Abstract
Yohimbine is an alpha-2 adrenoceptor antagonist that has been used in numerous studies as a pharmacological stressor in rodents, monkeys, and humans. Recently, yohimbine has become the most common stress manipulation in studies on reinstatement of drug and food seeking. However, the wide range of conditions under which yohimbine promotes reward seeking is significantly greater than that of stressors like intermittent footshock. Here we addressed two fundamental questions regarding yohimbine’s effect on reinstatement of reward seeking: (1) whether the drug’s effect on operant responding is dependent on previous reward history or cue contingency, and (2) whether yohimbine is aversive or rewarding under conditions typically used in reinstatement studies. We also used in vivo microdialysis to determine yohimbine’s effect on dopamine levels in nucleus accumbens (NAc) and medial prefrontal cortex (mPFC).
We found that the magnitude of yohimbine-induced (0.5, 1.0, 2.0 mg/kg) operant responding during the reinstatement tests was critically dependent on the contingency between lever-pressing and discrete tone-light cue delivery but not the previous history with food reward during training. We also found that yohimbine (2 mg/kg) did not cause conditioned place aversion. Finally, we found that yohimbine modestly increased dopamine levels in mPFC but not NAc.
Results suggest that yohimbine’s effects on operant responding in reinstatement studies are likely independent of the history of contingent self-administration of food or drug rewards and may not be related to the commonly assumed stress-like effects of yohimbine.
Keywords: Conditioned place preference/aversion, dopamine, reinstatement, stress, sensory seeking, yohimbine
Introduction
Yohimbine is an alpha-2 adrenoceptor antagonist that increases brain noradrenaline cell firing and release (Abercrombie et al., 1988; Aghajanian and VanderMaelen, 1982). In psychiatric research, yohimbine is the most commonly used pharmacological stressor, because it induces anxiety- and stress-like responses in humans, monkeys, dogs, and rodents (Bremner et al., 1996a, b). Based on this literature, and the finding that alpha-2 adrenoceptor agonists (which decrease noradrenaline cell firing and release) block intermittent footshock stress-induced reinstatement of drug seeking (Erb et al., 2000; Shaham et al., 2000a), a decade ago three laboratories determined yohimbine’s effect on reinstatement of drug seeking (Le et al., 2005; Lee et al., 2004; Shepard et al., 2004). Lee et al. found that yohimbine reinstates cocaine seeking in monkeys, an effect associated with increased plasma levels of the stress hormone cortisol and species-typical stress-related behaviors. Shepard et al. and Le et al. found that yohimbine reliably reinstates methamphetamine and alcohol seeking in rats, mimicking the effect of intermittent footshock stress on reinstatement.
Subsequent studies provided additional evidence to support the notion that, like intermittent footshock, yohimbine induces a stress-like state that leads to reinstatement of drug seeking. Both intermittent footshock- and yohimbine-induced reinstatement are critically dependent on activation of extrahypothalamic corticotropin-releasing factor (CRF) (Hansson et al., 2006; Le et al., 2013; Marinelli et al., 2007; Shalev et al., 2010). Both intermittent footshock and yohimbine increase resistance to extinction of drug seeking when administered prior to the daily extinction sessions (Highfield et al., 2000; Kupferschmidt et al., 2009). Both intermittent footshock and yohimbine increase levels of the stress hormone corticosterone; yohimbine also induces stress-like responses in the rat social interaction test that are reversed by a CRF1 receptor antagonist (Ghitza et al., 2006; Marinelli et al., 2007; Shaham et al., 1997). Based on these findings, and the findings that the effect of yohimbine on reinstatement is more robust and less variable than that of intermittent footshock (Le et al., 2005; Shepard et al., 2004), yohimbine has recently become the most commonly used stressor in studies on reinstatement of drug or food seeking (Calu et al., 2014; Mantsch et al., 2014; See and Waters, 2010).
However, with the increasing use of yohimbine in reinstatement studies, evidence emerges for notable behavioral and neurobiological differences between the effects of intermittent footshock and yohimbine on reward seeking. Unlike footshock (Ahmed and Koob, 1997; Mantsch and Goeders, 1999), yohimbine potently reinstates food seeking and also increases alcohol and food self-administration (Ayanwuyi et al., 2013; Cifani et al., 2012; Le et al., 2005; Marinelli et al., 2007; Noori et al., 2014). Unlike intermittent footshock, yohimbine does not induce stress-related 22 kHz ultrasonic distress vocalizations (Mahler et al., 2013). Unlike intermittent footshock (Shaham et al., 2000b), yohimbine’s effect on reinstatement of food and cocaine seeking is not blocked by the alpha-2 adrenoceptor agonist clonidine (Brown et al., 2009; Nair et al., 2009). These behavioral and neuropharmacological differences between yohimbine and intermittent footshock raise the possibility that yohimbine’s effect on reinstatement of reward seeking might not be due to its ability to induce a stress-like state that motivates drug or food seeking.
In the present study, we addressed two fundamental questions regarding yohimbine’s effect on reinstatement of reward seeking: (1) whether yohimbine’s effect on operant responding is dependent on previous reward history or cue contingency, and (2) whether yohimbine (at a dose typically used in reinstatement studies) induces an aversive state, as assessed by a conditioned place preference/aversion procedure (Tzschentke, 1998). We also used in vivo microdialysis to determine yohimbine’s effect on dopamine levels in nucleus accumbens (NAc) and medial prefrontal cortex (mPFC), brain areas important for food and drug reward (Wise, 2004) and reinstatement of drug and food seeking (Bossert et al., 2013; Calu et al., 2014).
Materials and methods
See supplemental online material section for description of subjects and apparatus, drugs, training and extinction phase, reinstatement testing, in vivo microdialysis surgery, and microdialysis procedure.
Specific experiments
Exp. 1. Effect of reward history and cue contingency on pellet priming and yohimbine-induced reinstatement of lever responding
In Exp. 1 we determined the effect of previous food pellet history and cue contingency during food self-administration training on yohimbine-induced reinstatement of lever pressing after extinction. For comparison purposes, we also determined whether the different training histories affect reinstatement of lever responding induced by pellet priming, a manipulation known to selectively reinstate food seeking (de Wit, 1996; Nair et al., 2009). We used four groups of rats (n=8–12 per group) that during the training phase were exposed to the following conditions: (1) lever response-contingent pellet+cue, (2) non-contingent pellet+cue (yoked to group 1), (3) lever response-contingent cue-only, and (4) non-contingent cue-only (yoked to group 3). In order to isolate the impact of lever response contingency during training on subsequent lever presses during the reinstatement tests, we installed in the self-administration chambers one retractable lever instead of both ‘active’ and ‘inactive’ levers, as we and others commonly use in reinstatement studies (Nair et al., 2009; Shalev et al., 2002).
During the tests for pellet-priming-induced reinstatement, we first gave the rats a 30 min acclimation period in the operant chamber, after which we delivered 0 (no pellet), 1, 2, or 4 non-contingent pellets (spaced 20 s apart) within 1 min after the start of the sessions. During the tests for yohimbine-induced reinstatement, we injected the rats with water (vehicle) or yohimbine (0.5, 1.0 and 2.0 mg/kg) 30 min before the start of the test sessions. We performed the test sessions for pellets and yohimbine during a consecutive 5-day period, with one regular extinction session between test days 2 and 3, in which we delivered no pellets or we gave sham injections of water (vehicle). We included the regular extinction session in the middle of testing to ensure that baseline lever pressing is not affected by the repeated testing procedure.
[Note that from a drug/food reinstatement literature terminology perspective, during testing only the behavior of the rats in Group 1 but not Groups 2–4 (which did not undergo formal operant training and extinction training) can be termed reinstatement of reward seeking (Shaham et al., 2003). However, for the sake of consistency with the reinstatement literature we refer to the different phases of the experiment as training, extinction, and reinstatement testing.]
Unexpectedly, we found that during the reinstatement test yohimbine strongly increased lever pressing in both the contingent pellet+cue group and the contingent cue-only group (see Results), suggesting that some non-specific arousal or locomotor activating effects of yohimbine promote lever responding. Therefore, we also determined in sub-groups of rats previously tested for reinstatement induced by pellet priming and yohimbine whether this effect of yohimbine would be mimicked by methamphetamine, a psychostimulant that increases arousal and locomotor activity (Berridge, 2006). The sub-groups were: contingent pellet+cue: n=4; contingent cue-only: n=4; non-contingent cue-only: n=8). One day after the last yohimbine test, we injected all 16 rats with methamphetamine (1.0 mg/kg, i.p.) 15 min prior to the start of the session. We compared their lever pressing after methamphetamine to their lever pressing during the last extinction session, as well as to their lever pressing during the 2.0 mg/kg yohimbine reinstatement session.
Exp. 2. Effect of yohimbine on locomotor activity
In Exp. 2 we directly tested whether yohimbine, at a dose that induced robust reinstatement, also increases locomotor activity. We used 15 rats from Exp. 1 (water: n=7; yohimbine: n=8). We injected the rats with water or yohimbine (2.0 mg/kg, i.p.) 30 min before the locomotor activity test. In the beginning of the test, we placed the rats in the center of the chamber and allowed them to explore the apparatus for 1 hr under white light. We recorded the total distance traveled and the number of vertical movements. In Exp. 2–4 we used rats that have been previously used in Exp. 1 that we repeatedly tested for locomotor activity, CPP, and microdialysis dopamine levels in NAc and mPFC, because we wanted to determine yohimbine’s effect on these measures in rats with a history of chronic food restriction that underwent the experimental procedure of Exp. 1.
Exp. 3. Effect of yohimbine on conditioned place preference/aversion (CPP/CPA)
We used 15 rats from Exp. 2 and prior to CPP/CPA conditioning we divided the rats into two groups. We injected one group of rats (n=8) with yohimbine (2.0 mg/kg, i.p.), pairing it with one of the two end compartments, and in separate conditioning sessions injected water, pairing it with the opposite end compartment (counterbalanced). We injected the other group of rats (n=7) with water throughout the experiment, and confined them to each of the two end compartments during conditioning. We conducted all sessions under white light. The CPP/CPA procedure consisted of three phases: pre-exposure (1 day), conditioning (8 days), tests 1 and 2 (conducted on separate days). During the pre-exposure phase, we placed the rats in the center choice compartment and immediately opened the guillotine doors, which remained open for the duration of the pre-exposure session, allowing the rats to explore the entire apparatus for 15 min. We recorded time spent in each compartment to assess unconditioned compartment preferences.
For the conditioning phase, we confined all rats to a single compartment during a given session, which lasted for 60 min. We gave rats in the yohimbine-paired group yohimbine injections paired with one of the two end compartments and water with the other compartment (in a counterbalanced manner). We gave rats in the water-only group water injections paired with both end compartments in a counter balanced order. We injected all rats with either yohimbine (2.0 mg/kg, i.p.) or water (0.5 ml/kg) 30 min before each conditioning session started. The daily conditioning sessions lasted for 60 min, during which time the center choice compartment remained closed off by the guillotine door.
We conducted the first CPP/CPA test two days after the last conditioning session. We placed rats in the center choice compartment and opened the guillotine doors immediately after the test started, permitting access to all three chambers. The test lasted for 15 min. We recorded time spent in each chamber to assess individual preference. We conducted the second CPP/CPA test two weeks after the first test and the procedure for this test was identical to the first test.
Exp. 4. Effect of yohimbine on dopamine levels in the mPFC and NAc
We used six rats from Exp. 1–3 to determine the changes in dopamine levels in the mPFC and NAc after yohimbine injections. We first obtained stable dopamine values (less than 10% variability) for 2–3 consecutive samples (typically after about 1–2 hr), after which we injected rats with yohimbine (2.0 mg/kg) and monitored the changes in dopamine concentrations for 90 min after the injection. We obtained microdialysis measurements in a single session and euthanized the rats at the end of testing.
Results
Exp. 1. Effect of reward history and cue contingency on pellet priming and yohimbine-induced reinstatement of lever responding
We determined the effect of food reward history and compound cue contingency during the training phase on pellet-priming and yohimbine-induced lever pressing during the reinstatement test. We used four groups of rats that during the training phase were exposed to: (1) response-contingent pellet+cue, (2) non-contingent pellet+cue (yoked to group 1), (3) response-contingent cue-only, and (4) non-contingent cue-only (yoked to group 3).
Training and extinction phases
Training
We found that operant responding during the training phase was significantly higher in the response-contingent pellet+cue group than in the other three groups (Fig. 1A). The mixed ANOVA using the between-subjects factor of Group and the within-subjects factor of Training session showed a significant main effect of Group (F(3,36)=70.4, p<0.01), but no effect of Training session (p=0.58) or interaction between the two factors (p=0.64).
Figure 1. Lever responding during the training and extinction phases in rats trained under different food reward availability and cue contingency conditions.
Left panel: response-contingent pellet+cue (n=12) and non-contingent pellet+cue (n=8, yoked condition). Right panel: response-contingent cue-only (n=12) and non-contingent cue-only (n=8). A. Training phase. B. Extinction phase. * Different from the respective non-contingent group within each day, p<0.05. Note: the y-axis is drawn on a different scale for the pellet+cue (contingent and non-contingent) groups and the cue-only (contingent and non-contingent) groups. Data are mean±SEM.
Extinction
We found that lever presses during the extinction phase decreased over time in the response-contingent pellet+cue group, while lever presses in the other 3 groups remained relatively stable (Fig. 1B). The mixed ANOVA using the between-subjects factor of Group and the within-subjects factor of Extinction session showed a significant interaction between the two factors (F(30,360)=19.1, p<0.01).
Pellet priming reinstatement test
Non-contingent delivery of pellets at the start of the test sessions increased lever responding during testing in the response-contingent pellet+cue group but not in the other groups (Fig. 2A). The mixed ANOVA using the between-subjects factor of Group and the within-subjects factor of Pellet number (0, 1, 2, 4 pellets) showed a significant interaction between the two factors (F(9,108)=5.1, p<0.01). These data demonstrate that the reinstatement effect of pellet priming is critically dependent on prior contingent pellet delivery during the training phase.
Figure 2. Effect of different reward history and cue contingency during training on pellet priming and yohimbine-induced lever pressing during reinstatement tests.
Left panel: contingent pellet+cue (n=12) and non-contingent pellet+cue (n=7–8). Right panel: contingent cue-only (n=12) and non-contingent cue-only (n=8). A. Pellet priming-induced reinstatement. B. Yohimbine-induced reinstatement. Different from the control condition (0 pellet or water vehicle injection), * p<0.05. Data are mean±SEM.
Yohimbine reinstatement test
Unlike pellet priming, during testing yohimbine significantly increased lever presses in all groups, an effect that was substantially stronger in rats with a history of response contingent delivery of pellet+cue or cue alone during the training phase (Fig. 2B). The mixed ANOVA using the between-subjects factor of Group and the within-subjects factor of Yohimbine dose (0, 0.5, 1.0, 2.0 mg/kg) showed significant main effects of Group (F(3,35)=4.3, p<0.05) and Yohimbine dose (F(3,105)=19.0, p<0.01), and an approaching significant interaction between the two factors (F(9,105)=1.97, p=0.051). These data demonstrate that the effect of yohimbine on lever pressing during the yohimbine-induced reinstatement tests is independent of the history of food self-administration. Further, the different magnitude of the yohimbine-induced responding between groups suggests that the effect of the drug on lever pressing during testing is strongly potentiated when lever-pressing had been previously paired with cue delivery.
Methamphetamine reinstatement test
In a subgroup of rats (response-contingent pellet+cue: n=4; response-contingent cue-only: n=4; non-contingent cue-only: n=8), we found that methamphetamine injections (1.0 mg/kg) increased lever presses during the reinstatement test, an effect that was more pronounced in the response-contingent cue+pellet and response-contingent cue-only groups than in the non-contingent cue-only group (Fig. 3A). The mixed ANOVA using the between-subjects factor of Group and the within-subjects factor of Test session (last extinction session, methamphetamine session) showed significant main effects of Group (F(2,13)=4.6, p<0.05) and Test session (F(1,13)=34.7, p<0.01), but no interaction between the two factors (p>0.1). Post-hoc differences are shown in Fig. 3, which also shows the correlation (r=0.48, p=0.06) between lever presses induced by yohimbine (2 mg/kg) and methamphetamine during testing. These data suggest that, like yohimbine, the effect of methamphetamine on lever pressing during testing is dependent on the cue contingency but not history of food delivery during training. However, the methamphetamine data should be interpreted with caution because we only assessed the drug’s effect on lever pressing in the reinstatement test in three of the four groups of rats from Exp. 1 and in a small n per condition.
Figure 3. Effect of different reward history and cue contingency during training on methamphetamine-induced lever pressing in the reinstatement tests.
A. Methamphetamine-induced reinstatement (contingent pellet+cue: n=4; contingent cue-only: n=4; non-contingent cue-only: n=8). * Different from the baseline extinction session, p<0.05; Data are mean±SEM. B. Correlation plot of lever pressing induced by methamphetamine (1 mg/kg) and yohimbine (2 mg/kg) during the reinstatement tests.
Exp. 2. Effect of yohimbine on locomotor activity
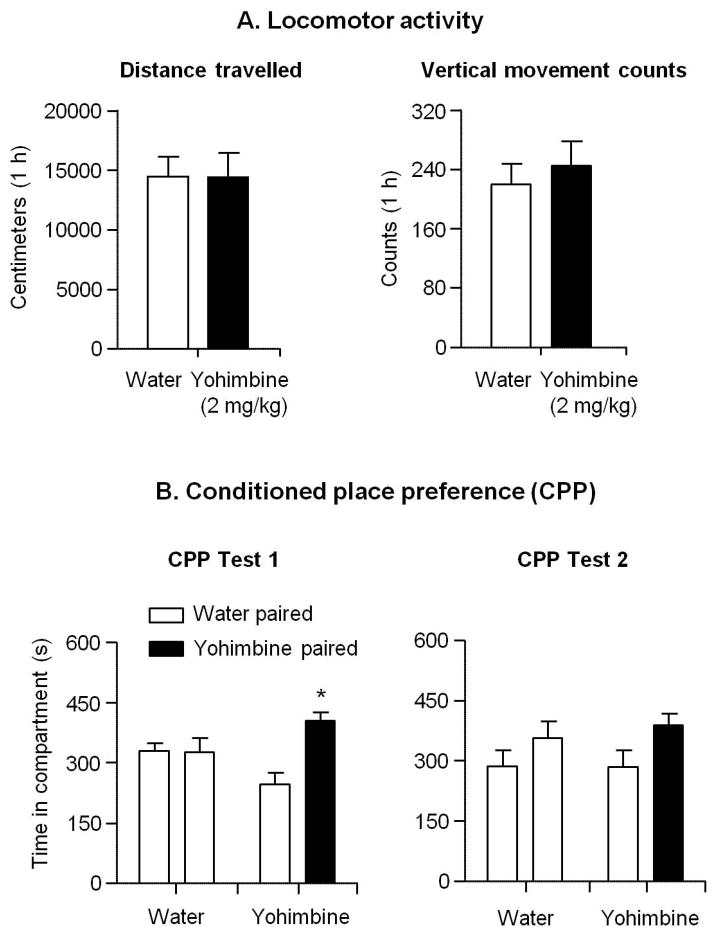
The goal of this experiment was to test whether the effect of yohimbine on lever presses during the reinstatement test was due to non-specific increases in locomotor activity. We found that yohimbine (2 mg/kg) had no effect on locomotor activity (Fig. 4A). The ANOVA using the between-subjects factor of Yohimbine dose (0, 2 mg/kg) showed no significant effects for distance traveled (p=0.98) or vertical movement counts (p=0.58). These data suggest that yohimbine’s effect on lever pressing during the reinstatement tests in Exp. 1 is not due to non-selective locomotor activation.
Figure 4. Effect of yohimbine on locomotor activity and conditioned place preference/aversion.
A–B. Locomotor activity. C–D. Conditioned place preference/aversion on tests 1 and 2 conducted 2 days and 16 days after conditioned place preference training. * Different from water-paired compartment, p<0.05 (n=7–8 per group). Data are mean±SEM.
Exp. 3. Effect of yohimbine on conditioned place preference/aversion (CPP/CPA)
In Exp. 3 we tested whether yohimbine (at a dose that strongly increases lever pressing during the reinstatement test) is rewarding or aversive, as assessed in the CPP/CPA procedure. We found that under our experimental conditions, yohimbine caused a weak CPP that diminished over time (Fig. 4B). The pre-exposure test showed that the yohimbine-paired group (n=8) and water-only group (n=7) had similar preference prior to conditioning. The dependent measure was a pre-exposure vs. post-training change score (yohimbine paired - water paired for the yohimbine group or randomly chosen compartment 1 - compartment 2 for the water group). We analyzed the change score using a paired t-test (Pre- vs. Post-conditioning) for each group. For the initial test, which occurred 2 days after the last conditioning session, the results showed a significant effect for yohimbine-paired group (pre-exposure: 14.4 ± 33.6; post-conditioning test 1: 157.0 ± 42.9; t7=2.5, p<0.05), suggesting that these rats developed modest preference to the yohimbine-paired side regardless of their initial preference. This preference was not evident 16 days after CPP/CPA training (Fig. 4B). There was no difference in place preference in the water-only group (post-conditioning test 1: t6=0.8, p=0.46; post-conditioning test 2: t6=0.0, p=0.99). Together, our results indicate that yohimbine, at a dose typically used in reinstatement studies, is not an aversive stimulus in the CPP/CPA procedure.
Exp. 4. Effect of yohimbine on extracellular dopamine levels in mPFC and NAc
In a previous study we found that mPFC dopamine plays a critical role in yohimbine-induced reinstatement of food seeking (Nair et al., 2011) and in Exp. 1 we found that the effect of yohimbine on lever pressing during the reinstatement tests is mimicked by methamphetamine, which increases dopamine levels in mPFC (Staiti et al., 2011). In Exp. 4 we determined whether yohimbine would increase mPFC dopamine levels and also determined the drug’s effect on NAc dopamine levels. We found that yohimbine (2 mg/kg) increased dopamine levels in mPFC but not NAc (Fig. 5). We analyzed the data in 30 min time-bins, using the within-subjects factor of Session time (pre-treatment, yohimbine 0–30 min, yohimbine 30–60 min, yohimbine 60–90 min). The analysis showed a significant effect of Session time for mPFC (F(3,6)=11.0, p<0.01) but not NAc (p=0.61).
Figure 5. Effect of yohimbine on the extracellular dopamine levels in NAc and mPFC.
A. Coronal sections (Paxinos, 2005) showing the microdialysis probe placements. B. Percent change from baseline dopamine levels after yohimbine (2 mg/kg) injections. Each time block represents average data in 30 min from samples collected every 10 min. * Different from baseline (block 1), p<0.05. Data are mean±SEM.
Discussion
We found that the effect of yohimbine on reinstatement of operant responding is critically dependent on the history of response-contingent cue delivery during training but not response-contingent food delivery. In contrast, pellet priming-induced reinstatement is critically dependent on the history of response-contingent food delivery during training but not response-contingent cue delivery. Additionally, yohimbine at a dose commonly used in reinstatement studies of food or drug seeking did not induce an aversive state, as assessed in the CPP/CPA procedure. Furthermore, yohimbine had no effect on locomotor activity, suggesting that the drug’s effect on lever-pressing during reinstatement is not due to non-specific hyperactivity. Finally, in agreement with a previous study (Tanda et al., 1996), yohimbine increased dopamine levels in mPFC but not NAc. Our results suggest that the effect of yohimbine on operant responding in reinstatement studies in rats is independent of the history of contingent self-administration of food reward and may not be related to the commonly assumed stress-like effects of yohimbine.
Psychological and neuropharmacological mechanisms of the reward-independent effects of yohimbine
Yohimbine has been used in many studies on reinstatement of drug and food seeking (Calu et al., 2014; Mantsch et al., 2014; See and Waters, 2010). A common assumption is that yohimbine-induced reinstatement of lever-pressing after extinction is due to stress-induced reinstatement of the previously reinforced drug- or food-associated operant response. Our results challenge this assumption by demonstrating that the contingent cue-only training group had a similar dose-response curve for the effect of yohimbine on ‘reinstatement’ as that of the response-contingent pellet+cue group (Fig. 2B). What psychological and neuropharmacological mechanisms might account for the reward-independent effects of yohimbine?
Psychological perspective
We propose that the primary action of yohimbine in reinstatement studies is to invigorate responding for visual or auditory stimuli/cues that under normal conditions have weak or moderate rewarding effects in rodents (Deroche-Gamonet et al., 2002; Donny et al., 2003; Olsen and Winder, 2009; Shin et al., 2010; Stewart, 1960). We see evidence for weak sensory seeking in the higher lever pressing of the contingent cue-only versus non-contingent cue-only groups (Fig. 1), an effect that is potentiated by yohimbine (Fig. 2B). The findings that yohimbine strongly enhances cue-induced reinstatement of drug seeking (Banna et al., 2010; Feltenstein et al., 2012; Feltenstein and See, 2006) support the notion that the primary effect of yohimbine in reinstatement studies is to potentiate cue responding.
Our finding that yohimbine also somewhat increased lever pressing in the yoked response-non-contingent pellet+cue or response-non-contingent cue-only training groups seems inconsistent with the idea that the primary action of yohimbine in reinstatement studies is to enhance cue responding. However, it is challenging to make operant lever-pressing a cue-free manipulation, because pressing an operant lever results in a clicking noise that is itself a sensory cue and the lever-pressing action may have some sensory properties. Thus, we speculate that yohimbine-induced increases in lever pressing during testing in the yoked training groups are also likely due to potentiation of the motivational effects of sensory cues that are distinct from the tone-light cue.
Other studies using delay discounting and five-choice serial reaction time tasks suggest alternative psychological effects of yohimbine, which act either to increase preservative choice of previously learned behaviors (Schwager et al., 2014) or to increase motor impulsivity (Sun et al., 2010). While yohimbine’s effect on lever pressing during the reinstatement tests in the present study may be due to the drug’s ability to increase motor impulsivity, it is difficult to assess the impact of yohimbine on perseverative choice since across all phases of Exp. 1 the response contingency on the single lever remained unchanged for the contingent cue-only and non-contingent pellet+cue and cue-only groups.
Neuropharmacological perspective
We propose that yohimbine-induced potentiation of responding to sensory cues is mediated by mPFC dopamine transmission. Several lines of evidence support this notion. First, operant responding for sensory cues in rats is increased by the indirect dopamine agonists amphetamine (Keller et al., 2014; Winterbauer and Balleine, 2007) or methamphetamine (Fig. 3 and (Gancarz et al., 2012)), and is decreased by knockdown of D1-dopamine receptors in mice (Olsen and Winder, 2009). Second, yohimbine-induced reinstatement of food seeking is decreased by systemic or dorsal mPFC injections of the D1-family receptor antagonist SCH 23390 (Nair et al., 2011). Third, as previously reported (Tanda et al., 1996) and replicated in our study, yohimbine increases mPFC dopamine release (Fig. 5).
Another possibility is that yohimbine-induced potentiation of responding to sensory cues is mediated by noradrenaline transmission, as suggested by the finding that the beta adrenoceptor antagonist, propranolol, decreases yohimbine-induced reinstatement of cocaine CPP in mice (Mantsch et al., 2010). However, two lines of evidence are inconsistent with this possibility. First, lesions of the ventral or dorsal noradrenergic bundles have no effect on yohimbine-induced reinstatement of alcohol seeking (Le et al., 2009). Additionally, yohimbine-induced reinstatement of food or cocaine seeking is not blocked by the alpha-2 adrenoceptor agonist clonidine (Brown et al., 2009; Nair et al., 2009). However, these negative results should be interpreted with caution, because they may due to higher alpha-2 receptor occupancy by yohimbine versus clonidine within the dose range used in these studies.
Alternatively, there is evidence that yohimbine reduces dorsal raphe cell firing and serotonin release (Millan et al., 2000). Thus, yohimbine may induce sensory-seeking behavior by suppressing the serotonergic system. Indeed, this system has been implicated in sensory reinforcement, as inactivation of median and dorsal raphe increases visual stimulus seeking (Vollrath-Smith et al., 2012). Our previous study showing that serotonin releaser fenfluramine reduced yohimbine-induced reinstatement of food seeking (Pickens et al., 2012) further supports this hypothesis.
Yohimbine and place preference/aversion
We found that yohimbine did not cause the expected conditioned place aversion (CPA) as previously reported by File (1986). The present results, which are in agreement with a previous report (Morales et al., 2001), stands in contrast to the stress-like effects of yohimbine that have been documented in many studies using different behavioral measures (Bhattacharya et al., 1997; Chopin et al., 1986; Davis et al., 1979; Ghitza et al., 2006; Johnston et al., 1988; Pellow et al., 1985). The reasons for the different results between our study and the Morales et al. study versus the File study are unknown, and may be due to the yohimbine dose, feeding conditions, and prior history (our rats were food restricted and had prior experience with yohimbine), and the use of a biased CPP/CPA procedure in the File study. Overall, our results extend previous findings with cocaine, caffeine, and nicotine, demonstrating that anxiogenic agents that induce stress-like response are not invariably aversive (Brockwell et al., 1991; Ettenberg, 2009; File et al., 2000; Sarnyai et al., 2001; Trigo et al., 2009).
Implications of the present finding to studies on reinstatement of food and drug seeking
We found that both yohimbine and methamphetamine caused ‘reinstatement’ in rats that did not undergo formal contingent operant training and extinction training for food reward. These results have implications for future reinstatement studies for which investigators commonly assume that reinstatement of reward seeking after extinction is due to the prior history of food or drug self-administration. One implication is that the ‘selectivity’ of different pharmacological and neurobiological manipulations in reinstatement studies should be assessed using a contingent cue-only group, instead of the commonly used ‘inactive’ lever control condition. Unlike the inactive lever control condition, the contingent cue-only control condition is identical to the experimental group in all aspects except for the omission of the critical variable under study (the food or drug reward). Another implication is that results from studies using psychostimulant priming or yohimbine manipulations should be interpreted with caution, because they may not necessary demonstrate evidence for ‘reinstatement of reward seeking.’
An issue to consider based on the current results is whether the effect of intermittent footshock on reinstatement of food or drug seeking is also independent of the self-administration training history. We believe that this not the case, because several studies have reported that footshock-induced reinstatement is observed in rats with a history of cocaine (Ahmed and Koob, 1997; Mantsch and Goeders, 1999), nicotine (Buczek et al., 1999), or alcohol (Le et al., 1998) self-administration, but not food self-administration. Since footshock does not reinstate food-seeking under conditions equivalent to the pellet+cue group in our study, it is unlikely that footshock would reinstate lever-pressing in the cue-only condition.
Finally, our data do not rule out the contribution of stress-like effects of yohimbine on reinstatement. Indeed, there is evidence that both extrahypothalamic CRF and the bed nucleus of the stria terminalis, which play critical roles in mediating both stress responses (Davis et al., 2009) and footshock stress-induced reinstatement (Shaham et al., 2000a; Shalev et al., 2010), also contribute to yohimbine-induced reinstatement (Buffalari and See, 2011; Hansson et al., 2006; Le et al., 2013; Marinelli et al., 2007; Shalev et al., 2010). Additionally, human studies indicate that yohimbine induces both physiological and psychological stress-like responses and increases opiate craving and intake (Greenwald et al., 2013; Stine et al., 2002), as well as alcohol and cocaine craving (Moran-Santa Maria et al., 2014; Umhau et al., 2011).
Concluding remarks
Since 2004 (Lee et al., 2004; Shepard et al., 2004), yohimbine has been used in many studies as a pharmacological stressor to study the mechanisms of stress-induced reinstatement of drug and food seeking (Bossert et al., 2013; Calu et al., 2014; See and Waters, 2010). Our results challenge two commonly accepted assumptions in reinstatement studies: that yohimbine-induced reinstatement of lever-pressing after extinction primarily reflects stress-induced reinstatement of responding for the previously reinforced drug or food reward and that psychostimulant priming-induced reinstatement of reward seeking is primarily controlled by the prior history response-contingent drug self-administration. Finally, our results extend previous results on the profound effect of conditioned sensory stimuli/cues on operant responding commonly assumed to be controlled by current or past history of experience with unconditioned food or drug rewards (Bastle et al., 2012; Caggiula et al., 2002).
Supplementary Material
Acknowledgments
We would like to acknowledge Mark Coggiano for his technical assistance with the microdialysis experiments. The work was supported by the Intramural Research Program of the National Institute on Drug Abuse.
Footnotes
Authors’ contribution: YWC, YS, and DJC contributed to the design of the experiments and the data analysis; YWC, KAF, and SZB conducted all behavioral experiments; GT conducted the microdialysis experiments and related analysis; YWC, YS, and DJC wrote the manuscript. All authors reviewed the content and approved the final version of the manuscript.
The authors declare that they do not have any conflicts of interest (financial or otherwise) related to the data presented in this manuscript.
References
- Abercrombie ED, Keller RW, Jr, Zigmond MJ. Characterization of hippocampal norepinephrine release as measured by microdialysis perfusion: pharmacological and behavioral studies. Neuroscience. 1988;27:897–904. doi: 10.1016/0306-4522(88)90192-3. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, VanderMaelen CP. alpha 2-adrenoceptor-mediated hyperpolarization of locus coeruleus neurons: intracellular studies in vivo. Science. 1982;215:1394–1396. doi: 10.1126/science.6278591. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Cocaine- but not food-seeking behavior is reinstated by stress after extinction. Psychopharmacology. 1997;132:289–295. doi: 10.1007/s002130050347. [DOI] [PubMed] [Google Scholar]
- Ayanwuyi LO, Carvajal F, Lerma-Cabrera JM, Domi E, Bjork K, Ubaldi M, Heilig M, Roberto M, Ciccocioppo R, Cippitelli A. Role of a genetic polymorphism in the corticotropin-releasing factor receptor 1 gene in alcohol drinking and seeking behaviors of marchigian sardinian alcohol-preferring rats. Front Psychiatry. 2013;4:23. doi: 10.3389/fpsyt.2013.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banna KM, Back SE, Do P, See RE. Yohimbine stress potentiates conditioned cue-induced reinstatement of heroin-seeking in rats. Behav Brain Res. 2010;208:144–148. doi: 10.1016/j.bbr.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastle RM, Kufahl PR, Turk MN, Weber SM, Pentkowski NS, Thiel KJ, Neisewander JL. Novel cues reinstate cocaine-seeking behavior and induce Fos protein expression as effectively as conditioned cues. Neuropsychopharmacology. 2012;37:2109–2120. doi: 10.1038/npp.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW. Neural substrates of psychostimulant-induced arousal. Neuropsychopharmacology. 2006;31:2332–2340. doi: 10.1038/sj.npp.1301159. [DOI] [PubMed] [Google Scholar]
- Bhattacharya SK, Satyan KS, Chakrabarti A. Anxiogenic action of caffeine: an experimental study in rats. J Psychopharmacol. 1997;11:219–224. doi: 10.1177/026988119701100304. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Marchant NJ, Calu DJ, Shaham Y. The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology. 2013;229:453–476. doi: 10.1007/s00213-013-3120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: I. Preclinical studies. Synapse. 1996a;23:28–38. doi: 10.1002/(SICI)1098-2396(199605)23:1<28::AID-SYN4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: II. Clinical studies. Synapse. 1996b;23:39–51. doi: 10.1002/(SICI)1098-2396(199605)23:1<39::AID-SYN5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Brockwell NT, Eikelboom R, Beninger RJ. Caffeine-induced place and taste conditioning: production of dose-dependent preference and aversion. Pharmacol Biochem Behav. 1991;38:513–517. doi: 10.1016/0091-3057(91)90006-n. [DOI] [PubMed] [Google Scholar]
- Brown ZJ, Tribe E, D’Souza NA, Erb S. Interaction between noradrenaline and corticotrophin-releasing factor in the reinstatement of cocaine seeking in the rat. Psychopharmacology. 2009;203:121–130. doi: 10.1007/s00213-008-1376-4. [DOI] [PubMed] [Google Scholar]
- Buczek Y, Le AD, Wang A, Stewart J, Shaham Y. Stress reinstates nicotine seeking but not sucrose solution seeking in rats. Psychopharmacology. 1999;144:183–188. doi: 10.1007/s002130050992. [DOI] [PubMed] [Google Scholar]
- Buffalari DM, See RE. Inactivation of the bed nucleus of the stria terminalis in an animal model of relapse: effects on conditioned cue-induced reinstatement and its enhancement by yohimbine. Psychopharmacology. 2011;213:19–27. doi: 10.1007/s00213-010-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Environmental stimuli promote the acquisition of nicotine self-administration in rats. Psychopharmacology. 2002;163:230–237. doi: 10.1007/s00213-002-1156-5. [DOI] [PubMed] [Google Scholar]
- Calu DJ, Chen YW, Kawa AB, Nair SG, Shaham Y. The use of the reinstatement model to study relapse to palatable food seeking during dieting. Neuropharmacology. 2014;76(Pt B):395–406. doi: 10.1016/j.neuropharm.2013.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopin P, Pellow S, File SE. The effects of yohimbine on exploratory and locomotor behaviour are attributable to its effects at noradrenaline and not at benzodiazepine receptors. Neuropharmacology. 1986;25:53–57. doi: 10.1016/0028-3908(86)90058-4. [DOI] [PubMed] [Google Scholar]
- Cifani C, Koya E, Navarre BM, Calu DJ, Baumann MH, Marchant NJ, Liu QR, Khuc T, Pickel J, Lupica CR, Shaham Y, Hope BT. Medial prefrontal cortex neuronal activation and synaptic alterations after stress-induced reinstatement of palatable food seeking: a study using c-fos-GFP transgenic female rats. J Neurosci. 2012;32:8480–8490. doi: 10.1523/JNEUROSCI.5895-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Redmond DE, Jr, Baraban JM. Noradrenergic agonists and antagonists: effects on conditioned fear as measured by the potentiated startle paradigm. Psychopharmacology. 1979;65:111–118. doi: 10.1007/BF00433036. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2009;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H. Priming effects with drugs and other reinforcers. Exp Clin Psychopharmacol. 1996;4:5–10. [Google Scholar]
- Deroche-Gamonet V, Piat F, Le Moal M, Piazza PV. Influence of cue-conditioning on acquisition, maintenance and relapse of cocaine intravenous self-administration. Eur J Neurosci. 2002;15:1363–1370. doi: 10.1046/j.1460-9568.2002.01974.x. [DOI] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Clements LA, Sved AF. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology. 2003;169:68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Erb S, Hitchcott PK, Rajabi H, Mueller D, Shaham Y, Stewart J. Alpha-2 adrenergic receptor agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology. 2000;23:138–150. doi: 10.1016/S0893-133X(99)00158-X. [DOI] [PubMed] [Google Scholar]
- Ettenberg A. The runway model of drug self-administration. Pharmacol Biochem Behav. 2009;91:271–277. doi: 10.1016/j.pbb.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, Ghee SM, See RE. Nicotine self-administration and reinstatement of nicotine-seeking in male and female rats. Drug Alcohol Depend. 2012;121:240–246. doi: 10.1016/j.drugalcdep.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. Potentiation of cue-induced reinstatement of cocaine-seeking in rats by the anxiogenic drug yohimbine. Behav Brain Res. 2006;174:1–8. doi: 10.1016/j.bbr.2006.06.039. [DOI] [PubMed] [Google Scholar]
- File SE. Aversive and appetitive properties of anxiogenic and anxiolytic agents. Behav Brain Res. 1986;21:189–194. doi: 10.1016/0166-4328(86)90236-6. [DOI] [PubMed] [Google Scholar]
- File SE, Kenny PJ, Cheeta S. The role of the dorsal hippocampal serotonergic and cholinergic systems in the modulation of anxiety. Pharmacol Biochem Behav. 2000;66:65–72. doi: 10.1016/s0091-3057(00)00198-2. [DOI] [PubMed] [Google Scholar]
- Gancarz AM, Ashrafioun L, San George MA, Hausknecht KA, Hawk LW, Jr, Richards JB. Exploratory studies in sensory reinforcement in male rats: effects of methamphetamine. Exp Clin Psychopharmacol. 2012;20:16–27. doi: 10.1037/a0025701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza UE, Gray SM, Epstein DH, Rice KC, Shaham Y. The anxiogenic drug yohimbine reinstates palatable food seeking in a rat relapse model: a role of CRF(1) receptors. Neuropsychopharmacology. 2006;31:2188–2196. doi: 10.1038/sj.npp.1300964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald MK, Lundahl LH, Steinmiller CL. Yohimbine increases opioid-seeking behavior in heroin-dependent, buprenorphine-maintained individuals. Psychopharmacology. 2013;225:811–824. doi: 10.1007/s00213-012-2868-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson AC, Cippitelli A, Sommer WH, Fedeli A, Bjork K, Soverchia L, Terasmaa A, Massi M, Heilig M, Ciccocioppo R. Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proc Natl Acad Sci U S A. 2006;103:15236–15241. doi: 10.1073/pnas.0604419103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highfield D, Clements A, Shalev U, McDonald R, Featherstone R, Stewart J, Shaham Y. Involvement of the medial septum in stress-induced relapse to heroin seeking in rats. Eur J Neurosci. 2000;12:1705–1713. doi: 10.1046/j.1460-9568.2000.00037.x. [DOI] [PubMed] [Google Scholar]
- Johnston AL, Baldwin HA, File SE. Measures of anxiety and stress in the rat following chronic treatment with yohimbine. J Psychopharmacol. 1988;2:33–38. doi: 10.1177/026988118800200106. [DOI] [PubMed] [Google Scholar]
- Keller KL, Vollrath-Smith FR, Jafari M, Ikemoto S. Synergistic interaction between caloric restriction and amphetamine in food-unrelated approach behavior of rats. Psychopharmacology. 2014;231:825–840. doi: 10.1007/s00213-013-3300-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupferschmidt DA, Tribe E, Erb S. Effects of repeated yohimbine on the extinction and reinstatement of cocaine seeking. Pharmacol Biochem Behav. 2009;91:473–480. doi: 10.1016/j.pbb.2008.08.026. [DOI] [PubMed] [Google Scholar]
- Le AD, Funk D, Coen K, Li Z, Shaham Y. Role of corticotropin-releasing factor in the median raphe nucleus in yohimbine-induced reinstatement of alcohol seeking in rats. Addict Biol. 2013;18:448–451. doi: 10.1111/j.1369-1600.2011.00374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Funk D, Harding S, Juzytsch W, Fletcher PJ. The role of noradrenaline and 5-hydroxytryptamine in yohimbine-induced increases in alcohol-seeking in rats. Psychopharmacology. 2009;204:477–488. doi: 10.1007/s00213-009-1481-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Funk D, Shaham Y. Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology. 2005;179:366–373. doi: 10.1007/s00213-004-2036-y. [DOI] [PubMed] [Google Scholar]
- Le AD, Quan B, Juzystch W, Fletcher PJ, Joharchi N, Shaham Y. Reinstatement of alcohol-seeking by priming injections of alcohol and exposure to stress in rats. Psychopharmacology. 1998;135:169–174. doi: 10.1007/s002130050498. [DOI] [PubMed] [Google Scholar]
- Lee B, Tiefenbacher S, Platt DM, Spealman RD. Pharmacological blockade of alpha2-adrenoceptors induces reinstatement of cocaine-seeking behavior in squirrel monkeys. Neuropsychopharmacology. 2004;29:686–693. doi: 10.1038/sj.npp.1300391. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Moorman DE, Feltenstein MW, Cox BM, Ogburn KB, Bachar M, McGonigal JT, Ghee SM, See RE. A rodent “self-report” measure of methamphetamine craving? Rat ultrasonic vocalizations during methamphetamine self-administration, extinction, and reinstatement. Behav Brain Res. 2013;236:78–89. doi: 10.1016/j.bbr.2012.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Goeders NE. Ketoconazole blocks the stress-induced reinstatement of cocaine-seeking behavior in rats: relationship to the discriminative stimulus effects of cocaine. Psychopharmacology. 1999;142:399–407. doi: 10.1007/s002130050905. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Vranjkovic O, Twining RC, Gasser PJ, McReynolds JR, Blacktop JM. Neurobiological mechanisms that contribute to stress-related cocaine use. Neuropharmacology. 2014;76(Pt B):383–394. doi: 10.1016/j.neuropharm.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Weyer A, Vranjkovic O, Beyer CE, Baker DA, Caretta H. Involvement of noradrenergic neurotransmission in the stress- but not cocaine-induced reinstatement of extinguished cocaine-induced conditioned place preference in mice: role for beta-2 adrenergic receptors. Neuropsychopharmacology. 2010;35:2165–2178. doi: 10.1038/npp.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Juzytsch W, Harding S, Rice KC, Shaham Y, Le AD. The CRF1 receptor antagonist antalarmin attenuates yohimbine-induced increases in operant alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology. 2007;195:345–355. doi: 10.1007/s00213-007-0905-x. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Newman-Tancredi A, Audinot V, Cussac D, Lejeune F, Nicolas JP, Coge F, Galizzi JP, Boutin JA, Rivet JM, Dekeyne A, Gobert A. Agonist and antagonist actions of yohimbine as compared to fluparoxan at alpha(2)-adrenergic receptors (AR)s, serotonin (5-HT)(1A), 5-HT(1B), 5-HT(1D) and dopamine D(2) and D(3) receptors. Significance for the modulation of frontocortical monoaminergic transmission and depressive states. Synapse. 2000;35:79–95. doi: 10.1002/(SICI)1098-2396(200002)35:2<79::AID-SYN1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Morales L, Perez-Garcia C, Alguacil LF. Effects of yohimbine on the antinociceptive and place conditioning effects of opioid agonists in rodents. Br J Pharmacol. 2001;133:172–178. doi: 10.1038/sj.bjp.0704057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran-Santa Maria MM, McRae-Clark A, Baker NL, Ramakrishnan V, Brady KT. Yohimbine administration and cue-reactivity in cocaine-dependent individuals. Psychopharmacology. 2014 doi: 10.1007/s00213-014-3555-9. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SG, Adams-Deutsch T, Epstein DH, Shaham Y. The neuropharmacology of relapse to food seeking: methodology, main findings, and comparison with relapse to drug seeking. Prog Neurobiol. 2009;89:18–45. doi: 10.1016/j.pneurobio.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SG, Navarre BM, Cifani C, Pickens CL, Bossert JM, Shaham Y. Role of dorsal medial prefrontal cortex dopamine D1-family receptors in relapse to high-fat food seeking induced by the anxiogenic drug yohimbine. Neuropsychopharmacology. 2011;36:497–510. doi: 10.1038/npp.2010.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noori HR, Helinski S, Spanagel R. Cluster and meta-analyses on factors influencing stress-induced alcohol drinking and relapse in rodents. Addict Biol. 2014;19:225–232. doi: 10.1111/adb.12125. [DOI] [PubMed] [Google Scholar]
- Olsen CM, Winder DG. Operant sensation seeking engages similar neural substrates to operant drug seeking in C57 mice. Neuropsychopharmacology. 2009;34:1685–1694. doi: 10.1038/npp.2008.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos GWC. The rat brain in stereotaxic coordinates. 5. Elsevier Academic Press; Amsterdam: 2005. [Google Scholar]
- Pellow S, Chopin P, File SE. Are the anxiogenic effects of yohimbine mediated by its action at benzodiazepine receptors? Neurosci Lett. 1985;55:5–9. doi: 10.1016/0304-3940(85)90303-9. [DOI] [PubMed] [Google Scholar]
- Pickens CL, Cifani C, Navarre BM, Eichenbaum H, Theberge FR, Baumann MH, Calu DJ, Shaham Y. Effect of fenfluramine on reinstatement of food seeking in female and male rats: implications for the predictive validity of the reinstatement model. Psychopharmacology. 2012;221:341–353. doi: 10.1007/s00213-011-2585-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnyai Z, Shaham Y, Heinrichs SC. The role of corticotropin-releasing factor in drug addiction. Pharmacol Rev. 2001;53:209–244. [PubMed] [Google Scholar]
- Schwager AL, Haack AK, Taha SA. Impaired flexibility in decision making in rats after administration of the pharmacological stressor yohimbine. Psychopharmacology. 2014 doi: 10.1007/s00213-014-3529-y. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE, Waters RP. Pharmacologically-induced stress: a cross-species probe for translational research in drug addiction and relapse. Am J Transl Res. 2010;3:81–89. [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev. 2000a;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Funk D, Erb S, Brown TJ, Walker CD, Stewart J. Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. J Neurosci. 1997;17:2605–2614. doi: 10.1523/JNEUROSCI.17-07-02605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Highfield D, Delfs J, Leung S, Stewart J. Clonidine blocks stress-induced reinstatement of heroin seeking in rats: an effect independent of locus coeruleus noradrenergic neurons. Eur J Neurosci. 2000b;12:292–302. doi: 10.1046/j.1460-9568.2000.00899.x. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shalev U, Erb S, Shaham Y. Role of CRF and other neuropeptides in stress-induced reinstatement of drug seeking. Brain Res. 2010;1314:15–28. doi: 10.1016/j.brainres.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Shin R, Cao J, Webb SM, Ikemoto S. Amphetamine administration into the ventral striatum facilitates behavioral interaction with unconditioned visual signals in rats. PLoS One. 2010;5:e8741. doi: 10.1371/journal.pone.0008741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staiti AM, Morgane PJ, Galler JR, Grivetti JY, Bass DC, Mokler DJ. A microdialysis study of the medial prefrontal cortex of adolescent and adult rats. Neuropharmacology. 2011;61:544–549. doi: 10.1016/j.neuropharm.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J. Reinforcing effects of light as a function of intensity and reinforcement schedule. J Comp Physiol Psychol. 1960;53:187–193. doi: 10.1037/h0047315. [DOI] [PubMed] [Google Scholar]
- Stine SM, Southwick SM, Petrakis IL, Kosten TR, Charney DS, Krystal JH. Yohimbine-induced withdrawal and anxiety symptoms in opioid-dependent patients. Biol Psychiatry. 2002;51:642–651. doi: 10.1016/s0006-3223(01)01292-6. [DOI] [PubMed] [Google Scholar]
- Sun H, Green TA, Theobald DE, Birnbaum SG, Graham DL, Zeeb FD, Nestler EJ, Winstanley CA. Yohimbine increases impulsivity through activation of cAMP response element binding in the orbitofrontal cortex. Biol Psychiatry. 2010;67:649–656. doi: 10.1016/j.biopsych.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Bassareo V, Di Chiara G. Mianserin markedly and selectively increases extracellular dopamine in the prefrontal cortex as compared to the nucleus accumbens of the rat. Psychopharmacology. 1996;123:127–130. doi: 10.1007/BF02246169. [DOI] [PubMed] [Google Scholar]
- Trigo JM, Zimmer A, Maldonado R. Nicotine anxiogenic and rewarding effects are decreased in mice lacking beta-endorphin. Neuropharmacology. 2009;56:1147–1153. doi: 10.1016/j.neuropharm.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Umhau JC, Schwandt ML, Usala J, Geyer C, Singley E, George DT, Heilig M. Pharmacologically induced alcohol craving in treatment seeking alcoholics correlates with alcoholism severity, but is insensitive to acamprosate. Neuropsychopharmacology. 2011;36:1178–1186. doi: 10.1038/npp.2010.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollrath-Smith FR, Shin R, Ikemoto S. Synergistic interaction between baclofen administration into the median raphe nucleus and inconsequential visual stimuli on investigatory behavior of rats. Psychopharmacology. 2012;220:15–25. doi: 10.1007/s00213-011-2450-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbauer NE, Balleine BW. The influence of amphetamine on sensory and conditioned reinforcement: evidence for the re-selection hypothesis of dopamine function. Front Integr Neurosci. 2007;1:9. doi: 10.3389/neuro.07.009.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.