Abstract
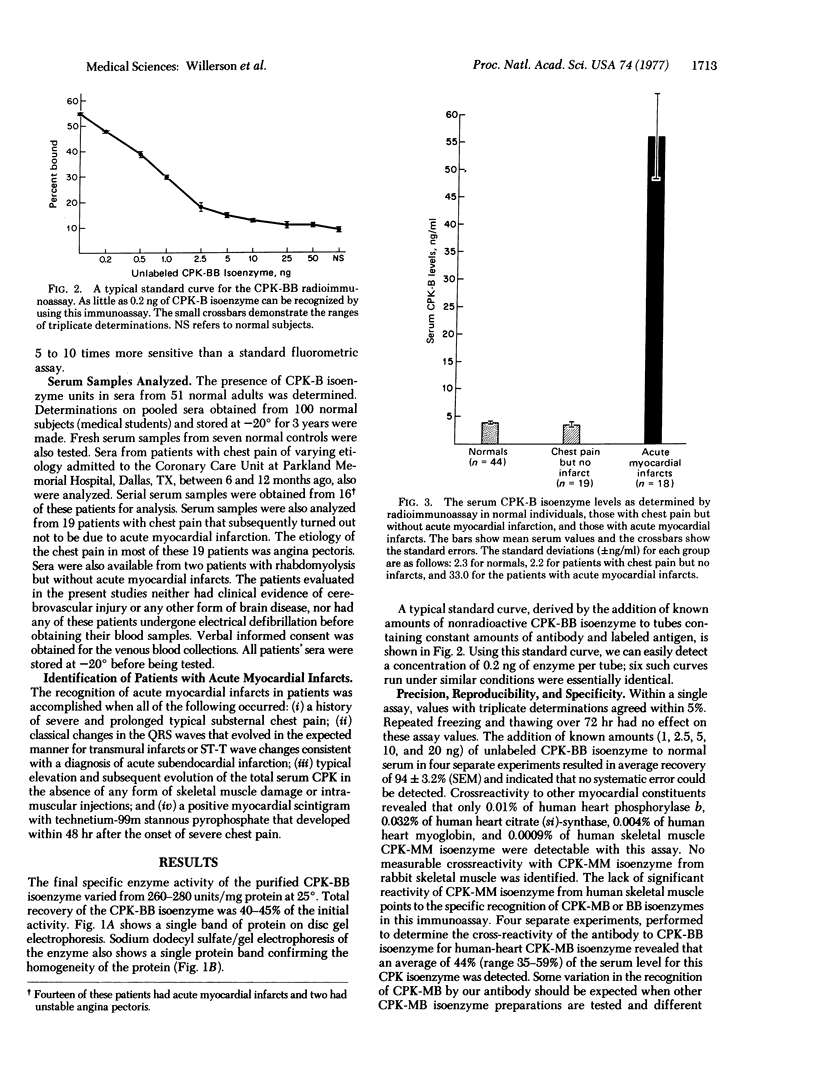
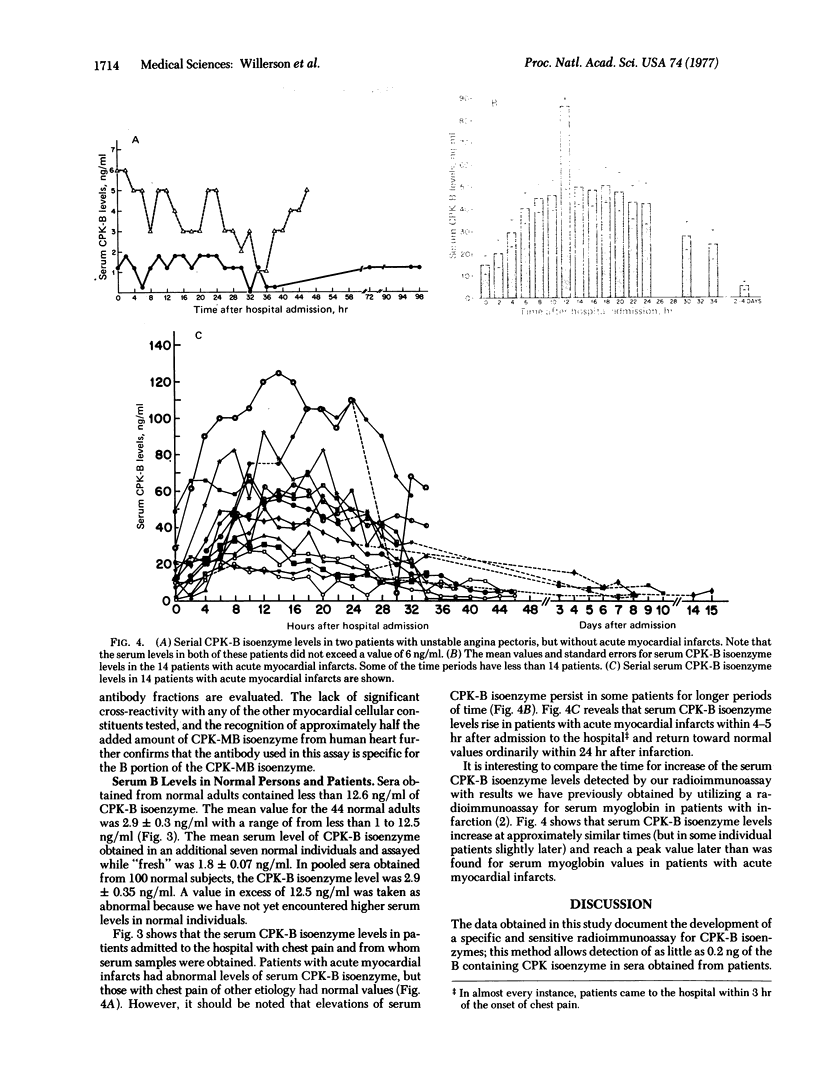
A radioimmunoassay was developed to measure serum levels of the B isoenzyme of creatine kinase(ATP: creatine N-phosphotransferase, EC 2.7.3.2) (CPK) in order to evaluate the time course and frequency of MB isoenzyme elevation in patients with acute myocardial infarction. The method can identify as little as 0.2 ng of the B portion of the CPK-MB isoenzyme, does not significantly crossreact with CPK-MM isoenzyme, and is not affected by storage of serum at --20 degrees CPK isoenzyme containing B subunits was detected in 48 out of 51 sera from normal adults; serum levels in these individuals ranged between 1.2 and 12.5 ng/ml [mean +/- SEM was 2.7 +/- 0.30 ng/ml]. The mean serum level of CPK-B isoenzyme in a pool of sera obtained from 100 normal subjects was 2.9 +/- 0.35 ng/ml; two patients with rhabdomyolysis that were studied had serum CPK-B isoenzyme levels of 2.5 and 3.5 ng/ml, respectively. In contrast, serum levels of the CPK-B isoenzyme were markedly elevated in sera from 18 patients with acute myocardial infarcts when obtained within 12 hr after hospital admission; the mean +/- SEM concentration was 56 +/- 7.8 ng/ml. We performed serial determinations on 14 patients with acute myocardial infarcts and demonstrated that maximal serum CPK-B levels occurred within the first 12 hr after admission and were lower thereafter. The serum concentration of B-containing CPK isoenzyme in 19 additional patients admitted with chest pain but without acute myocardial infarction was 3.4 +/- 0.50 ng/ml. Thus, radioimmunoassay measurement of CPK-B isoenzyme appears to be a useful and sensitive test for the detection of acute myocardial infarcts in patients.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson E., Roberts R., Sobel B. E. Preparation of individual CPK isoenzymes from myocardium and brain. J Mol Cell Cardiol. 1976 Feb;8(2):159–167. doi: 10.1016/0022-2828(76)90027-4. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Davis C. H., Schliselfeld L. H., Wolf D. P., Leavitt C. A., Krebs E. G. Interrelationships among glycogen phosphorylase isozymes. J Biol Chem. 1967 Oct 25;242(20):4824–4833. [PubMed] [Google Scholar]
- Henry P. D., Roberts R., Sobel B. E. Rapid separation of plasma creatine kinase isoenzymes by batch adsorption on glass beads. Clin Chem. 1975 Jun;21(7):844–849. [PubMed] [Google Scholar]
- Keutel H. J., Okabe K., Jacobs H. K., Ziter F., Maland L., Kuby S. A. Studies on adenosine triphosphate transphosphorylases. XI. Isolation of the crystalline adenosine triphosphate-creatine transphosphorylases from the muscle and brain of man, calf, and rabbit; and a preparation of their enzymatically active hybrids. Arch Biochem Biophys. 1972 Jun;150(2):648–678. doi: 10.1016/0003-9861(72)90085-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mukherjee A., Srere P. A. Purification of and mechanism studies on citrate synthase. Use of biospecific adsorption-elution techniques. J Biol Chem. 1976 Mar 10;251(5):1476–1480. [PubMed] [Google Scholar]
- Roberts R., Sobel B. E. Ediortial: Isoenzymes of creatine phosphokinase and diagnosis of myocardial infarction. Ann Intern Med. 1973 Nov;79(5):741–743. doi: 10.7326/0003-4819-79-5-741. [DOI] [PubMed] [Google Scholar]
- Roberts R., Sobel B. E., Parker C. W. Radioimmunoassay for creatine kinase isoenzymes. Science. 1976 Nov 19;194(4267):855–857. doi: 10.1126/science.982049. [DOI] [PubMed] [Google Scholar]
- Rosalki S. B. An improved procedure for serum creatine phosphokinase determination. J Lab Clin Med. 1967 Apr;69(4):696–705. [PubMed] [Google Scholar]
- Shell W. E., Kjekshus J. K., Sobel B. E. Quantitative assessment of the extent of myocardial infarction in the conscious dog by means of analysis of serial changes in serum creatine phosphokinase activity. J Clin Invest. 1971 Dec;50(12):2614–2625. doi: 10.1172/JCI106762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel B. E., Bresnahan G. F., Shell W. E., Yoder R. D. Estimation of infarct size in man and its relation to prognosis. Circulation. 1972 Oct;46(4):640–648. doi: 10.1161/01.cir.46.4.640. [DOI] [PubMed] [Google Scholar]
- Stone M. J., Willerson J. T., Gomez-Sanchez C. E., Waterman M. R. Radioimmunoassay of myoglobin in human serum. Results in patients with acute myocardial infarction. J Clin Invest. 1975 Nov;56(5):1334–1339. doi: 10.1172/JCI108211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner G. S., Roe C. R., Limbird L. E., Rosati R. A., Wallace A. G. The importance of identification of the myocardial-specific isoenzyme of creatine phosphokinase (MB form) in the diagnosis of acute myocardial infarction. Circulation. 1973 Feb;47(2):263–269. doi: 10.1161/01.cir.47.2.263. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Willerson J. T., Parkey R. W., Bonte F. J., Meyer S. L., Atkins J. M., Stokley E. M. Technetium stannous pyrophosphate myocardial scintigrams in patients with chest pain of varying etiology. Circulation. 1975 Jun;51(6):1046–1052. doi: 10.1161/01.cir.51.6.1046. [DOI] [PubMed] [Google Scholar]