transfusion.
Footnotes
Conflicts of Interest: None
Disclosures
Financial support for the preparation of this manuscript was provided from departmental funds of the Department of Anesthesiology, Vanderbilt University School of Medicine. Dr. Austin is supported by NIH grant T32GM108554.
References
- 1.White N, Marcus R, Dover S, et al. Predictors of blood loss in fronto-orbital advancement and remodeling. Journal of Craniofacial Surgery. 2009;20:378–381. doi: 10.1097/SCS.0b013e31819b9429. [DOI] [PubMed] [Google Scholar]
- 2.Dadure C, Sauter M, Bringuier S, et al. Intraoperative tranexamic acid reduces blood transfusion in children undergoing craniosynostosis surgery: a randomized double-blind study. Anesthesiology. 2011;114:856–861. doi: 10.1097/ALN.0b013e318210f9e3. [DOI] [PubMed] [Google Scholar]
- 3.Goobie SM, Meier PM, Pereira LM, et al. Efficacy of tranexamic acid in pediatric craniosynostosis surgery: a double-blind, placebo-controlled trial. Anesthesiology. 2011;114:862–871. doi: 10.1097/ALN.0b013e318210fd8f. [DOI] [PubMed] [Google Scholar]
- 4.Haas T, Goobie S, Spielmann N, et al. Improvements in patient blood management for pediatric craniosynostosis surgery using a ROTEM((R)) -assisted strategy - feasibility and costs. Paediatr Anaesth. 2014;24:774–780. doi: 10.1111/pan.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Redlin M, Kukucka M, Boettcher W, et al. Blood transfusion determines postoperative morbidity in pediatric cardiac surgery applying a comprehensive blood-sparing approach. J Thorac Cardiovasc Surg. 2013;146:537–542. doi: 10.1016/j.jtcvs.2012.09.101. [DOI] [PubMed] [Google Scholar]
- 6.Guzzetta NA. Benefits and risks of red blood cell transfusion in pediatric patients undergoing cardiac surgery. Paediatr Anaesth. 2011;21:504–511. doi: 10.1111/j.1460-9592.2010.03464.x. [DOI] [PubMed] [Google Scholar]
- 7.Stricker PA, Shaw TL, Desouza DG, et al. Blood loss, replacement, and associated morbidity in infants and children undergoing craniofacial surgery. Paediatr Anaesth. 2010;20:150–159. doi: 10.1111/j.1460-9592.2009.03227.x. [DOI] [PubMed] [Google Scholar]
- 8.Stricker PA, Fiadjoe JE, Kilbaugh TJ, et al. Effect of transfusion guidelines on postoperative transfusion in children undergoing craniofacial reconstruction surgery. Pediatr Crit Care Med. 2012;13:e357–362. doi: 10.1097/PCC.0b013e31825b561b. [DOI] [PubMed] [Google Scholar]
- 9.Kearney RA, Rosales JK, Howes WJ. Craniosynostosis: an assessment of blood loss and transfusion practices. Can J Anaesth. 1989;36:473–477. doi: 10.1007/BF03005352. [DOI] [PubMed] [Google Scholar]
- 10.Schwarz G. Estimating the dimension of a model. The annals of statistics. 1978;6:461–464. [Google Scholar]
- 11.Hoeting JA, Madigan D, Raftery AE, et al. Bayesian model averaging: a tutorial. Statistical science. 1999:382–401. [Google Scholar]
- 12.Raftery AE, Painter IS. BMA: an R package for Bayesian model averaging. R news. 2005;5:2–8. [Google Scholar]
- 13.Stricker PA, Cladis FP, Fiadjoe JE, et al. Perioperative management of children undergoing craniofacial reconstruction surgery: a practice survey. Paediatr Anaesth. 2011;21:1026–1035. doi: 10.1111/j.1460-9592.2011.03619.x. [DOI] [PubMed] [Google Scholar]
- 14.Jeevasankar M, Agarwal R, Chawla D, et al. Polycythemia in the newborn. Indian J Pediatr. 2008;75:68–72. doi: 10.1007/s12098-008-0010-0. [DOI] [PubMed] [Google Scholar]
- 15.Lavoie J. Blood transfusion risks and alternative strategies in pediatric patients. Paediatr Anaesth. 2011;21:14–24. doi: 10.1111/j.1460-9592.2010.03470.x. [DOI] [PubMed] [Google Scholar]
- 16.Wood A, Wilson N, Skacel P, et al. Reducing donor exposure in preterm infants requiring multiple blood transfusions. Arch Dis Child Fetal Neonatal Ed. 1995;72:F29–33. doi: 10.1136/fn.72.1.f29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts DO, Covert B, Lindsey T, et al. Directed blood donor program decreases donor exposure for children with sickle cell disease requiring chronic transfusion. Immunohematology. 2012;28:7–12. [PubMed] [Google Scholar]
- 18.Minifee PK, Daeschner CW, 3rd, Griffin MP, et al. Decreasing blood donor exposure in neonates on extracorporeal membrane oxygenation. J Pediatr Surg. 1990;25:38–42. doi: 10.1016/s0022-3468(05)80161-7. [DOI] [PubMed] [Google Scholar]
- 19.Lee DA, Slagle TA, Jackson TM, et al. Reducing blood donor exposures in low birth weight infants by the use of older, unwashed packed red blood cells. J Pediatr. 1995;126:280–286. doi: 10.1016/s0022-3476(95)70561-9. [DOI] [PubMed] [Google Scholar]
- 20.Alves VM, Martins PR, Soares S, et al. Alloimmunization screening after transfusion of red blood cells in a prospective study. Rev Bras Hematol Hemoter. 2012;34:206–211. doi: 10.5581/1516-8484.20120051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blumberg N. Deleterious clinical effects of transfusion immunomodulation: proven beyond a reasonable doubt. Transfusion. 2005;45:33S–39S. doi: 10.1111/j.1537-2995.2005.00529.x. discussion 39S–40S. [DOI] [PubMed] [Google Scholar]