Abstract
Vasculature response is a hallmark for most inflammatory skin disorders. Tape stripping on human skin causes a minor inflammation which leads to changes in microvasculature. In this study, optical microangiography (OMAG), noninvasive volumetric microvasculature in vivo imaging method, has been used to track the vascular responses after tape stripping. Vessel density has been quantified and used to correlate with the degree of skin irritation. The proved capability of OMAG technique in visualizing the microvasculature network under inflamed skin condition can play an important role in clinical trials of treatment and diagnosis of inflammatory skin disor-ders.
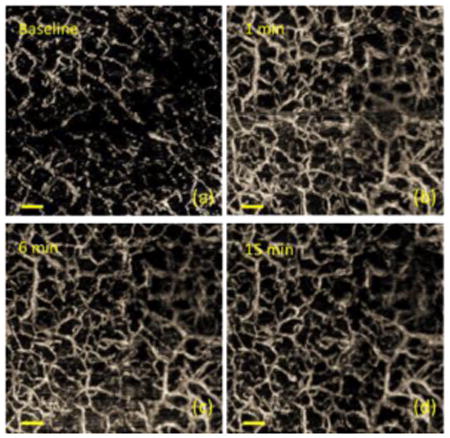
UHS-OMAG images of the skin at (a) before tape stripping, (b) 1 min, (c) 6 min, and (d) 15 min after stripping. Scale bar: 0.3 mm.
Keywords: Optical coherence tomography, Optical microangiography, Skin microcirculation, Capillary blood flow, Tape stripping, Inflammatory skin disorders
1. Introduction
It is known that many skin diseases are linked to either insufficient or overactive vasculature [1]. Among them, skin inflammatory disorders, which represent a large number of clinical cases in dermatology, are common examples. Representative inflammatory skin diseases associated with prominent remodeling in microvasculature include psoriasis [2], rosacea [3], atopic dermatitis [4], UV damage [5], bullous pemphigoid [6], and systemic sclerosis [7]. Other than skin inflammatory disorders, some systemic diseases, such as inflammatory bowel disease [8], rheumatoid arthritis [9], atherosclerosis [10] and asthma [11], have also shown to be associated with peripheral microvascular modifications. Due to the easy accessibility of skin, the cutaneous microvasculature can provide a unique opportunity for the measurement of microcirculation status. Therefore, skin microvascular function can be considered as a prognostic marker when evaluating certain diseases and treatment effects.
Topical drug delivery has become an increasingly common strategy for local therapy and to reduce systemic adverse effects in the treatment of skin diseases. However, the barrier function of stratum corneum (SC) results in poor penetration of drugs into the skin, which often limits the efficacy of topical formulations. Topical therapeutics through skin is limited in efficiency, usually only 50% of the therapeutics can reach to the target site. [12] In order to deliver the therapeutic drug concentrations into the viable epidermal layer, improved drug permeation across SC is required. Tape stripping is a commonly used method to increase the penetration depth of drug delivery [13]. It has also been used to investigate the SC physiology as well as the kinetics and penetration depth of topical drugs [14-17]. During the tape stripping process, the skin is usually applied with appropriate adhesive tapes, and the SC is sequentially removed by a serial number of stripping procedures [18]. This is considered as a minimally invasive technique and is the basis of the FDA's so-called dermatopharmacokinetic (DPK) approach to the assessment of topical bioavailability and bioequivanlence [19].
On the other hand, tape stripping induces mechanical disruptions of the epidermal barrier that lead to skin inflammation [20, 21]. Similar to many other inflammatory skin disorders, microvascular remodeling is usually involved [22]. Surprisingly, despite the popularity of tape stripping in dermatological research and practices, as well as its potential of causing skin inflammation, little work has been done on revealing the microvasculature changes and/or to correlate the degree of the vascular reactions with the degree of damage produced. To our knowledge, there is only one prior study that has recorded the skin capillary changes after tape stripping procedure, and the authors have concluded that there is not always a correlation between the macroscopic appearance of the stimulated area and the microscopic anatomic vascular changes [21]. However, this study was performed using capillaroscopy, which can only image the very shallow capillaries and may not be able to provide a complete microvascular network. Therefore, there is a need to visualize the detailed and complete microvasculature changes after tape stripping process.
A variety of non-invasive techniques have been developed to explore skin microcirculation. Capillaroscopy, which is essentially a method of looking at the skin capillaries under a microscope, has been used to directly visualize the skin surface of nailfold area [23-24]. However, being a traditional light microscopy, capillaroscopy has limited light penetration, especially for dark skin subjects. Therefore, capillaroscopy is restricted to image thin skin area such as nail-fold, and it is difficult to visualize the complete microvascular network. Other techniques such as orthogonal polarization spectral imaging (OPSI), which uses polarized light at λ=550 nm and collects the reflected light orthogonal to the emitted light, can be used to image small blood vessels of nail bed or lip [25]. To date, it has been used in applications such as assessment of the sublingual microcirculation in critical care patients as a strong predictor of outcome in severe sepsis [26]. However, OPSI does not allow easy studies on blood perfusion or microvascular dysfunction. Laser Doppler imaging (LDI) and laser Doppler flowmetry (LDF) are more recently developed imaging techniques, which utilize the Doppler effect and provide a direct measure of microcirculatory flow [27-28]. Laser Doppler based techniques have been previously used to evaluate burns [29], dermal inflammation [30], wound healing [31], and cutaneous ulceration [32]. Although they can provide large-scale relative changes in skin blood perfusion, they have limited image resolution to resolve some of the small capillaries and cannot provide absolute velocity of blood flow [27].
Optical coherence tomography (OCT), an interferometric method, is another non-invasive imaging technique capable of producing large-scale (millimeters) cross-sectional morphological views of tissue microstructures in vivo with a micron-level imaging resolution [33-34]. OCT detects the scattered photons and the imaging contrast is originated from the variance of refractive index within heterogeneous tissue [35-36]. Due to the relatively high resolution, deep imaging depth (1-3 mm), and the real-time image acquisition, OCT has gained more and more attention in the field of dermatology. So far, OCT has been successfully used to study non-melanoma basal cell carcinoma [37], actinic keratosis [38], inflammatory diseases [39-40], to quantify skin changes and monitor therapeutic effects [41-42]. Although of great value, traditional OCT technique is difficult, if not impossible, to provide blood vessel imaging, therefore, limiting it from studying blood perfusion status. Fortunately, by analyzing both the intensity and the phase information embedded in the OCT spectral interferograms, a new technique named optical microangiography (OMAG), has been developed to provide volumetric vasculature image and 3D blood perfusion map in microcirculatory tissue beds in vivo [43]. To improve the sensitivity to image capillaries, ulatrahigh-sensitive OMAG (UHS-OMAG) has been recently proposed [44]. It is a variation of OMAG technique, which allows for imaging the complete microvasculature network down to capillary level by differentiating signals scattered off static tissues from signals scattered off dynamic components such as moving red blood cells (RBC) within patent vessels. During the last few years, OMAG technique has been intensively used to study microvasculature of a variety of biological tissues in vivo. For example, UHS-OMAG has been used to study the mouse cerebral microvasculature and its responses to systemic hypoxia, normoxia and hyperoxia [45], where OMAG is demonstrated to have capability to visualize the acute hypoxia and hyperoxia on microhemodynamic activities, including the passive and active modulation of microvascular density and flux regulation in vivo. UHS-OMAG has also been used to provide depth-resolved retinal microvasculature images within human retina, promising a non-invasive tool for the diagnosis of eye diseases with significant vascular involvement, such as diabetic retinopathy and age-related macular degeneration [46]. For skin applications, Doppler-OMAG (DOMAG) [47], which uses Doppler principle has been combined with UHS-OMAG to image capillary morphology in human finger cuticle [48] and to study skin wound healing process in mouse pinna in vivo [49]. UHS-OMAG system has also been used to investigate the vascular abnormalities in psoriasis [50], where the microcirculation within the normal skin sites can be differentiated from that in the psoriatic skin sites.
Based on these previous results, we believe that OMAG technique is advantageous for visualizing functional microvasculature within skin tissue beds. In this paper, we propose to apply tape stripping on in vivo human skin to produce a simple but representative skin inflammation condition and utilize OMAG to track the microvascular changes after this procedure. Our aim is to explore the feasibility of OMAG to detect changes in microvasculature under skin irritation and to introduce microvasculature-based biomarkers in evaluating the severity of skin inflammation. We believe that the acquired high-quality, detailed microvascular images of the skin will help understanding the microvasculature responses to tape stripping. In addition, the capability of visualizing the complete microvasculature network under inflamed skin condition may significantly broaden the potential applications of OMAG in skin disease evaluations and in other fields of biomedical research.
2. System and methods
OMAG System Setup
All the measurements of this study were performed using a fiber-based spectral-domain OCT system developed in house. The detailed information of this system has been previously described in reference [51]. Briefly, the system employed an extended broadband superluminescent diode (Thorlabs Inc.) as the light source, which has the central wavelength of 1340 nm and bandwidth of 110 nm. The axial resolution of the system was measured to be ∼7 μm in the air. To focus the light onto the sample, a 10× microscope objective lens with a focal length of 18 mm was used to achieve a lateral resolution of ∼7 μm. The output signals from the interferometer was directed to a home-made spectrometer system, which provides spectral resolution of ∼0.141 nm with a detectable depth range of ∼3 mm on each side of the zero delay line. The InGaAs linescan camera (Goodrich Inc.) used in the system had an acquisition rate of 92 kHz. With a 3.5 mW illumination power exposed at the sample surface, the developed system was measured to have a dynamic range of 105 dB. To visualize the volumetric microvasculature, a unique UHS-OMAG scanning protocol was applied [52]. Each B-frame consists of 400 A-lines covering a distance of ∼3.0 mm. The imaging rate was 180 fps. In the slow axis (C-scan), a total number of 2000 B-frames with 5 repetitions in each location were performed also covering a distance of ∼2.8 mm. ED-based clutter filtering algorithm [53] was implemented in MATLAB® to suppress the effect of tissue motion and extract microvasculature. For Doppler OMAG measurement to quantify the velocity of blood flow, 200 B-scans and 5000 A-lines covering 1.5 mm× 1.5 mm range were performed. The imaging speed was set at 1.5 fps, which is optimized for imaging blood flow for the finger skin. Details of this imaging protocol can be found in [45, 54]. During each measurement, the volunteer was sitting on a chair with his elbow supported and his finger was gently fixed in a home-made finger holder. For the DOMAG experiments, the stage was tilted 20° with respect to the optical table in order to have a detectable axial component of the RBC velocity. Images are obtained by Doppler processing of complex signals among A-lines. Phase variance mask is used to separate Doppler flow signals from noisy phase background [54].
Tape Stripping Procedure
A customized hand holder, which was used to support the volunteer's hand and to reduce the involuntary body movement, was designed and employed. Dorsal skin of the second finger from the right hand of a 26-year-old male volunteer was measured. In order to remove the surface reflection, a thin layer of mineral oil was applied the skin surface with a glass coverslip covered on the top. For each stripping procedure, the tape was applied on the finger skin for 5 s and was removed rapidly. This procedure was repeated for15 times. Since the experiment conditions (movement from the volunteer, the amount of oil, etc.) are difficult to keep stable, data acquired after 15 min is disregarded. UHS-OMAG images were acquired at every 40 s for the first 10 min after the tape stripping procedure, and then continued to acquire data at every 2.5 min till 15 min after the stripping procedure. For Doppler OMAG measurement, images were acquired at every 5 min till 15 min after the procedure.
3. Results and discussion
With the superior lateral and axial resolutions of the system, microvascular network even at capillary level can be visualized. In order to determine the degree of stimulation and its recovery characteristics, UHS-OMAG imaging was used to monitor the microvasculature changes after applying tape stripping procedure. Figure 1 compares the microvasculature responses at different time points after applying tape stripping. Figure 1(a) shows a representative UHS-OMAG image acquired from the finger skin before tape stripping. The reserve blood vessels were not activated with most area shown as black in the OMAG image. At 1 min after the procedure, the reserve blood vessels were strongly activated with numerous newly recruited capillaries as shown in Figure 1(b). This activation effect lasted for ∼2-3 min and started to decrease ∼5 min after stripping. At 15 min after the procedure, the blood flow seemed to get close to its normal physiology status.
Figure 1.
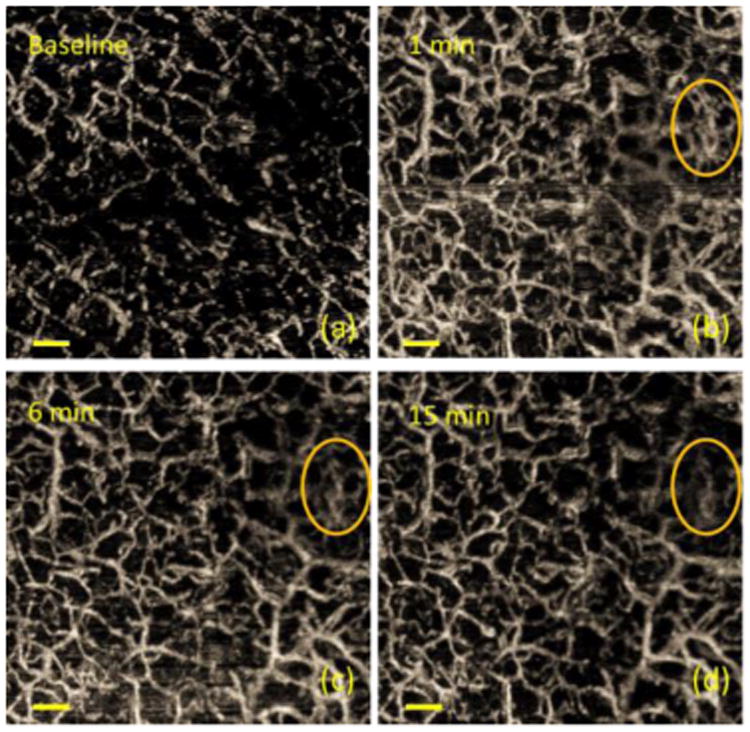
Monitor the skin after applying tape stripping at different time points using UHS-OMAG. (a)-(d) were acquired at before tape stripping, 1 min, 6 min, and 15 min after tape stripping procedure, respectively. Yellow circle in (b)-(d) labels the area where obvious decrease in blood flow was observed. Scale bar is 0.3 mm.
The degree of stimulation should decrease with time after applying tape stripping. To investigate whether the imaging device has the capability to differentiate degrees of stimulation which potentially implies the degree of inflammation, we monitored the vessel density changes over time. To calculate the vessel density for each image, segmentation algorithm is applied. Briefly in this method, images are binarized with an adaptive threshold technique specifically tailored for quantifying OMAG results [54], and vessel density is calculated by dividing the number of ones with the total pixel number. Figure 2 shows the changes in vessel density after the tape stripping procedure was performed 15 times on the finger skin of a 26-year-old male volunteer using an electrical tape. Stars are measured data points while the curve shows the polynomial fitting result. We can see that the vessel density decreases rapidly from ∼0.17 to ∼0.10 within the first 8 min. And then the skin gradually stabilizes and the vessel density reaches ∼0.09 at 15 min after the procedure.
Figure 2.
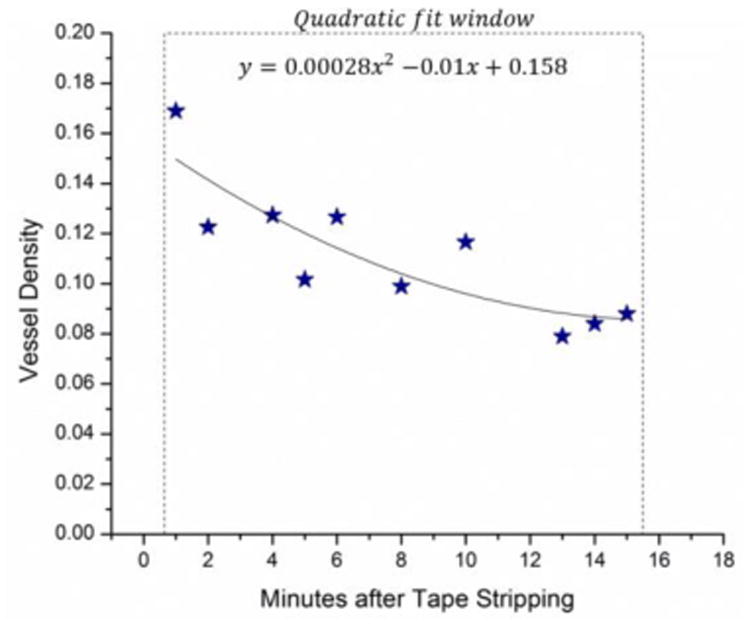
Quantification of the recovery trend after tape stripping. Tape: Scotch 3M 10 mm width electrical tape; number of stripping: 15.
To better quantify the changes in blood flow velocity after applying tape stripping, Doppler OMAG images were acquired. Figure 3 shows the comparison of blood flow at 1 min, 5 min and 13 min after the procedure. The velocity range is ±0.3 mm/s. As shown in Figure 3(a) and (b), compared to 1 min after the procedure, the vessels became less dense with a decrease in both the diameter and the velocity at 5 min after tape stripping. At 13 min after the procedure (Figure 3(c)), the vessels appeared to be similar compared to at 5 min, indicating that the blood vessel response has been stabilized and the skin has gone almost back to normal physiological state. Moreover, in order to quantify the overall blood flow changes in the measured skin area, total blood flow in the corresponding area was calculated for each time point using the method described in [54]. Results indicated that total blood flow was ∼0.023 mm3/s at 1 min after the tape stripping and it dropped to ∼0.017 mm3/s and ∼0.014 mm3/s at 5 min and 13 min after the tape stripping, respectively.
Figure 3.
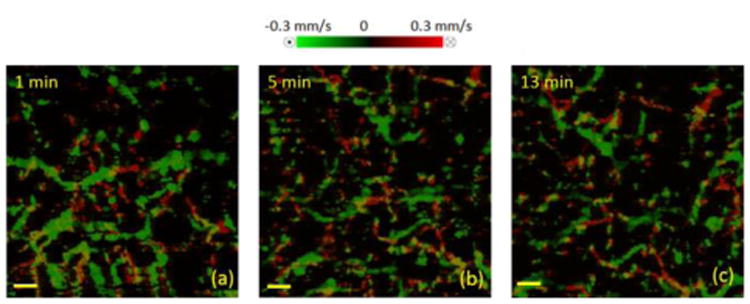
Changes in blood flow velocity caused by tape stripping. (a) Doppler OMAG image of the skin at 1 min; (b) 5 min; and (c) 13 min after applying tape stripping. Stripping was performed using Scotch 3M 10 mm width electrical tape for 25 times. Red represents flow against (arterial) and green represents flow towards (venous) the incident beam. Scale bar is 0.15 mm.
To identify the location of the blood vessels and to better correlate the vessel response with the vessel location, UHS-OMAG images were merged with structural OCT images for both before and after tape stripping. Figure 4 (a) and (b) are UHS-OMAG images taken at 15 min and 1 min after tape stripping. The corresponding OCT structural images taken at the cross section labeled in yellow dash lines in (a) and (b) are shown in Figure 4 (c) and (d). Figure 4 (e) shows the merged image taken at 15 min after tape stripping, where we can see that the main flows come from the blood vessels located at the dermis of the skin. However, at 1 min after the stripping procedure where the skin is still under irritation status, significantly increased blood flow of vessels located at the dermal epidermal junction (DEJ) and papillary dermis layers was observed as shown in Figure 4(f).
Figure 4.
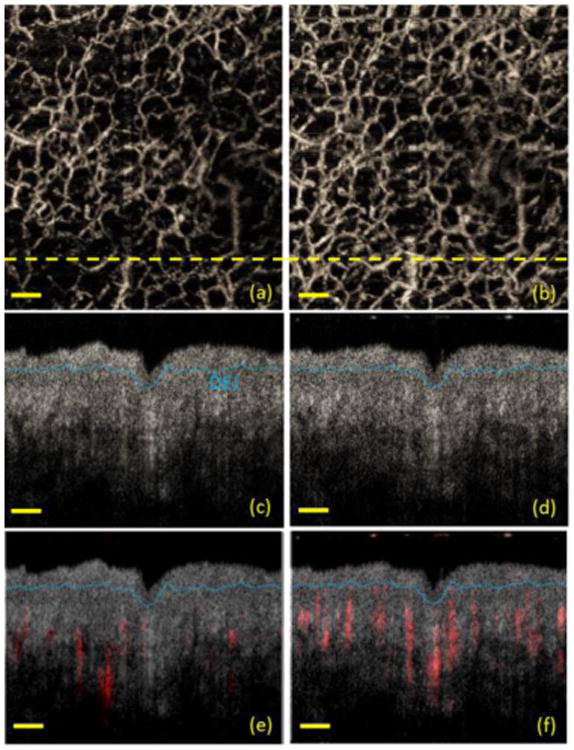
OCT structural image merged with OMAG flow image. Maximal projection view of UHS-OMAG images taken at (a) 15 min and (b) 1 min after tape stripping; (c) and (d) are corresponding OCT structural images taken at the cross section labeled in yellow dash lines in (a) and (b), respectively; (e) and (f) are merged images of the OCT structural image and the OMAG flow image taken at 15 min and 1 min after tape stripping. The OMAG flow information is labeled in red, while the OCT structural image was displayed in grayscale. The blue dash line in (c)-(f) marks the dermal-epidermal junction (DEJ). Scale bar is 0.3 mm.
Progression of various connective tissue diseases, diabetes, and RP are reflected in morphological and functional changes in microvasculature. The ability of visualizing structural alterations of capillaries with RBC velocity mapping within a human finger cuticle might be a critical aid in the treatment or diagnosis of these diseases. The presented imaging modality enables imaging large number of capillaries in one 3D scan which is crucial to decrease subjectivity and to locate the same set of capillaries of patients at each visit. The velocity range is easily adjustable by slightly changing the frame rate [54] for various clinical studies. Moreover, independent of patient's skin characteristics, the cutaneous microcirculation in human finger nail-fold area with different depths can also be investigated which is not possible with alternative methods. We believe there are certain reserve blood vessels existed in the tissue, which are usually not functional but can be activated to supply the needs of affected tissue when stimulation happens. Vascular reserve originates from the description of coronary autoregulation by Mosher in 1964 and the control of coronary blood flow by an autoregulatory mechanism is explained in [57]. The authors claimed that blood vessel reserves exist in healthy subjects, and they identified that coronary blood flow at rest depends on the determinants of myocardial oxygen demand. Previous literature also suggests that the main functions of skin microcirculation are also guaranteed by its functional reserve [58]. Therefore, we believe that the procedure of tape stripping may activate these reserve blood vessels. In order to compare the vessel response within the same area, image registration was performed for images acquired at different time points. The post-registered images shown in Figure 2 represent blood vessels from exactly the same area on the volunteer's finger skin. From the results, we can see that the overall activated reserve blood vessels decreased with time. In addition, the decrease in blood vessel density was not uniform within the imaging area. For example, the area labeled by a yellow circle in Figure 1(b), (c) and (d) showed a more obvious decrease compared to the rest area. This could be due to the non-uniform vessel stimulation caused by tape stripping.
The image registration steps performed in the post-processing is crucial to have a reliable quantitative comparison between the images of the same area in different time points. We also tried our best to keep all the crucial parameters, such as the focus of the probe beam and positioning and orientation of the target to be the same throughout the entire measurement period. Since it is not possible to keep the measurement area and all the above-mentioned parameters to be the same, it is not practical to obtain the basal vessel density before the stripping procedure is applied. Therefore, the imaging area in Figure 1(b)-(d) may not be exactly the same area compared to baseline (Figure 1(a)). Moreover, due to increased discomfort in time significant body movement from the volunteer starts to occur after 15-20 min. Therefore, our monitoring period has been limited to ∼15-20 min after tape stripping. After applying the stripping procedure, it takes ∼1 min to apply oil, put coverslip on, and adjust the focus. Therefore, the first data point that we managed to acquire is 1 min after the tape stripping. From the curves shown in Figure 2, the skin stabilizes at around 15 min after applying 15 times tape stripping, and the vessel density well characterizes the degree of irritation. Other parameters, such as vessel diameter, may also be used to evaluate the degree of stimulation, which may correspond to the degree of inflammation. Moreover, with the newer development of ultrafast swept source OCT system, the imaging time will be significantly reduced, which may promote OCT system to be applied clinically.
From Figure 3, because of the same limitations mentioned above, it was not practical to acquire a comparable baseline Doppler OMAG image before the tape stripping. Based on the results shown in Figure 1-3, we believe that the skin should have already settled down and gone back to its normal physiological condition at 15 min after tape stripping. Therefore, Figure 4(a), (c) and (e) can be considered as baseline images representing normal skin condition. We can see that the blood flow at finger skin was not very active as shown in Figure 4(a) and (e). However, a dramatic increase in the number of functional capillaries and vessels was observed at 1 min after the procedure as shown in Figure 4(b) and (f). This is because at 1 min after the procedure, the skin is still under irritation, where blood vessel responses are still active.
Our results in general show that OMAG imaging technique is capable of monitoring microcirculation responses for skin under inflamed condition. OMAG can also be used to monitor changes in the degree of inflammation. Inflammatory skin conditions such as psoriasis and rosacea are considered to be associated with microvascular modifications [2-3]. Therefore, OMAG has great potential to be applied to investigate for these skin orders. Moreover, tape stripping procedure is heavily involved in drug delivery research because the removal of stratum corneum allows better penetration of the drugs. From our result, there are clear vessel responses associated with tape stripping procedure which may cause different penetration efficiencies when delivering the drug at different time points. Therefore, OMAG may be used to help determine the optimal time point for drug delivery after tape stripping and eventually achieve the best efficacy. In the future, we plan to study inflammatory diseases covering different degrees of inflammation using OMAG to further confirm the capability of our technique in studying inflammatory skin disorders and its related diseases. More quantification parameters/indices will also be derived to better correlate the inflammation/irritation status.
4. Conclusion
In summary, tape stripping is a common procedure used both in clinic and in research. Characterization of the detailed effects especially on the microcirculation changes after tape stripping will help us determine the degree of inflammation caused by the procedure. Our results have shown that OMAG is able to detect microvasculature changes followed by tape stripping, therefore, indicated that the OMAG technique may potentially be used to detect and differentiate between normal and irritated skin. Moreover, the presented results have improved the understanding on the microcirculation dynamics before and after tape stripping. In general, appropriate tape stripping causes only minimal inflammation to human skin. It should be considered as a relatively safe method to study drug delivery and related topics.
Acknowledgments
The work was supported in part by National Institutes of Health grants (R01HL093140, and R01EB009682).
References
- 1.Carmeliet P. Nat Med. 2003;9(6):653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 2.Braverman IM. J Invest Dermatol. 1972;59(1):91–98. doi: 10.1111/1523-1747.ep12625852. [DOI] [PubMed] [Google Scholar]
- 3.Gomaa AH, Yaar M, Eyada MM, Bhawan J. J Cutan Pathol. 2007;34(10):748–753. doi: 10.1111/j.1600-0560.2006.00695.x. [DOI] [PubMed] [Google Scholar]
- 4.Agha-Majzoub R, Becker RP, Schraufnagel DE, Chan LS. Microcirculation. 2005;12(6):455–476. doi: 10.1080/10739680591003297. [DOI] [PubMed] [Google Scholar]
- 5.Yano K, Kajiya K, Ishiwata M, Hong YK, Miyakawa T, Detmar M. J Invest Dermatol. 2004;122(1):201–208. doi: 10.1046/j.0022-202X.2003.22101.x. [DOI] [PubMed] [Google Scholar]
- 6.Brown LF, Harrist TJ, Yeo KT, Ståhle-Bäckdahl M, Jackman RW, Berse B, Tognazzi K, Dvorak HF, Detmar M. J Invest Dermatol. 1995;104(5):744–749. doi: 10.1111/1523-1747.ep12606974. [DOI] [PubMed] [Google Scholar]
- 7.Distler O, Distler JH, Scheid A, Acker T, Hirth A, Rethage J, Michel BA, Gay RE, Müller-Ladner U, Matucci-Cerinic M, Plate KH, Gassmann M, Gay S. Circ Res. 2004;95(1):109–116. doi: 10.1161/01.RES.0000134644.89917.96. [DOI] [PubMed] [Google Scholar]
- 8.Chidlow JH, Shukla D, Grisham MB, Kevil CG. Am J Physiol Gastrointest Liver Physiol. 2007;293(1):G5–G18. doi: 10.1152/ajpgi.00107.2007. [DOI] [PubMed] [Google Scholar]
- 9.Paleolog EM. Arthritis Res. 2002;4(Suppl 3):S81–S90. doi: 10.1186/ar575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansson GK. N Engl J Med. 2005;352(16):1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 11.Ribatti D, Puxeddu I, Crivellato E, Nico B, Vacca A, Levi-Schaffer F. Clin Exp Allergy. 2009;39(12):1815–1821. doi: 10.1111/j.1365-2222.2009.03385.x. [DOI] [PubMed] [Google Scholar]
- 12.Cevc G. Expert Opin Inv Drug. 1997;6(12):1887–1937. doi: 10.1517/13543784.6.12.1887. [DOI] [PubMed] [Google Scholar]
- 13.Escobar-Chavez JJ, Merino-Sanjuán V, López-Cervantes M, Urban-Morlan Z, Piñón-Segundo E, Quintanar-Guerrero D, Ganem-Quintanar A. J Pharm Pharm Sci. 2008;11(1):104–130. doi: 10.18433/j3201z. [DOI] [PubMed] [Google Scholar]
- 14.Bommannan D, Potts RO, Guy RH. J Invest Dermatol. 1990;95(4):403–408. doi: 10.1111/1523-1747.ep12555503. [DOI] [PubMed] [Google Scholar]
- 15.Higo N, Naik A, Bommannan DB, Potts RO, Guy RH. Pharm Res. 1993;10(10):1500–1506. doi: 10.1023/a:1018987612155. [DOI] [PubMed] [Google Scholar]
- 16.Tojo K, Lee ARC. J Invest Dermatol. 1989;92(1):105–108. doi: 10.1111/1523-1747.ep13071314. [DOI] [PubMed] [Google Scholar]
- 17.Escobar-Chávez JJ, Quintanar-Guerrero D, Ganem-Quintanar A. Drug Dev Ind Pharm. 2005;31(4-5):447–454. doi: 10.1080/03639040500214662. [DOI] [PubMed] [Google Scholar]
- 18.Pinkus H, H J Invest Derm. 1951;16:383–386. doi: 10.1038/jid.1951.45. [DOI] [PubMed] [Google Scholar]
- 19.Shah VP. US Dept of Health and Human Services, Rockville. 1998:1–19. [Google Scholar]
- 20.Holzmann S, Tripp CH, Schmuth M, Janke K, Koch F, Saeland S, Stoitzner P, Romanil N. J Invest Derm. 2004;122(5):1165–1174. doi: 10.1111/j.0022-202X.2004.22520.x. [DOI] [PubMed] [Google Scholar]
- 21.Rapp Y, Glickman FS, Frank L. Arch Dermatol. 1963;88(3):257–266. doi: 10.1001/archderm.1963.01590210015002. [DOI] [PubMed] [Google Scholar]
- 22.Huggenberger R, Detmar M. J Invest Dermatol Symp P. 2011;15(1):24–32. doi: 10.1038/jidsymp.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shore AC. Brit J Clin Pharmaco. 2000;50(6):501–513. doi: 10.1046/j.1365-2125.2000.00278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giuggioli D, Manfredi A, Colaci M, Manzini CU, Antonelli A, Ferri C. Autoimmun Rev. 2013;12(11):1058–1063. doi: 10.1016/j.autrev.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 25.Groner W, Winkelman JW, Harris AG, Ince C, Bouma GJ, Messmer K, Nadeau RG. Nat Med. 1999;5(10):1209–1212. doi: 10.1038/13529. [DOI] [PubMed] [Google Scholar]
- 26.De Backer D, Donadello K, Sakr Y, Ospina-Tascon G, Salgado D, Scolletta S, Vincent JL. Crit Care Med. 2013;41(3):791–799. doi: 10.1097/CCM.0b013e3182742e8b. [DOI] [PubMed] [Google Scholar]
- 27.Clark S, Campbell F, Moore T, Jayson M, IV, King TA, Herrick AL. Microvasc Res. 1999;57(3):284–291. doi: 10.1006/mvre.1998.2124. [DOI] [PubMed] [Google Scholar]
- 28.Debbabi H, Bonnin P, Ducluzeau PH, Lefthériotis G, Levy BI. Am J Hypertens. 23(5):541–546. doi: 10.1038/ajh.2010.10. [DOI] [PubMed] [Google Scholar]
- 29.Riordan CL, McDonough M, Davidson JM, Corley R, Perlov C, Barton R, Guy J, Nanney LB. J Burn Care Rehabil. 2003;24(4):177–186. doi: 10.1097/01.BCR.0000075966.50533.B0. [DOI] [PubMed] [Google Scholar]
- 30.Clough GF. J Physiol. 1999;516(2):549–557. doi: 10.1111/j.1469-7793.1999.0549v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ljung P, Bornmyr S, Svensson H. Acta Orthop. 1995;66(1):59–63. doi: 10.3109/17453679508994642. [DOI] [PubMed] [Google Scholar]
- 32.Newton DJ, Khan F, Belch JJF, Mitchell MR, Leese GP. J Foot Ankle Surg. 2002;41(4):233–237. doi: 10.1016/s1067-2516(02)80020-5. [DOI] [PubMed] [Google Scholar]
- 33.Huang D, Swanson E, Lin C, Schuman J, Stinson W, Chang W, Hee M, Flotte T, Gregory K, Puliafito CA. Science. 1991;254(5035):1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tomlins PH, Wang RK. J Phys D Appl Phys. 2005;38(15):2519–2535. [Google Scholar]
- 35.Schmitt JM. IEEE J Sel Top Quant. 1999;5(4):1206–1215. [Google Scholar]
- 36.Wang RK, Ma Z. Opt Lett. 2006;31:3001–3003. doi: 10.1364/ol.31.003001. [DOI] [PubMed] [Google Scholar]
- 37.Gambichler T, Orlikov A, Vasa R, Moussa G, Hoffmann K, Stücker M, Altmeyer P, Bechara FG. J Dermatol Sci. 2007;45(3):167–173. doi: 10.1016/j.jdermsci.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 38.Mogensen M, Nürnberg BM, Forman JL, Thomsen JB, Thrane L, Jemec GBE. Brit J Dermatol. 160(5):1026–1033. doi: 10.1111/j.1365-2133.2008.09003.x. [DOI] [PubMed] [Google Scholar]
- 39.Yano K, Kajiya K, Ishiwata M, Hong YK, Miyakawa T, Detmar M. J Invest Dermatol. 2004;122(1):201–208. doi: 10.1046/j.0022-202X.2003.22101.x. [DOI] [PubMed] [Google Scholar]
- 40.Welzel J, Bruhns M, Wolff HH. Arch Dermatol Res. 2003;295(2):50–55. doi: 10.1007/s00403-003-0390-y. [DOI] [PubMed] [Google Scholar]
- 41.Gambichler T, Boms S, Stücker M, Moussa G, Kreuter A, Sand M, Sand D, Altmeyer P, Hoffmann K. Arch Dermatol Res. 2005;297(5):218–225. doi: 10.1007/s00403-005-0604-6. [DOI] [PubMed] [Google Scholar]
- 42.Themstrup L, Mogensen M, Jemec GBE. 22nd World Congress of Dermatology. 2011 [Google Scholar]
- 43.Wang RK, Jacques SL, Ma Z, Hurst S, Hanson SR, Gruber A. Opt Express. 2007;15(7):4083–4097. doi: 10.1364/oe.15.004083. [DOI] [PubMed] [Google Scholar]
- 44.An L, Qin J, Wang RK. Opt Express. 2010;18:8220–8228. doi: 10.1364/OE.18.008220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jia Y, Li P, Wang RK. J Biomed Opt. 2011;16(9):096019. doi: 10.1117/1.3625238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.An L, Shen TT, Wang RK. J Biomed Opt. 2011;16(10):106013. doi: 10.1117/1.3642638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang RK, An L. Opt Express. 2009;17(11):8926–8940. doi: 10.1364/oe.17.008926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baran U, Shi L, Wang RK. J Biophotonics. 2013 doi: 10.1002/jbio.201300154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jung Y, Dziennis S, Zhi Z, Reif R, Zheng Y, Wang RK. PloS one. 2013;8(2):e57976. doi: 10.1371/journal.pone.0057976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qin J, Jiang J, An L, Gareau D, Wang RK. Laser Surg Med. 2011;43(2):122–129. doi: 10.1002/lsm.20977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jia Y, Wang RK. J Neurosci Meth. 2010;194:108–115. doi: 10.1016/j.jneumeth.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang RK, An L, Francis P, Wilson D. Opt Lett. 2010;35:1467–1469. doi: 10.1364/OL.35.001467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yousefi S, Zhi Z, Wang RK IEEE T. Biomed Eng. 2011;58(8):2316–2323. doi: 10.1109/TBME.2011.2152839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shi L, Qin J, Reif R, Wang RK. J Biomed Opt. 2013;18(10):106015–106015. doi: 10.1117/1.JBO.18.10.106015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reif R, Qin J, An L, Zhi Z, Dziennis S, Wang RK. J Biomed Imaging. 2012;2012:509783. doi: 10.1155/2012/509783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Srinivasan VJ, Sakadžič S, Gorczynska SI, Ruvinskaya S, Wu W, Fujimoto JG, Boas DA. Opt Express. 2010;18(3):2477–2494. doi: 10.1364/OE.18.002477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mosher P, Ross J, Mcfate PA, Shaw RF. Circ Res. 1964;14(3):250–259. doi: 10.1161/01.res.14.3.250. [DOI] [PubMed] [Google Scholar]
- 58.Rossi M, Carpi A. Biomed Pharmacother. 2004;58(8):427–431. doi: 10.1016/j.biopha.2004.08.004. [DOI] [PubMed] [Google Scholar]


