Abstract
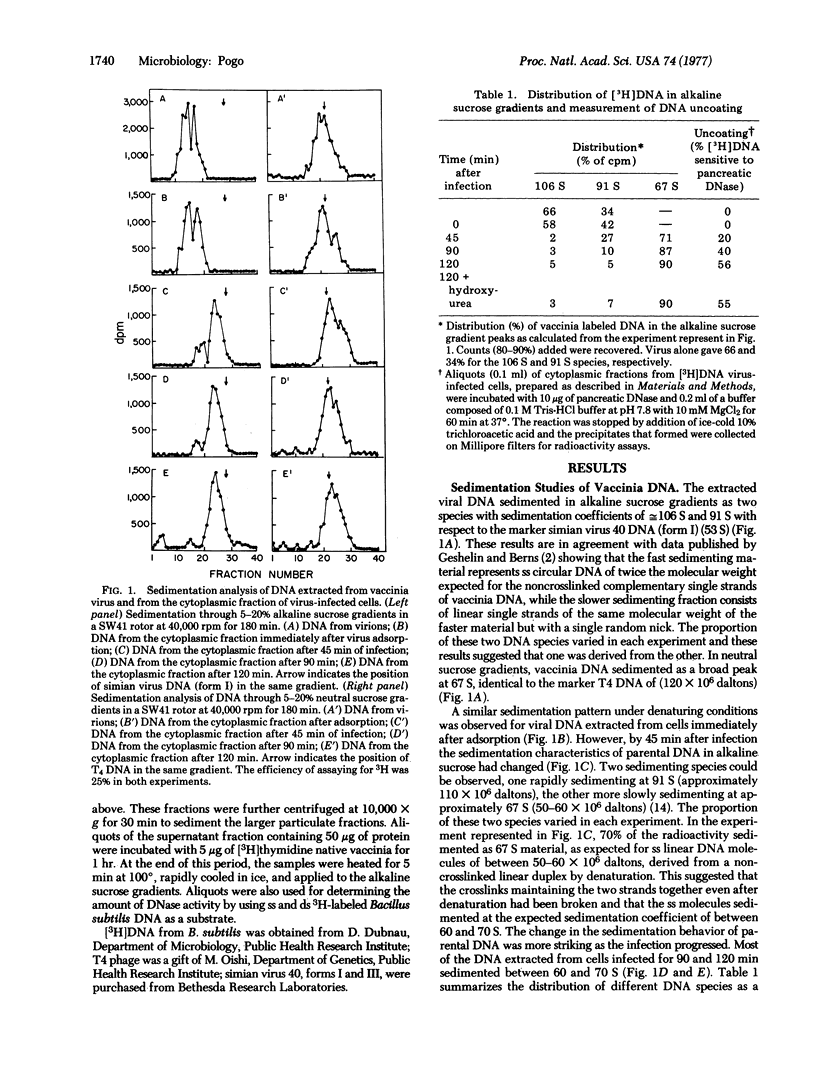
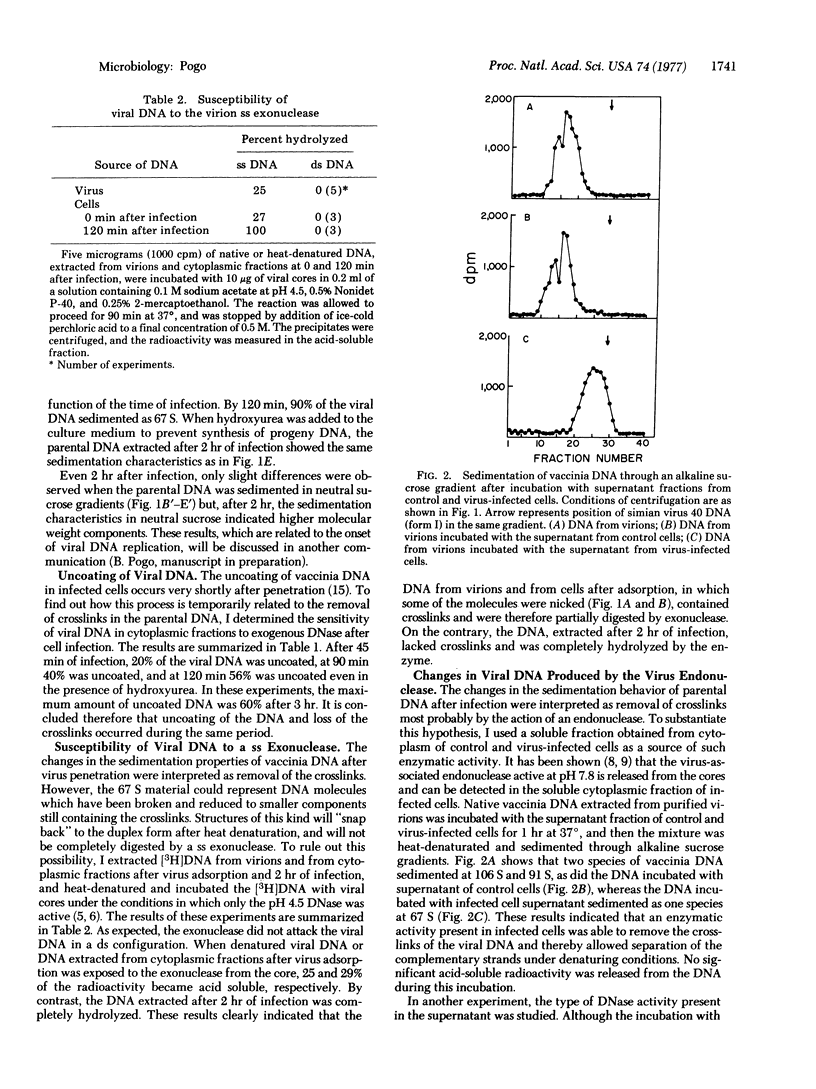
Vaccinia virus DNA, extracted from purified virus or from the cytoplasmic fraction of virus-infected cells very shortly after infection, was analyzed by sedimentation in alkaline and neutral sucrose gradients. The sedimentation properties of vaccinia DNA under denaturing conditions changed, immediately after penetration into the cell, from the characteristic circular viral DNA (crosslinked double-stranded linear DNA) to nicked circular DNA or to single-stranded molecules. This transition occurred at the time of uncoating of the virus and with a slight change in the DNA size, as judged by sedimentation in neutral sucrose. These results indicate that the crosslinks, that held the complementary strands of the genome together, are removed after penetration. When vaccinia DNA was incubated with the supernatant fraction of virus-infected cells, a similar change in the sedimentation properties of the DNA under denaturing conditions was observed. It is concluded that the endonuclease present in the supernatant of infected cells eliminated the crosslinks in the DNA, and that this enzymatic hydrolysis may be the mechanism by which crosslinks are removed prior to DNA replication.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B. M., Doty P. Characterization of a naturally occurring, cross-linked fraction of DNA. 1. Nature of the cross-linkage. J Mol Biol. 1968 Mar 14;32(2):379–403. doi: 10.1016/0022-2836(68)90017-x. [DOI] [PubMed] [Google Scholar]
- Barzilai R., Thomas C. A., Jr Spontaneous renaturation of newly-synthesized bacteriophage T7 deoxyribonucleic acid. J Mol Biol. 1970 Jul 14;51(1):145–155. doi: 10.1016/0022-2836(70)90276-7. [DOI] [PubMed] [Google Scholar]
- Berns K. I., Silverman C. Natural occurrence of cross-linked vaccinia virus deoxyribonucleic acid. J Virol. 1970 Mar;5(3):299–304. doi: 10.1128/jvi.5.3.299-304.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALES S. The uptake and development of vaccinia virus in strain L cells followed with labeled viral deoxyribonucleic acid. J Cell Biol. 1963 Jul;18:51–72. doi: 10.1083/jcb.18.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dales S., Mosbach E. H. Vaccinia as a model for membrane biogenesis. Virology. 1968 Aug;35(4):564–583. doi: 10.1016/0042-6822(68)90286-9. [DOI] [PubMed] [Google Scholar]
- FURLONG N. B. A SIMPLE AND RAPID METHOD FOR DETERMINING THE CHAIN LENGTH OF OLIGONUCLEOTIDES RANDOMLY LABELED WITH P32. Anal Biochem. 1965 Aug;12:349–356. doi: 10.1016/0003-2697(65)90102-8. [DOI] [PubMed] [Google Scholar]
- Forte M. A., Fangman W. L. Naturally occurring cross-links in yeast chromosomal DNA. Cell. 1976 Jul;8(3):425–431. doi: 10.1016/0092-8674(76)90155-0. [DOI] [PubMed] [Google Scholar]
- Geshelin P., Berns K. I. Characterization and localization of the naturally occurring cross-links in vaccinia virus DNA. J Mol Biol. 1974 Oct 5;88(4):785–796. doi: 10.1016/0022-2836(74)90399-4. [DOI] [PubMed] [Google Scholar]
- Holowczak J. A., Diamond L. Poxvirus DNA. II. Replication of vaccinia virus DNA in the cytoplasm of HeLa cells. Virology. 1976 Jul 1;72(1):134–146. doi: 10.1016/0042-6822(76)90318-4. [DOI] [PubMed] [Google Scholar]
- Holowczak J. A. Poxvirus DNA. I. Studies on the structure of the vaccinia genome. Virology. 1976 Jul 1;72(1):121–133. doi: 10.1016/0042-6822(76)90317-2. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K., BECKER Y. THE REPLICATION AND COATING OF VACCINIA DNA. J Mol Biol. 1964 Dec;10:452–474. doi: 10.1016/s0022-2836(64)80066-8. [DOI] [PubMed] [Google Scholar]
- PFAU C. J., MCCREA J. F. STUDIES ON THE DEOXYRIBONUCLEIC ACID OF VACCINIA VIRUS. III. CHARACTERIZATION OF DNA ISOLATED BY DIFFERENT METHODS AND ITS RELATION TO VIRUS STRUCTURE. Virology. 1963 Nov;21:425–435. doi: 10.1016/0042-6822(63)90204-6. [DOI] [PubMed] [Google Scholar]
- Pogo B. G., Dales S. Biogenesis of poxviruses: further evidence for inhibition of host and virus DNA synthesis by a component of the invading inoculum particle. Virology. 1974 Apr;58(2):377–386. doi: 10.1016/0042-6822(74)90073-7. [DOI] [PubMed] [Google Scholar]
- Pogo B. G., Dales S. Biogenesis of vaccinia: separation of early stages from maturation by means of hydroxyurea. Virology. 1971 Jan;43(1):144–151. doi: 10.1016/0042-6822(71)90232-7. [DOI] [PubMed] [Google Scholar]
- Pogo B. G., Dales S. Regulation of the synthesis of nucleotide phosphohydrolase and neutral deoxyribonuclease: two activities present within purified vaccina virus. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1297–1303. doi: 10.1073/pnas.63.4.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogo B. G., Dales S. Two deoxyribonuclease activities within purified vaccinia virus. Proc Natl Acad Sci U S A. 1969 Jul;63(3):820–827. doi: 10.1073/pnas.63.3.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]



