Abstract
Introduction
A custom designed HD exposure system was used to deliver controlled inhaled doses to an animal model through an endotracheal tube.
Methods
Target HD vapor challenges were generated by a temperature controlled bubbler/aerosol trap, while concentration was monitored near real-time by gas chromatography. Animal breathing parameters were monitored real-time by an in-line pneumotach, pressure transducer, and Buxco pulmonary analysis computer/software. For each exposure, the challenge atmosphere was allowed to stabilize at the desired concentration while the anesthetized animal was provided humidity controlled clean air. Once the target concentration was achieved and stable, a portion of the challenge atmosphere was drawn past the endotracheal tube, where the animal inhaled the exposure ad libitum. During the exposure, HD vapor concentration and animal weight were used to calculate the needed inhaled volume to achieve the target inhaled dose (μg/kg). The exposures were halted when the inhaled volume was achieved.
Results
The exposure system successfully controlled HD concentrations from 22.2 to 278 mg/m3 and accurately delivered inhaled doses between 49.3 and 1120 μg/kg with actual administered doses being within 4% of the target level.
Discussion
This exposure system administers specific HD inhaled doses to evaluate physiological effects and for evaluation of potential medical countermeasure treatments.
Keywords: Dosimetry, Inhalation exposure, Inhaled dose, Methods, Sulfur mustard vapor
1. Introduction
Sulfur mustard (HD) is a vesicant agent that has been and still is a major military threat since it was first used in World War I. Commonly known as “mustard gas”, HD is a liquid at room temperature. Liquid or vapor contact to skin or mucous membranes induces blistering and necrosis. Signs and symptoms are delayed (appearing hours after exposure) and include dermal (skin erythema and blistering), respiratory (cough, dyspnea, pneumonitis, and acute lung injury), ocular (conjunctivitis and burns), and gastrointestinal (vomiting) effects (Centers for Disease Control and Prevention [CDC], 2013; U.S. Army Medical Research Institute of Chemical Defense [USAMRICD], 2007).
Although primarily referred to as an incapacitating agent, inhaled HD vapor exposure can cause life-threatening injuries. The extent of lung injury depends on the exposure concentration and duration, with higher exposures leading to earlier onset and greater severity of the toxic effects (CDC, 2013; USAMRICD, 2007; Eisenkraft, Tashma, & Laria, 2003). Early respiratory effects (4–6 hours after exposure) include inflammation of mucous membranes of the respiratory tract. Later, the respiratory epithelium becomes necrotic, resulting in epithelial sloughing, pseudomembrane formation, and airway occlusion. These respiratory effects were documented in Iranian veterans exposed to HD during the Iran-Iraq conflict in 1980–1988 (Balali-Mood & Hefazi, 2006).
There have been a limited number of HD inhalation studies. The effect of inhaled HD on dog lung was studied by Winternitz and Finney (1920), where they found HD-exposed lung tissue lost elasticity, formation of membrane casts in the bronchial tree, and epithelial necrosis of the upper respiratory passages. Cameron, Gaddum, and Short (1946) found that 80–90% of HD was absorbed in the oral-nasal region of the respiratory tract in rabbits and monkeys; inducing inflammation associated with hemorrhage and necrosis of lymphoid tissue. Box and Cullumbine (1947) found a quantitative relationship between inhaled dose and survival time in the mouse model. Lung lavage fluid from rats that received HD inhalation exposures was biochemically analyzed by Cowan, Anderson, Broomfield, Byers, and Smith (1997), where they found time-dependent increases in proteolytic activity. Anderson et al. (1996) described the pathogenesis of HD-induced lesions in the rat respiratory tract. More recent studies in the pig model, Fairhall et al. (2010) found significant changes in shunt fraction from 3 to 6 hours post-exposure, increased hypoxia, respiratory acidosis, and pathological finding of necrosis and erosions in the tracheal epithelium.
There currently is no established antidote for inhalational HD exposure (USAMRICD, 2007; Papirmeister, Feister, Robinson, & Ford, 2000) though several therapeutic approaches are currently under investigation (Papirmeister et al., 2000; Anderson, Byers, & Vesely, 2000; Anderson, Taylor, Fetterer, & Holmes, 2009; O’Neill, et al., 2010; O’Neill, et al., 2011; Rancourt et al., 2012; Veress, et al., 2013; Jugg et al., 2013). In order to properly evaluate the efficacy of potential treatments for this route of exposure, animal models that accurately replicate the physiological injuries induced by HD inhalation are needed. Consequently, the need for an exposure system that can precisely and reproducibly deliver HD by the inhalation route is critical to this goal. For inhalation testing, it is critical to have stable HD vapor concentration control and at least near real-time concentration monitoring capabilities to verify consistent HD exposure conditions. In addition, animal respiratory parameters during HD exposures must be monitored real-time to ensure accurate inhaled dose delivery. Described here is a novel inhalation exposure system developed to deliver a controlled inhaled dose of HD vapor through an endotracheal tube to a mid to large sized animal model to evaluate potential medical countermeasures.
2. Methods
2.1. Animals
This exposure system was designed to be used for any non-rodent/non-obligate nasal breather animal models. The inhalation exposure system evaluation data presented here is for a mid to large size animal model (10 to 25 kg). Due to contractual and nondisclosure restrictions, the specific animal model used during the development of this system cannot be disclosed
Animals were quarantined in accordance with (IAW) Battelle Standard Operating Procedure (SOP) timelines and screened for disease prior to use. The animals were maintained under Battelle animal care and use program accredited by the Association for Assessment and Accreditation for Laboratory Animal Care International. This care and use program was in accordance with guidelines set forth in the “Guide for the Care and Use of Laboratory Animals”, National Research Council, and/or the regulations and standards promulgated by the Agricultural Research Service, United States Department of Agriculture (USDA), pursuant to the Laboratory Animal Welfare Act of August 24, 1966, as amended. The Battelle Institutional Animal Care and Use Committee at Battelle, Columbus, OH approved the experimental protocol. Animals were maintained on a 12-h light/dark cycle with no twilight. Air temperature in animal rooms was maintained within a 16 to 27°C range, with relative humidity maintained between 30 and 70 percent. The animals were fed twice daily (PMI Feeds, Inc.; St. Louis, MO), water was provided ad libitum, and housed individually IAW Battelle SOP.
Anesthesia in preparation for HD inhalation exposure was accomplished by the intramuscular (IM) administration of a 0.044 mL/kg mixture of tiletamine (250 mg) and zolazepam (Telazol®, 250 mg) reconstituted with 5 mL of a 100 mg/mL xylazine hydrochloride solution. The animals were orally intubated with a 5.0 mm cuffed endotracheal tube. The placement of the endotracheal tube was confirmed via endoscope to ensure the tube was positioned anterior to the carina.
Animals surviving to the end of the 24 hours post-exposure observation period were humanely euthanized with Fetal Plus (Vortech Pharmaceuticals, Dearborn, MI) administered via intravenous (IV) injection.
2.2. Sulfur Mustard (HD)
HD (96% pure) was acquired through the U.S. Army Edgewood Chemical and Biological Center (Aberdeen Proving Ground, MD) as part of an Interagency Agreement between the National Institutes of Health (NIH) and the Department of Defense (DOD). Table 1 lists selected HD information.
Table 1.
Selected HD information
2.3. Exposure system
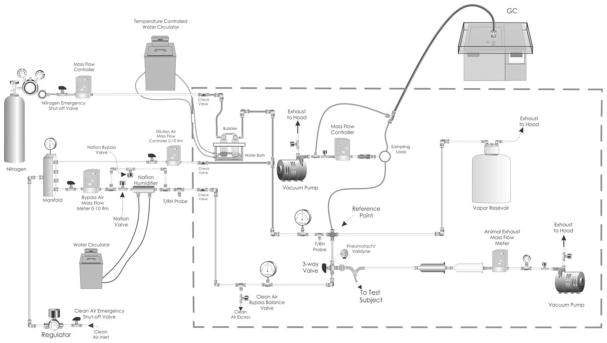
Briefly, the exposure system was designed to deliver controlled inhaled doses to a mid to large size animal model through a cuffed endotracheal tube. Target HD vapor challenges were generated by a temperature controlled bubbler/aerosol trap, while the vapor concentration was monitored near real-time via gas chromatography. Animal breathing parameters (respiratory rate, tidal volume, and minute volume) were monitored real-time via an in-line pneumotach, pressure transducer, and Buxco pulmonary analysis computer/software. For each exposure, the HD concentration was allowed to stabilize at the desired concentration while the anesthetized animal was provided humidity controlled clean air. Once the target concentration was achieved and stable, a portion of the HD vapor was drawn past the endotracheal tube, where the animal inhaled the exposure ad libitum. During the exposure, HD vapor concentration measurements and animal weight were used to calculate the needed inhaled volume to achieve the target inhaled dose. A schematic of the exposure system is presented in Figure 1 and the procedures are summarized below.
Fig. 1.
Schematic of the HD vapor inhalation exposure system.
2.3.1. HD vapor generation
HD vapor was generated by metering nitrogen through a custom bubbler (Fig. 2) containing 1.5 to 5 mL of liquid HD. A temperature controlled water bath maintained the bubbler at the desired temperature (between 35 and 65°C), while any generated aerosols were removed by the aerosol trap and heat traced delivery lines prevented vapor condensation. Target vapor concentrations were achieved by controlling bubbler temperature. Bubbler flow rate was 0.35 L/min and the total source flow rate was 27 L/min.
Fig. 2.

Custom bubbler required less than 5 mL HD to generated controlled vapor concentrations.
The theoretical basis of vapor generation approach was to generate near-saturated HD vapor concentrations from the temperature controlled bubbler, which could then be diluted to the target exposure concentration. In this approach, dry carrier gas (nitrogen) bubbled through the liquid HD becomes saturated with HD vapor in the process. The degree of bubble saturation depended upon bubble size, vapor diffusivity, and the residence time of the bubbles in the liquid (Mayer, Collins, & Walton, 2001). Under ideal conditions, the HD vapor concentration exiting the bubbler would be near the saturated vapor concentration (or volatility) associated with the water bath temperature.
To estimate the HD saturated vapor concentration, the HD vapor pressure was calculated using Antoine’s equation (below) and constants (Table 1), where Pv is the vapor pressure and T is the temperature in degrees Celsius.
An example calcualtion at 35°C is shown below:
The calculated HD vapor pressures are shown in Figure 3, with the lower and upper bubbler temperature lines corresponding with the temperature range used to generate the HD vapor (35 to 65°C, respectively).
Fig. 3.
HD vapor pressure vs. temperature plot (calculated by Antoine’s equation).
2.3.2. HD exposure concentration monitoring
Challenge concentration was monitored at the reference point (Fig. 1) near real-time using a Hewlett Packard 5890 Series II gas chromatogram (Palo Alto, CA) equipped with a flame ionization detector (GC/FID) and Restek RTX-5 column (15m × 0.32mm × 0.53μm; Bellefonte, PA). The oven temperature program was 40°C for 0.5 min followed by 20°C/min to 160°C (6.5 min run time). An inline six-port gas sampling loop (Valco Instruments Co. Inc., Houston, TX) captured challenge samples (0.57 or 1.0 mL) to quantify the HD concentration. The 0.57 mL sampling loop was used during high concentration (nominally > 200 mg/m3) exposures, while the 1.0 mL sampling loop was used during the low concentration (nominally 20 to 40 mg/m3) exposures. Figure 4 illustrates a representative calibration curve used during the low HD-concentration exposures.
Fig. 4.
Representative GC/FID calibration curve.
2.3.3. Respiratory monitoring
Animal breathing parameters were monitored real-time via an in-line pneumotach (Series 4500, Hans Rudolph Inc., Shawnee, KS), pressure transducer (DP45, Validyne Engineering Corp., Northridge, CA), signal amplifier (Buxco Max II, DSI, St. Paul, MN), and pulmonary analysis computer/software (Version 2.5.0 Buxco Biosystem XA, DSI, St. Paul, MN). System calibration was performed using a 500 mL gas-tight syringe followed by 50 mL calibration checks at the beginning of every exposure day. The in-line pneumotach is shown in the Figure 5 photograph (pressure transducer not shown). Real-time respiratory data and HD vapor concentration data were used to ensure animals received their target inhaled dose.
Fig. 5.
Exposure system photograph showing in-line pneumotach.
2.3.4. Exposure procedures
Prior to loading the animal into the system, the HD vapor concentration was permitted to stabilize at the target concentration. When the HD concentration was stable, the anesthetized animal was positioned within the chemical fume hood in dorsal recumbency and the endotracheal tube secured to a “Y” fitting downstream from the pneumotach. Clean humidified air (50 – 60% relative humidity) was drawn through the “Y” fitting and the pneumotach before and after exposure. The exposure start time was defined as the time when the three -way valve was switched, diverting a portion of the HD vapor flow through the pneumotach and “Y” fitting. During the exposure, HD vapor concentration measurements and animal weight were used to calculate the needed inhaled volume to achieve the target inhaled dose (mg/kg). Once the inhaled volume was reached (monitored real-time by the Buxco system), the exposure was terminated by switching the three-way valve to clean air. A post-exposure air wash-out was carried out for a minimum of 10 minutes.
2.4. Inhaled dose calculation
The GC/FID data was used to document the HD vapor concentration while the Buxco respiratory data documented the animal breathing parameters. The mean HD concentration was calculated from the GC/FID results just prior to the start of exposure, results during the exposure, and the next result after the exposure was stopped. Total inhaled dose (mg/kg) was calculated from the mean HD vapor concentration, total inhaled volume, and animal weight using the following equation.
An example calcualtion a low HD-concentration exposure is shown below:
3. Results
3.1. Exposure system characterization
3.1.1. HD vapor delivery efficiency
To assess delivery efficiency prior to animal testing, the HD concentration at the reference point was compared to the HD concentration at the “Y” fitting connected to the endotracheal tube (Fig. 1, 5). HD concentration was monitored at the reference point until it was stable. Once stable, the concentration was monitored by alternating between the “Y” fitting and the reference point. To simulate animal breathing, a 50 mL syringe was connected to the “Y” fitting and manually actuated. A two-tailed t-test analysis of the concentration results provided a p-value > 0.3, indicating that the reference point concentration was not significantly different than the concentration delivered to the animal.
3.1.2. HD vapor concentration stability and control
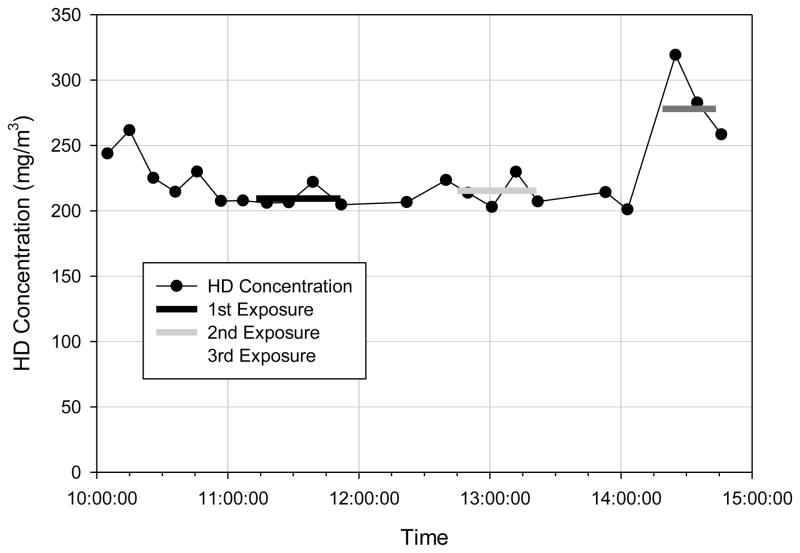
Once the bubbler water bath reached the desired temperature, the HD vapor concentration was shown to be stable within 30 minutes. The concentration was probably stable earlier, but it required 30 minutes to obtain three measurements on the GC/FID (6.5 minute run time + oven cool down time). The system successfully generated controlled exposure concentrations between 22 and 278 mg/m3 that were stable up to 5 hours. A representative HD concentration profile during low HD-concentration exposures is shown in Figure 7, while a profile for high HD-concentration exposures is shown in Figure 8.
Fig. 7.
Representative concentration profile during low HD-concentration exposures.
Fig. 8.
Representative concentration profile during the high HD-concentration exposures.
3.2. Animal exposures
3.2.1 Respiratory monitoring
During 18 HD exposure tests, real-time respiratory data was used to track the total inhaled volume to determine when to stop the exposure (procedure described above). Breathing frequency, tidal volume, and minute volume results are summarized in Table 2. Representative respiratory data is shown in Figure 9.
Table 2.
Respiratory monitoring results (N=18)
| Parameter | Mean (1 SD) | Range |
|---|---|---|
| Frequency (breaths/min) | 55.1 (20.6) | 21.5 – 96.6 |
| Tidal Volume (mL/breath) | 54.2 (17.3) | 28.8 – 84.4 |
| Minute Ventilation (mL/min) | 2680 (553) | 1700 – 3780 |
Fig. 9.
Representative respiratory data.
3.2.2 HD exposures
Mean HD vapor concentrations ranged from 22.2 to 278 mg/m3, exposure durations ranged from 12.6 to 49.3 minutes, and inhaled doses ranged from 49.3 to 1120 μg/kg.
Target inhaled doses were 50, 100, 150, 175, 200, and 1100 μg/kg. During the low HD-dose exposures (50 to 200 μg/kg), animals were exposed to mean HD vapor concentrations ranging from 22.2 to 38.4 mg/m3, exposure durations ranged from 12.6 to 41.8 minutes, and inhaled doses ranged from 49.3 to 197.9 μg/kg. During the high HD-dose exposures (target inhaled dose of 1100 μg/kg), animals were exposed to mean HD vapor concentrations ranging from 220 to 278 mg/m3, exposure durations from 24.5 to 38.9 minutes, and inhaled doses from 1080 to 1120 μg/kg. All inhaled doses were within 4% of the target. Table 3 summarizes the individual animal exposure results and Table 4 summarizes inhaled dose results for each target dose level. There were no significant differences among the target dose levels (p=0.6005) in a one-way analysis of variance (ANOVA) of inhaled dose expressed as a percentage of target dose. The model-estimated coefficient of variance was 1.53%.
Table 3.
Individual animal exposure results
| Target Dose (μg/kg) | Wt (kg) | Mean HD Conc (mg/m3) | Inhaled Vol (L) | Exposure Dur (min) | Inhaled Dose (μg/kg) | Percent of Target Dose |
|---|---|---|---|---|---|---|
| 50 | 13.4 | 22.7 | 29.2 | 13.0 | 49.3 | 98.6% |
| 100 | 15.7 | 22.2 | 70.4 | 32.1 | 99.5 | 99.5% |
| 11.3 | 35.3 | 32.5 | 15.6 | 101 | 101% | |
| 11.4 | 35.7 | 32.1 | 12.6 | 100 | 100% | |
| 19.6 | 32.6 | 61.6 | 17.0 | 102 | 102% | |
| 150 | 13.2 | 28.4 | 70.6 | 41.8 | 152 | 101% |
| 13.9 | 28.6 | 71.0 | 22.8 | 147 | 97.7% | |
| 12.2 | 34.7 | 52.7 | 23.2 | 150 | 100% | |
| 12.3 | 33.6 | 55.7 | 14.7 | 152 | 101% | |
| 175 | 12.4 | 30.5 | 71.3 | 25.8 | 175 | 99.9% |
| 13.6 | 30.8 | 76.3 | 30.3 | 174 | 99.2% | |
| 13.1 | 34.8 | 66.2 | 27.5 | 176 | 101% | |
| 12.8 | 33.6 | 64.5 | 19.2 | 169 | 96.7% | |
| 200 | 13.0 | 37.1 | 69.5 | 23.1 | 198 | 99.0% |
| 14.5 | 38.4 | 73.9 | 27.0 | 196 | 98.1% | |
| 1100 | 19.3 | 209 | 103 | 38.9 | 1120 | 101% |
| 20.2 | 215 | 104 | 36.4 | 1110 | 101% | |
| 13.4 | 278 | 51.9 | 24.5 | 1080 | 97.9% |
Table 4.
Summary of inhaled dose results for each target dose level
| Inhaled HD Dose (μg/kg) | Percent difference from Target | N | |
|---|---|---|---|
| Target | Mean Achieved (SD) | ||
| 50 | 49.3 (−) | −1.4% | 1 |
| 100 | 101 (1.3) | 1.0% | 4 |
| 150 | 150 (2.6) | 0.2% | 4 |
| 175 | 173 (2.9) | −0.9% | 4 |
| 200 | 197 (1.2) | −1.5% | 2 |
| 1100 | 1101 (21) | 0.1% | 3 |
4. Discussion
The objective of this effort was to develop a novel HD exposure system capable of administering accurate inhaled doses to a mid to large sized animal model. The HD exposure system functioned as designed and all performance objectives were achieved or exceeded. Eighteen animals were successfully exposed to controlled inhaled HD doses ranging from 49.3 to 1120 μg/kg, with actual administered doses being within 4% of the target level.
During the exposure system development phase, the HD vapor generation approach and system flow rates were adjusted to achieve the desired performance capabilities. Specifically, the custom vapor generation approach was developed to provide a controlled and stable HD vapor concentration between 20 and 200 mg/m3 using 1.2 to 5 mL of HD liquid. In addition, challenge flow and bias flow rates were increased to prevent animal rebreathing of the challenge atmosphere.
The HD vapor generation approach and equipment required two modifications before the final bubbler configuration was identified. The initial approach identified the diffusion cell as a potential HD vapor generation approach. Upon further investigation of diffusion cell theory, it was determined that this approach would not have sufficient HD output to achieve the needed HD exposure concentrations at the target challenge air flow rate (initially set at 10 L/min).
A bubbler was selected as the optimum method for generating the higher HD vapor concentrations. Based on theory, a temperature controlled bubbler can generate near saturated vapor concentrations which can be diluted to the target exposure concentrations. This approach was successfully used in recent testing at Battelle’s Hazardous Materials and Research Center (unpublished data). The initial bubbler approach included a midget impinger bottle (Model 7531-02, Ace Glass, Inc., Vineland, NJ) and low jet velocity stem (Model 7533-08, Ace Glass, Inc.) followed by an aerosol trap with ball/socket connections. Due to HD volume constraints, the use of commercially available midget bubblers (e.g., Model 7532-06, Ace Glass, Inc.) was not feasible. These bubblers require >10 mL of liquid to cover the sparger in order to effectively generate HD vapor. The jet stem was selected because it formed bubbles below the HD liquid level. However, test results indicated that the jet stem generated bubbles too large for efficient HD vapor generation.
To improve the HD vapor generation efficiency, a custom bubbler stem was fabricated (PhotoVac Laser Corp., Grove City, OH) to generate smaller bubbles and longer bubble transition time in the limited HD volume. The stem design had a wider diameter and a fritted end with all other dimensions and connections remaining the same as the initial bubbler setup (Fig. 2). During the initial assessment of the custom bubbler (using water), small bubbles were found to coalesce between the stem and bubbler wall, forming large bubbles that would not improve the HD liquid to vapor diffusion efficiency. Upon further investigation, it was found that reversing the air flow (up through the stem) produced the desired fine bubbles within the custom stem tube. In addition, the custom stem diameter was smaller than the bubbler bottle diameter, resulting in a longer bubble transition time. HD characterization test results indicated that the custom stem and the reverse flow pattern efficiently generated a near saturated HD vapor that could be diluted to the target HD challenge concentrations needed.
Accurate control of the exposure system flow rates was critical for controlling the HD challenge, maintaining proper system pressures, and monitoring animal respiratory parameters. The initial system design had the HD challenge flow at 10 L/min and the animal bias flow at 8 L/min. These setting were selected to keep HD challenge flow to a minimum, provide sufficient flow for the expected ventilation rate, and to maintain a slight positive pressure at reference point. During the initial exposures, animal breathing induced intermittent negative pressures at the reference point (ranging from −1 to 4 inches of water), presenting a challenge rebreathing concern. To mitigate this problem, the total challenge flow rate was increased to 27 L/min and animal bias flow rate was increased to 15 L/min. These settings provided sufficient challenge flow to keep the reference point pressures during animal exposures between 0 to 4 inches of water and eliminated the challenge rebreathing concern.
In conclusion, the custom bubbler and temperature/flow control approach used in this exposure system can efficiently generate controlled HD vapor concentrations between 22 and 278 mg/m3 using as little as 2.7 mL of neat HD. The generated HD vapor can reach the target concentration in less than 30 minutes and remained stable for up to 5 hours. The in-line sampling loop and GC/FID approach can provide near real-time HD vapor concentration measurements. A mid to large animal model can easily be integrated into the exposure system and real-time respiratory monitoring facilitates accurate delivery of target inhaled doses. The exposure system described here can be used to administer specific inhaled HD doses to a variety of mid to large size animal models. This, in turn, should allow the characterization of the physiological and biological responses to inhaled HD and the evaluation of potential medical counter measures to mitigate these toxic effects.
Fig. 6.
HD vapor delivery efficiency.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) Office of the Director through an interagency agreement (OD#: Y1-OD-0387-01) between the National Institute of Allergy and Infectious Diseases (NIAID) and Department of Defense (DoD) and prepared under the auspices of the NIH, NIAID, National Institute of Neurological Disorders and Stroke (NINDS), and the DoD Defense Technical Information Center (DTIC) under the Chemical, Biological, Radiological & Nuclear Defense Information Analysis Center (CBRNIAC) program, Contract No. SP0700-00-D-3180, Delivery Order Number 0687, CBRNIAC Task 832/CB-IO-OOI2.
Footnotes
The views expressed in this article are those of the authors and do not reflect the official policy of the NIH, Department of Health and Human Services, or the U.S. Government. No official support or endorsement of this article by the NIAID, NINDS, or NIH is intended or should be inferred. The experimental protocol was approved by the Institutional Animal Care and Use Committee at Battelle. All procedures were conducted in accordance with the principles stated in the Guide for the Care and Use of Laboratory Animals and the Animal Welfare Act of 1966 (P.L. 89-544), as amended.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson DR, Byers SL, Vesely KR. Treatment of sulfur mustard (HD)-induced lung injury. Journal of Applied Toxicology. 2000;20:129–132. doi: 10.1002/1099-1263(200012)20:1+<::aid-jat670>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Anderson DR, Taylor SL, Fetterer DP, Holmes WW. Evaluation of protease inhibitors and an antioxidant for treatment of sulfur mustard-induced toxic lung injury. Toxicology. 2009;263:41–46. doi: 10.1016/j.tox.2008.08.025. [DOI] [PubMed] [Google Scholar]
- Anderson DR, Yourick JJ, Moeller RB, Petrali JP, Young GD, Byers SL. Pathologic changes in rat lungs following acute sulfur mustard inhalation. Inhalation Toxicology. 1996;8:285–297. [Google Scholar]
- Balali-Mood M, Hefazi M. Comparison of early and late toxic effects of sulfur mustard in Iranian veterans. Basic & Clinical Pharmacology & Toxicology. 2006;99:273–282. doi: 10.1111/j.1742-7843.2006.pto_429.x. [DOI] [PubMed] [Google Scholar]
- Box GEP, Cullumbine H. The relationship between survival time and dosage with certain toxic agents. British Journal of Pharmacology. 1947;2:27–37. doi: 10.1111/j.1476-5381.1947.tb00318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron GR, Gaddum JH, Short RHD. The absorption of war gases by the nose. Journal of Pathology & Bacteriology. 1946;58:449–455. doi: 10.1002/path.1700580315. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Case Definition: Vesicant (Mustards, Dimethyl Sulfate, and Lewisite) 2013 Jun 18; Retrieved May 8, 2014, from http://www.bt.cdc.gov/agent/vesicants/casedef.asp.
- Cowan FM, Anderson DR, Broomfield CA, Byers SL, Smith WJ. Biochemical alterations in rat lung lavage fluid following acute sulfur mustard inhalation: II. Increases in proteolytic activity. Inhalation Toxicology. 1997;9:53–61. [Google Scholar]
- Eisenkraft A, Tashma Z, Luria S. Mustard gas - clinical implications and management. In: Shemer J, Shoenfeld Y, editors. Terror and medicine: Medical aspects of biological, chemical and radiological terrorism. Lengerich Germany: Pabst Science Publishers; 2003. pp. 172–182. [Google Scholar]
- Fairhall SJ, Jugg BJA, Read RW, Stubbs SJ, Rutter SJ, Smith AJ, Mann TM, Jenner J, Sciuto AM. Exposure-response effects of inhaled sulfur mustard in a large porcine model: a 6-h study. Inhalation Toxicology. 2010;22(14):1135–1143. doi: 10.3109/08958378.2010.527398. [DOI] [PubMed] [Google Scholar]
- Jugg B, Fairhall S, Smith A, Rutter S, Mann T, Perrott R, Jenner J, Salguero J, Shute J, Sciuto A. N-acetyl-L-cysteine protects against inhaled sulfur mustard poisoning in the large swine. Clinical Toxicology. 2013;51:216–224. doi: 10.3109/15563650.2013.780208. [DOI] [PubMed] [Google Scholar]
- Mayer B, Collins CC, Walton M. Transient analysis of carrier gas saturation in liquid source vapor generators. Journal of Vacuum Science and Technology. 2001;19(1):329–344. [Google Scholar]
- O’Neill HC, Orlicky DJ, Hendry-Hofer TB, Loader JE, Day BJ, White CW. Role of reactive oxygen and nitrogen species in olfactory epithelial injury by the sulfur mustard analogue 2-chloroethly ethyl sulfide. American Journal of Respiratory Cell and Molecular Biology. 2011;45:323–331. doi: 10.1165/rcmb.2010-0214OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill HC, White CW, Veress LA, Hendry-Hofer TB, Loader JE, Min E, Huang J, Rancourt RC, Day BJ. Treatment with the catalytic metalloporphyrin AEOL 10150 reduces inflammation and oxidative stress due to inhalation of the sulfur mustard analog 2-chloroethyl ethyl sulfide. Free Radical Biology & Medicine. 2010;48:1118–1196. doi: 10.1016/j.freeradbiomed.2010.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papirmeister B, Feister AJ, Robinson SI, Ford RD. Medical defense against mustard gas: Toxic mechanisms and pharmacological implications. Boca Raton, FL: CRC Press, Inc; 2000. Pretreatments and therapies; pp. 243–296. [Google Scholar]
- Penski EC. The properties of Di-(2-chloroethyl) sulfide. 1. Vapor pressure review and analysis. Aberdeen Proving Ground, MD: Edgewood Research, Development & Engineering Center; 1993. [Google Scholar]
- Rancourt RC, Veress LA, Guo X, Jones TN, Hendry-Hofer TB, White CW. Airway tissue factor-dependent coagulation activity in response to sulfur mustard analog 2-chloroethyl ethyl sulfide. American Journal of Physiology – Lung Cellular and Molecular Physiology. 2012;302:82–92. doi: 10.1152/ajplung.00306.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt DH, Small MJ, Kimmell TA, Anderson AW. Background chemistry for chemical warfare agents and decontamination processes in support of delisting waste streams at the US Army Dugway Proving Ground, Utah. Argonne, IL: Argonne National Laboratory, Environmental Assessment Division; 1996. [Google Scholar]
- U.S. Army Medical Research Institute of Chemical Defense. Medical management of chemical casualties handbook. 4. Aberdeen Proving Ground, MD: U.S. Army Medical Research Institute of Chemical Defense, Chemical Casualty Care Division; 2007. Mustard HD, H; pp. 64–106. [Google Scholar]
- Veress LA, Hendry-Hofer TB, Loader JE, Rioux JS, Garlick RB, White CW. Tissue plasminogen activator prevents mortality from sulfur mustard analog-induced airway obstruction. American Journal of Respiratory Cell and Molecular Biology. 2013;48(4):439–447. doi: 10.1165/rcmb.2012-0177OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winternitz MC, Finney WP., Jr . The pathology of mustard poisoning. In: Winternitz MC, editor. Collected studies on the pathology of war gas poisoning. New Haven, CT: Yale University Press; 1920. pp. 101–113. [Google Scholar]