SUMMARY
How control of subcellular events in single cells determines morphogenesis on the scale of the tissue is largely unresolved. The stereotyped cross-midline mitoses of progenitors in the zebrafish neural keel [1–4] provide a unique experimental paradigm for defining the role and control of single cell orientation for tissue-level morphogenesis in vivo.
We show that the coordinated orientation of individual progenitor cell division in the neural keel is the cellular determinant required for morphogenesis into a neural tube epithelium with a single straight lumen. We find that Scribble is required for oriented cell division and that its function in this process is independent of canonical apico-basal and planar polarity pathways. We identify a role for Scribble in controlling clustering of α-Catenin foci in dividing progenitors. Loss of either Scrib or of N-cadherin results in abnormally oriented mitoses, reduced cross-midline cell divisions and similar neural tube defects. We propose that Scribble-dependent nascent cell-cell adhesion clusters between neuroepithelial progenitors contribute to define orientation of their cell division. Finally, our data demonstrate that while oriented mitoses of individual cells determine neural tube architecture, the tissue can in turn feed back on its constituent cells to define their polarization and cell division orientation to ensure robust tissue morphogenesis.
RESULTS
To identify mechanisms underlying the establishment of epithelial tissue form and shape, we focus on the immature epithelium of the zebrafish neural keel whose morphogenesis results in the formation of the lumenized neuroepithelial tube. Neural keel development is characterized by stereotyped oriented mitoses that generate two bilaterally distributed neural progenitors (Fig.1A,B) [1–4]. We focus on the symmetric oriented cell divisions of the neural keel in order to uncouple morphogenesis from cell fate. Here we set out to address whether oriented cell divisions are a determinant for morphogenesis of the zebrafish neural tube formation, and to define the underlying mechanisms that control the orientation of cell division in vivo.
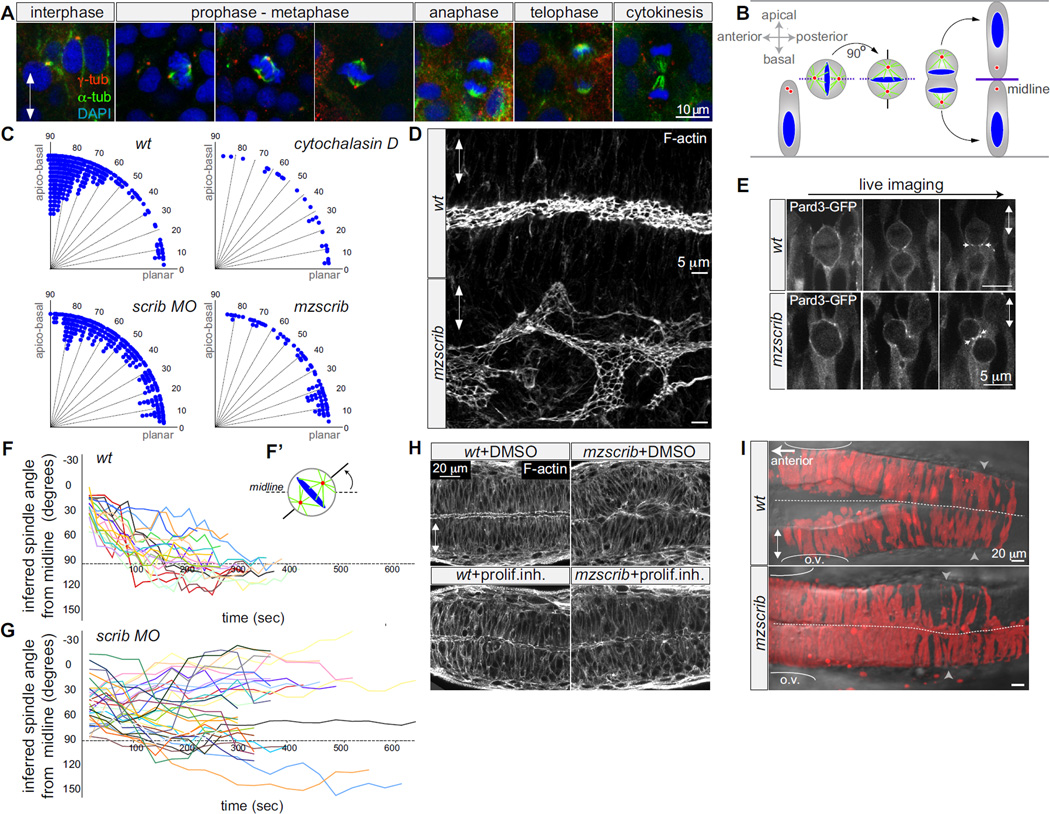
Figure 1. Scrib regulates mitotic spindle orientation and neural tube morphogenesis.
A) Immunostainings of neural keel progenitors showing that the orientation of the mitotic spindle changes over the course of mitosis. γ-tubulin (centrosomes; red), α-tubulin (spindle; green), and DAPI (DNA, blue). This process results in the bilateral distribution of daughter cells as schematized in (B). (C) Quantification of mitosis orientation at anaphase. Chi-square analysis shows that the three distributions shown are highly significantly different from wt (n=269): 3 µg/ml Cytochalasin-D treated (n=28, Χ2=51, 1 degree of freedom (df); p<0.001); scrib morphant (n=235, Χ2=306, 8 df; p<<0.001); mzscrib mutant (n=76, Χ2=201, 2 df; p<<0.001). While all three distributions are more homogeneous than wt, only Cytochalasin-D treatment results in a statistically randomized distribution (Χ2=2.7, 3 df; p=0.44). (D) Posterior hindbrain lumen morphology, defined by apical F-actin, with an abnormal, branched organization in the mzscrib mutant compared to wt. (E) Pard3-GFP localization to subapical foci upon cytokinesis (arrows) occurs normally in wt and misoriented mzscrib mutant neural keel progenitors. (F,G) Representation of mitotic spindle rotation in live (F) wt (n=20) and (G) abberantly rotating scrib morphant (n=32) progenitors. Plots present the angle between inferred mitotic spindle axis and the midline over time. (F’) Scheme of spindle rotation in a mitotic progenitor. Mitotic spindle axis as solid line, midline as dashed line. (H) Inhibition of cell proliferation results in diminished neural tube morphogenesis defects in mzscrib embryos. (I) Requirement of Scrib for cross-midline cell divisions in the neural keel. Labeled cells in a 22 hpf wt embryo have a bilateral distribution, but have a predominantly unilateral distribution in the posterior hindbrain/anterior spinal cord region of an mzscrib mutant. Arrowheads indicate the position of the first somite (left is hindbrain, spinal cord is to the right), ovals mark the otic vesicle, dotted line indicates the midline. In all panels, anterior is to the left and double arrowheads indicate the apico-basal axis of neuroepithelial progenitors. See also Supp.Fig.1.
We first analyzed wt mitotic spindle kinetics in keel stage progenitors and find that the mitotic spindle apparatus assembles with the spindle poles aligning with the anterior-posterior (planar) body axis and subsequently rotates about 90° into an apico-basal orientation (Fig.1A,B)[4]. Inferring the mitotic spindle orientation as orthogonal to the metaphase plate, we determined that in wt embryos the spindle orientation is on average 16.2 +/−14.6° from the planar axis at the onset of mitosis and 70 +/−21° after rotation (Fig.1F).
Mitotic spindle positioning can be regulated by the interaction of astral microtubules with the cell cortex and with the cortical actin-based network [5, 6]. Using a potent actin dynamics inhibitor, cytochalasin-D [7, 8], we find that perturbing actin dynamics influences the orientation of mitosis in the neural keel, resulting in a distribution of anaphase angles that is statistically similar to random (Fig.1C).
The molecular machinery that mediates the interactions between the cortical cues and actin to locally stabilize spindle position is not well understood. Scribble functions in other systems in apico-basal epithelial polarity and junction formation [9–11], in planar cell polarity (PCP)-dependent processes [12–14] and in mitotic spindle asymmetry in Drosophila neuroblasts [15]. Scrib has also been indirectly implicated in actin organization [16]. Therefore we tested whether the single zebrafish scribble gene [13], scrib, plays a role in orienting mitoses in the neural keel. We analyzed spindle orientation in live embryos lacking maternal and zygotic scrib (mzscrib) and in Tg(h2a.f/z-GFP) transgenics injected with scrib morpholinos. We find that the cell division axes of mzscrib mutants and scrib morphants display abnormally homogeneous distributions, very different from wt (Fig.1C).
This finding allowed us to ask whether the oriented mitosis in the neural keel is required for normal neural tube morphogenesis. We find that in mzscrib the apical cell surfaces that constitute the epithelial neural tube lumen are not aligned along the neural axis (Fig.1D, Suppl.Fig.1A,B). However the general apical cortical organization remains intact in mzscrib (Suppl.Fig.1A,B). Consistent with this, apical Pard3, visualized as a GFP fusion protein [17], is correctly localized near the cleavage plane of misoriented mzscrib mutant cells (Fig.1E). Subsequent sub-apical Pard3 localization and junction formation between neighbours generated by such misoriented divisions would be expected to result in the disorganized neural tube lumen such as that observed in mzscrib mutants. We observe the strongest morphogenetic perturbations in mzscrib mutants in more dorsal regions of the neural tube (Suppl.Fig.1C) and at posterior hindbrain and anterior spinal cord levels, resulting in disorganized neuronal architecture there (Suppl.Fig.1D).
The morphogenesis defects we observe in mzscrib mutants is not due to abnormalities in the neural plate before the onset of cross-midline divisions or in convergent extension movements that would result in abnormally positioned mitoses in the lateral neural keel (Suppl. Fig.2A-D). However the mzscrib neural tube phenotype is rooted in a defect in cell division, since blocking cell proliferation rescues it. Applying inhibitors of cell proliferation [17, 18] decreases morphogenetic defects from 87% in untreated mzscrib embryos to 30.5% in treated mzscrib embryos (Fig.1H). Since it is the orientation of cell division, not its timing or position, that is affected in mzscrib mutants, we conclude that correct spindle orientation is essential for the morphogeneis of a straight neural tube and lumen.
Abnormally oriented mitoses in mzscrib could be due to abnormal planar spindle orientation at the entry into mitosis and/or due to abnormal subsequent spindle rotation. While 90% of wt progenitors enter mitosis in the planar orientation (mitotic spindle angle of less than 30° from the midline), only 25% of mzscrib progenitors do so. Furthermore wt spindles rotate on average for 72° (±15°) into the apicobasal orientation at a rate of 24°/minute while in mzscrib embryos the spindle rotates less (44°±34°) and slower (15°/minute)(Fig.1F,G). We observe that the 25% of mzscrib mutant cells that are correctly oriented at the onset of mitosis do not enter anaphase correctly with any higher probability than mzscrib mutant cells that are incorrectly oriented at the onset of mitosis (Fig.1F,G and data not shown). Thus Scribble is required to control both initial spindle positioning and rotation into the final correct apico-basal orientation.
In the wt zebrafish neural keel, only daughters of dividing cells cross the midline [3, 17–19]. To address whether correct orientation of mitoses in the neural keel is required for the bilateral daughter cell distribution in the neural tube, we labeled neural progenitors on one side of gastrulating embryos and assessed the subsequent distribution of labeled cells in the neural tube. Whereas in wt embryos cells show a typical bilateral distribution, in the posterior hindbrain/anterior spinal cord region of mzscrib mutants where morphogenesis is most severely disrupted, labeled cells are predominantly unilateral (Fig.1I, n=30).
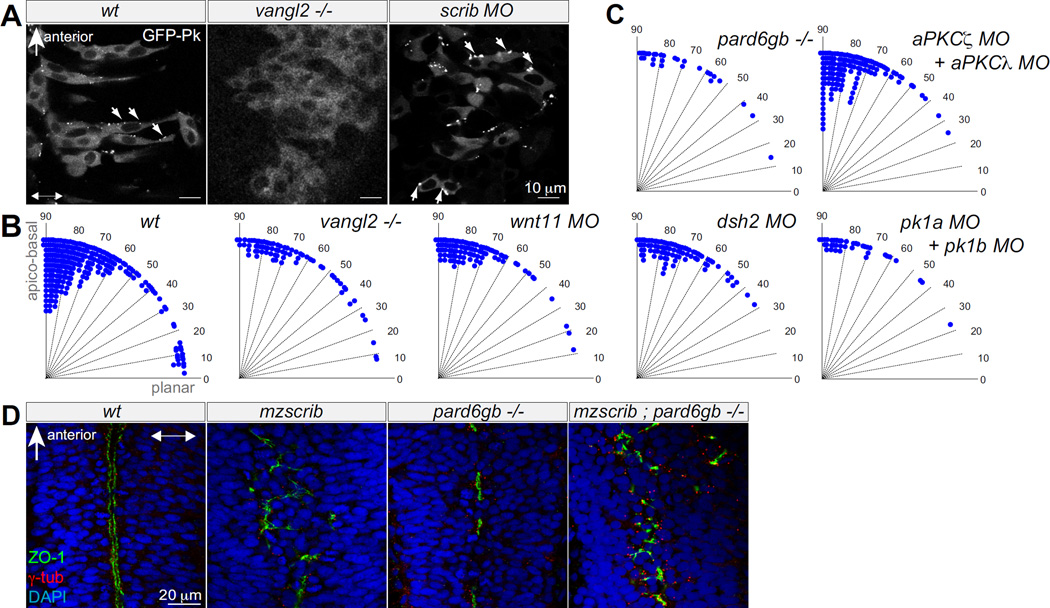
Vertebrate Scrib has been demonstrated to be functional in tissues that require PCP [13][12]. Furthermore, non-canonical Wnts and Vangl2, a core PCP component, are essential for normal neural tube morphogenesis in zebrafish [17, 18]. Scrib could thus potentially act in the PCP pathway, which could provide the cue for oriented cell divisions in the neural keel. The PCP reporter GFP-Prickle (GFP-Pk) [18] is localized in foci predominantly at the anterior sides of progenitors in wt. GFP-Pk puncta are lost in vangl2−/− mutants [18](Fig.2A). In mzscrib embryos, while the ratio of anterior to posterior positioned foci is lower (1.7:1) than in wt (3.1:1), the GFP-Pk protein still localizes to distinct foci (Fig.2A). Furthermore, we note that the anterior localization of GFP-Pk is lost in wt neural keel progenitors during mitosis (Suppl.Fig.3A), further suggesting that PCP might not be directing the axis of neural keel mitoses. Consistent with this, the distribution of mitosis orientation in live Tg(h2a.z/f-GFP) Wnt11, Dsh2, Pk1a and Pk1b morphants or vangl2−/− mutants are all statistically similar to the wt distribution (Fig.2B). Comparing mzscrib and vangl2 phenotypes more closely, there are multiple differences: vangl2 morphants have slower neuroepithelial convergence movements resulting in ectopic laterally positioned mitoses and duplicated neural tube lumina, whereas mzscrib mutants have midline mitoses and a single, though branched, neural tube lumen (Suppl.Fig.2B-E) [17]. The phenotypic differences between mzscrib and PCP mutants/morphants thus suggest that Scrib does not function in the context of PCP to orient mitoses in the neural keel.
Figure 2. Neither PCP components nor Par complex are required for proper spindle orientation in the neural keel.
(A) GFP-Prickle positive foci (arrows) in posterior hindbrain neural keel progenitors of wt, vangl2−/− mutant and scrib morphant embryos. (B,C) Quantification of anaphase orientation without functional PCP and Par complex components. The distributions of the various mutant or morphant conditions shown are not significantly different from wt (n=269): vangl2−/− mutant (n=72; Χ2=5.1; 4 df; p=0.28); wnt11 morphant (n=74; Χ2=2.6, 3 df; p=0.41); dsh2 morphant (n=86, Χ2=9.6, 4 df; p=0.05), pk1a+pk1b morphant (n=38, Χ2=2.6, 3 df; p=0.46); pard6gb−/− mutant (n=28, Χ2=2.7, 3 df; p=0.43); the distribution of mitotic angles in aPKCζ+aPKCλ double morphants is slightly more biased toward apicobasal than wt (n=139, Χ2=14.4, 4 df; p=0.006). (D) The branched, disorganized neural tube lumen of mzscrib and mzscrib;pard6gb−/− double mutants. Dorsal optical sections at 18 hpf immunostained with γ-tubulin (red), ZO-1 (green), and DAPI (blue). In A and D, anterior is to the top, double arrowheads indicate the apico-basal axis. See also Suppl.Fig.2.
Scrib can restrict the Par complex to the apical cortical domain of polarized cells [9]. We asked whether the function of Scrib in oriented mitoses in the neural keel depends on apical Par components. Scrib is not polarized in mitotic progenitors, and Pard3-GFP localization is normal in mzscrib (Fig. 1E, Suppl.Fig.3B). Furthermore, pard6gb−/− mutant and aPKCζ/aPKCλ morphant progenitors divide with normal orientation (Fig.2C). Finally, mzscrib;pard6gb−/− double mutants have a branched, disorganized midline similar to mzscrib single mutants (Fig.2D). Thus, these results suggest that Scrib’s function in orienting cell division in the neural keel is distinct from a role in establishing or maintaining apico-basal polarity.
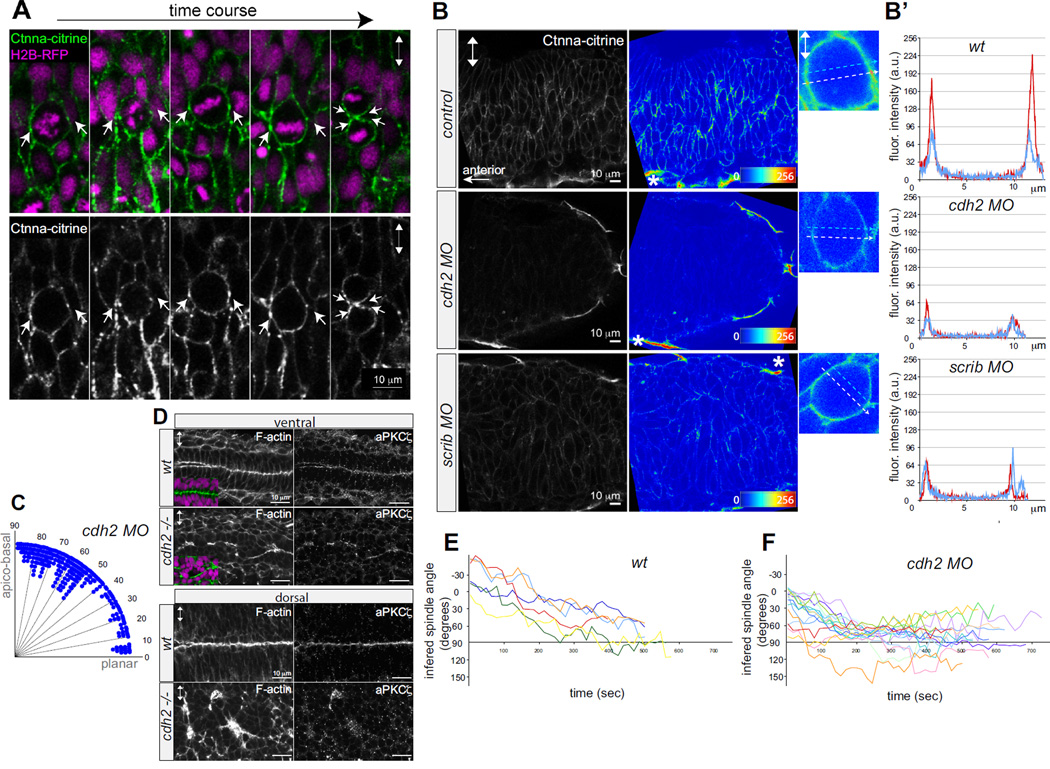
Since Scribble polarizes epithelial non-dividing cells by regulating adhesion junctional complexes [10, 11, 20], Scrib might affect the organization of cell-cell adhesion complexes in the context of the neural keel. We analyzed the subcellular distribution of adhesion complexes with an α-Catenin (Ctnna) transgenic gene trap line in which citrine is expressed as a fusion protein with α-Catenin (Ctnna), Gt(Ctnna-citrine)ct3a, reflecting endogenous Ctnna protein localization (Suppl.Fig.3C). In dividing wt progenitors the Ctnna-citrine is predominantly cortical. Importantly for this work, we noted that Ctnna-citrine is also enriched in cortical foci that lie at the presumptive cleavage plane and which, upon cytokinesis, segregate into subapical structures (Fig.3A, Suppl.Fig.3D). We discovered that overall cortical Ctnna-citrine, as well as enrichment at these cortical foci, was reduced in scrib morphants. Ctnna-citrine localization was similarly affected in N-cadherin/cdh2 morphants, as would be predicted for loss of an essential component of the adherens junction (Fig.3B,B’). Whereas in wt progenitors the ratio of fluorescence intensity at the cortical foci compared to the adjacent cortex is 2.2, the ratio in cdh2 and scrib morphants was on average 1.8 and 1.4, respectively (data not shown). This requirement for Scrib in the localization of Ctnna is restricted to the neural keel stage, since Ctnna-citrine is localized normally in the mature (but disorganized) neural tube epithelium of scrib morphants (Suppl.Fig.3D).
Figure 3. Reduction of α-Catenin foci in the neural keel correlates with aberrant mitotic orientation and neural tube architecture defects.
(A) Equatorially positioned cortical Ctnna-citrine foci (α-Catenin, green, arrows) are stationary while chromosomes (H2B-RFP, purple) rotate in a timelapse of a dividing neural keel progenitor. Upper panel merged, lower panel showing Ctnna-citrine alone. (B) Scrib and Ncad/cdh2 are required for Ctnna-citrine abundance and the localization of equatorial cortical Ctnna-citrine foci in neural keel mitotic progenitors. Posterior hindbrain at 6–8 somites with Ctnna-citrine (left), fluorescence intensity in pseudocolors (middle), and in single mitotic progenitors (right). Localization of Ctnna-citrine in wt (upper row), cdh2 morphants (middle row) and scrib morphants (lower row). The unchanged Ctnna-citrine signal in non-neural peridermal cells is marked by an asterisk. (B’) Fluorescence intensity plots of Ctnna-citrine levels in wt, cdh2 MO, scrib MO mitotic cells (shown in B) with the y-axis displaying arbitrary gray values along a line across a mitotic cell at equatorial (white dashed arrow in pictures, red line in plots) and lateral (blue dashed arrow in pictures, blue line in plots) positions averaged over 8 pixels in width. (C) Quantification of mitosis orientation at anaphase in cdh2 morphants. This distribution is highly significantly different from wt (n=267, Χ2=224; 8 df; p<<0.001). For wt controls compare to Fig.1C. (D) Disorganized, branched neural tube midline in the cdh2−/− neural tube in horizontal cryo-sections. F-actin/ phalloidin (green), aPKCζ and DAPI (purple). (E,F) Representation of mitotic spindle rotation in live wt (n=6) (E) and cdh2 morphant (n=16) (F) neural keel progenitors. Spindle orientation is inferred from the orientation of the chromosomes marked with H2B-GFP. Images in all panels are in dorsal view. Double arrowheads indicate the apico-basal cell axis. See also Supp.Fig.3.
Since cadherin-mediated cell-cell contacts can be instructive in spindle orientation in other contexts [22, 23], we considered that Scrib might be regulating oriented mitoses in the neural keel via regulation of α-Catenin-based cell-cell adhesion. If this is the case, cdh2−/− mutants would be expected to have defects similar to mzscrib mutants. Focusing on the ventral neural keel of cdh2 morphant and cdh2−/− mutants (since dorsal regions are severely disorganized [21]), we find that mitotic angles in cdh2 morphants indeed show a more homogeneous distribution, different from the wt distribution (Fig.3C). Mitotic spindle kinetics in cdh2 morphants were also similar to scrib morphants, with only 19% of cells oriented correctly at the onset of mitosis, and with prolonged, slow rotation thereafter (Fig.3.E,F). The subsequent alignment of neuroepithelial apical surfaces in the ventral hindbrain is disorganized in the absence of Cdh2 (Fig.3D). The similar consequences of loss of Scrib and loss of Cdh2 on α-Catenin localization, oriented cell division and neural tube morphogenesis suggest that Scrib controls oriented mitosis, at least in part, through control of cell adhesion in the neural keel.
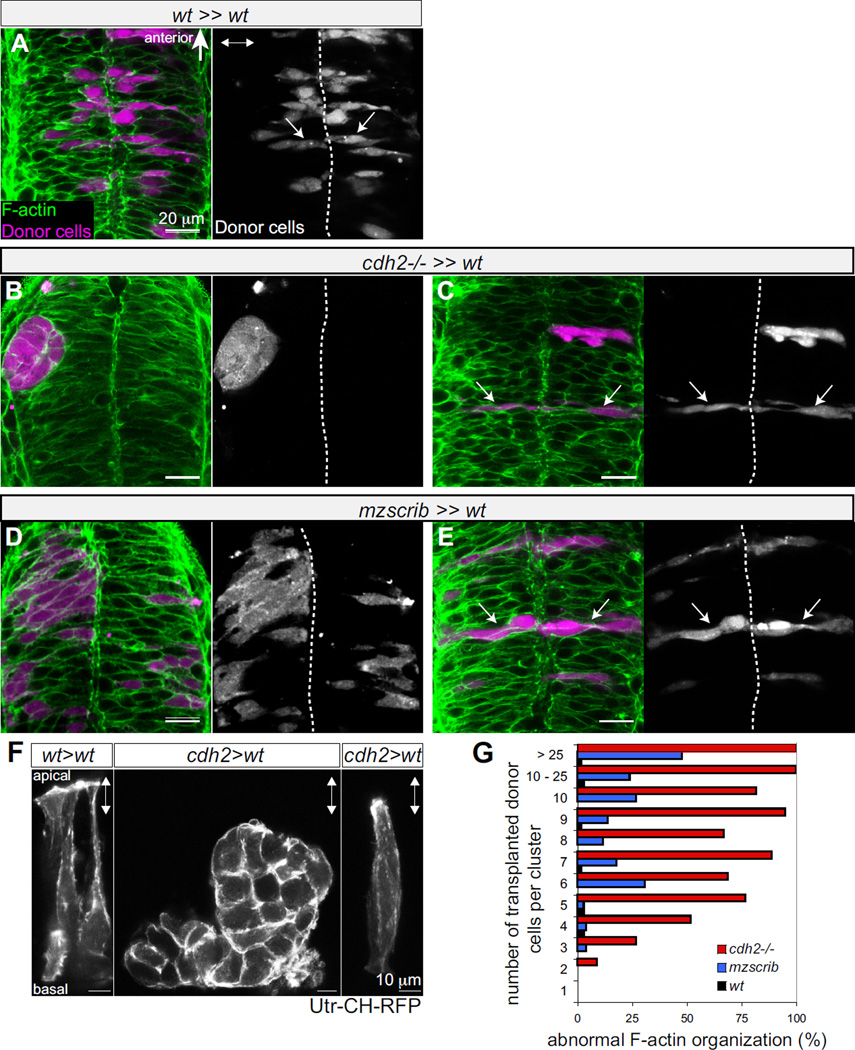
Finally, we used genetic mosaics to determine whether oriented cross-midline mitosis is the result of a genetically encoded program in a single cell or a property that emerges only in the tissue. Whereas wt cells distribute daughters to both sides of the midline (Fig.4A), cdh2−/− transplanted cells in a wt environment form tight unilateral clusters (Fig.4B). Interestingly, when four or fewer cdh2−/− cells are surrounded by wt cells, they acquire a normal cell shape and are positioned as bilateral pairs (Fig.4C). Similar observations were made for mzscrib mutant cells (Fig. 4D). In both instances, isolated mutant cells that are rescued by surrounding wt cells acquire normal subcellular organization of F-actin visualized by phalloidin (Fig.4A-E) and 3D-live imaging of the Utr-CH-RFP reporter [24] (Fig.4F). Additionally, in vivo timelapse analyses of mosaic embryos confirm that single cdh2−/− as well as single isolated mzscrib cells predominantly divide with correct orientation (Suppl.Fig.4 and data not shown). Our study thus reveals a previously unrecognized cellular community effect that ensures cytoskeletal polarization and orientation of mitosis in individual neural progenitor cells.
Figure 4. Non-autonomous rescue of oriented cross-midline cell division of single cdh2−/− and mzscrib mutant cells.
(A-E) Genetic mosaics at neural tube stage (21 hpf) in which donor derived cells (purple) were transplanted into the presumptive posterior hindbrain of wt host embryos (F-actin in green). (A) Bilateral distribution of wt cells (arrows) in a wt environment. (B) cdh2−/− cells form unilateral aggregates in a wt environment. (C) In contrast, isolated cdh2−/− progenitors show rescued bilateral distribution and normal cell shape. (D) A large group of mzscrib mutant cells in a wt environment with unilateral cell distribution. (E) Isolated mzscrib cells in a wt environment have rescued shape and bilateral distribution. (F) Utr-CH-RFP reporter in mosaic embryos showing normal apical enrichment in wt control transplants, loss of apical enrichment in a cluster of transplanted cdh2−/− cells, and rescue in a single cdh2−/− cell that is surrounded by wt host cells. Images represent maximal intensity projections of optical sections. (G) Quantification of occurrence of abnormal F-actin organization in mosaics. wt black (n=5 experiments, 13 embryos), mzscrib blue (n=3 experiments, 21 embryos), cdh2−/− in red (n=2 experiments, 15 embryos). Embryos in dorsal view with anterior to the top. Dotted white line indicates the neural tube midline, double arrowheads indicate the apico-basal axis of the neuroepithelium. See also Supp.Fig.4.
DISCUSSION
The goal of this work was to determine whether oriented cell division patterns per se could control tissue morphogenesis, and which cues regulate the highly stereotyped oriented mitoses in the zebrafish neural keel. Most oriented cell divisions studied to date occur in the context of mature epithelia (fly imaginal disc, chick neural tube, mammalian cortex, retina and kidney). In contrast, our work suggests that the well-established mechanisms that determine spindle orientation [6] in mature epithelia may not apply in epithelia undergoing junctional establishment. We have shown that Scrib is required for oriented mitoses in the zebrafish neural keel and that this results in abnormal morphogenesis of the neural tube.
How does Scrib control the orientation of cell division in the neural keel? In different contexts, Scrib has been implicated in PCP and apico-basal polarity, both of which can be instructive in oriented mitoses [25–29]. We find that Scrib functions in neither of these processes for mitosis orientation in the neural keel. Taking into account that Scrib functions as a tumor suppressor by promoting Cadherin-dependent cell-cell adhesion [30, 31] and is required for E-cadherin-mediated cell adhesion in cultured epithelial cells [20], we considered a function in regulating nascent adhesive structures in mitotic progenitors in the neural keel. We find that scrib knock-down prevents efficient recruitment of α-Catenin to equatorial cortical foci in mitotic cells that correspond to the future subapical junctional complexes of the mature epithelium. We argue that this function is responsible for the spindle orientation defects of mzscrib mutants because we find that cdh2 mutants have similar defects in α-Catenin localization, spindle orientation and rotation in dividing progenitors.
How might Scrib control the establishment and localization of equatorial adherens complexes in dividing cells? Cadherin-mediated cell-cell contacts are instructive in spindle orientation in cultured epithelial cells and in the fly sensory organ lineage [22, 32]. Since the mitotic spindle is initially aligned with the equatorial α-Catenin foci, Scrib-dependent equatorial α-Catenin might tether a pool of astral microtubules at the onset of mitosis. Mechanistically, initial positioning could depend on Scrib engaging Cadherin and α-Catenin at the cortex [20] and functioning via regulation of the β-catenin-Dynein complex [33], or through the APC (Adenomatous Polyposis Coli) complex [34, 35]. Since the equatorial adherens foci do not rotate, one possibility is that during the second phase Scrib prevents planar positioning of astral microtubules. Alternatively, the equatorially positioned α-Catenin foci could form a barrier restricting dynamic microtubule-mediated search-and-capture only to the apical- and basal-most cell hemispheres. Since cortical dynein anchor sites can be determined by actin organization [5] and we show that disruption of actin impairs the orientation of mitosis, Scrib might establish a barrier function via influencing the actin network. An intriguing possibility is that Scrib controls spindle orientation through Cdc42-dependent reorganization of the actin cytoskeleton [36, 37] as observed in the Xenopus [38]. However expressing a dominant negative form of Cdc42 does not disrupt the orientation of mitosis in the zebrafish neural keel (data not shown).
Unexpectedly, we find that cell shape, cytoskeletal organization and oriented cell division could be rescued if single mzscrib or cdh2 mutant cells are surrounded by wt cells in mosaic embryos. One possibility is that external physical forces arising as community effect in the wildtype tissue could feed back into redirecting or reinforcing the cellular polarity in neighboring cells. The only similar feedback loop to our knowledge between cell shape and cytoskeletal organization for mitosis in vivo has been proposed for the fission yeast [39], and very recently for cell orientation by anisotropic tension in the Drosophila pupal wing epithelium [40]. It is therefore tempting to ask whether shape-dependent polarity control is an evolutionary ancient property that has been adapted in multicellular organisms for correct morphogenesis of complex forms.
We propose that the zebrafish neural keel represents an important model system for how single cell polarity sculpts developing tissue structure and the tissue structure in turn instructs individual cell polarization and cell division orientation in vivo. In the future, it will be essential to integrate cell-cell, cell-extracellular matrix interactions as well as biophysical properties as possible non-exclusive regulatory regimes that reinforce robust intracellular decisions in single cells to effect morphogenesis at the tissue level.
Supplementary Material
ACKNOWLEDGMENTS
We thank L. Solnica-Krezel and H. Okamoto for zebrafish lines and B. Ciruna, H. Wada, M. Halloran and S. Megason for plasmids. We are grateful for Katherine Guthrie for her help with statistical analysis. Alexander Kohlmaier, Charisios Tsiairis, Valera Vasioukhin and members of the Moens lab are acknowledged for their comments on the manuscript. The work in the S.E.F. lab was supported by NHGRI Center of Excellence in Genomic Science grant P50HG004071. This work was supported by NIH grant HD37909 to C.B.M.. C.B.M. is an investigator with the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kimmel CB, Warga RM, Kane DA. Cell cycles and clonal strings during formation of the zebrafish central nervous system. Development. 1994;120:265–276. doi: 10.1242/dev.120.2.265. [DOI] [PubMed] [Google Scholar]
- 2.Papan C, Campos-Ortega JA. A clonal analysis of spinal cord development in the zebrafish. Dev Genes Evol. 1997;207:71–81. doi: 10.1007/s004270050093. [DOI] [PubMed] [Google Scholar]
- 3.Concha ML, Adams RJ. Oriented cell divisions and cellular morphogenesis in the zebrafish gastrula and neurula: a time-lapse analysis. Development. 1998;125:983–994. doi: 10.1242/dev.125.6.983. [DOI] [PubMed] [Google Scholar]
- 4.Geldmacher-Voss B, Reugels AM, Pauls S, Campos-Ortega JA. A 90-degree rotation of the mitotic spindle changes the orientation of mitoses of zebrafish neuroepithelial cells. Development. 2003;130:3767–3780. doi: 10.1242/dev.00603. [DOI] [PubMed] [Google Scholar]
- 5.Kunda P, Baum B. The actin cytoskeleton in spindle assembly and positioning. Trends Cell Biol. 2009;19:174–179. doi: 10.1016/j.tcb.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Siller KH, Doe CQ. Spindle orientation during asymmetric cell division. Nat Cell Biol. 2009;11:365–374. doi: 10.1038/ncb0409-365. [DOI] [PubMed] [Google Scholar]
- 7.Zalik SE, Lewandowski E, Kam Z, Geiger B. Cell adhesion and the actin cytoskeleton of the enveloping layer in the zebrafish embryo during epiboly. Biochem Cell Biol. 1999;77:527–542. [PubMed] [Google Scholar]
- 8.Carvalho L, Stuhmer J, Bois JS, Kalaidzidis Y, Lecaudey V, Heisenberg CP. Control of convergent yolk syncytial layer nuclear movement in zebrafish. Development. 2009;136:1305–1315. doi: 10.1242/dev.026922. [DOI] [PubMed] [Google Scholar]
- 9.Bilder D, Perrimon N. Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature. 2000;403:676–680. doi: 10.1038/35001108. [DOI] [PubMed] [Google Scholar]
- 10.Bilder D, Schober M, Perrimon N. Integrated activity of PDZ protein complexes regulates epithelial polarity. Nat Cell Biol. 2003;5:53–58. doi: 10.1038/ncb897. [DOI] [PubMed] [Google Scholar]
- 11.Legouis R, Gansmuller A, Sookhareea S, Bosher JM, Baillie DL, Labouesse M. LET-413 is a basolateral protein required for the assembly of adherens junctions in Caenorhabditis elegans. Nat Cell Biol. 2000;2:415–422. doi: 10.1038/35017046. [DOI] [PubMed] [Google Scholar]
- 12.Montcouquiol M, Rachel RA, Lanford PJ, Copeland NG, Jenkins NA, Kelley MW. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature. 2003;423:173–177. doi: 10.1038/nature01618. [DOI] [PubMed] [Google Scholar]
- 13.Wada H, Iwasaki M, Sato T, Masai I, Nishiwaki Y, Tanaka H, Sato A, Nojima Y, Okamoto H. Dual roles of zygotic and maternal Scribble1 in neural migration and convergent extension movements in zebrafish embryos. Development. 2005;132:2273–2285. doi: 10.1242/dev.01810. [DOI] [PubMed] [Google Scholar]
- 14.Courbard JR, Djiane A, Wu J, Mlodzik M. The apical/basalpolarity determinant Scribble cooperates with the PCP core factor Stbm/Vang and functions as one of its effectors. Dev Biol. 2009;333:67–77. doi: 10.1016/j.ydbio.2009.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albertson R, Doe CQ. Dlg, Scrib and Lgl regulate neuroblast cell size and mitotic spindle asymmetry. Nat Cell Biol. 2003;5:166–170. doi: 10.1038/ncb922. [DOI] [PubMed] [Google Scholar]
- 16.Dow LE, Kauffman JS, Caddy J, Zarbalis K, Peterson AS, Jane SM, Russell SM, Humbert PO. The tumour-suppressor Scribble dictates cell polarity during directed epithelial migration: regulation of Rho GTPase recruitment to the leading edge. Oncogene. 2007;26:2272–2282. doi: 10.1038/sj.onc.1210016. [DOI] [PubMed] [Google Scholar]
- 17.Tawk M, Araya C, Lyons DA, Reugels AM, Girdler GC, Bayley PR, Hyde DR, Tada M, Clarke JD. A mirror-symmetric cell division that orchestrates neuroepithelial morphogenesis. Nature. 2007;446:797–800. doi: 10.1038/nature05722. [DOI] [PubMed] [Google Scholar]
- 18.Ciruna B, Jenny A, Lee D, Mlodzik M, Schier AF. Planar cell polarity signalling couples cell division and morphogenesis during neurulation. Nature. 2006;439:220–224. doi: 10.1038/nature04375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lyons DA, Guy AT, Clarke JD. Monitoring neural progenitor fate through multiple rounds of division in an intact vertebrate brain. Development. 2003;130:3427–3436. doi: 10.1242/dev.00569. [DOI] [PubMed] [Google Scholar]
- 20.Qin Y, Capaldo C, Gumbiner BM, Macara IG. The mammalian Scribble polarity protein regulates epithelial cell adhesion and migration through E-cadherin. J Cell Biol. 2005;171:1061–1071. doi: 10.1083/jcb.200506094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong E, Brewster R. N-cadherin is required for the polarized cell behaviors that drive neurulation in the zebrafish. Development. 2006;133:3895–3905. doi: 10.1242/dev.02560. [DOI] [PubMed] [Google Scholar]
- 22.Le Borgne R, Bellaiche Y, Schweisguth F. Drosophila E-cadherin regulates the orientation of asymmetric cell division in the sensory organ lineage. Curr Biol. 2002;12:95–104. doi: 10.1016/s0960-9822(01)00648-0. [DOI] [PubMed] [Google Scholar]
- 23.Lechler T, Fuchs E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature. 2005;437:275–280. doi: 10.1038/nature03922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burkel BM, von Dassow G, Bement WM. Versatile fluorescent probes for actin filaments based on the actin-binding domain of utrophin. Cell Motil Cytoskeleton. 2007;64:822–832. doi: 10.1002/cm.20226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gong Y, Mo C, Fraser SE. Planar cell polarity signalling controls cell division orientation during zebrafish gastrulation. Nature. 2004;430:689–693. doi: 10.1038/nature02796. [DOI] [PubMed] [Google Scholar]
- 26.Baena-Lopez LA, Baonza A, Garcia-Bellido A. The orientation of cell divisions determines the shape of Drosophila organs. Curr Biol. 2005;15:1640–1644. doi: 10.1016/j.cub.2005.07.062. [DOI] [PubMed] [Google Scholar]
- 27.Saburi S, Hester I, Fischer E, Pontoglio M, Eremina V, Gessler M, Quaggin SE, Harrison R, Mount R, McNeill H. Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat Genet. 2008;40:1010–1015. doi: 10.1038/ng.179. [DOI] [PubMed] [Google Scholar]
- 28.Bellaiche Y, Gho M, Kaltschmidt JA, Brand AH, Schweisguth F. Frizzled regulates localization of cell-fate determinants and mitotic spindle rotation during asymmetric cell division. Nat Cell Biol. 2001;3:50–57. doi: 10.1038/35050558. [DOI] [PubMed] [Google Scholar]
- 29.Walston TD, Hardin J. Wnt-dependent spindle polarization in the early C. elegans embryo. Semin Cell Dev Biol. 2006;17:204–213. doi: 10.1016/j.semcdb.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Pagliarini RA, Xu T. A genetic screen in Drosophila for metastatic behavior. Science. 2003;302:1227–1231. doi: 10.1126/science.1088474. [DOI] [PubMed] [Google Scholar]
- 31.Zhan L, Rosenberg A, Bergami KC, Yu M, Xuan Z, Jaffe AB, Allred C, Muthuswamy SK. Deregulation of scribble promotes mammary tumorigenesis and reveals a role for cell polarity in carcinoma. Cell. 2008;135:865–878. doi: 10.1016/j.cell.2008.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.den Elzen N, Buttery CV, Maddugoda MP, Ren G, Yap AS. Cadherin adhesion receptors orient the mitotic spindle during symmetric cell division in mammalian epithelia. Mol Biol Cell. 2009;20:3740–3750. doi: 10.1091/mbc.E09-01-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ligon LA, Karki S, Tokito M, Holzbaur EL. Dynein binds to beta-catenin and may tether microtubules at adherens junctions. Nat Cell Biol. 2001;3:913–917. doi: 10.1038/ncb1001-913. [DOI] [PubMed] [Google Scholar]
- 34.Yamashita YM, Jones DL, Fuller MT. Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science. 2003;301:1547–1550. doi: 10.1126/science.1087795. [DOI] [PubMed] [Google Scholar]
- 35.McCartney BM, McEwen DG, Grevengoed E, Maddox P, Bejsovec A, Peifer M. Drosophila APC2 and Armadillo participate in tethering mitotic spindles to cortical actin. Nat Cell Biol. 2001;3:933–938. doi: 10.1038/ncb1001-933. [DOI] [PubMed] [Google Scholar]
- 36.Chu YS, Thomas WA, Eder O, Pincet F, Perez E, Thiery JP, Dufour S. Force measurements in E-cadherin-mediated cell doublets reveal rapid adhesion strengthened by actin cytoskeleton remodeling through Rac and Cdc42. J Cell Biol. 2004;167:1183–1194. doi: 10.1083/jcb.200403043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nelson WJ. Regulation of cell-cell adhesion by the cadherin-catenin complex. Biochem Soc Trans. 2008;36:149–155. doi: 10.1042/BST0360149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kieserman EK, Wallingford JB. In vivo imaging reveals a role for Cdc42 in spindle positioning and planar orientation of cell divisions during vertebrate neural tube closure. J Cell Sci. 2009;122:2481–2490. doi: 10.1242/jcs.042135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Terenna CR, Makushok T, Velve-Casquillas G, Baigl D, Chen Y, Bornens M, Paoletti A, Piel M, Tran PT. Physical mechanisms redirecting cell polarity and cell shape in fission yeast. Curr Biol. 2008;18:1748–1753. doi: 10.1016/j.cub.2008.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aigouy B, Farhadifar R, Staple DB, Sagner A, Roper JC, Julicher F, Eaton S. Cell flow reorients the axis of planar polarity in the wing epithelium of Drosophila. Cell. 142:773–786. doi: 10.1016/j.cell.2010.07.042. [DOI] [PubMed] [Google Scholar]
- 41.Malicki J, Jo H, Pujic Z. Zebrafish N-cadherin, encoded by the glass onion locus, plays an essential role in retinal patterning. Dev Biol. 2003;259:95–108. doi: 10.1016/s0012-1606(03)00181-7. [DOI] [PubMed] [Google Scholar]
- 42.Stemple DL, Solnica-Krezel L, Zwartkruis F, Neuhauss SC, Schier AF, Malicki J, Stainier DY, Abdelilah S, Rangini Z, Mountcastle-Shah E, et al. Mutations affecting development of the notochord in zebrafish. Development. 1996;123:117–128. doi: 10.1242/dev.123.1.117. [DOI] [PubMed] [Google Scholar]
- 43.Grant PK, Moens CB. The neuroepithelial basement membrane serves as a boundary and a substrate for neuron migration in the zebrafish hindbrain. Neural Dev. 2010;5:9. doi: 10.1186/1749-8104-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pauls S, Geldmacher-Voss B, Campos-Ortega JA. A zebrafish histone variant H2A.F/Z and a transgenic H2A.F/Z:GFP fusion protein for in vivo studies of embryonic development. Dev Genes Evol. 2001;211:603–610. doi: 10.1007/s00427-001-0196-x. [DOI] [PubMed] [Google Scholar]
- 45.Megason SG, Fraser SE. Digitizing life at the level of the cell: high-performance laser-scanning microscopy and image analysis for in toto imaging of development. Mech Dev. 2003;120:1407–1420. doi: 10.1016/j.mod.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 46.Carmany-Rampey A, Moens CB. Modern mosaic analysis in the zebrafish. Methods. 2006;39:228–238. doi: 10.1016/j.ymeth.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 47.Kemp HA, Carmany-Rampey A, Moens C. Generating chimeric zebrafish embryos by transplantation. J Vis Exp. 2009 doi: 10.3791/1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park M, Moon RT. The planar cell-polarity gene stbm regulates cell behaviour and cell fate in vertebrate embryos. Nat Cell Biol. 2002;4:20–25. doi: 10.1038/ncb716. [DOI] [PubMed] [Google Scholar]
- 49.Carreira-Barbosa F, Concha ML, Takeuchi M, Ueno N, Wilson SW, Tada M. Prickle 1 regulates cell movements during gastrulation and neuronal migration in zebrafish. Development. 2003;130:4037–4046. doi: 10.1242/dev.00567. [DOI] [PubMed] [Google Scholar]
- 50.Rohrschneider MR, Elsen GE, Prince VE. Zebrafish Hoxb1a regulates multiple downstream genes including prickle1b. Dev Biol. 2007;309:358–372. doi: 10.1016/j.ydbio.2007.06.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.