Abstract
Background
The Amish have not been previously studied for cancer incidence, yet they have the potential to help in the understanding of its environmental and genetic contributions. The purpose of this study was to estimate the incidence of cancer among the largest Amish population.
Methods
Adults from randomly selected households were interviewed and a detailed cancer family history was taken. Using both the household interview data and a search of the Ohio cancer registry data, a total of 191 cancer cases were identified between the years 1996 and 2003.
Results
The age-adjusted cancer incidence rate for all cancers among the Amish adults was 60% of the age-adjusted adult rate in Ohio (389.5/105 vs. 646.9/105; p < 0.0001). The incidence rate for tobacco-related cancers in the Amish was 37% of the rate for Ohio adults (p < 0.0001). The incidence rate for non-tobacco-related cancers in the Amish was 72% of the age-adjusted adult rate in Ohio (p = 0.0001).
Conclusion
Cancer incidence is low in the Ohio Amish. These data strongly support reduction of cancer incidence by tobacco abstinence but cannot be explained solely on this basis. Understanding these contributions may help to identify additional important factors to target to reduce cancer among the non-Amish.
Keywords: Cancer incidence, Amish, Tobacco, Founder population
Introduction
The Amish are a unique group of people, not previously studied for cancer incidence, who could help in understanding the contributions of environment and genetics to cancer. Amish religious beliefs and traditions stress limited cultural contacts with mainstream society, and their marriage patterns have led to genetic isolation. The Amish have large families with extensive genealogical records, which serve to define the population [1, 2]. Their similar genetic background and lifestyle and dietary practices allow investigators to consider the contribution of genetic and non-genetic/environmental factors. The Amish represent a genetic founder population, a small population, which has been established in cultural or geographic isolation and demonstrates a lack of genetic diversity due to extremely limited mating outside the population. Genetic founder populations display allele frequencies different from more heterogeneous populations. Perhaps the most remarkable manifestations of this phenomenon are the observed overrepresentation of some disease alleles, both recessive and dominant, that lead to relatively high rates of certain otherwise rare disease phenotypes in the Ohio Amish [3–13].
To our knowledge, no systematic study of cancer incidence among the Amish has been attempted. Other founder populations have contributed to our understanding of hereditary cancer syndromes. For example, the Ashkenazi Jewish population has assisted in the study of hereditary breast/ovarian cancer syndrome caused by mutations in the BRCA1 and BRCA2 genes [14–16], as has the Finnish population in hereditary nonpolyposis colorectal cancer (HNPCC) caused by MLH1 gene mutations [17]. Although the Ashkenazi Jews have practiced cultural isolation because of religious beliefs and the Finns are geographically isolated in northern Europe, neither have remained isolated in the post-industrial revolution era nor have retained a unique lifestyle as have the Amish.
The objective of this study was to estimate the incidence of cancer among the largest Amish population in the world—the Amish of Holmes County, Ohio (referred to hereafter as the Ohio Amish). The incidence rates among the Amish were then compared to those observed among non-Amish whites in Ohio.
Methods
The research protocol was approved by the local institutional review board. Funding organizations did not have any role in the conduct of the study. Cancer incidence rates were estimated in a cohort of Amish during the years 1996–2003 and these rates were compared to the cancer rates among whites in Ohio during the same time period. However, only 87 out of the 88 counties were included in the calculation of the Ohio rate because one county, Holmes County, has a high percentage of Amish (nearly 40%) and therefore was not included in the Ohio rate estimate. The first year, 1996, was chosen because it was the first year that the Ohio Cancer Incidence and Surveillance System (OCISS) had high quality data to report and the last year, 2003, was chosen because it was the most current year data were available at the time of the analysis.
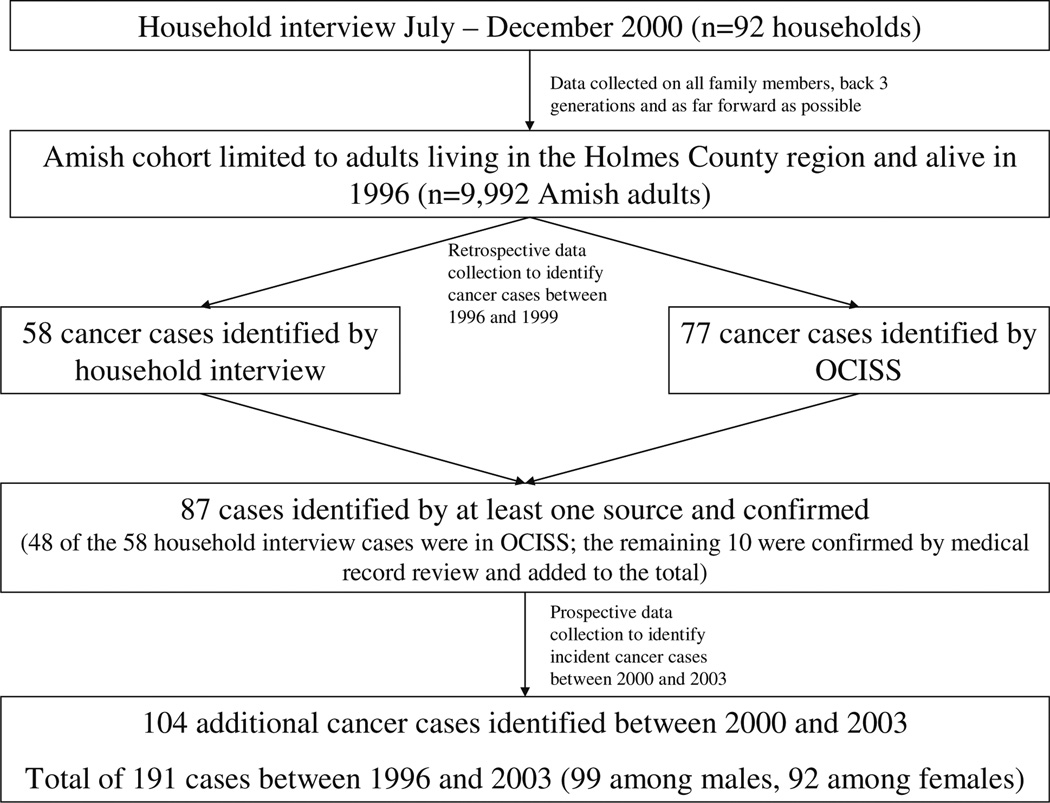
The study design included a cross-sectional household survey, and both retrospective and prospective data collection methods (see Fig. 1). The cross-sectional household survey was conducted between July and December 2000. Households were sampled from the 1996 Holmes County Amish Directory, which contained information on 4,512 homes (nearly all) in this Amish settlement. A random sample of 130 homes was drawn and a letter was sent to each that invited the male and female heads of the household to participate in the study. An interview was performed with the heads in the 92 households that agreed to participate and provided informed consent. A research assistant conducted a cancer family history interview with each participant, obtaining cancer information on all relatives back three generations and as far forward as possible. Each participant reported on family members within the immediate household and on relatives living in and outside the Holmes County Amish settlement. The cohort of family members was limited to Amish adults residing in the Holmes County area (n = 9,992) by using the Amish Directory in order to facilitate the search of OCISS for Amish cancer occurrences (described below). Each household reported an average of about 100 family members in a 3–4 generation history. While this number is high, it is not unusually high for the Amish given that these families have an average of six children.
Fig. 1.
Flow diagram depicting study design
Two methods were used to retrospectively collect data on cancer occurrences. First, cancer cases were identified by family report and medical records were requested for the cases that occurred between 1996 and 1999. In addition, death certificates were retrieved for all Ohio Amish individuals who died of any cause between 1996 and 1999. The death certificates among the Amish in our cohort were reviewed and information on medical history, cancer diagnosis, and age and year of diagnosis were checked for accuracy by a single investigator. The death certificates of Ohio Amish have been shown to have no systematic or significant differences from non-Amish death certificates [18].
The second retrospective data collection method involved searching OCISS. The database was initially searched for cases that had occurred during the years 1996–1999 to both confirm the family reports and also to obtain information on additional cases that were not identified during the interview. Standard search methods, including the matching of birthdays and names, were used to find Amish cohort members in OCISS.
The prospective data collection also occurred with an OCISS search. The database was searched for incident cases of cancer among the Amish cohort members between the years 2000 and 2003. Cohort members were not contacted after the cross-sectional survey in 2000. Therefore, to know who died during the 2000–2003 period, the social security death index (http://stevemorse.org/ssdi/ssdi.html) and Brother’s Keeper, an Amish genealogy software program (Brother’s Keeper for Windows version 6.1.25; Rockford, MI; 2004), were searched to determine which cohort members died, and thus no longer contributed person– time, which is necessary to know for the incidence rate calculation.
Statistical analysis
Age-adjusted cancer incidence rates were calculated for all cancer types, tobacco-related cancers, and non-tobacco-related cancers for the individuals of age 20 and older in the Amish cohort. The incidence rate for each type of cancer was calculated by dividing the number of incident cases within an age group by the amount of person–time observed within that age stratum. Individuals were assumed to contribute half a year of follow-up during years in which they developed cancer or died. To avoid underestimating the Amish cancer incidence rates, loss to follow-up percentages were estimated using information in the Amish Directory. The person–time contribution in each 10-year age stratum was then reduced by the appropriate amount to account for the fact that some individuals moved out of the Holmes County settlement and thus could not be counted as a cancer case even if cancer had occurred.
The cancer incidence rates among the Amish were compared to the age-adjusted incidence rates among whites in Ohio (minus Holmes County) using a two-sided Z-test. These Ohio rates came from OCISS and are published annually.
Standardized incidence ratios (SIR), defined as the ratio of the number of observed cases in the Amish to the number of expected cases, were calculated for individual cancer types. Cancer types were defined using the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) program classifications. The expected number of cases was calculated as the number that would have occurred if cancer incidence in the Amish had followed that of whites in Ohio (minus Holmes County). Exact Poisson confidence intervals, which take into account both the observed and the expected number of cases, were calculated for each SIR.
In a secondary analysis, we attempted to determine whether any differences between the Amish and non- Amish in Ohio might be due to a difference in screening rates. To do this, we calculated the SIR for cancer sites for which screening is not generally performed. As a consequence, we excluded prostate, breast, colorectal, and cervical cancers from the calculation.
Results
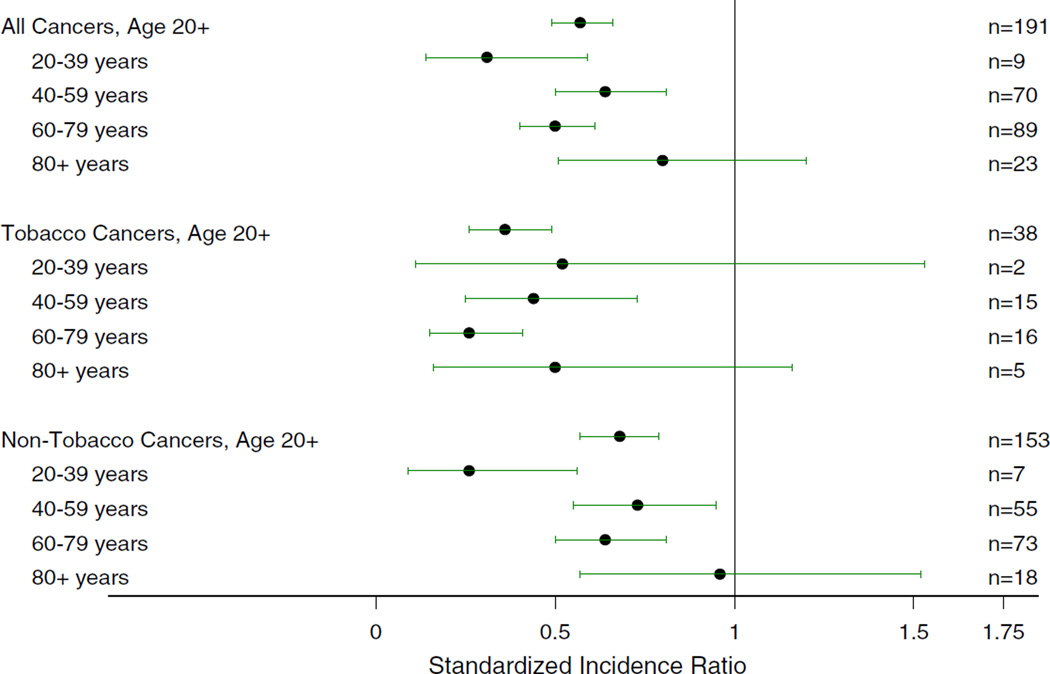
The 9,992 Amish individuals in the sample contributed a total of 74,727 person–years of follow-up time. This estimate of person–time, which is adjusted to account for individuals who may no longer be in the Holmes County Amish settlement, is 1% less than the unadjusted estimate; thus, few people moved out of the Amish community during follow-up. Between 1996 and 2003, there were 191 incident cancer cases identified through family report and/ or OCISS (99 cases among males and 92 among females; see Fig. 1 for details). Table 1 contains the estimated cancer incidence rates among the Amish and in Ohio (minus Holmes County). The estimated age-adjusted cancer incidence rate for all cancers among the Amish adults was 389.5 ± 37.7/105 person–years. This rate was 60% (p < 0.0001) of the age-adjusted rate among adults in Ohio during the same time period, 646.9 ± 1.0/105 person– years. Standardized incidence ratios are presented in Fig. 2, using the 191 reported cancers during the study period of 1996–2003. The SIR is significantly less than 1.0 for all cancers, and for all age groups from 20 to 79 years, and it approaches 1.0 for individuals older than 80 years. Thus, the number of cancers observed in the Amish was significantly lower than the number expected based on the Ohio cancer incidence rate. We also compared the Amish incidence rates to national cancer incidence rates in order to more directly compare our results to other published reports. The Amish rate of cancer occurrences was 56% (p < 0.0001) of the national rate, showing that comparisons of the Amish data to Ohio and national data are similar.
Table 1.
Cancer incidence rates in the Ohio Amish and in Ohio (minus Holmes County) between 1996 and 2003
| Cancer type | Amish rate ± SE | Ohio rate ± SE | Amish rate/Ohio rate | p-Valuea |
|---|---|---|---|---|
| All cancer | ||||
| Male and female (n = 191) | 389.5 ± 37.7 | 646.9 ± 1.0 | 0.60 | <0.0001 |
| Male (n = 99) | 449.5 ± 62.4 | 762.0 ± 1.7 | 0.59 | <0.0001 |
| Female (n = 92) | 344.3 ± 47.7 | 574.8 ± 1.3 | 0.60 | <0.0001 |
| Tobacco-related cancersb | ||||
| Male and female (n = 40) | 81.6 ± 23.9 | 221.9 ± 0.6 | 0.37 | <0.0001 |
| Male (n = 23) | 98.2 ± 40.0 | 309.5 ± 1.1 | 0.32 | <0.0001 |
| Female (n = 17) | 67.3 ± 25.6 | 159.7 ± 0.6 | 0.42 | 0.0003 |
| Non-tobacco-related cancers | ||||
| Male and female (n = 151) | 307.9 ± 30.4 | 425.0 ± 1.0 | 0.72 | 0.0001 |
| Male (n = 76) | 351.3 ± 47.9 | 452.5 ± 1.6 | 0.78 | 0.034 |
| Female (n = 75) | 277.0 ± 40.3 | 415.1 ± 1.4 | 0.67 | 0.0006 |
New cases of cancer/105 person–years. Rates are age-adjusted to match the year 2000 United States Standard Population of persons aged 20 years and older
Two-sided p-values corresponding to the observed Z statistic comparing the two rates
Tobacco-related cancers: oral cavity, pharynx, larynx, lung, esophagus, pancreas, stomach, liver, cervix, kidney, bladder, and myeloid leukemia [41]
Fig. 2.
Standardized incidence ratios and 95% exact Poisson confidence intervals comparing Amish to Ohio (minus Holmes County) incidence rates, for all cancers, tobacco-related cancers, and non-tobacco-related cancers overall and by age group. The number of cancer cases in each category is listed on the right side of the figure
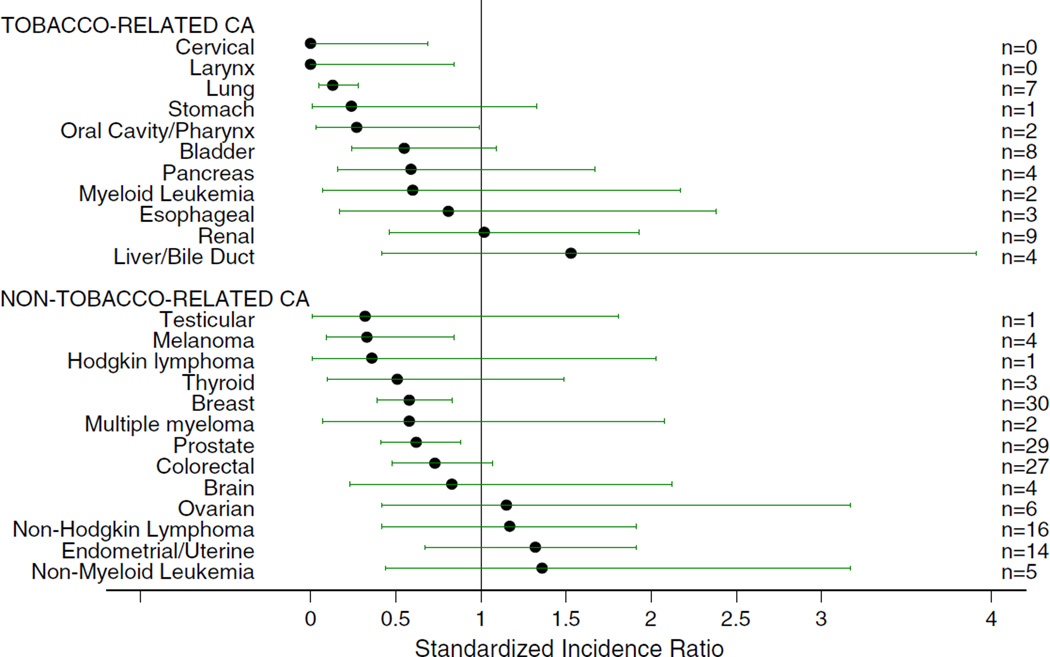
The rate for tobacco-related cancers among the Amish was 37% of the age-adjusted rate in Ohio (p < 0.0001) with Amish males showing the most significant difference in rate (32%, p < 0.0001). The Amish SIR was significantly less than 1.0 for all tobacco-related cancers combined and for ages 40–79. The SIRs for cervical (SIR 0, 95% CI 0–0.69), laryngeal (SIR 0, 95% CI 0–0.84), lung (SIR 0.13, 95% CI 0.05–0.28), and oral cavity/pharyngeal (SIR 0.26, 95% CI 0.03–0.99) cancer were significantly less than 1.0 (Fig. 3). All tobacco-related cancer SIRs were less than 1.0 except for renal and liver/bile duct, which were not significantly increased.
Fig. 3.
Standardized incidence ratios and 95% exact Poisson confidence intervals comparing Amish to Ohio (minus Holmes County) incidence rates for individual cancer types. The number of cancer cases in each category is listed on the right side of the figure. The number of non-tobacco-related cancers in this figure does not add up to the numbers presented in Fig. 2 and Table 1 because the SEER category “Other types/sites” is included in the overall nontobacco rate and SIR. Due to the mix in this category, we did not calculate an individual SIR
The estimated age-adjusted cancer incidence rate for non-tobacco-related cancers among the Amish was also significantly lower than in non-Amish Ohio adults (72%, p = 0.0001) with Amish females showing the most significant difference in rate (67%, p = 0.0006) (Table 1). The SIR for all non-tobacco-related cancers was also significantly less than 1.0 (Fig. 2). All non-tobacco-related cancer SIRs were less than 1.0 in the Amish except for ovarian, non-Hodgkin lymphoma, endometrial/uterine, and non-myelogenous leukemia, which were not significantly increased. SIRs for three non-tobacco-related cancers were significantly less than 1.0: melanoma (SIR 0.33, 95% CI 0.09–0.84), breast (SIR 0.58, 95% CI 0.39–0.83), and prostate (SIR 0.62, 95% CI 0.41–0.88) (Fig. 3). The decreased rate of melanoma may be accounted for by the Amish customs of dress where broad-brimmed hats and long sleeves are used throughout the year. The decreased rates of prostate and breast cancers do not appear to be accounted for by the fact that the Amish participate in breast and prostate screening programs at a lower rate than non-Amish in the same region [19]. When screened cancers were excluded (prostate, breast, colorectal, and cervical), the SIR of all non-tobacco-related cancers was still significantly less than 1.0. The SIR for males was 0.48 (95% CI 0.36–0.63) and the SIR for females was 0.57 (95% CI 0.43–0.75), supporting a minimal effect of decreased cancer screening on the observed low cancer incidence in the Amish.
No known hereditary cancer syndromes were identified. One family reported a family member with a de novo mutation of the APC gene, which was associated with desmoid tumor formation rather than cancer [20].
Discussion
The incidence of all cancers in the Ohio Amish is 60% of that in the general population of Ohio and is significantly lower for both tobacco-related cancers (37%) and nontobacco-related cancers (72%). Twenty-four cancer types were evaluated, 18 of which had a SIR less than 1.0 in the Amish. The SIRs for seven cancer types (cervical, laryngeal, lung, oral cavity/pharyngeal, melanoma, breast, and prostate) were significantly less than 1.0.
The low cancer incidence in the Ohio Amish may be partially explained by lifestyle factors such as limited tobacco consumption and lack of sexual promiscuity. We are aware of only two cancer-specific studies previously conducted in the Amish; one was a study of cervical cancer incidence in the Ohio Amish [21, 22] and the other was a comparison of lung cancer incidence in the Amish and non- Amish of Lancaster County, Pennsylvania [23]. Both studies found extremely low cancer incidence rates among the Amish as would be expected based on monogamous sexual practices and low tobacco usage. In a separate study, we examined lifestyle behaviors among the Amish and non-Amish living in the Holmes County region and we found a low prevalence of smoking and total tobacco use among the Amish [24]. Importantly, in the present study we found lower than expected rates (37%) of tobacco-related cancers overall, and lung cancers in particular. About 75–90% of lung cancer is thought to be tobacco-related [25, 26]. The absence of any cervical cancer in the Ohio Amish confirms previously published accounts [21, 22]. The absence of laryngeal cancer in the Amish is consistent with the finding that 95% of laryngeal cancer is thought to be tobacco related [27].
The 2004 Surgeon General’s report [28] strengthens the causal relationship between tobacco use and cancer. According to this report, the number of cancers directly caused by tobacco use has increased dramatically from those described in the original 1964 report. Similarly, the current study with Ohio Amish provides important evidence that smoking-attributable mortality can be reduced by preventing the initiation of tobacco use.
Our findings in the Amish are in accord with those for two other groups, the Mormons of Utah and Seventh-Day Adventists of California, who have the same religious proscriptions on tobacco, alcohol, and sexual promiscuity [29–34]. However, these groups are not genetically isolated because of their strong efforts to recruit additional members. For all three religious groups, the incidence rate for all cancer types is reduced from the national rate (32–75% of national rate) in both genders except for female Seventh- Day Adventists (92% of national rate). The decrease in the Mormon population is accounted for primarily by the lack of smoking in Mormon males and differences in sexual and reproductive behaviors in Mormon females [34]. The decrease in incidence rates in the Amish is seen not only in the tobacco-related cancers but also in the non-tobacco-related cancers.
The reduced rate of non-tobacco-related cancers is only partially explained by sexual and reproductive behaviors of Amish females. Amish multiparity may account for much of the observed SIR for breast cancer of 0.58. The Collaborative Group on Hormonal Factors in Breast Cancer found a 7% reduction in breast cancer relative risk for each birth with a maximum of five births considered [35]. The typical Amish family in our study had an observed average of six children. The Utah Mormons have a similar level of multiparity and a low breast cancer incidence [36].
Additional lifestyle differences present in the Amish may contribute to the reduction in cancer incidence and are under investigation. While little exists in the published literature, there is at least one older study of Amish in Holmes County that examined dietary intake [37]. The authors found that the Amish were less likely to drink alcohol and salt their food, and more likely to take vitamin supplements than the non-Amish Holmes County comparison group [37]. However, other dietary factors did not differ between the groups.
Incomplete ascertainment through under-reporting to OCISS is unlikely to be responsible for the observed difference due to the use of both family history and OCISS information to screen for cancer diagnoses, at least during the first 4 years. To determine if the different methodology used during the 2000–2003 period, which relied solely on an OCISS search, resulted in a different rate we estimated age-adjusted incidence rates for each 4-year period. The rates varied very little (381/105 for the 1996–1999 period versus 397/105 for the 2000–2003 period). Missed diagnoses in OCISS are not likely as the Amish in Ohio have an established history of participation in medical care, medical research, and disclosure of medical information [18, 37–39].
In addition to lifestyle factors, the special genetic makeup of the Ohio Amish may also contribute substantially to the low cancer incidence. To what extent does it explain or relate to our findings regarding cancer incidence? First, we studied enough pedigrees and covered a large enough proportion of all the Amish in the region to reveal cancer caused by known inherited cancer syndromes and present in multiple generations (dominant inheritance) had it existed. No such families were found. Only one case report of familial cancer in the Amish is present in the literature [40]. This study reported three separate occurrences of Hodgkin lymphoma in one extended Amish kindred in Wisconsin and Missouri. Other inherited cancer syndromes may be recessively inherited and appear as multiple affected siblings rather than multiple affected generations. This was not observed in the extended family histories taken. We, therefore, tentatively conclude that neither dominant nor recessive inherited cancer syndromes, nor the genetic mutations associated with them, occur with appreciable frequency in the relatively small population of the Holmes county Amish.
Possible genetic mechanisms to explain the low cancer incidence include a low frequency of genetic mutations predisposing to cancer and a high frequency of genetic polymorphisms protecting against cancer. The founder population status of the Amish could lead to both situations; moreover, such polymorphisms would in all likelihood be modified substantially by other genetic polymorphisms and environmental factors and therefore difficult to detect.
We believe that the Ohio Amish comprise the first genetically isolated population with good reporting, recordkeeping, and reasonable access to medical care that has been described with low cancer rates. Decreased use of tobacco results in approximately a 63% reduction in tobacco-related cancers for both Amish males and females. In addition to decreased use of tobacco and decreased sexual promiscuity, it is possible that the Ohio Amish have genetic “protective” factors that reduce their cancer susceptibility to all cancer types. Recent research in cancer genetics has focused primarily on factors that increase cancer risk. Genetic factors that reduce cancer risk may be extremely difficult to identify in a typical heterogeneous population. These protective genetic factors, whether they are monogenic, multigenic, or multifactorial (genetic and environmental), may be easier to find and characterize in a genetically homogeneous ethnic group like the Ohio Amish. Lifestyle factors other than tobacco use may also contribute to the low incidence rate of cancer and require further in-depth epidemiologic studies.
Acknowledgments
The authors gratefully acknowledge the assistance of Maurice Mullett MD, Holmes County Health Commissioner, and his staff; Jay Fisher PhD for provision of data from the Ohio Cancer Incidence Surveillance System; Harold Cross MD for providing introduction to the Ohio Amish community; Elizabeth Stover MD, PhD for conducting the pre-study field work; and James Hostetler for developing the computerized form of the Ohio Amish genealogy.
Financial support This work was supported by Ohio Division of the American Cancer Society, National Institutes of Health (P30 CA16058), Leukemia Clinical Research Foundation.
Contributor Information
Judith A. Westman, Division of Human Genetics, The Ohio State University, Columbus, OH, USA
Amy K. Ferketich, Email: aferketich@cph.osu.edu, Division of Epidemiology, The Ohio State University, 320 West 10th Avenue, Columbus, OH 43210, USA.
Ross M. Kauffman, School of Nursing, Indiana University, Indianapolis, IN, USA
Steven N. MacEachern, Department of Statistics, The Ohio State University, Columbus, OH, USA
J. R. Wilkins, III, Division of Epidemiology, The Ohio State University, Columbus, OH, USA.
Patricia P. Wilcox, Division of Epidemiology, The Ohio State University, Columbus, OH, USA
Robert T. Pilarski, Division of Human Genetics, The Ohio State University, Columbus, OH, USA
Rebecca Nagy, Division of Human Genetics, The Ohio State University, Columbus, OH, USA.
Stanley Lemeshow, Division of Biostatistics, The Ohio State University, Columbus, OH, USA.
Albert de la Chapelle, Department of Molecular Virology, Immunology, and Medical Genetics, The Ohio State University, Columbus, OH, USA.
Clara D. Bloomfield, Comprehensive Cancer Center and James Cancer Hospital and Solove Research Institute, The Ohio State University, Columbus, OH, USA
References
- 1.Wengerd M. Ohio Amish Directory: Holmes County and Vicinity 1996 revised ed. Sugarcreek, OH: Carlisle Press; 1997. [Google Scholar]
- 2.Wengerd M. Ohio Amish Directory: Holmes County and Vicinity. Sugarcreek, OH: Carlisle Press; 2000. [Google Scholar]
- 3.McKusick V, Eldridge R, Hostetler JA, et al. Dwarfism in the Amish. II. Cartilage-hair hypoplasia. Bull Johns Hopkins Hosp. 1965;116:285–326. [PubMed] [Google Scholar]
- 4.McKusick V, Cross H. Ataxia-telangiectasia and Swiss-type agammaglobulinemia. Two genetic disorders of the immune mechanism in related Amish sibships. JAMA. 1966;195:739–745. doi: 10.1001/jama.195.9.739. [DOI] [PubMed] [Google Scholar]
- 5.Telatar M, Teraoka S, Wang Z, et al. Ataxia-telangiectasia: identification and detection of founder-effect mutations in the ATM gene in ethnic populations. Am J Hum Genet. 1998;62:86–97. doi: 10.1086/301673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wall R, McConnell J, Moore D, et al. Christmas disease, color-blindness and blood group Xga. Am J Med. 1967;43:214–226. doi: 10.1016/0002-9343(67)90166-0. [DOI] [PubMed] [Google Scholar]
- 7.Cross H, McKusick V. The Troyer syndrome: a recessive form of spastic paraplegia with distal muscle wasting. Arch Neurol. 1967;16:473–485. doi: 10.1001/archneur.1967.00470230025003. [DOI] [PubMed] [Google Scholar]
- 8.Cross H, McKusick V. The Mast syndrome: a recessively inherited form of presenile dementia with motor disturbances. Arch Neurol. 1967;16:1–13. doi: 10.1001/archneur.1967.00470190005001. [DOI] [PubMed] [Google Scholar]
- 9.Klinger K. Cystic fibrosis in the Ohio Amish: gene frequency and founder effect. Hum Genet. 1983;65:94–98. doi: 10.1007/BF00286641. [DOI] [PubMed] [Google Scholar]
- 10.Westman J, Stover E, Singley C. Microcephalic osteodysplastic primordial dwarfism type I in the Amish. Am J Hum Genet. 1999;65:A348. [Google Scholar]
- 11.Proukakis C, Cross H, Patel H, et al. Troyer syndrome revisited. A clinical and radiological study of a complicated hereditary spastic paraplegia. J Neurol. 2004;251:1105–1110. doi: 10.1007/s00415-004-0491-3. [DOI] [PubMed] [Google Scholar]
- 12.Patel H, Cross H, Proukakis C, et al. SPG20 is mutated in Troyer syndrome, an hereditary spastic paraplegia. Nat Genet. 2002;31:347–348. doi: 10.1038/ng937. [DOI] [PubMed] [Google Scholar]
- 13.Simpson M, Cross H, Proukakis C, et al. Maspardin ismutated in Mast syndrome, a complicated form of hereditary spastic paraplegia associated with dementia. Am J Hum Genet. 2003;73:1147–1156. doi: 10.1086/379522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Struewing J, Abeliovich D, Peretz T, et al. The carrier frequency of the BRCA1 185delAG mutation is approximately 1 percent in Ashkenazi Jewish individuals. Nat Genet. 1995;11:198–200. doi: 10.1038/ng1095-198. [DOI] [PubMed] [Google Scholar]
- 15.Neuhausen S, Gilewski T, Norton L, et al. Recurrent BRCA2 6174delT mutations in Ashkenazi Jewish women affected by breast cancer. Nat Genet. 1996;13:126–128. doi: 10.1038/ng0596-126. [DOI] [PubMed] [Google Scholar]
- 16.Tonin P, Weber B, Offit K, et al. Frequency of recurrent BRCA1 and BRCA2 mutations in Ashkenazi Jewish breast cancer families. Nat Med. 1996;2:1179–1183. doi: 10.1038/nm1196-1179. [DOI] [PubMed] [Google Scholar]
- 17.Nystrom-Lahti M, Kristo P, Nicolaides N, et al. Founding mutations and Alu-mediated recombination in hereditary colon cancer. Nat Med. 1995;1:1203–1206. doi: 10.1038/nm1195-1203. [DOI] [PubMed] [Google Scholar]
- 18.Hamman R, Barancik J, Lilienfeld A. Patterns of mortality in the Old Order Amish. I. Background and major causes of death. Am J Epidemiol. 1982;114:845–861. doi: 10.1093/oxfordjournals.aje.a113255. [DOI] [PubMed] [Google Scholar]
- 19.Katz M, Ferketich A, Harley A, et al. Cancer screening among Amish adults. 29th annual meeting of the American Society of Preventive Oncology; San Francisco, California. 2005. 2005. [Google Scholar]
- 20.Halling K, Lazzaro C, Honchel R, et al. Hereditary desmoid disease in a family with a germline Alu I repeat mutation of the APC gene. Hum Hered. 1999;49:97–102. doi: 10.1159/000022852. [DOI] [PubMed] [Google Scholar]
- 21.Cross H, Kennel E, Lilienfeld A. Cancer of the cervix in an Amish population. Cancer. 1968;2:102–108. doi: 10.1002/1097-0142(196801)21:1<102::aid-cncr2820210116>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 22.Cross H, Kennel E, Lilienfeld A, McKusick V. Cancer of the cervix in the Amish. Trans Assoc Am Physicians. 1967;80:133–141. [PubMed] [Google Scholar]
- 23.Miller G. Lung cancer: a comparison of incidence between the Amish and non-Amish in Lancaster County. J Indiana State Med Assoc. 1983;76:121–123. [PubMed] [Google Scholar]
- 24.Ferketich AK, Katz ML, Kauffman RM, et al. Tobacco use among the Amish in Holmes County, Ohio. J Rural Health. 2008;24:84–90. doi: 10.1111/j.1748-0361.2008.00141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ginsberg RJ, Vokes EE, Rosenzweig K. Non-small cell lung cancer. In: DeVita VTJ, Hellman S, Rosenberg SA, editors. Cancer: principles and practice of oncology. 6th edn. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. pp. 925–983. [Google Scholar]
- 26.Tyczynski J, Bray F, Parkin D. Lung cancer in Europe in 2000: epidemiology, prevention, and early detection. Lancet Oncol. 2003;4:45–55. doi: 10.1016/s1470-2045(03)00960-4. [DOI] [PubMed] [Google Scholar]
- 27.Zatonski W, Becher H, Lissowska J, Wahrendorf J. Tobacco, alcohol, and diet in the etiology of laryngeal cancer: a population-based case–control study. Cancer Causes Control. 1991;2:3–10. doi: 10.1007/BF00052355. [DOI] [PubMed] [Google Scholar]
- 28.Pittsburgh, PA: Government Printing Office; 2004. The health consequences of smoking: a report of the surgeon general. [Google Scholar]
- 29.Mills P, Beeson W, Phillips R, Fraser G. Cancer incidence among California Seventh-Day Adventists, 1976–1982. Am J Clin Nutr. 1994;59(5 Suppl):1136S–1142S. doi: 10.1093/ajcn/59.5.1136S. [DOI] [PubMed] [Google Scholar]
- 30.Lyon J, Gardner K, Gress R. Cancer incidence among Mormons and non-Mormons in Utah (United States) 1971–85. Cancer Causes Control. 1994;5:149–156. doi: 10.1007/BF01830261. [DOI] [PubMed] [Google Scholar]
- 31.Gardner J, Lyon J. Cancer in Utah Mormon men by lay priesthood level. Am J Epidemiol. 1982;116:243–257. doi: 10.1093/oxfordjournals.aje.a113409. [DOI] [PubMed] [Google Scholar]
- 32.Gardner J, Lyon J. Cancer in Utah Mormon women by church activity level. Am J Epidemiol. 1982;116:258–265. doi: 10.1093/oxfordjournals.aje.a113410. [DOI] [PubMed] [Google Scholar]
- 33.Gardner J, Sanborn J, Slattery M. Behavioral factors explaining the low risk for cervical carcinoma in Utah Mormon women. Epidemiology. 1995;6:187–189. doi: 10.1097/00001648-199503000-00019. [DOI] [PubMed] [Google Scholar]
- 34.Merrill R, Lyon J. Cancer incidence among Mormons and non-Mormons in Utah (United States) 1995–1999. Prev Med. 2005;40:535–541. doi: 10.1016/j.ypmed.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 35.Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50302 women with breast cancer and 96973 women without the disease. Lancet. 2002;360:187–195. doi: 10.1016/S0140-6736(02)09454-0. [DOI] [PubMed] [Google Scholar]
- 36.Daniels M, Merrill R, Lyon J, Stanford J, White GJ. Associations between breast cancer risk factors and religious practices in Utah. Prev Med. 2004;38:28–38. doi: 10.1016/j.ypmed.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 37.Levinson R, Fuchs J, Stoddard R, Jones D, Mullet M. Behavioral risk factors in an Amish community. Am J Prev Med. 1989;5:150–156. [PubMed] [Google Scholar]
- 38.Fuchs J, Levinson R, Stoddard R, Mullet M, Jones D. Health risk factors among the Amish: results of a survey. Health Educ Q. 1990;17:197–211. doi: 10.1177/109019819001700206. [DOI] [PubMed] [Google Scholar]
- 39.von Gruenigen V, Showalter A, Gil K, Frasure H, Hopkins M, Jenison E. Complementary and alternative medicine use in the Amish. Complement Ther Med. 2001;9:232–233. doi: 10.1054/ctim.2001.0485. [DOI] [PubMed] [Google Scholar]
- 40.Halazun J, Kerr S, Lukens J. Hodgkin’s disease in three children from an Amish kindred. J Pediatr. 1972;80:289–291. doi: 10.1016/s0022-3476(72)80594-8. [DOI] [PubMed] [Google Scholar]
- 41.Vineis P, Alavanja M, Buffler P, et al. Tobacco and cancer: recent epidemiological evidence. J Natl Cancer Inst. 2004;96:99–106. doi: 10.1093/jnci/djh014. [DOI] [PubMed] [Google Scholar]