Abstract
A subset of liver transplant (LT) recipients who undergo transplant for hepatocellular carcinoma (HCC) will develop post-operative recurrence. There has yet to be a thorough investigation of donor factors influencing recurrence. Data regarding adult, primary LT recipients with HCC (n=5002) transplanted between January 1, 2006 and September 30, 2010 were extracted from United Network for Organ Sharing (UNOS) database, with subsequent estimation of the cumulative incidence of post-LT recurrence by donor factors. Of the HCC LT recipients, 324 (6.5%) developed recurrence. Analysis of donor characteristics demonstrated a higher cumulative incidence of recurrence within 4 years of transplant among recipients of donors ≥60 years old (versus donors <60 years old) (11.8% versus 7.3%, respectively; p<0.001), and donors from non-local share distribution (versus local share distribution) (10.6% versus 7.4%, respectively; p=0.004). The latter two findings held true on multivariable analysis with HCC recurrence risk increased by 70% for recipients of donor livers ≥60 years old (SHR=1.70, 95% CI 1.31–2.20, p<0.001) and by 42% for recipients of non-local share distribution (SHR=1.42, 95% CI 1.09–1.84, p=0.009) after adjusting for clinical characteristics. In conclusion, consideration of certain donor factors may reduce the cumulative incidence of post-transplant HCC recurrence, and thus improve long-term survival following LT.
Keywords: hepatocellular carcinoma, liver transplantation, recurrence, organ donor
Introduction
Liver transplantation has emerged as the optimal treatment for early stage hepatocellular carcinoma over the last two decades, achieving 5 year survival rates of 75%. (1) This success, fueled by the implementation of Model for End-Stage Liver Disease (MELD) allocation in 2002 and the associated priority listing for patients with HCC within Milan criteria, has resulted in a significant increase in the proportion of patients being listed and subsequently transplanted for HCC. (2)
Given the concern for systematic underreporting of national HCC recurrence data, our group sought to validate the OPTN HCC recurrence data and demonstrated that the observed HCC recurrence rate was not significantly lower than the expected rate at any center. (3) Within the aforementioned study, we demonstrated an increased risk of HCC recurrence or HCC related death in those recipients with increased tumor burden, a history of ablative therapy, an alpha-fetoprotein (AFP) level >500 ng/ml, and interestingly an increased Donor Risk Index (DRI).
With the progress in the application of liver transplantation as treatment for HCC, there has emerged a consistent observation of post-transplant HCC recurrence in up to 20% of recipients. (4) It has been demonstrated that the downstream effect of HCC recurrence is significantly lower rates of survival when compared to HCC liver transplant recipients who do not develop recurrence. (5) There have been many studies identifying or implicating various recipient-associated factors that portend a higher rate of post-transplant recurrence: including the presence of microvascular or macrovascular invasion, tumor size larger than 5 cm, tumor grade, bilobar disease, total number of lesions, and elevated serum α-fetoprotein levels. (4) Sharma et al, utilizing a single center retrospective review, demonstrated that the number of HCC lesions and size of the largest lesion were significant predictors of HCC recurrence. (6) Furthermore, they noted that the median donor age in their cohort of 94 liver transplant recipients was higher in those with HCC recurrence post-transplant (49 years old vs 36 years old, p=0.008) and HCC recurrence risk increased with donor age (adjusted hazard ratio 1.06 per year, p=0.002), representing the only evidence thus far suggesting a donor derived HCC recurrence effect.
As there has yet to be a thorough investigation attempting to understand the full scope of potential donor derived factors contributing to HCC recurrence, this study sought to serve as an initial examination of the potential role of the donor in HCC recurrence following liver transplantation through examination of the national registry.
Methods
Observational data on adults 18 years of age or older assigned an exception for HCC diagnosis meeting policy 3.6.4.4 criteria (Stage T2: one lesion between 2 and 5cm in size, or two lesions between 1 and 3cm in size, with late arterial enhancement on CT or MRI) and receiving a first liver transplant between January 1, 2006 and September 30, 2010, were obtained retrospectively from the United Network for Organ Sharing (UNOS) Standard Transplant Analysis Research (STAR) files (created 03/02/2012) to investigate predictors of post-transplant HCC recurrence. Patients with a cause of death from cholangiocarcinoma (HCC likely misdiagnosed on initial evaluation; n=11) or with laboratory MELD >=22 at transplant (likely other underlying liver complications in addition to HCC; n=25) were excluded from the analysis. As the UNOS dataset does not contain biopsy confirmation of HCC recurrences, the malignancy follow-up data file documenting post-transplant malignancies was utilized under the heading “post-transplant recurrence of pre-transplant malignancy”. Recurrence was defined as either a diagnosis of HCC recurrence or a post-transplant HCC-related death as determined by physician review (JPR) of (a) indication of recurrence in malignancy follow-up data or (b) primary and contributory causes of post-transplant death.
HCC was designated as the primary diagnosis for 34% of patients. To identify the underlying cause of liver disease in these patients, secondary diagnosis at listing and diagnosis at transplant (when secondary diagnosis was unavailable or also HCC) were evaluated. Patients with only a diagnosis of HCC and evidence of viral hepatitis (hepatitis C virus seropositive or hepatitis B virus surface antigen positive) were categorized to their respective viral hepatitis diagnosis.
We described recipient, tumor and donor characteristic with counts (proportions) and medians (interquartile ranges (IQR)) and evaluated differences by donor age (<60 versus >=60 years) and organ sharing (local versus non-local) using the chi-square and Wilcoxon rank sum tests. We calculated tumor volume in cm3 as the volume of a sphere ( * pi * tumor radius3) where the tumor radius was half of the reported tumor size and cumulated the tumor volumes for patients with multiple tumors. We calculated donor risk index (DRI) per Feng, et al. (7) Donor age was dichotomized at 60 years after evaluation of decade of age beyond 40 years showed similar risk of recurrence until age 60 years. Evaluation of organ sharing by local, regional and national categories resulted in similar risk estimates for regional and national share and was therefore dichotomized as local versus non-local (including both regional and national). A 120 day cut-off for time waiting from assignment of HCC exception to transplant was derived from cumulative incidence plots of HCC recurrence by 30 day increments of wait time. An AFP cut-off of 500 ng/uL was used in accordance with studies showing AFP of around 500 to be predictive of poor post-transplant survival (8) and increased waiting-list drop-out. (9)
We imputed missing values within the dataset. Missing cold ischemia time (3.7%) was imputed with the median time by transplant region and share distribution. Donor height and BMI were missing for 2 and 3 observations, respectively, and were imputed with the median by gender. Hepatitis B data were missing for 7 subjects and these patients were categorized to the reference group.
Risk of post-transplant HCC recurrence was evaluated using competing risks regression with the Fine and Gray model. (10) Post-transplant follow-up terminated with the event, HCC recurrence or HCC-related death, the competing risk, death due to other causes, or censoring at last follow-up. Time to event was measured in years from liver transplant to (a) date of diagnosis for HCC recurrence (if reported) or HCC-related death, (b) date of death from non-HCC causes for patients with a competing event, or (c) date of last follow-up for patients alive or lost to follow-up (censored). For patients subsequently receiving a second or third liver transplant, follow-up time was evaluated from the date of first transplant to death, recurrence or last follow-up after re-transplant. Post-transplant follow-up status and date were updated when valid Social Security death certificate master file data were available.
Observed cumulative incidence and 95% confidence intervals (95% CI) of post-transplant HCC recurrence was calculated while accounting for competing risks and evaluated by donor age and organ sharing. Single predictor estimates for risk of post-transplant HCC recurrence (subhazard ratios (SHR)) were first estimated by modeling the cumulative incidence function with competing risks regression for tumor, recipient and donor characteristics. Characteristics with p<0.1 were further evaluated in the multivariable model. The final model included factors where multivariable p values were less than 0.05, and accounted for center-level clustering of outcomes. We evaluated the assumption of proportional sub-distribution hazards and modeled covariates violating the assumption as time-varying covariates (wait time, AFP and diagnosis). We also evaluated potential interactions between donor characteristics (donor age and non-local organ sharing) and clinical characteristics (wait time, tumor size and number, ablation, and AFP) (p>0.05, data not shown).
Data manipulation and analysis were completed in SAS version 9.3 (SAS Institute, Cary, NC). Competing risks regression was completed in Stata/IC 11.1 (StataCorp, College Station, TX). Presented p values are for two-sided tests. This study received approval from the UCSF Committee on Human Research.
Results
The characteristics of the liver transplant recipients with HCC for the time period studied (n=5002) are presented in Table 1. The majority of recipients were male (77.4%), white race (67.4%), with a diagnosis of HCV (62.1%). The median age at transplant was 57, with more than half of the recipients undergoing transplant at a MELD score of 22, representing transplant at the initial application for HCC exception point listing. Only 3.5% of liver recipients received a split liver or living donor liver transplant.
Table 1.
Recipient characteristics of HCC liver transplant recipients.
| Total Population (n=5002) |
Donor Age <60 (n=4162) |
Donor Age ≥60 (n=840) |
Local Share (n=3957) |
Non-local (n=1045) |
|||
|---|---|---|---|---|---|---|---|
|
Recipient characteristics |
n (%) | n (%) | n (%) | p value | n (%) | n (%) | p value |
| Male | 3872 (77.4) | 3251 (78.1) | 621 (73.9) | 0.008 | 3068 (77.5) | 804 (76.9) | 0.68 |
| Ethnicity | |||||||
| White | 3373 (67.4) | 2814 (67.6) | 559 (66.6) | 0.55 | 2657 (67.2) | 716 (68.5) | 0.40 |
| Black | 447 (8.9) | 369 (8.9) | 78 (9.3) | 0.70 | 354 (9.0) | 93 (8.9) | 0.96 |
| Hispanic/Latino | 684 (13.7) | 567 (13.6) | 117 (13.9) | 0.81 | 550 (13.9) | 134 (12.8) | 0.37 |
| Asian | 435 (8.7) | 356 (8.6) | 79 (9.4) | 0.42 | 344 (8.7) | 91 (8.7) | 0.99 |
| Other/multi-race | 63 (1.3) | 56 (1.4) | 7 (0.8) | 0.22 | 52 (1.3) | 11 (1.0) | 0.50 |
| MELD at transplant | |||||||
| 22 | 2666 (53.3) | 2225 (53.5) | 441 (52.5) | 0.61 | 1997 (50.5) | 669 (64.0) | <0.001 |
| 23–24 | 44 (0.9) | 37 (0.9) | 7 (0.8) | 0.87 | 29 (0.7) | 15 (1.4) | 0.03 |
| 25 | 1199 (24.0) | 983 (23.6) | 216 (25.7) | 0.19 | 1031 (26.1) | 168 (16.1) | <0.001 |
| 26–27 | 110 (2.2) | 83 (2.0) | 27 (3.2) | 0.03 | 84 (2.1) | 26 (2.5) | 0.47 |
| 28 | 471 (9.4) | 399 (9.6) | 72 (8.6) | 0.36 | 407 (10.3) | 64 (6.1) | <0.001 |
| 29–30 | 245 (4.9) | 202 (4.8) | 43 (5.1) | 0.74 | 198 (5.0) | 47 (4.5) | 0.50 |
| 31 | 117 (2.3) | 98 (2.4) | 19 (2.3) | 0.87 | 96 (2.4) | 21 (2.0) | 0.43 |
| 32–40 | 150 (3.0) | 135 (3.2) | 15 (1.8) | 0.02 | 115 (2.9) | 35 (3.4) | 0.46 |
| ICU at transplant | 72 (1.4) | 61 (1.5) | 11 (1.3) | 0.73 | 52 (1.3) | 20 (1.9) | 0.15 |
| Diagnosis | |||||||
| HCV | 3108 (62.1) | 2662 (64.0) | 446 (53.1) | <0.001 | 2465 (62.3) | 643 (61.5) | 0.65 |
| Alcoholic cirrhosis | 422 (8.4) | 321 (7.7) | 101 (12.0) | <0.001 | 329 (8.3) | 93 (8.9) | 0.54 |
| Non-cholestatic cirrhosis | 287 (5.7) | 228 (5.5) | 59 (7.0) | 0.08 | 232 (5.9) | 55 (5.3) | 0.46 |
| HBV | 290 (5.8) | 232 (5.6) | 58 (6.9) | 0.13 | 233 (5.9) | 57 (5.4) | 0.59 |
| NASH | 215 (4.3) | 167 (4.0) | 48 (5.7) | 0.03 | 176 (4.4) | 39 (3.7) | 0.31 |
| Other | 680 (13.6) | 552 (13.3) | 128 (15.2) | 0.13 | 522 (13.2) | 158 (15.1) | 0.10 |
| Median (IQR) | Median (IQR) | Median (IQR) | p value | Median (IQR) | Median (IQR) | p value | |
| Age at transplant | 57 (53–62) | 57 (53–62) | 58 (54–64) | <0.001 | 57 (53–62) | 58 (53–63) | 0.16 |
| Waitlist time (days) | 77 (27–158) | 76 (26–157) | 80 (30–168) | 0.37 | 87 (28–165) | 53 (22–119) | <0.001 |
| Post-transplant follow-up (days) | 753 (383–1323) | 756 (385–1367) | 742 (369–1124) | 0.002 | 756 (383–1348) | 745 (381–1188) | 0.21 |
The tumor characteristics of recipients transplanted for HCC are detailed in Table 2. The majority of recipients (81.5%) were transplanted for a single tumor ≥ 2cm or multiple tumors with only 1 tumor ≥ 2cm, and with 98.9% of recipients being within Milan criteria at the time of exception point listing. Ablative therapy was pursued in 43.3% of recipients. Median (IQR) time to transplant from time of exception point listing was 77 (27–158) days. The median (IQR) duration of post-transplant follow up was 2.1 (1.0–3.6) years. During this time, 324 patients (6.5%) were documented to have HCC recurrence.
Table 2.
Tumor characteristics of HCC liver transplant recipients.
| Total Population (n=5002) |
Donor Age <60 (n=4162) |
Donor Age ≥60 (n=840) |
Local Share (n=3957) |
Non-local (n=1045) |
|||
|---|---|---|---|---|---|---|---|
|
Tumor Characteristics |
n (%) | n (%) | n (%) | p value | n (%) | n (%) | p value |
| Tumor number and size | |||||||
| >1 tumor all <2cm | 630 (12.6) | 504 (12.1) | 126 (15.0) | 0.02 | 493 (12.5) | 137 (13.1) | 0.57 |
| 1 tumor ≥2cm or multiple tumors with only 1 ≥2cm | 4077 (81.5) | 3424 (82.3) | 653 (77.7) | 0.002 | 3237 (81.8) | 840 (80.4) | 0.29 |
| 2–3 tumors ≥2cm | 295 (5.9) | 234 (5.6) | 61 (7.3) | 0.06 | 227 (5.7) | 68 (6.5) | 0.35 |
| Milan criteria at exception | 4946 (98.9) | 4117 (98.9) | 829 (98.7) | 0.57 | 3916 (99.0) | 1030 (98.6) | 0.28 |
| Ablative therapy at exception | 2167 (43.3) | 1827 (43.9) | 340 (40.5) | 0.07 | 1727 (43.6) | 440 (42.1) | 0.37 |
| AFP >500 ng/mL at exception | 293 (5.9) | 248 (6.0) | 45 (5.4) | 0.50 | 240 (6.1) | 53 (5.1) | 0.22 |
| HCC recurrence | 324 (6.5) | 244 (5.9) | 80 (9.5) | <0.001 | 236 (6.0) | 88 (8.4) | 0.004 |
| Non-HCC death | 866 (17.3) | 682 (16.4) | 184 (21.9) | <0.001 | 667 (16.9) | 199 (19.0) | 0.10 |
| Median (IQR) | Median (IQR) | Median (IQR) | p value | Median (IQR) | Median (IQR) | p value | |
| Total tumor volume at exception (cm3) | 9.2 (5.6–17.2) | 9.2 (5.6–17.2) | 9.7 (4.8–17.2) | 0.68 | 9.2 (5.6–17.2) | 9.4 (5.6–17.2) | 0.71 |
| Tumor size of main nodule (cm) | 2.4 (2.0–3.1) | 2.4 (2.0–3.1) | 2.3 (1.8–3.0) | 0.02 | 2.4 (2.0–3.1) | 2.4 (2.0–3.1) | 0.99 |
Donor characteristics for the HCC transplanted population are detailed in Table 3. The median [IQR] DRI was 1.37 [1.13–1.68]. The majority of donors were of white race (66%), and had suffered brain death (94.5%). Donors >=60 year old were utilized in 16.8% of the recipients transplanted. Non-local share distribution represented 20.9% of donors utilized. The overall median (IQR) cold ischemic time of 6.5 (5.0–8.2) hours; however, it should be noted that livers from non-local share distribution had longer median [IQR] cold ischemic times than liver grafts distributed from local share (7.7 (6.0–9.5) hours versus 6.2 (5.0–7.9) hours; p<0.001), a higher DRI (1.60 (1.30–1.97) versus 1.33 (1.09–1.61); p<0.001), a higher rate of HCV positive donors (8% versus 3%, p<0.001), and a higher median donor age (45 (29–58) years old versus 42 (26–54) years old; p<0.001). Furthermore, for non-local and local share distribution, there was no significant difference in median (IQR) donor BMI (26.3 (23.3–30.5) versus 26.2 (23.1–30.2); p=0.35), nor DCD utilization (7% versus 5%; p=0.06).
Table 3.
Donor characteristics of transplant recipients with HCC.
| Total Population (n=5002) |
|
|---|---|
| Donor characteristics | n (%) |
| Age >=60 | 840 (16.8) |
| Ethnicity | |
| White | 3300 (66.0) |
| Black | 831 (16.6) |
| Hispanic/Latino | 681 (13.6) |
| Asian | 149 (3.0) |
| Other/Multi-race | 41 (0.8) |
| DCD | 273 (5.5) |
| Partial or split liver | 119 (2.4) |
| Cause of death | |
| Anoxia | 983 (19.7) |
| Other | 137 (2.7) |
| Stroke | 2087 (41.7) |
| Head trauma | 1741 (34.8) |
| Live donors | 54 (1.1) |
| Non-local organ sharing | 1045 (20.9) |
| HBV core positive | 340 (6.8) |
| CDC high risk donor | 470 (9.4) |
| HCV positive | 187 (3.7) |
| BMI | |
| Underweight (BMI <18.5) | 159 (3.2) |
| Normal (BMI 18.5–24.9) | 1833 (36.6) |
| Overweight (BMI 25.0–29.9) | 1679 (33.6) |
| Obese (BMI >30) | 1331 (26.6) |
| Median (IQR) | |
| Height (cm) | 173 (165–180) |
| Cold ischemia time (hours) | 6.5 (5.0–8.2) |
| DRI | 1.37 (1.13–1.68) |
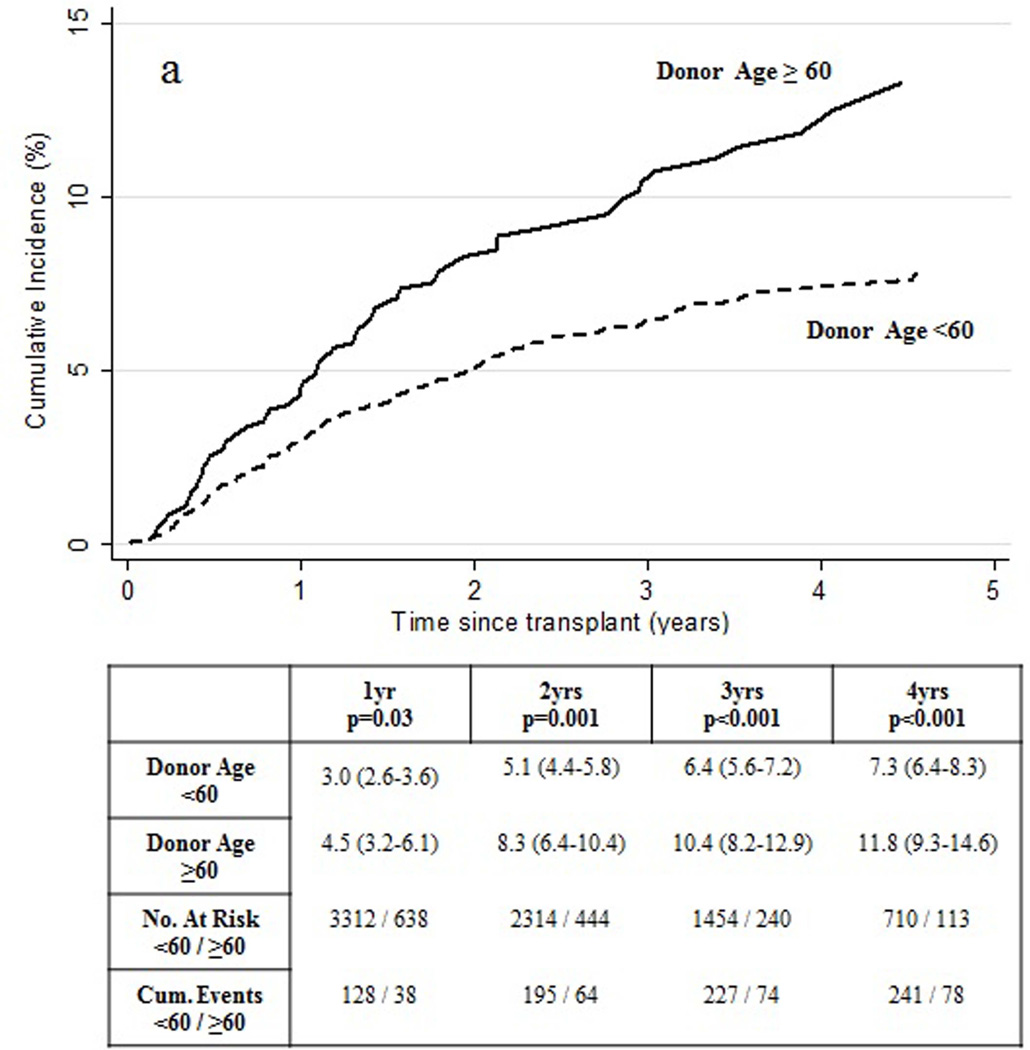
The cumulative incidence of HCC recurrence within 1 and 2 years of transplant were 3.3% (95% CI 2.8–3.8) and 5.6% (95% CI 5.0–6.3). Transplant recipients with a donor ≥60 years of age experienced significantly higher cumulative incidence of HCC recurrence compared to patients receiving a donor <60 years of age (Figure 1a). Comparison of HCC recipient and tumor characteristics for patients who received a donor allograft ≥60 years of age (versus <60 years of age) is shown in Table 1 and Table 2, respectively. Of note, HCC recipients transplanted with a donor allograft ≥60 years of age were more often female (26.1% vs 19.9; p=0.008), diagnosed with alcoholic liver disease (12.0% vs 7.7%; p<0.001) or NASH (5.7% vs 4.0%; p=0.03), but less often diagnosed with HCV (53.1% vs 64.0%; p<0.001). HCC recipients of an older donor allograft had a higher rate of non-HCC deaths (21.9% vs 16.4%; p<0.001). Recipients of older allografts had a smaller median (IQR) size of the main HCC nodule (2.3 cm (1.8–3.0) vs 2.4 cm (2.0–3.1); p=0.02).
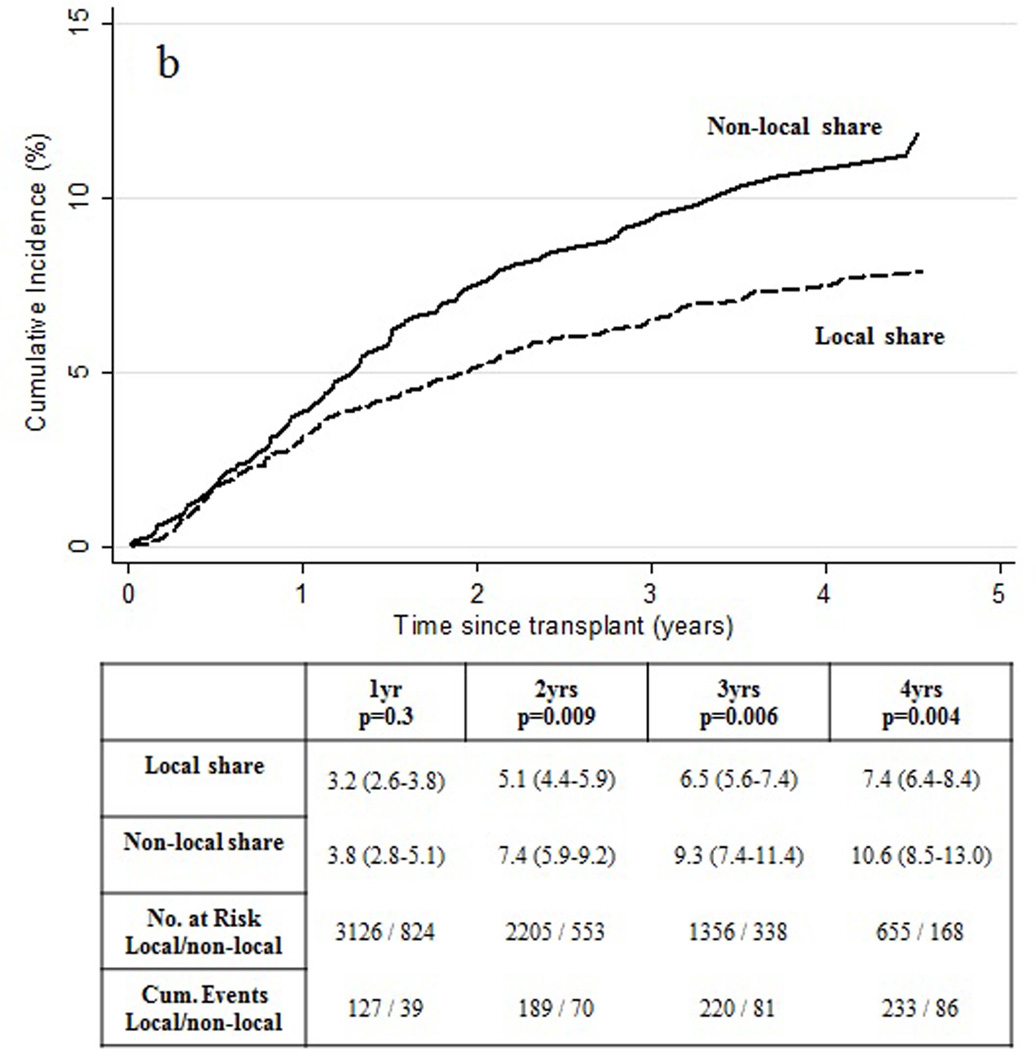
Figure 1.
Observed cumulative incidence of HCC recurrence by (a) donor age and (b) organ share type.
The cumulative incidence of HCC recurrence was also elevated for recipients of non-local, rather than local, organ share distribution achieving statistical significance at 2 years following transplant. (Figure 1b) Comparison of recipient and tumor characteristics based upon share type are shown in Table 1 and Table 2, respectively, and was notable for a shorter median [IQR] time from exception point listing to liver transplant for recipients of non-local share distribution (53 [22–119] days versus 87 [28–165] days; p<0.001).
Univariable and multivariable competing risks regression analyses are demonstrated in Table 4. Risk of post-transplant HCC recurrence was independently associated with donor age and type of organ sharing after adjusting for wait time, tumor size and volume, ablative therapy, AFP >500 ng/mL and diagnosis. Risk of post-transplant HCC recurrence was significantly increased in recipients of donor livers ≥60 years of age (versus <60 years of age) (SHR=1.70, 95% CI 1.31–2.20, p<0.001). This observation was independent of HCV status as in comparison to patients receiving an organ from a donor <60 years of age, the adjusted sub-hazard ratio for risk of recurrence for patients receiving donors >=60 years was 1.82 (95% CI 1.18–2.82; p=0.007) among non-HCV/HCC recipients and 1.62 (95% CI 1.16–2.27; p=0.005) among HCV/HCC recipients. Risk of post-transplant HCC recurrence was also significantly increased in recipients of donor livers of non-local share distribution (versus local share distribution) (SHR=1.42, 95% CI 1.09–1.84, p=0.009). Additional donor factors analyzed, including cold ischemic time and DCD donation, were not found to be associated with an increased risk of HCC recurrence.
Table 4.
Univariable and multivariable subhazard ratios (SHRs) and 95% confidence intervals (95% CI) for risk of post-transplant HCC recurrence with the Fine and Gray competing risks regression model.
| Univariable | Multivariable* | |||||
|---|---|---|---|---|---|---|
| Donor characteristics | SHR | 95% CI | p | SHR | 95% CI | p |
| Age >=60 (versus <60) | 1.66 | 1.28–2.14 | <0.001 | 1.70 | 1.31–2.20 | <0.001 |
| Ethnicity | ||||||
| White | 1 | |||||
| Black | 1.24 | 0.93–1.66 | 0.14 | |||
| Hispanic/Latino | 1.24 | 0.91–1.70 | 0.16 | |||
| Asian | 0.88 | 0.43–1.79 | 0.72 | |||
| Other/Multi-race | 1.64 | 0.62–4.36 | 0.32 | |||
| DCD | 1.14 | 0.71–1.80 | 0.54 | |||
| Partial or split liver (versus whole) | 1.61 | 0.88–2.91 | 0.12 | |||
| Cause of death | ||||||
| Anoxia | 1 | |||||
| Other | 1.26 | 0.64–2.48 | 0.50 | |||
| Stroke | 1.29 | 0.94–1.76 | 0.11 | |||
| Head trauma | 1.02 | 0.73–1.42 | 0.91 | |||
| Live donor (versus cadaveric) | 0.96 | 0.30–3.04 | 0.95 | |||
| Non-local organ sharing (versus local) | 1.45 | 1.13–1.85 | 0.003 | 1.42 | 1.09–1.84 | 0.009 |
| HBV core positive | 0.67 | 0.40–1.13 | 0.13 | |||
| CDC high risk donor | 1.08 | 0.75–1.55 | 0.69 | |||
| HCV positive | 0.77 | 0.40–1.49 | 0.43 | |||
| BMI | ||||||
| Underweight (BMI <18.5) | 1 | |||||
| Normal (BMI 18.5–24.9) | 1.28 | 0.63–2.62 | 0.49 | |||
| Overweight (BMI 25.0–29.9) | 1.25 | 0.61–2.56 | 0.54 | |||
| Obese (BMI >30) | 1.31 | 0.64–2.69 | 0.46 | |||
| Donor height (per cm increase) | 0.996 | 0.99–1.01 | 0.47 | |||
| CIT (per hour increase) | 0.997 | 0.95–1.04 | 0.89 | |||
Adjusted for wait time, tumor volume and size, ablative therapy, AFP>500 ng/mL and diagnosis as well as center clustering.
Discussion
We present here the first examination of the UNOS database evaluating the potential role of donor derived factors in post-transplant HCC recurrence. We demonstrate a higher cumulative incidence of recurrence associated with recipients who receive allografts from donors ≥60 years old, and with donors derived from distribution of non-local share. This donor profile associated with recipient HCC recurrence shares overlap with what have been often classified as expanded criteria donors, representing a broadening of what has previously been considered a usable liver donor. Utilization of these marginal donors has been based upon attempts of maintaining adequate supply for the growing demand of liver transplant candidates or at times expediting a candidate’s time to transplant. While there may be concern that recipients at higher risk for HCC recurrence are biased to receipt of these marginal allografts, our study accounted for potential center differences as well as recipient tumor variables related to recurrence, and still demonstrated that donor age ≥60 years and non-local organ share remained independently associated with HCC recurrence, suggesting a potential role for a donor specific effect.
Rodent models have demonstrated enhanced growth of intrahepatic tumors due to the oxidative stress and hepatocyte injury caused by the process of ischemia/reperfusion. Furthermore, this ischemia/reperfusion induced outgrowth of hepatic metastases has been demonstrated in rodent models to be accentuated in both the aged liver and the steatotic liver, allografts that in clinical transplantation are also associated with increased degree of ischemia/reperfusion injury. (11,12) Our data confirms the negative effect of advanced donor age on HCC recurrence initially observed by Sharma et al (6). We did not observe a difference associated with duration of cold ischemic time, which one could postulate would influence the degree of ischemia/reperfusion. However, we did demonstrate an association between HCC recurrence and organ share type and that there was a significant difference in cold ischemic times between the local share and non-local share distribution cohorts. It is largely believed that organs used for broader sharing reflect use of a more marginal organ, as validated through previous reports documenting a higher DRI associated with nationally versus locally placed livers (2.1 versus 1.4; p<0.001). (13) The latter finding held true in our study population as well (non-local share 1.60 versus local-share 1.33; p<0.001). Although the non-local share donor had a significantly higher median age, when compared to local share donors, this did not appear to have clinical significance (45 years old versus 42 years old; p<0.001). Additional variables analyzed that may lend to the marginal status of the non-local share donor, and/or lend to a potential for increased ischemia/reperfusion injury, including donor BMI and utilization of DCD allografts, did not demonstrate significance when compared to donors of local share. In contrast, there was a higher rate of HCV positive donors in the non-local share donor group. Unfortunately, attempts at comparing degree of macrosteatosis or microsteatosis within the liver grafts were limited by missing data within UNOS, with more than 65% of the population lacking this information. The latter, along with potentially additional donor factors contributing to the marginal nature of the non-local share allograft not captured in our donor analysis, may contribute individually or perhaps collectively to the higher rate of recurrence seen in the non-local share group.
It should be noted that there was no association between HCC recurrence and donor BMI; however, the latter is not a precise surrogate for degree of donor steatosis and thus may limit interpretation of the contribution of graft steatosis in HCC recurrence. Furthermore, our data does not support the recent assertion of a higher rate of recurrence associated with DCD allografts, as has been previously speculated to exist due to an observed inferior patient and graft survival seen in HCC recipients of DCD allografts. (14) Finally, we failed to identify significantly higher cumulative incidence of HCC recurrence among recipients of partial liver allografts (split livers and living donor liver transplants); however, the small number of subjects and events in these subcategories limited our analysis.
Akin to the application of the Milan Criteria for recipient candidate selection, we propose that a component of post-liver transplant outcomes optimization for HCC patients may depend upon improved donor selection. We acknowledge that recipient factors associated with the primary disease burden significantly contribute to the risk for HCC recurrence, as has been extensively investigated previously. However, despite controlling for potential recipient tumor variables that portend a higher rate of HCC recurrence, our data demonstrate a potential role for donor derived factors in HCC recurrence following liver transplantation.
The role of a donor profile influencing post-operative recurrence of disease has been previously demonstrated in regards to the influence of liver donor age in HCV positive recipients. Grafts from donors with advanced age have been shown to result in more rapid HCV fibrosis progression post-transplant - correlating with a subsequent decrease in graft and patient survival. (15,16) Indeed, the latter association has resulted in adoption of donor-age restriction policies for HCV positive recipients at the authors’ institutions in order to optimize outcomes in the HCV positive recipient population. Of note, the data presented here demonstrate a significantly reduced percentage of older donor utilization for the HCV positive recipient population. Application of the same type of donor restrictions for candidates with HCC may potentially allow for not only a reduction in the observed rate of post-transplant HCC recurrence, but also improved post-transplant survival. One could speculate that a more limited donor pool may result in prolongation in the waitlist time for candidates with HCC; however, recent data has shown that a donor restriction policy for HCV positive waitlisted patients in Region 5 was not associated with longer waiting times or a higher risk of wait-list mortality. (17) Utilization of an “ablate and wait” strategy for HCC candidates (18), in addition to optimal donor selection, may allow for a selection benefit to minimize the risk of post-transplant HCC recurrence and optimize long-term survival. Indeed, recent data has demonstrated a 40% reduction in recurrence within the first year following transplant for those HCC candidates with a wait time >120 days. (19)
This study is an observational, retrospective review utilizing a national registry database. The assimilated data were not collected for the sole purpose of investigating HCC recurrence, posing a limitation of this study. Reporting HCC recurrence to UNOS/OPTN is not mandated likely resulting in misclassification of some HCC recurrences to the competing event (non-HCC death) and underestimation of the cumulative incidence of HCC recurrence. However, we expect this misclassification occurs at random such that reporting of HCC recurrence is not systematic by donor age or donor share. Therefore, differences in HCC recurrence by donor factors should be valid but our risk ratio estimates are likely attenuated. This misclassification reduces our power to detect differences in HCC recurrence by donor subgroups. Although there is the potential for inaccuracies within the UNOS database, our group has specifically validated the OPTN HCC recurrence data to exclude systematic under- and over-reporting by centers. (3) Additional limitations are represented by the absence of specific information regarding the type and timing of the HCC recurrence, as this information would help in potentially differentiating between de novo disease and true recurrence.
In summary, we demonstrate that donors of older age (≥60 years old) and of non-local share distribution, when utilized for HCC liver transplant candidates, may portend a higher cumulative incidence of post-transplant HCC recurrence. Further investigation into the potential tumor enhancing effects associated with donor factors is warranted, as this may potentially allow a further reduction in the cumulative incidence of HCC recurrence, and thus improved long-term survival following liver transplantation.
Acknowledgements
This work was supported by the Biostatistics Core of the UCSF Liver Center (P30 DK026743).
Abbreviations
- AHN
acute hepatic necrosis
- CDC
Center for Disease Control
- CIT
cold ischemic time
- DCD
donation after cardiac death
- DRI
donor risk index
- ESLD
end-staged liver disease
- HBV
Hepatitis B Virus
- HCC
hepatocellular carcinoma
- HCV
Hepatitis C Virus
- LT
liver transplant
- MELD
Model for End-Staged Liver Disease
- NT
no transplant
- PBC
primary biliary cirrhosis
- PSC
primary sclerosing cholangitis
- SHR
subhazard ratio
Footnotes
Disclosures: The authors have no conflicts of interest to declare.
References
- 1.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;11:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 2.Massie AB, Caffo B, Gentry SE, Hall EC, Axelrod DA, Lentine KL, et al. MELD Exceptions and Rates of Waiting List Outcomes. Am J Transplant. 2011 Nov;11(11):2362–2371. doi: 10.1111/j.1600-6143.2011.03735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samoylova ML, Dodge JL, Vittinghoff E, Yao FY, Roberts JP. Validating posttransplant hepatocellular carcinoma recurrence data in the united network for organ sharing database. Liver Transpl. 2013;19:1318–1323. doi: 10.1002/lt.23735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zimmerman MA, Ghobrial RM, Tong MJ, Hiatt JR, Cameron AM, Hong J, et al. Recurrence of hepatocellular carcinoma following liver transplantation: a review of preoperative and postoperative prognostic indicators. Arch Surg. 2008 Feb;143(2):182–188. doi: 10.1001/archsurg.2007.39. [DOI] [PubMed] [Google Scholar]
- 5.Roayaie S, Schwartz JD, Sung MW, Emre SH, Miller CM, Gondolesi GE, et al. Recurrence of hepatocellular carcinoma after liver transplant: patterns and prognosis. Liver Transpl. 2004;10(4):534–540. doi: 10.1002/lt.20128. [DOI] [PubMed] [Google Scholar]
- 6.Sharma P, Welch K, Hussain H, Pelletier SJ, Fontana RJ, Marrero J, et al. Incidence and risk factors of hepatocellular carcinoma recurrence after liver transplantation in the MELD era. Dig Dis Sci. 2012 Mar;57(3):806–812. doi: 10.1007/s10620-011-1910-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2006;6(4):783–790. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 8.Ioannou GN, Perkins JD, Carithers RL., Jr Liver transplantation for hepatocellular carcinoma: impact of the MELD allocation system and predictors of survival. Gastroenterology. 2008;134(5):1342–1351. doi: 10.1053/j.gastro.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Washburn K, Edwards E, Harper A, Freeman R. Hepatocellular Carcinoma Patients Are Advantaged in the Current Liver Transplant Allocation System. Am J Transplant. 2010;10(7):1643–1648. doi: 10.1111/j.1600-6143.2010.03127.x. [DOI] [PubMed] [Google Scholar]
- 10.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc. 1999;94(446):496. [Google Scholar]
- 11.van der Bilt JD, Kranenburg O, Nijkamp MW, Smakman N, Veenendaal LM, Te Velde EA, et al. Ischemia/reperfusion accelerates the outgrowth of hepatic micrometastases in a highly standardized murine model. Hepatology. 2005 Jul;42(1):165–175. doi: 10.1002/hep.20739. [DOI] [PubMed] [Google Scholar]
- 12.van der Bilt JD, Kranenburg O, Borren A, van Hillegersberg R, Borel Rinkes IH. Ageing and hepatic steatosis exacerbate ischemia/reperfusion-accelerated outgrowth of colorectal micrometastases. Ann Surg Oncol. 2008 May;15(5):1392–1398. doi: 10.1245/s10434-007-9758-0. [DOI] [PubMed] [Google Scholar]
- 13.Lai JC, Roberts JP, Vittinghoff E, Terrault NA, Feng S. Patient, center and geographic characteristics of nationally placed livers. Am J Transplant. 2012 Apr;12(4):947–953. doi: 10.1111/j.1600-6143.2011.03962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Croome KP, Wall W, Chandok N, Beck G, Marotta P, Hernandez-Alejandro R. Inferior survival in liver transplant recipients with hepatocellular carcinoma receiving donation after cardiac death liver allografts. Liver Transpl. 2013 Nov;19(11):1214–1223. doi: 10.1002/lt.23715. [DOI] [PubMed] [Google Scholar]
- 15.Berenguer M, Prieto M, San Juan F, Rayon JM, Martinez F, Carrasco D, et al. Contribution of donor age to the recent decrease in patient survival among HCV-infected liver transplant recipients. Hepatology. 2002 Jul;36(1):202–210. doi: 10.1053/jhep.2002.33993. [DOI] [PubMed] [Google Scholar]
- 16.Wali M, Harrison RF, Gow PJ, Mutimer D. Advancing donor liver age and rapid fibrosis progression following transplantation for hepatitis C. Gut. 2002 Aug;51(2):248–252. doi: 10.1136/gut.51.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flemming JA, Vagefi PA, Freise CE, Yao FY, Terrault NA. Restricting liver transplant recipients to younger donors does not increase wait-list time or drop-out rate: The hepatitis C experience. Liver Transpl. doi: 10.1002/lt.23937. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts JP, Venook A, Kerlan R, Yao F. Hepatocellular carcinoma: Ablate and wait versus rapid transplantation. Liver Transpl. 2010 Aug;16(8):925–929. doi: 10.1002/lt.22103. [DOI] [PubMed] [Google Scholar]
- 19.Samoylova ML, Dodge JL, Yao FY, Roberts JP. Time to transplantation as a predictor of hepatocellular carcinoma recurrence after liver transplantation. Liver Transpl. doi: 10.1002/lt.23902. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]