Abstract
Astrocytic glutamate transporters, the excitatory amino acid transporter (EAAT) 2 and EAAT1 [glutamate transporter 1 (GLT-1) and glutamate aspartate transporter (GLAST) in rodents, respectively], are the main transporters for maintaining optimal glutamate levels in the synaptic clefts by taking up more than 90% of glutamate from extracellular space thus preventing excitotoxic neuronal death. Reduced expression and function of these transporters, especially EAAT2, has been reported in numerous neurological disorders, including amyotrophic lateral sclerosis, Alzheimer’s disease, Parkinson’s disease, schizophrenia and epilepsy. The mechanism of down-regulation of EAAT2 in these diseases has yet to be fully established. Genetic as well as transcriptional dys-regulation of these transporters by various modes, such as single nucleotide polymorphisms (SNPs) and epigenetics, resulting in impairment of their functions, might play an important role in the etiology of neurological diseases. Consequently, there has been an extensive effort to identify molecular targets for enhancement of EAAT2 expression as a potential therapeutic approach. Several pharmacological agents increase expression of EAAT2 via NF-κB and CREB at the transcriptional level. However, the negative regulatory mechanisms of EAAT2 have yet to be identified. Recent studies, including those from our laboratory, suggest that the transcriptional factor yin yang 1 (YY1) plays a critical role in the repressive effects of various neurotoxins, such as manganese (Mn), on EAAT2 expression. In this review, we will focus on transcriptional epigenetics, and translational regulation of EAAT2.
Keywords: manganese, EAAT2, GLT-1, single nucleotide polymorphisms, RNA splicing, transcription, epigenetic, NF-κB, YY1, HDACs
1. Introduction
Astrocytes are critically involved in neuronal function and survival, as they produce neurotrophic factors, such as brain-derived neurotrophic factor (BDNF) and glia-derived neurotrophic factor (GDNF), as well as express two main glutamate transporters responsible for the removal of excessive glutamate from the synaptic clefts [1, 2]. Glutamate is the major excitatory neurotransmitter in the central nervous system (CNS), playing a major role in memory and cognitive function [3], and glutamate transporters as such prevent the overstimulation of post-synaptic glutamate receptors that lead to excitotoxic neuronal injury [4, 5]. Among the five subtypes of glutamate transporters identified, glutamate aspartate transporter (GLAST) and glutamate transporter-1 (GLT-1) [excitatory amino acid transporter (EAAT) 1 and 2 in humans, respectively], are predominantly expressed in astrocytes. They are responsible for the uptake of excess glutamate from the extracellular space [6-8], supported by the fact that knockdown of either GLT-1 or GLAST in mice increases extracellular glutamate levels, leading to excitotoxicity related neurodegeneration and progressive paralysis [9]. In the adult brain, that EAAT2 accounts for >90% of the extracellular glutamate clearance [10-12], since the genetic deletion of both alleles of GLT-1 in mice led to the development of lethal seizures [13]. On the other hand, EAAT1 plays a major role during development [14]. Notably, reduction of EAAT2 expression and function is associated with numerous neurological disorders including amyotrophic lateral sclerosis (ALS), Alzheimer’s disease (AD), Parkinson’s disease (PD), schizophrenia and epilepsy [2, 15]. For example, EAAT2 protein expression is decreased in ALS [12] and AD patients [16], and cultured astrocytes from AD patients also show reduction of both EAAT1 and EAAT2 protein levels along with attenuated glutamate uptake [17]. Accordingly, potential drugs that target to enhance the expression and function of these transporters may serve as efficient therapeutics modalities to combat these diseases [2, 11].
Dysregulation of EAAT1 and EAAT2 expression and function occurs at multiple levels from abnormal genetic coding to altered posttranslational modifications. Genetic dysregulation of EAAT2, such as single nucleotide polymorphisms (SNPs) and aberrant mRNA splicing of EAAT2 are known to impair EAAT2 expression and function, and are linked to several neurological diseases [18, 19]. Several pharmacological agents, such as ceftriaxone [20], estrogen [21], tamoxifen [21, 22] and riluzole [23] increase EAAT1 and EAAT2 expression at the transcription level via activation of nuclear factor κB (NF-κB) [22, 24]. Negative regulatory mechanisms of EAAT1 and EAAT2 at the transcription level have been linked to the transcription factor yin yang 1(YY1) [25] and YY2 [26]. Manganese (Mn) and tumor necrosis factor-α (TNF-α) decreased EAAT2 via activation of YY1 [27]. Herein, we discuss the genetic and transcriptional modulatory mechanisms of EAAT2 linked to neurological disorders.
2. Genetic regulation of EAAT2 associated with neurological disorders
2.1. EAAT2 regulation by RNA splicing
Altered EAAT2 splice variants have been found in ALS as well as in other diseases, such as AD, and this abnormal splicing of EAAT2 mRNA contributes to the loss of EAAT2 protein in these diseases [28, 29]. An AD mouse model expresses altered EAAT2 splice variants in response to hypoxia [30]. Treatment with 3-nitropropionic acid (a chemical hypoxic agent) prior to deposition of amyloid altered the expression of the 5′-splice forms of mouse EAAT2/5UT3, EAAT2/5UT4, and EAAT2/5UT5 in the frontal cortex, hippocampus and cerebellum of the APP23 transgenic mouse model [30]. This indicates that hypoxia facilitates alternative splicing of EAAT2 in an AD model, providing a possible molecular mechanism linking higher vascular risk to early pathophysiology of AD. The splicing variants of EAAT2 mRNA containing a long 5′-UTR are associated with increased EAAT2 protein expression at the translational level in response to extracellular factors such as corticosterone and retinol [31]. Alternative splicing also occurs in the C-terminal of EAAT2, resulting in three different variants, referred to as GLT-1a, GLT-1b and GLT-1c [32]. GLT-1a is a normal form, containing 11 exons, while GLT-1b and GLT-1c terminate at exon 10 by generating a new C-terminus sequence. EAAT2 RNA splicing events regulated by 5′-regulatory sequences are impaired in astrocytic tumors [33] as human glioma cells U251 express aberrant EAAT2 mRNA, resulting in reduction of EAAT2 protein levels [33]. These observations indicate that the alternate RNA splicing variations of EAAT2 are linked to several neurological diseases, including ALS, AD and glioma.
2.2. EAAT2 regulation by single nucleotide polymorphisms (SNPs)
The EAAT2 promoter contains consensus sites for several transcription factors (TFs) and thus, SNPs in these regions could alter TF bindings to the EAAT2 promoter, resulting in dysregulation of EAAT2 expression and function. Nucleotide change from A to C in −181 position of the EAAT2 promoter transform the consensus sequences of activator protein-2 (AP-2) (a positive TF) to GC-binding factor-2 (GCF-2) (a negative TF), resulting in decreased EAAT2 expression and glutamate uptake [18]. Increased plasma glutamate levels associated with this SNP might trigger strokes. Moreover, the same A to C SNPs on −181 position of the EAAT2 promoter decreases EAAT2 expression and increases plasma glutamate levels during relapse in multiple sclerosis (MS) patients [34]. Another study conducted in a healthy Japanese population revealed that the −181 A to C SNPs in the EAAT2 promoter affects the personality trait of reward dependence [35]. Recently, this SNP variant rs4354668 (-181 A to C) in EAAT2 gene has gained more attention regarding its role in various neurological disorders. For example, EAAT2 −181 A to C variant that causes lower EAAT2 expression and leads to higher prefrontal cortex glutamate levels is associated with impaired prefrontal cognitive performance during schizophrenia [36]. Moreover, this EAAT2 SNP variant has been reported to be responsible for increased recurrence of episodes in bipolar disorder and lower gray matter volumes with poorer working memory performance in schizophrenic patients [37, 38]. The SNPs in EAAT2 gene are linked to higher susceptibility to schizophrenia in the Japanese population [39]. Another study reported the EAAT2 SNP variant rs1885343 in which the GG genotype decreases EAAT2 protein expression compared to AA or AG genotypes in the nucleus accumbens [40].
On the other hand, polymorphism in coding regions of EAAT2, resulting from replacement of the amino acid glycine with arginine (EAAT2 G603A variant), confer vulnerability to risk-taking behavior in alcoholics and is also associated with alcoholic cirrhosis [41, 42]. DNA demethylation on selective DNA demethylation on selective
3. Transcriptional regulation of EAAT2
The EAAT2 promoter contains cis-elements for several transcription factors, such as NF-κB, Sp1, N-myc, NFAT and YY1 [44]. Several pharmacological agents, such as epidermal growth factor (EGF), transforming growth factor-α (TGF-α), dibutyryl-AMP increase EAAT2 promoter activity, mRNA and protein levels, whereas tumor necrosis factor-α (TNF-α) decrease EAAT2 expression in primary human fetal astrocytes [44].
3.1. Positive transcriptional regulation of EAAT2
Several studies have shown that the NF-κB pathway is critical for positive transcription of EAAT2. EGF, ceftriaxone and estrogenic compounds including 17β-estradiol as well as tamoxifen and raloxifene (selective estrogen receptor modulators [SERMs]) activate the NF-κB pathway to enhance EAAT2 expression at the transcriptional level [22, 24, 44, 45]. The EAAT2 promoter contains at least three NF-κB binding motifs at −583, −272 and −251 in the promoter sequences and mutations in any of these sites significantly decrease EAAT2 promoter activity [22, 46]. EGF enhances expression of EAAT2 mRNA and protein levels via activation of NF-κB binding to −583 site of the EAAT2 promoter [46]. EGF increases phosphorylation of MEK1/2 rather than activating the conventional IκB pathway in order to activate NF-κB and subsequent increase of EAAT2 mRNA levels. Ceftriaxone, a β–lactam antibiotic, enhances EAAT2 expression and function in the brain, exerting neuroprotective effects in an ALS mouse model [20]. NF-κB binding site at −272 of the EAAT2 promoter is critical for the ceftriaxone-induced increase in EAAT2 promoter activity [24]. Ceftriaxone activates the conventional NF-κB pathway with degradation of IκBα and nuclear translocation of p65 isoform of NF-κB [24]. Moreover, neurons enhance EAAT2 expression in astrocytes when they are co-cultured [47, 48]. Although factors released from neurons that are responsible for increasing astrocytic EAAT2 expression are not well understood, NF-κB appears to be critically involved in neuronal activation of EAAT2 [49]. NF-κB binding sites at −583 or −251 of the EAAT2 promoter are important for neuronal activation of EAAT2 promoter activity and both NF-κB isoforms, p65 and p50, interact with these sites to enhance EAAT2 promoter activity [49]. In addition, the activation of kappa B-motif binding phospho-protein (KBPP) is involved in neuronal activation of EAAT2 promoter activity [50]. Reduced KBPP expression is correlated with transcriptional dysfunction of EAAT2, decreasing EAAT2 mRNA and protein levels. We have reported that 17β-estradiol via GPR30 [51] and tamoxifen as well as raloxifene, can all exert neuroprotection [52-56] and enhance EAAT2 expression by activation of the NF-κB pathway [22, 27]. These observations suggest that NF-κB serves as a critical transcription factor mediating the effects of positive modulators of EAAT2. Nonetheless, the disturbed positive NF-κB regulation of EAAT2 associated with lower EAAT2 expression under neuropathologic condition remains to be established.
Other transcription factors, such as cAMP response element binding protein (CREB) might also positively regulate EAAT2 promoter activity. We have reported that CREB plays a critical role in tamoxifen-induced up-regulation of EAAT2 in in vitro culture of rat primary astrocytes [22]. Mutation of CREB binding site at −308 of the EAAT2 promoter significantly decreases EAAT2 promoter activity. Tamoxifen activates both NF-κB and CREB to increase EAAT2 promoter activity, establishing that both factors are critical in tamoxifen-induced enhancement of EAAT2 expression [22].
PI3K/Akt is also positively modulating transcriptional regulation of EAAT2 [44, 57]. Overexpression of Akt increases EAAT2 mRNA levels and mediates EGF-enhanced EAAT2 expression [57]. The protein kinase A (PKA) also mediates dbcAMP- and tamoxifen-enhanced EAAT2 promoter activity [22, 44].
3.2. Negative transcriptional regulation of EAAT2
Most of the studies on the mechanisms of EAAT2 regulation have been directed at positive regulation. Few have addressed negative regulatory mechanisms of EAAT2 expression. One such study reported that a negative regulatory mechanism of EAAT2 is mediated by TNF-α where the latter decreases EAAT2 mRNA expression by co-activation of both NF-κB and N-myc concurrently [46].
The transcription factor yin yang 1(YY1) is a critical negative regulator of astrocytic glutamate transporters. YY1 is a multifunctional transcription factor, acting as a transcriptional initiator, activator or repressor, depending on its interaction with available cellular co-factors [58]. YY1 is a critical transcription factor in regulating a variety of biological processes such as cell proliferation and differentiation, DNA repair, and apoptosis [59], regulating multiple genes involved in cell cycle transitions, many of which are oncogenes and tumor-suppressor genes [58]. YY1 also plays an important role in the brain, as it is involved in neural development, neuronal function, developmental myelination, yet it may also contribute to neurological diseases [60]. For example, YY1 might be involved in the pathogenesis of AD by beta-site precursor protein-cleaving enzyme 1 (BACE1) promoter in neurons and astrocytes [61]. BACE1 cleaves amyloid precursor protein (APP) to produce β-amyloid, which deposits in the AD brain and is one of the major hallmarks of AD. YY1 has also been reported to play a role in the regulation of genes that are involved in heritable neurodegenerative disease Charcot-Marie-Tooth disease and in a severe neurodevelopmental disorder called Rett syndrome [62, 63]. In addition, a role for YY1 in the negative regulation of EAAT2 has been implicated given its ability to serve as a co-repressor of astrocyte elevated gene-1 (AEG-1) to repress EAAT2 at the transcriptional level, resulting in reduced glutamate uptake in astrocytes [26]. We have also reported that YY1 is a critical repressor of the EAAT2 promoter, as overexpression of YY1 decreases, whereas knockdown of YY1 or mutation of YY1 binding site in the EAAT2 promoter increases EAAT2 promoter activity [27].
4. Epigenetic deregulation in neurological disorders
Epigenetic modifications such as methylation or acetylation of histones and methylation of DNA are altered in several genes including GLT-1 (EAAT2) associated with neurodegeneration [64]. Epigenetic DNA methylation involve DNA methyltransferases (DNMT), an enzyme transferring a methyl group from S-adenosyl-l-methionine to the carbon 5 position of cytosine resulting in gene silencing [65]. Methylation of the SNCA gene, coding for alpha-synuclein, which is involved in formation of Lewy body in PD, is known to take place, leading to a decrease of gene expression in PD patients [66]. DNA methylation modification is also found in postmortem frontal cortex tissue derived from bipolar disorder (BD) and AD patients, showing hypomethylation of cyclooxygenase-2 (COX-2) and hypermethylation of the BDNF promoter regions in these patients [67]. DNMT mRNA expression is altered in suicide brains, and this change is associated with increased methylation of a gamma-aminobutyric acid (GABA) A type receptor (GABAA) alpha1 subunit gene whose mRNA expression is reduced in the cortex [68]. Human subjects who experience childhood abuse show increased methylation of a stress responder the glucocorticoid receptor (GR) promoter along with reduced expression of GR mRNA levels in the hippocampus [69]. Chronic social stress induces histone modifications in the BDNF promoter along with reduced BDNF mRNA levels [70]. These observations suggest that alteration in epigenetic regulation mechanisms are closely associated with altered gene expression in neurological disorders.
4.1 Epigenetic dysregulation of EAAT2
Histone modification by acetylation also plays a major role in the epigenetic regulation of EAAT2 expression. Histone acetylation modification is characterized by the addition and removal of acetyl moiety from acetyl-coenzyme A to the ε-amino group of lysine residue; this reaction is carried out by two enzymes, histone acetyltransferases (HATs) and histone deacetylases (HDACs) [71]. Histone deacetylases (HDACs) inhibitors, such as TSA and valproic acid (VPA) increase EAAT2 mRNA and protein levels, indicating the role of acetylation in regulating EAAT2 expression [64, 72, 73]. In addition, CREB-binding protein (CBP), a HAT, has been reported to contribute as a co-repressor of YY1 in the negative regulation of EAAT2 [26].
DNA demethylation on selective CpG sites of the GLT-1 (EAAT2) promoter is highly correlated to increased GLT-1 mRNA levels in mouse brain astrocytes in response to neuronal stimulation [74]. However, low level of methylation was found on CpG sites of EAAT2 promoter from postmortem motor cortex of human ALS patients. Nonetheless, the limitations of using human postmortem tissues could compromise the methylation analysis of EAAT2 promoter for several reasons; (1) detection of the methylation changes in bulk tissue may not represent the loss of EAAT2/GLT1 in the limited area in patient with ALS or transgenic rodent models of ALS, (2) quantification of EAAT2 mRNA may not be accurate due to the repeated freeze-thaw cycle for tissue sample storage which might lead to unstable RNA, and (3) a small sample size for control and patients with ALS. The same authors suggested that a large-scale whole genome DNA methylation analysis of the pathological tissues of larger number of patients with ALS and controls is warranted to reveal the possible epigenetic changes involved in ALS astrocytes in the future [74].
Moreover, DNA methylation analysis in human glioma cell lines and human brain tissue has shown that increased methylation in the EAAT2 promoter is associated with reduced EAAT2 expression [75]. The inhibition of DNMT restores EAAT2 transcription, suggesting a role for methylation in reduction of EAAT2 transcription [75]. Region-specific expression of EAAT2 appears to be associated with the methylation status of the EAAT2 promoter, since higher methylation is detected in the cerebellum compared to the cortex, and is inversely correlated with the region-specific EAAT2 expression [76]. Dexamethasone increases EAAT2 expression in the cortex, but its effect in the cerebellum is minimal due to hypermethylation of EAAT2 in that region [77]. Moreover, neuronal regulation of EAAT2 expression in neuronal-astrocyte co-cultures induces hypomethylation of CpG sites on the EAAT2 promoter, resulting in increased EAAT2 mRNA levels [62].
5. Post translational deregulation of EAAT2
Palmitoylation is one of the posttranslational modifications of proteins in which palmitate is attached to cysteine residues via a thioester linkage by palmitoyl acyl transferases (PATs) (reviewed in [78]). Palmitoylation at cysteine38 (C38) is required for normal EAAT2 (GLT-1) function [79], thus inhibition of palmitoylation severely impairs glutamate uptake. Palmitoylation of EAAT2 (GLT-1) has been shown to be reduced in the YAC128 HD mouse model along with decrease of glutamate uptake, suggesting the role of palmitoylation in EAAT2 (GLT-1) function [79].
Sumoylation is also playing a role in EAAT2 posttranslational modulation. Sumoylated EAAT2 localizes to intracellular compartments, while non-sumoylated EAAT2 resides on the plasma membrane, consistent with the results that desumoylation increases EAAT2-mediated glutamate uptake in primary astrocytes [80]. Moreover, caspase-3 cleaved EAAT2 generates sumoylated proteolytic fragment (CTE), followed by intracellular accumulation of sumoylated CTE in organelles, such as the nucleus and endosome in spinal cord astrocytes of ALS mice [80, 81]. Prolonged nuclear accumulation of CTE induces neuronal toxicity by axonal growth impairment in primary motor neurons, suggesting that sumoylated proteolytic fragment of the astroglial glutamate transporter EAAT2 could participate to the pathogenesis of ALS [81]. Based on these recent findings, sumoylation has been considered as an important pathway in ALS [82].
Ubiquitination of the C-terminal tail of EAAT2 (GLT-1) has also been reported. Ubiquitination mediates internalization and degradation of EAAT2 (GLT-1) via PKC, resulting in decrease of glutamate uptake in C6 glioma cells or primary cortical cultures, suggesting that this ubiquitin-mediated PKC-dependent degradation of EAAT2 might possibly increase under pathological conditions [83]. Activation of PKC increases the ubiquitination of EAAT2 (GLT-1) both in vitro and in vivo experimental conditions, leading to accumulation of ubiquitinated EAAT2 (GLT-1) in the intracellular compartment [84]. Accordingly, inhibition of the ubiquitin-activating enzyme E1 promotes the retention of GLT-1 at the plasma membrane. The translocation of EAAT2 (GLT-1) from the recycling endosomes to the plasma membrane is blocked by inhibition of the deubiquitinating enzyme (DUB) ubiquitin C-terminal hydrolase-L1, suggesting the existence of specific ubiquitination/deubiquitination cycles in regulating optimal concentrations of GLT-1 at the cell surface [85].
6. Mn-induced transcriptional reduction of EAAT2
Manganese (Mn) is well known to decrease expression of EAAT1 as well as EAAT2 with consequential reduction of glutamate uptake [45, 53, 86, 87]. However, the mechanisms of Mn-induced reduction of EAAT1 and 2 at the transcriptional level remain to be established. We have reported that YY1 might be the critical transcription factor in mediating Mn’s effect on reduced EAAT2 expression and function [27].
6.1. Role of YY1 in Mn-induced repression of EAAT2
Although Mn is an essential trace element in the body, serving as a cofactor for enzymes such as MnSOD and glutamine synthetase, its chronic excessive accumulation in the brain from environmental or occupational sources leads to a neurological disorder called manganism that shares similar pathological features with PD [88, 89]. Moreover, Mn neurotoxicity is also known to contribute to the development of multiple neurodegenerative disorders including AD, PD, ALS and Huntington disease (HD) [90]. Despite of its significant impact on multiple neurodegenerative diseases, the mechanisms of Mn-induced neurotoxicity are not completely understood. Several mechanisms, including oxidative stress and mitochondrial impairment have been reported [91-93]. Mn-induced excitotoxic neuronal injury is also considered to be a critical mechanism involved in Mn neurotoxicity. MK801, an N-methyl-D-aspartate (NMDA) antagonist blocks excitotoxic lesions in the striatum of Mn-injected rats [94]. Moreover, Mn decreases the expression and function of both astrocytic glutamate transporters, EAAT1 and EAAT2 [45, 53, 86, 87], representing a critical mechanism for Mn-induced neurotoxicity. Since there is no direct binding sites for Mn at the DNA levels identified, it is likely that Mn-induced oxidative stress [95] and inflammation [96] might mediate its repressive actions on glutamate transporters. We have found that YY1 mediates Mn-induced inhibitory effects on EAAT2. Mn increases YY1 promoter activity, mRNA and protein levels [27]. Mn enhances YY1 binding to its consensus sites in the EAAT2 promoter and accordingly, mutation of YY1 binding sites attenuate the Mn-induced decrease in EAAT2 promoter activity, indicating that YY1 is a critical transcriptional mediator in Mn-induced repression of EAAT2 [27].
6.2. Mechanism of Mn-induced repression of EAAT2 via YY1
Mn likely activates YY1 via proinflammatory mediators, as it potentiates the release of several inflammatory molecules including prostaglandins, cytokines, such as TNF-α, interleukin (IL)-6, IL-1β, as well as nitric oxide from activated glial cells [96-99]. TNF-α and IL-1β are negative regulators of EAAT2 and they decrease EAAT2 mRNA and protein levels in astrocytes [44, 46, 100-102]. Findings from our studies indicate that Mn increases production of TNF-α which, in turn, increases YY1 promoter activity, mRNA and protein levels in astrocytes [27], suggesting that TNF-α mediates Mn effects on reduction of EAAT2 expression via YY1.
NF-κB is involved also in Mn-induced repression of EAAT2 via YY1. Although NF-κB is a major positive regulator of EAAT2, resembling other inflammatory cytokines, such as TNF-α, Mn activates the NF-κB pathway [103], it represses EAAT2. We have shown that Mn activates YY1 via activation of NF-κB, and moreover, Mn-induced activation of the YY1 pathways is dominant over its activation of NF-κB, overriding the positive effects of NF-κB on EAAT2 [27].
7. Conclusion
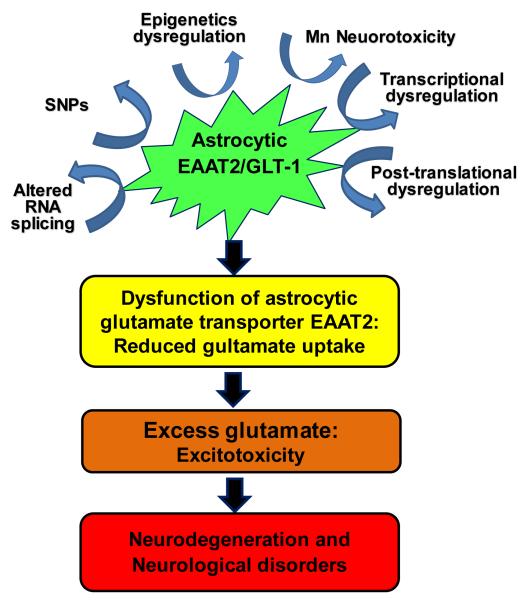
The reduced expression and function of astrocytic glutamate transporter EAAT1 and EAAT2 has been associated with numerous neurodegenerative diseases. Accordingly, understanding the precise molecular mechanisms involved in the transcriptional and translational dys-regulation of EAAT2, as well as other altered genetic regulatory mechanisms, such as SNPs and epigenetics is critical for the development of efficacious drugs for treatment of the neurological disorders associated with impairment of EAAT2 expression (Fig. 1). At the transcriptional level, the NF-κB and CREB pathways play critical roles in enhancing EAAT2 expression, mediating the effects of positive modulators of EAAT2. In addition, delineating the negative regulatory mechanisms of EAAT2 will be highly beneficial, because targeting this pathway can rescue and reverse the reduced expression and function and potentially delay the progression of neurodegenerative diseases. The YY1 pathway contributes to negative regulation of EAAT2, mediating TNF-α- and Mn-induced inhibitory effects on EAAT2 expression and function. Taken together, understanding positive and negative regulatory mechanisms of EAAT2 expression will offer novel therapeutic approaches to treat neurological disorders associated with excitotoxic neuronal injury.
Fig. 1. Proposed mechanisms of dysregulated EAAT2 expression and function that lead to excitotoxic neurodegeneration at multiple gene regulation levels.
At genetic level, altered RNA splicing and SNPs play, while at transcriptional level, YY1 and NF-κB as a repressor and an activator of EAAT2, respectively. Moreover, YY1 mediates the inhibitory modulation of EAAT2 induced by TNF-α as well as Mn as a part of its neurotoxicity mechanism. Epigenetic modifiers such as HDACs and post-translational modulators such as ubiquitin also play roles in the modulation of EAAT2 expression and function. The dysregulation of any of aforementioned mechanisms might lead to a decrease of EAAT2 expression and function resulting in triggering the excitotoxic neuropathological changes in many neurological disorders.
Acknowledgements
The present study was supported in part by NIH grants, NIGMS SC1089630 (EL) and R01ES10563 and R01ES10563S1 (MA).
References
- 1.Chai L, Guo H, Li H, Wang S, Wang YL, Shi F, Hu LM, Liu Y, Adah D. Scutellarin and caffeic acid ester fraction, active components of Dengzhanxixin injection, upregulate neurotrophins synthesis and release in hypoxia/reoxygenation rat astrocytes. Journal of ethnopharmacology. 2013;150:100–107. doi: 10.1016/j.jep.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 2.Sheldon AL, Robinson MB. The role of glutamate transporters in neurodegenerative diseases and potential opportunities for intervention. Neurochemistry international. 2007;51:333–355. doi: 10.1016/j.neuint.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Platt SR. The role of glutamate in central nervous system health and disease--a review. Veterinary journal (London, England : 1997) 2007;173:278–286. doi: 10.1016/j.tvjl.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Sattler R, Tymianski M. Molecular mechanisms of glutamate receptor-mediated excitotoxic neuronal cell death. Molecular neurobiology. 2001;24:107–129. doi: 10.1385/MN:24:1-3:107. [DOI] [PubMed] [Google Scholar]
- 5.Doble A. The role of excitotoxicity in neurodegenerative disease: implications for therapy. Pharmacology & therapeutics. 1999;81:163–221. doi: 10.1016/s0163-7258(98)00042-4. [DOI] [PubMed] [Google Scholar]
- 6.Furuta A, Rothstein JD, Martin LJ. Glutamate transporter protein subtypes are expressed differentially during rat CNS development. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:8363–8375. doi: 10.1523/JNEUROSCI.17-21-08363.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lehre KP, Levy LM, Ottersen OP, Storm-Mathisen J, Danbolt NC. Differential expression of two glial glutamate transporters in the rat brain: quantitative and immunocytochemical observations. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1995;15:1835–1853. doi: 10.1523/JNEUROSCI.15-03-01835.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanaka K. Functions of glutamate transporters in the brain. Neuroscience research. 2000;37:15–19. doi: 10.1016/s0168-0102(00)00104-8. [DOI] [PubMed] [Google Scholar]
- 9.Bristol LA, Rothstein JD. Glutamate transporter gene expression in amyotrophic lateral sclerosis motor cortex. Annals of neurology. 1996;39:676–679. doi: 10.1002/ana.410390519. [DOI] [PubMed] [Google Scholar]
- 10.Danbolt NC. Glutamate uptake. Progress in neurobiology. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 11.Kim K, Lee SG, Kegelman TP, Su ZZ, Das SK, Dash R, Dasgupta S, Barral PM, Hedvat M, Diaz P, Reed JC, Stebbins JL, Pellecchia M, Sarkar D, Fisher PB. Role of excitatory amino acid transporter-2 (EAAT2) and glutamate in neurodegeneration: opportunities for developing novel therapeutics. Journal of cellular physiology. 2011;226:2484–2493. doi: 10.1002/jcp.22609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rothstein JD, Van Kammen M, Levey AI, Martin LJ, Kuncl RW. Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. Annals of neurology. 1995;38:73–84. doi: 10.1002/ana.410380114. [DOI] [PubMed] [Google Scholar]
- 13.Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 14.Regan MR, Huang YH, Kim YS, Dykes-Hoberg MI, Jin L, Watkins AM, Bergles DE, Rothstein JD. Variations in promoter activity reveal a differential expression and physiology of glutamate transporters by glia in the developing and mature CNS. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:6607–6619. doi: 10.1523/JNEUROSCI.0790-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin CL, Kong Q, Cuny GD, Glicksman MA. Glutamate transporter EAAT2: a new target for the treatment of neurodegenerative diseases. Future medicinal chemistry. 2012;4:1689–1700. doi: 10.4155/fmc.12.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdul HM, Sama MA, Furman JL, Mathis DM, Beckett TL, Weidner AM, Patel ES, Baig I, Murphy MP, LeVine H, 3rd, Kraner SD, Norris CM. Cognitive decline in Alzheimer’s disease is associated with selective changes in calcineurin/NFAT signaling. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:12957–12969. doi: 10.1523/JNEUROSCI.1064-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang Z, Valla J, Sefidvash-Hockley S, Rogers J, Li R. Effects of estrogen treatment on glutamate uptake in cultured human astrocytes derived from cortex of Alzheimer’s disease patients. Journal of neurochemistry. 2002;80:807–814. doi: 10.1046/j.0022-3042.2002.00779.x. [DOI] [PubMed] [Google Scholar]
- 18.Mallolas J, Hurtado O, Castellanos M, Blanco M, Sobrino T, Serena J, Vivancos J, Castillo J, Lizasoain I, Moro MA, Davalos A. A polymorphism in the EAAT2 promoter is associated with higher glutamate concentrations and higher frequency of progressing stroke. The Journal of experimental medicine. 2006;203:711–717. doi: 10.1084/jem.20051979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyer T, Munch C, Liebau S, Fromm A, Schwalenstocker B, Volkel H, Ludolph AC. Splicing of the glutamate transporter EAAT2: a candidate gene of amyotrophic lateral sclerosis. Journal of neurology, neurosurgery, and psychiatry. 1998;65:954. doi: 10.1136/jnnp.65.6.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes Hoberg M, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su ZZ, Gupta P, Fisher PB. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- 21.Lee E, Sidoryk-Wegrzynowicz M, Yin Z, Webb A, Son DS, Aschner M. Transforming growth factor-alpha mediates estrogen-induced upregulation of glutamate transporter GLT-1 in rat primary astrocytes. Glia. 2012;60:1024–1036. doi: 10.1002/glia.22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karki P, Webb A, Smith K, Lee K, Son DS, Aschner M, Lee E. cAMP response element-binding protein (CREB) and nuclear factor kappaB mediate the tamoxifen-induced up-regulation of glutamate transporter 1 (GLT-1) in rat astrocytes. The Journal of biological chemistry. 2013;288:28975–28986. doi: 10.1074/jbc.M113.483826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carbone M, Duty S, Rattray M. Riluzole elevates GLT-1 activity and levels in striatal astrocytes. Neurochemistry international. 2012;60:31–38. doi: 10.1016/j.neuint.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee SG, Su ZZ, Emdad L, Gupta P, Sarkar D, Borjabad A, Volsky DJ, Fisher PB. Mechanism of ceftriaxone induction of excitatory amino acid transporter-2 expression and glutamate uptake in primary human astrocytes. The Journal of biological chemistry. 2008;283:13116–13123. doi: 10.1074/jbc.M707697200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosas S, Vargas MA, Lopez-Bayghen E, Ortega A. Glutamate-dependent transcriptional regulation of GLAST/EAAT1: a role for YY1. Journal of neurochemistry. 2007;101:1134–1144. doi: 10.1111/j.1471-4159.2007.04517.x. [DOI] [PubMed] [Google Scholar]
- 26.Lee SG, Kim K, Kegelman TP, Dash R, Das SK, Choi JK, Emdad L, Howlett EL, Jeon HY, Su ZZ, Yoo BK, Sarkar D, Kim SH, Kang DC, Fisher PB. Oncogene AEG-1 promotes glioma-induced neurodegeneration by increasing glutamate excitotoxicity. Cancer research. 2011;71:6514–6523. doi: 10.1158/0008-5472.CAN-11-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karki P, Webb A, Smith K, Johnson J, Jr., Lee K, Son DS, Aschner M, Lee E. Yin Yang 1 Is a Repressor of Glutamate Transporter EAAT2, and It Mediates Manganese-Induced Decrease of EAAT2 Expression in Astrocytes. Molecular and cellular biology. 2014;34:1280–1289. doi: 10.1128/MCB.01176-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Honig LS, Chambliss DD, Bigio EH, Carroll SL, Elliott JL. Glutamate transporter EAAT2 splice variants occur not only in ALS, but also in AD and controls. Neurology. 2000;55:1082–1088. doi: 10.1212/wnl.55.8.1082. [DOI] [PubMed] [Google Scholar]
- 29.Meyer T, Fromm A, Munch C, Schwalenstocker B, Fray AE, Ince PG, Stamm S, Gron G, Ludolph AC, Shaw PJ. The RNA of the glutamate transporter EAAT2 is variably spliced in amyotrophic lateral sclerosis and normal individuals. Journal of the neurological sciences. 1999;170:45–50. doi: 10.1016/s0022-510x(99)00196-3. [DOI] [PubMed] [Google Scholar]
- 30.Munch C, Zhu BG, Mink A, Seefried U, Riepe MW, Ludolph AC, Meyer T. Chemical hypoxia facilitates alternative splicing of EAAT2 in presymptomatic APP23 transgenic mice. Neurochemical research. 2008;33:1005–1010. doi: 10.1007/s11064-007-9540-5. [DOI] [PubMed] [Google Scholar]
- 31.Tian G, Lai L, Guo H, Lin Y, Butchbach ME, Chang Y, Lin CL. Translational control of glial glutamate transporter EAAT2 expression. The Journal of biological chemistry. 2007;282:1727–1737. doi: 10.1074/jbc.M609822200. [DOI] [PubMed] [Google Scholar]
- 32.Holmseth S, Scott HA, Real K, Lehre KP, Leergaard TB, Bjaalie JG, Danbolt NC. The concentrations and distributions of three C-terminal variants of the GLT1 (EAAT2; slc1a2) glutamate transporter protein in rat brain tissue suggest differential regulation. Neuroscience. 2009;162:1055–1071. doi: 10.1016/j.neuroscience.2009.03.048. [DOI] [PubMed] [Google Scholar]
- 33.Munch C, Penndorf A, Schwalenstocker B, Troost D, Ludolph AC, Ince P, Meyer T. Impaired RNA splicing of 5′-regulatory sequences of the astroglial glutamate transporter EAAT2 in human astrocytoma. Journal of neurology, neurosurgery, and psychiatry. 2001;71:675–678. doi: 10.1136/jnnp.71.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pampliega O, Domercq M, Villoslada P, Sepulcre J, Rodriguez-Antiguedad A, Matute C. Association of an EAAT2 polymorphism with higher glutamate concentration in relapsing multiple sclerosis. Journal of neuroimmunology. 2008;195:194–198. doi: 10.1016/j.jneuroim.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 35.Matsumoto Y, Suzuki A, Ishii G, Oshino S, Otani K, Goto K. The −181 A/C polymorphism in the excitatory amino acid transporter-2 gene promoter affects the personality trait of reward dependence in healthy subjects. Neuroscience letters. 2007;427:99–102. doi: 10.1016/j.neulet.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 36.Spangaro M, Bosia M, Zanoletti A, Bechi M, Cocchi F, Pirovano A, Lorenzi C, Bramanti P, Benedetti F, Smeraldi E, Cavallaro R. Cognitive dysfunction and glutamate reuptake: effect of EAAT2 polymorphism in schizophrenia. Neuroscience letters. 2012;522:151–155. doi: 10.1016/j.neulet.2012.06.030. [DOI] [PubMed] [Google Scholar]
- 37.Dallaspezia S, Poletti S, Lorenzi C, Pirovano A, Colombo C, Benedetti F. Influence of an interaction between lithium salts and a functional polymorphism in SLC1A2 on the history of illness in bipolar disorder. Molecular diagnosis & therapy. 2012;16:303–309. doi: 10.1007/s40291-012-0004-5. [DOI] [PubMed] [Google Scholar]
- 38.Poletti S, Radaelli D, Bosia M, Buonocore M, Pirovano A, Lorenzi C, Cavallaro R, Smeraldi E, Benedetti F. Effect of glutamate transporter EAAT2 gene variants and gray matter deficits on working memory in schizophrenia. European psychiatry : the journal of the Association of European Psychiatrists. 2014;29:219–225. doi: 10.1016/j.eurpsy.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 39.Deng X, Shibata H, Ninomiya H, Tashiro N, Iwata N, Ozaki N, Fukumaki Y. Association study of polymorphisms in the excitatory amino acid transporter 2 gene (SLC1A2) with schizophrenia. BMC psychiatry. 2004;4:21. doi: 10.1186/1471-244X-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kerdsan W, Thanoi S, Nudmamud-Thanoi S, Reynolds GP. An association between genotypic variations and protein expression of the glial glutamate transporter 2 in the human nucleus accumbens. Neuroscience letters. 2012;523:108–110. doi: 10.1016/j.neulet.2012.06.052. [DOI] [PubMed] [Google Scholar]
- 41.Foley PF, Loh EW, Innes DJ, Williams SM, Tannenberg AE, Harper CG, Dodd PR. Association studies of neurotransmitter gene polymorphisms in alcoholic Caucasians. Annals of the New York Academy of Sciences. 2004;1025:39–46. doi: 10.1196/annals.1316.005. [DOI] [PubMed] [Google Scholar]
- 42.Sander T, Ostapowicz A, Samochowiec J, Smolka M, Winterer G, Schmidt LG. Genetic variation of the glutamate transporter EAAT2 gene and vulnerability to alcohol dependence. Psychiatric genetics. 2000;10:103–107. doi: 10.1097/00041444-200010030-00001. [DOI] [PubMed] [Google Scholar]
- 43.Wu J, Lee MR, Choi S, Kim T, Choi DS. ENT1 regulates ethanol-sensitive EAAT2 expression and function in astrocytes. Alcoholism, clinical and experimental research. 2010;34:1110–1117. doi: 10.1111/j.1530-0277.2010.01187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Su ZZ, Leszczyniecka M, Kang DC, Sarkar D, Chao W, Volsky DJ, Fisher PB. Insights into glutamate transport regulation in human astrocytes: cloning of the promoter for excitatory amino acid transporter 2 (EAAT2) Proceedings of the National Academy of Sciences of the United States of America. 2003;100:1955–1960. doi: 10.1073/pnas.0136555100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karki P, Webb A, Zerguine A, Choi J, Son DS, Lee E. Mechanism of raloxifene-induced upregulation of glutamate transporters in rat primary astrocytes. Glia. 2014 doi: 10.1002/glia.22679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sitcheran R, Gupta P, Fisher PB, Baldwin AS. Positive and negative regulation of EAAT2 by NF-kappaB: a role for N-myc in TNFalpha-controlled repression. The EMBO journal. 2005;24:510–520. doi: 10.1038/sj.emboj.7600555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gegelashvili G, Danbolt NC, Schousboe A. Neuronal soluble factors differentially regulate the expression of the GLT1 and GLAST glutamate transporters in cultured astroglia. Journal of neurochemistry. 1997;69:2612–2615. doi: 10.1046/j.1471-4159.1997.69062612.x. [DOI] [PubMed] [Google Scholar]
- 48.Schlag BD, Vondrasek JR, Munir M, Kalandadze A, Zelenaia OA, Rothstein JD, Robinson MB. Regulation of the glial Na+-dependent glutamate transporters by cyclic AMP analogs and neurons. Molecular pharmacology. 1998;53:355–369. doi: 10.1124/mol.53.3.355. [DOI] [PubMed] [Google Scholar]
- 49.Ghosh M, Yang Y, Rothstein JD, Robinson MB. Nuclear factor-kappaB contributes to neuron-dependent induction of glutamate transporter-1 expression in astrocytes. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:9159–9169. doi: 10.1523/JNEUROSCI.0302-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang Y, Gozen O, Watkins A, Lorenzini I, Lepore A, Gao Y, Vidensky S, Brennan J, Poulsen D, Won Park J, Li Jeon N, Robinson MB, Rothstein JD. Presynaptic regulation of astroglial excitatory neurotransmitter transporter GLT1. Neuron. 2009;61:880–894. doi: 10.1016/j.neuron.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee E, Sidoryk-Wegrzynowicz M, Wang N, Webb A, Son DS, Lee K, Aschner M. GPR30 regulates glutamate transporter GLT-1 expression in rat primary astrocytes. The Journal of biological chemistry. 2012;287:26817–26828. doi: 10.1074/jbc.M112.341867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Callier S, Morissette M, Grandbois M, Pelaprat D, Di Paolo T. Neuroprotective properties of 17beta-estradiol, progesterone, and raloxifene in MPTP C57Bl/6 mice. Synapse (New York, NY) 2001;41:131–138. doi: 10.1002/syn.1067. [DOI] [PubMed] [Google Scholar]
- 53.Lee ES, Yin Z, Milatovic D, Jiang H, Aschner M. Estrogen and tamoxifen protect against Mn-induced toxicity in rat cortical primary cultures of neurons and astrocytes. Toxicological sciences : an official journal of the Society of Toxicology. 2009;110:156–167. doi: 10.1093/toxsci/kfp081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Neill K, Chen S, Brinton RD. Impact of the selective estrogen receptor modulator, raloxifene, on neuronal survival and outgrowth following toxic insults associated with aging and Alzheimer’s disease. Experimental neurology. 2004;185:63–80. doi: 10.1016/j.expneurol.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 55.Tian DS, Liu JL, Xie MJ, Zhan Y, Qu WS, Yu ZY, Tang ZP, Pan DJ, Wang W. Tamoxifen attenuates inflammatory-mediated damage and improves functional outcome after spinal cord injury in rats. Journal of neurochemistry. 2009;109:1658–1667. doi: 10.1111/j.1471-4159.2009.06077.x. [DOI] [PubMed] [Google Scholar]
- 56.Wakade C, Khan MM, De Sevilla LM, Zhang QG, Mahesh VB, Brann DW. Tamoxifen neuroprotection in cerebral ischemia involves attenuation of kinase activation and superoxide production and potentiation of mitochondrial superoxide dismutase. Endocrinology. 2008;149:367–379. doi: 10.1210/en.2007-0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li LB, Toan SV, Zelenaia O, Watson DJ, Wolfe JH, Rothstein JD, Robinson MB. Regulation of astrocytic glutamate transporter expression by Akt: evidence for a selective transcriptional effect on the GLT-1/EAAT2 subtype. Journal of neurochemistry. 2006;97:759–771. doi: 10.1111/j.1471-4159.2006.03743.x. [DOI] [PubMed] [Google Scholar]
- 58.Gordon S, Akopyan G, Garban H, Bonavida B. Transcription factor YY1: structure, function, and therapeutic implications in cancer biology. Oncogene. 2006;25:1125–1142. doi: 10.1038/sj.onc.1209080. [DOI] [PubMed] [Google Scholar]
- 59.Shi Y, Lee JS, Galvin KM. Everything you have ever wanted to know about Yin Yang 1. Biochimica et biophysica acta. 1997;1332:F49–66. doi: 10.1016/s0304-419x(96)00044-3. [DOI] [PubMed] [Google Scholar]
- 60.He Y, Casaccia-Bonnefil P. The Yin and Yang of YY1 in the nervous system. Journal of neurochemistry. 2008;106:1493–1502. doi: 10.1111/j.1471-4159.2008.05486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nowak K, Lange-Dohna C, Zeitschel U, Gunther A, Luscher B, Robitzki A, Perez-Polo R, Rossner S. The transcription factor Yin Yang 1 is an activator of BACE1 expression. Journal of neurochemistry. 2006;96:1696–1707. doi: 10.1111/j.1471-4159.2006.03692.x. [DOI] [PubMed] [Google Scholar]
- 62.Forlani G, Giarda E, Ala U, Di Cunto F, Salani M, Tupler R, Kilstrup-Nielsen C, Landsberger N. The MeCP2/YY1 interaction regulates ANT1 expression at 4q35: novel hints for Rett syndrome pathogenesis. Human molecular genetics. 2010;19:3114–3123. doi: 10.1093/hmg/ddq214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ratajewski M, Pulaski L. YY1-dependent transcriptional regulation of the human GDAP1 gene. Genomics. 2009;94:407–413. doi: 10.1016/j.ygeno.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 64.Perisic T, Zimmermann N, Kirmeier T, Asmus M, Tuorto F, Uhr M, Holsboer F, Rein T, Zschocke J. Valproate and amitriptyline exert common and divergent influences on global and gene promoter-specific chromatin modifications in rat primary astrocytes. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:792–805. doi: 10.1038/npp.2009.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Johnson AA, Akman K, Calimport SR, Wuttke D, Stolzing A, de Magalhaes JP. The role of DNA methylation in aging, rejuvenation, and age-related disease. Rejuvenation research. 2012;15:483–494. doi: 10.1089/rej.2012.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jowaed A, Schmitt I, Kaut O, Wullner U. Methylation regulates alpha-synuclein expression and is decreased in Parkinson’s disease patients’ brains. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:6355–6359. doi: 10.1523/JNEUROSCI.6119-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rao JS, Keleshian VL, Klein S, Rapoport SI. Epigenetic modifications in frontal cortex from Alzheimer’s disease and bipolar disorder patients. Translational psychiatry. 2012;2:e132. doi: 10.1038/tp.2012.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Poulter MO, Du L, Weaver IC, Palkovits M, Faludi G, Merali Z, Szyf M, Anisman H. GABAA receptor promoter hypermethylation in suicide brain: implications for the involvement of epigenetic processes. Biological psychiatry. 2008;64:645–652. doi: 10.1016/j.biopsych.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 69.McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonte B, Szyf M, Turecki G, Meaney MJ. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature neuroscience. 2009;12:342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nature neuroscience. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- 71.Kuo MH, Allis CD. Roles of histone acetyltransferases and deacetylases in gene regulation. BioEssays : news and reviews in molecular, cellular and developmental biology. 1998;20:615–626. doi: 10.1002/(SICI)1521-1878(199808)20:8<615::AID-BIES4>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 72.Itoh M, Hiroi T, Nishibori N, Sagara T, Her S, Lee MS, Morita K. Trichostatin A enhances glutamate transporter GLT-1 mRNA levels in C6 glioma cells via neurosteroid-mediated cell differentiation. Journal of molecular neuroscience : MN. 2013;49:21–27. doi: 10.1007/s12031-012-9842-1. [DOI] [PubMed] [Google Scholar]
- 73.Hobo S, Eisenach JC, Hayashida K. Up-regulation of spinal glutamate transporters contributes to anti-hypersensitive effects of valproate in rats after peripheral nerve injury. Neurosci Lett. 2011;502:52–55. doi: 10.1016/j.neulet.2011.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang Y, Gozen O, Vidensky S, Robinson MB, Rothstein JD. Epigenetic regulation of neuron-dependent induction of astroglial synaptic protein GLT1. Glia. 2010;58:277–286. doi: 10.1002/glia.20922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zschocke J, Allritz C, Engele J, Rein T. DNA methylation dependent silencing of the human glutamate transporter EAAT2 gene in glial cells. Glia. 2007;55:663–674. doi: 10.1002/glia.20497. [DOI] [PubMed] [Google Scholar]
- 76.Perisic T, Holsboer F, Rein T, Zschocke J. The CpG island shore of the GLT-1 gene acts as a methylation-sensitive enhancer. Glia. 2012;60:1345–1355. doi: 10.1002/glia.22353. [DOI] [PubMed] [Google Scholar]
- 77.Zschocke J, Bayatti N, Clement AM, Witan H, Figiel M, Engele J, Behl C. Differential promotion of glutamate transporter expression and function by glucocorticoids in astrocytes from various brain regions. The Journal of biological chemistry. 2005;280:34924–34932. doi: 10.1074/jbc.M502581200. [DOI] [PubMed] [Google Scholar]
- 78.Linder ME, Deschenes RJ. Palmitoylation: policing protein stability and traffic. Nat Rev Mol Cell Biol. 2007;8:74–84. doi: 10.1038/nrm2084. [DOI] [PubMed] [Google Scholar]
- 79.Huang K, Kang MH, Askew C, Kang R, Sanders SS, Wan J, Davis NG, Hayden MR. Palmitoylation and function of glial glutamate transporter-1 is reduced in the YAC128 mouse model of Huntington disease. Neurobiology of disease. 2010;40:207–215. doi: 10.1016/j.nbd.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 80.Foran E, Rosenblum L, Bogush A, Pasinelli P, Trotti D. Sumoylation of the astroglial glutamate transporter EAAT2 governs its intracellular compartmentalization. Glia. 2014;62:1241–1253. doi: 10.1002/glia.22677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Foran E, Bogush A, Goffredo M, Roncaglia P, Gustincich S, Pasinelli P, Trotti D. Motor neuron impairment mediated by a sumoylated fragment of the glial glutamate transporter EAAT2. Glia. 2011;59:1719–1731. doi: 10.1002/glia.21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dangoumau A, Veyrat-Durebex C, Blasco H, Praline J, Corcia P, Andres CR, Vourc’h P. Protein SUMOylation, an emerging pathway in amyotrophic lateral sclerosis. The International journal of neuroscience. 2013;123:366–374. doi: 10.3109/00207454.2012.761984. [DOI] [PubMed] [Google Scholar]
- 83.Sheldon AL, Gonzalez MI, Krizman-Genda EN, Susarla BT, Robinson MB. Ubiquitination-mediated internalization and degradation of the astroglial glutamate transporter, GLT-1. Neurochemistry international. 2008;53:296–308. doi: 10.1016/j.neuint.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gonzalez-Gonzalez IM, Garcia-Tardon N, Gimenez C, Zafra F. PKC-dependent endocytosis of the GLT1 glutamate transporter depends on ubiquitylation of lysines located in a C-terminal cluster. Glia. 2008;56:963–974. doi: 10.1002/glia.20670. [DOI] [PubMed] [Google Scholar]
- 85.Martinez-Villarreal J, Garcia Tardon N, Ibanez I, Gimenez C, Zafra F. Cell surface turnover of the glutamate transporter GLT-1 is mediated by ubiquitination/deubiquitination. Glia. 2012;60:1356–1365. doi: 10.1002/glia.22354. [DOI] [PubMed] [Google Scholar]
- 86.Erikson K, Aschner M. Manganese causes differential regulation of glutamate transporter (GLAST) taurine transporter and metallothionein in cultured rat astrocytes. Neurotoxicology. 2002;23:595–602. doi: 10.1016/s0161-813x(02)00012-8. [DOI] [PubMed] [Google Scholar]
- 87.Hazell AS, Norenberg MD. Manganese decreases glutamate uptake in cultured astrocytes. Neurochemical research. 1997;22:1443–1447. doi: 10.1023/a:1021994126329. [DOI] [PubMed] [Google Scholar]
- 88.Aschner JL, Aschner M. Nutritional aspects of manganese homeostasis. Molecular aspects of medicine. 2005;26:353–362. doi: 10.1016/j.mam.2005.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huang CC, Chu NS, Lu CS, Wang JD, Tsai JL, Tzeng JL, Wolters EC, Calne DB. Chronic manganese intoxication. Archives of neurology. 1989;46:1104–1106. doi: 10.1001/archneur.1989.00520460090018. [DOI] [PubMed] [Google Scholar]
- 90.Bowman AB, Kwakye GF, Herrero Hernandez E, Aschner M. Role of manganese in neurodegenerative diseases. Journal of trace elements in medicine and biology : organ of the Society for Minerals and Trace Elements (GMS) 2011;25:191–203. doi: 10.1016/j.jtemb.2011.08.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Milatovic D, Yin Z, Gupta RC, Sidoryk M, Albrecht J, Aschner JL, Aschner M. Manganese induces oxidative impairment in cultured rat astrocytes. Toxicological sciences : an official journal of the Society of Toxicology. 2007;98:198–205. doi: 10.1093/toxsci/kfm095. [DOI] [PubMed] [Google Scholar]
- 92.Milatovic D, Zaja-Milatovic S, Gupta RC, Yu Y, Aschner M. Oxidative damage and neurodegeneration in manganese-induced neurotoxicity. Toxicology and applied pharmacology. 2009;240:219–225. doi: 10.1016/j.taap.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rao KV, Norenberg MD. Manganese induces the mitochondrial permeability transition in cultured astrocytes. The Journal of biological chemistry. 2004;279:32333–32338. doi: 10.1074/jbc.M402096200. [DOI] [PubMed] [Google Scholar]
- 94.Brouillet EP, Shinobu L, McGarvey U, Hochberg F, Beal MF. Manganese injection into the rat striatum produces excitotoxic lesions by impairing energy metabolism. Experimental neurology. 1993;120:89–94. doi: 10.1006/exnr.1993.1042. [DOI] [PubMed] [Google Scholar]
- 95.Chen CJ, Liao SL. Oxidative stress involves in astrocytic alterations induced by manganese. Experimental neurology. 2002;175:216–225. doi: 10.1006/exnr.2002.7894. [DOI] [PubMed] [Google Scholar]
- 96.Filipov NM, Dodd CA. Role of glial cells in manganese neurotoxicity. Journal of applied toxicology : JAT. 2012;32:310–317. doi: 10.1002/jat.1762. [DOI] [PubMed] [Google Scholar]
- 97.Filipov NM, Seegal RF, Lawrence DA. Manganese potentiates in vitro production of proinflammatory cytokines and nitric oxide by microglia through a nuclear factor kappa B-dependent mechanism. Toxicological sciences : an official journal of the Society of Toxicology. 2005;84:139–148. doi: 10.1093/toxsci/kfi055. [DOI] [PubMed] [Google Scholar]
- 98.Liu M, Cai T, Zhao F, Zheng G, Wang Q, Chen Y, Huang C, Luo W, Chen J. Effect of microglia activation on dopaminergic neuronal injury induced by manganese, and its possible mechanism. Neurotoxicity research. 2009;16:42–49. doi: 10.1007/s12640-009-9045-x. [DOI] [PubMed] [Google Scholar]
- 99.Liu X, Sullivan KA, Madl JE, Legare M, Tjalkens RB. Manganese-induced neurotoxicity: the role of astroglial-derived nitric oxide in striatal interneuron degeneration. Toxicological sciences : an official journal of the Society of Toxicology. 2006;91:521–531. doi: 10.1093/toxsci/kfj150. [DOI] [PubMed] [Google Scholar]
- 100.Korn T, Magnus T, Jung S. Autoantigen specific T cells inhibit glutamate uptake in astrocytes by decreasing expression of astrocytic glutamate transporter GLAST: a mechanism mediated by tumor necrosis factor-alpha. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2005;19:1878–1880. doi: 10.1096/fj.05-3748fje. [DOI] [PubMed] [Google Scholar]
- 101.Szymocha R, Akaoka H, Dutuit M, Malcus C, Didier-Bazes M, Belin MF, Giraudon P. Human T-cell lymphotropic virus type 1-infected T lymphocytes impair catabolism and uptake of glutamate by astrocytes via Tax-1 and tumor necrosis factor alpha. Journal of virology. 2000;74:6433–6441. doi: 10.1128/jvi.74.14.6433-6441.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang Z, Pekarskaya O, Bencheikh M, Chao W, Gelbard HA, Ghorpade A, Rothstein JD, Volsky DJ. Reduced expression of glutamate transporter EAAT2 and impaired glutamate transport in human primary astrocytes exposed to HIV-1 or gp120. Virology. 2003;312:60–73. doi: 10.1016/s0042-6822(03)00181-8. [DOI] [PubMed] [Google Scholar]
- 103.Lee E, Yin Z, Sidoryk-Wegrzynowicz M, Jiang H, Aschner M. 15-Deoxy-Delta12,14-prostaglandin J(2) modulates manganese-induced activation of the NF-kappaB, Nrf2, and PI3K pathways in astrocytes. Free radical biology & medicine. 2012;52:1067–1074. doi: 10.1016/j.freeradbiomed.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]