Abstract
We evaluated Eastern Cooperative Group phase II and III trials E2696 and E1694 to assess the incidence and prognostic significance of autoimmunity induced by adjuvant high-dose interferon-α2b (HDI). In E2696, patients with resectable high-risk melanoma were randomized to receive vaccination with GM2-KLH/QS-1 (GMK) plus concurrent HDI, GMK plus sequential HDI, or GMK alone. E1694 randomized patients to either HDI or GMK. Sera from 103 patients in E2696 and 691 patients in E1694 banked at baseline and up to three subsequent time points were tested by ELISA for the development of five autoantibodies. In E2696, autoantibodies were induced in 16 patients (23.2%; n= 69) receiving HDI and GMK and two patients (5.9%; n =34) receiving GMK alone (P = 0.031). Of 691 patients in E1694, 67 (19.1%) who received HDI (n= 350) developed autoantibodies, but only 16 patients (4.7%) developed autoantibodies in the vaccine group (n= 341; P < 0.001). Almost all induced autoantibodies were detected at ≥ 12 weeks after the initiation of therapy. A 1-year landmark analysis among resected stage III patients treated with HDI in E1694 showed a trend toward a survival advantage associated with HDI-induced autoimmunity (hazard ratio = 0.80; 95% confidence interval: 0.50–1.98; P = 0.33). Therefore, adjuvant HDI therapy is associated with the induction of autoimmunity that should be further investigated prospectively as a surrogate marker of adjuvant therapeutic benefit. This potential biomarker develops over the course of up to 1 year, and cannot be used to alter the course of therapy. Studies of the genetic determinants of this response may better discriminate patients more likely to benefit from HDI immunomodulatory therapy.
Keywords: adjuvant, autoimmunity, interferon, melanoma
Introduction
In the USA, the incidence of melanoma has increased steadily over the past several decades. In 2013, it was estimated that 76 790 Americans will have developed melanoma and 9480 will die from the disease [1]. The AJCC staging system for melanoma classifies patients into four main stages. Patients with melanoma of stages I and II have thinner and thicker melanoma confined to the skin. Stage III patients have regional lymph node or other lymphatic metastasis, with or without in-transit metastasis or satellite lesions. Stage IV patients have distant meta-static melanoma [2]. The category of patients defined as being high risk following surgical resection had a 5-year recurrence and/or mortality risk estimated to be 40% or more, and were therefore candidates for the adjuvant interventions studied in the E2696 and E1694 trials.
High-risk resected melanoma patients have been the target of multiple adjuvant clinical trials testing various chemo-therapeutic and biological agents. Among these studies, only adjuvant high-dose interferon-α2b (HDI) as tested in four Eastern Cooperative Group (ECOG) and intergroup trials has consistently shown a significant and reproducible impact on relapse-free survival (RFS) and overall survival (OS). The three phase III ECOG and intergroup trials (E1684/E1690/E1694) of adjuvant HDI enrolled patients who had recurrence and/or mortality risk equivalent to stages IIB–III as defined by the current seventh edition AJCC system. Patients with lymph node disease with extracapsular extension, or evidence of satellite disease in the vicinity of the primary, or clinically apparent in-transit metastasis, were excluded [3,4]. The regimen defined as HDI tested in these trials was administered as 20 million international units (MIU)/m2/day intravenously, 5 days/ week for 4 weeks (induction), followed by 10MIU/m2/day, three times a week subcutaneously for 48 weeks (maintenance). Overall, these studies have shown significant and reproducible benefits of HDI therapy as measured in terms of the hazard of relapse, which was reduced by 24–38% (E1684, E1690, and E1694). In addition, the hazard of mortality was reduced by 22–32% in E1684 compared with observation and E1690 compared with GMK vaccination (GM2-KLH/QS-21, GMK; Progenics Pharmaceuticals, Tarrytown, New York, USA) [5–8].
E2696 was a randomized phase II adjuvant trial that assigned patients to GMK plus concurrent HDI (Arm A), GMK plus sequential HDI (Arm B), or GMK alone (Arm C). Eligible patients had resected disease equivalent to the most recent AJCC stages IIB–III, including patients with resectable in-transit metastases or extracapsular extension of nodal disease (IIIC). It also allowed patients with resectable distant metastatic (M1, stage IV) disease [9]. At a median follow-up of 24 months, there was a significant reduction in the risk of relapse for patients receiving the combination of GMK plus HDI compared with GMK alone [hazard ratio (HR)=1.75 for Arm C vs. Arm A; HR=1.96 for Arm C vs. Arm B] [10]. This treatment benefit was statistically significant after adjusting for sex, performance status, time to resection, nodal status, and age (P=0.016 for Arm A vs. Arm C; P=0.03 for Arm B vs. Arm C).
E1694 accrued 880 patients between 1996 and 1999 and was designed to test whether GMK was superior to HDI. This trial was closed early on the basis of an interim analysis in April 2000 indicating that GMK was significantly inferior to HDI for both relapse and mortality endpoints. The results of this trial were reported in 2001 on the basis of a final analysis in June 2000, with a median follow-up interval of 16 months [7]. Among eligible patients in this trial, HDI provided a statistically significant RFS benefit (HR=1.47; P= 0.0015) and OS benefit (HR=1.52; P=0.009) compared with GMK. A similar benefit was observed in the intent-to-treat analysis of RFS (HR=1.49) and OS (HR=1.38). Cox regression analysis adjusting for sex, ECOG performance status, nodal status, and age also showed a statistically significant RFS and OS benefit for HDI versus GMK.
Recent evidence suggested that the benefits of interferon-α2b (IFN-α2b) therapy in patients with melanoma [similar to the observations reported with respect to two other active immunotherapeutic agents: interleukin-2 (IL-2) and anti-CTLA4 monoclonal antibodies] are associated with the induction of autoimmunity [11–22]. High-risk melanoma patients who participated in a trial of 4 weeks (intravenous induction) or 52 weeks (1 month of intravenous induction plus 11 months of subcutaneous maintenance) of a modified 25–33% reduced dose regimen of adjuvant HDI who derived most of the benefit of this treatment were those who developed clinical and/or serological manifestations of autoimmunity during the interval of 1 year of therapy. These patients showed markedly improved clinical outcomes measured both in terms of time to progression and mortality compared with patients who did not develop manifestations of autoimmunity [23]. However, the absence of a control group in this initial study of autoimmunity associated with IFN-α2b has made it difficult to quantify this association. We have therefore evaluated the sera collected in the context of the phase II E2696 trial (103 patients) and the phase III E1694 trial (691 patients) to assess the incidence of HDI-induced serological manifestations of autoimmunity and the prognostic significance of the serological development of evidence of auto-immunity in patients with high-risk surgically resected melanoma.
Patients and methods
Patients
Banked sera from 103 patients participating in the E2696 trial were utilized for the first phase of this project [10]. These included 35 patients in Arm A (GMK plus concurrent HDI), 34 in Arm B (GMK plus sequential HDI), and 34 in Arm C (GMK alone).
After reviewing preliminary data obtained from E2696, we proceeded with the second phase of our project utilizing banked sera from 691 patients participating in E1694, including 341 patients in Arm A (GMK) and 350 patients in Arm B (HDI) [7].
Collection and storage of blood serum
Using standardized phlebotomy procedures, 10 or 20 ml of peripheral blood was drawn from each of the patients. Samples were obtained from the patients before treatment (baseline) and after initiation of therapy at weeks 4–6, weeks 12–14, and weeks ≥ 48 (both E2696 and E1694). Blood samples were collected without anti-coagulant into red top vacutainers and allowed to coagulate for 20–30 min at room temperature. Sera were separated by centrifugation and all specimens were immediately aliquoted, frozen, and stored in a dedicated −80°C freezer. No more than two freeze–thaw cycles were allowed before testing for each sample.
Serologic assays
Sera from 103 patients enrolled in E2696 and 691 patients enrolled in E1694, banked at baseline, and at three subsequent time points (weeks 4–6, weeks 12–14, and weeks ≥ 48 after initiation of therapy) were used for this analysis using a qualitative enzyme-linked immunosorbent assay (DIASTAT; Euro-Diagnostica, Malmö, Sweden) following the manufacturer’s protocol. Patient serum samples were tested for the presence of the following autoantibodies: antinuclear antibody screen [positive absorbance ratio (sample absorbance value/mean reference control absorbance value) ≥ 1.0], antithyroglobulin antibody (positive>1.0), antithyroperoxidase antibody (positive>1.0), antimitochondrial antibody (positive> 1.0), and anticardiolipin antibody (total: IgA+IgM+IgG; positive concentration>21 phospholipid U/ml).
Samples were thawed at room temperature and diluted 1/101 with the appropriate diluent as indicated. The wells of the microtiter strips were coated with highly purified HEp-2 cell extract, purified human Tg antigen, recombinant human (rTPO), M2 antigen, or purified cardiolipin antigen. During the first incubation, specific antibodies in diluted serum bind to the antigen-coated surface. The wells are then washed to remove unbound components. In the second incubation, the conjugate, enzyme-labeled antibodies to human, binds any surface-bound autoantibodies. After further washing, specific autoantibodies are traced by incubation with the substrate. The addition of stop solution terminates the reaction, resulting in a colored end product. The amount of conjugate bound is measured in absorbance units on a microtiter plate reader at 550 nm and compared with the absorbance bound by the reference control.
Statistical analysis
Data from E2696 and E1694 were analyzed separately to evaluate the incidence of autoimmunity developing subsequent to treatment in patients treated with or without HDI, and the effect of autoimmunity on RFS and OS in each of these studies. We defined autoimmunity as the presence of antibody above the threshold to any of five different antigens. If a patient had an antibody that was positive at baseline, that specific antibody was excluded from the analysis. Taking ‘induced autoimmunity’ as the primary endpoint, we assessed the effects of induced autoimmunity during and after HDI on OS and RFS as the secondary endpoints of this study. The method of Kaplan and Meier [24] was used to evaluate OS and RFS in univariate analysis. The difference in RFS or OS by the autoimmunity status was compared using the log-rank test. Cox proportional hazards regression models [25] were used to evaluate the significance of autoimmunity status in the HDI group (univariate Cox model) and in the overall study population adjusting for treatment assignment (HDI and GMK).
Results
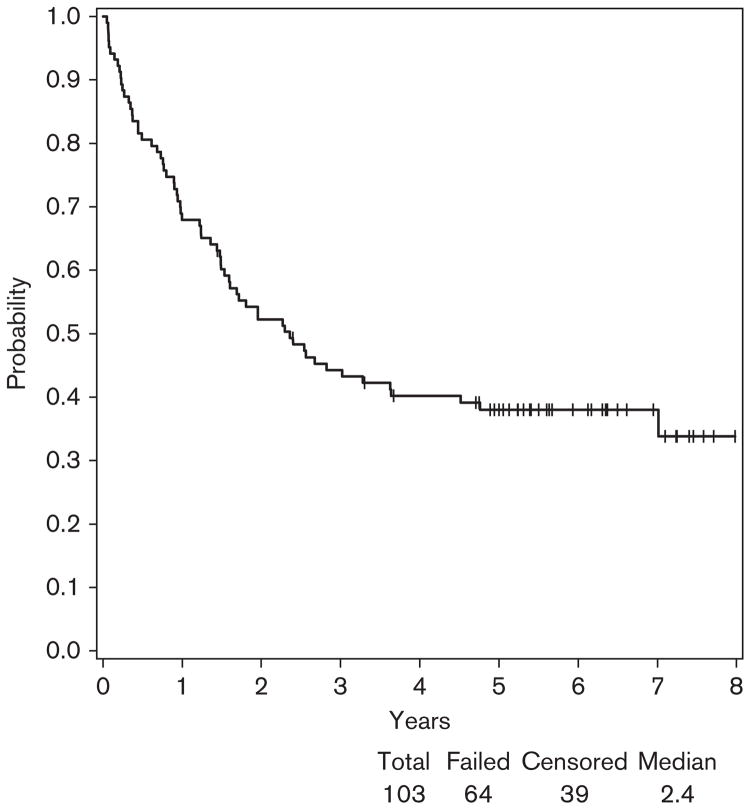
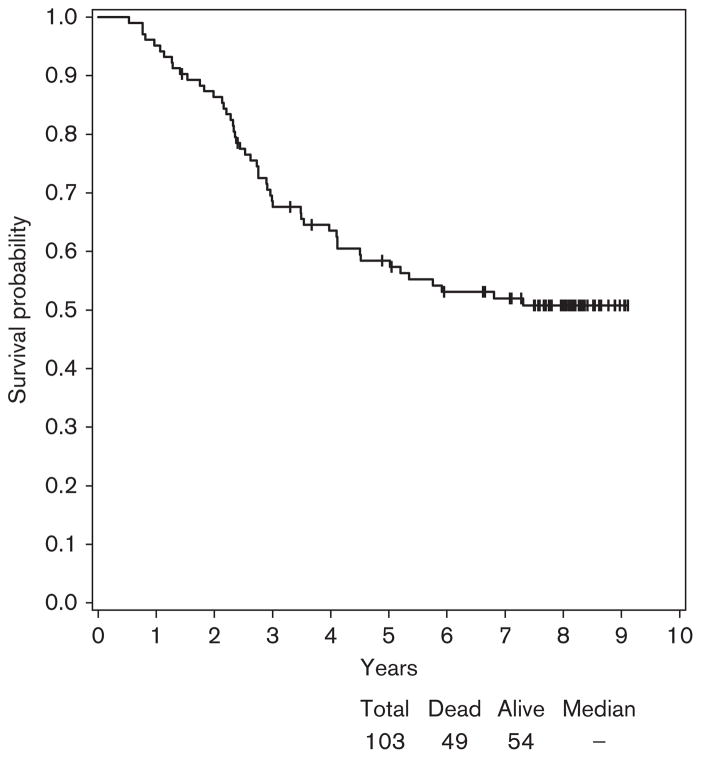
In E2696, at a median follow-up of 8 years (range: 1.4–9.1), the median RFS was 2.4 years with a 95% confidence interval (CI) (1.5–3.6) (Fig. 1). Of the 103 patients tested for autoantibodies in this study, 64 patients developed a relapse and 54 were alive (Fig. 2).
Fig. 1.
Relapse-free survival (RFS) for patients participating in E2696. At a median follow-up of 8 years (range: 1.4–9.1), the median RFS was 2.4 years with a 95% confidence interval (1.5–3.6). Among 103 patients, 64 patients developed relapse.
Fig. 2.
Overall survival for patients participating in E2696. At a median follow-up of 8 years, 54 patients were alive of the 103 patients tested for autoantibodies.
Autoantibodies were induced in 10 patients in ArmA (28.6%; n=35), six in Arm B (17.6%; n=34), and two in Arm C (5.9%; n=34). Therefore, autoantibodies developed in 16 patients (23.2%; n=69) receiving HDI and GMK versus two (5.9%; n=34) receiving GMK without HDI (Fisher, P=0.031). In the HDI arms, all induced autoantibodies were detected at ≥ 12 weeks after initiation of therapy. The two patients in the non-HDI arm who had induced autoantibodies were detected at the ≥ 6 weeks (Table 1).
Table 1.
Incidence of autoantibodies at different time points after the initiation of adjuvant therapy in E2696
HDI, high-dose interferon-α2b.
N=total number of patients who developed autoantibodies.
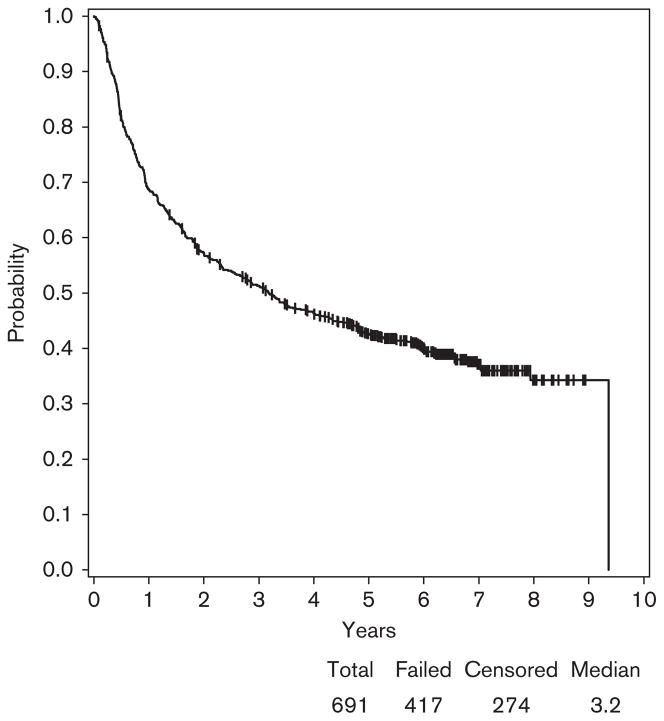
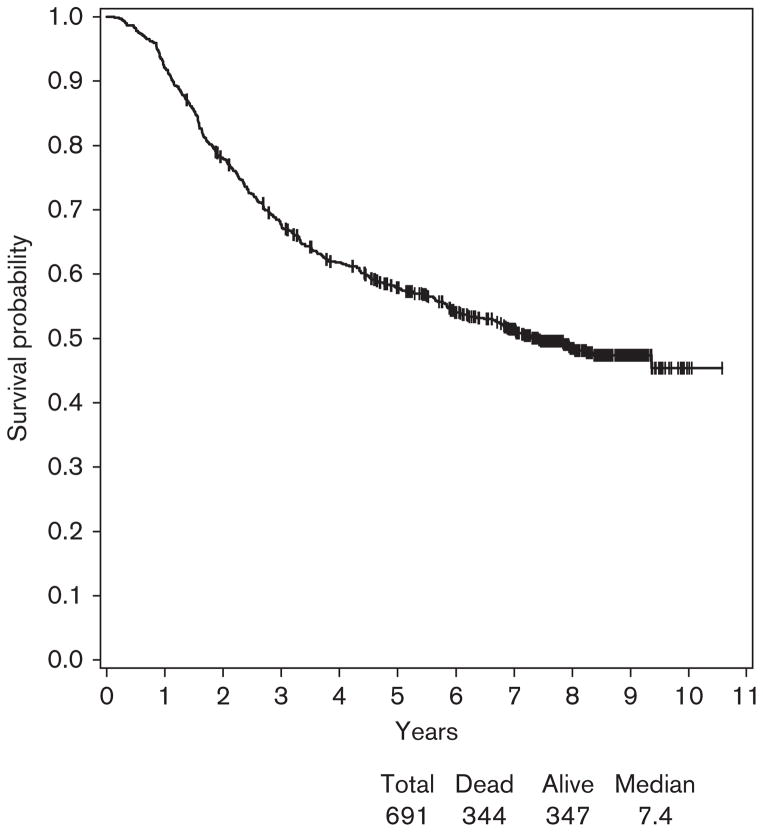
Among 691 patients enrolled in E1694 who had blood serum samples banked and available for autoantibody testing, 341 patients received GMK (Arm A) and 350 patients received HDI (Arm B). At a median follow-up of 7.8 years (range: 1.4–10.6), the median RFS was 3.2 years with 95% CI (2.5–4.0) (Fig. 3). Among 691 patients, 417 patients developed relapse. The median survival was 7.4 years with 95% CI (6.1, –) and among 691 patients, 317 were alive (Fig. 4).
Fig. 3.
Relapse-free survival (RFS) for patients participating in E1694. At a median follow-up of 7.8 years (range: 1.4–10.6), the median RFS was 3.2 years with a 95% confidence interval (2.5–4.0). Among 691 patients, 417 developed relapse.
Fig. 4.
Overall survival for patients participating in E1694. At a median follow-up of 7.8 years, the median survival was 7.4 years with 95% confidence interval (6.1 to −) and among 691 patients, 347 were alive.
A significantly greater number of patients developed autoantibodies on the HDI arm (67 patients, 19.1%) compared with the control (GMK) arm (16 patients, 4.7%) (P<0.001). In the HDI arm, four patients had induced autoantibodies detected at the 4–6 week time point, 20 at the 12–14 week time point, and 43 at ≥ 48 week time point (Table 2).
Table 2.
Incidence of autoantibodies at different time points after the initiation of adjuvant therapy in E1694
| Arm | n | Weeks 4–6 | Weeks 12–14 | Week ≥ 48 |
|---|---|---|---|---|
| A (GMK) | 16 | 7 | 4 | 5 |
| B (HDI) | 67 | 4 | 20 | 43 |
GMK, GM2-KLH/QS-21vaccine (Progenics Pharmaceuticals); HDI, high-dose interferon-α2b.
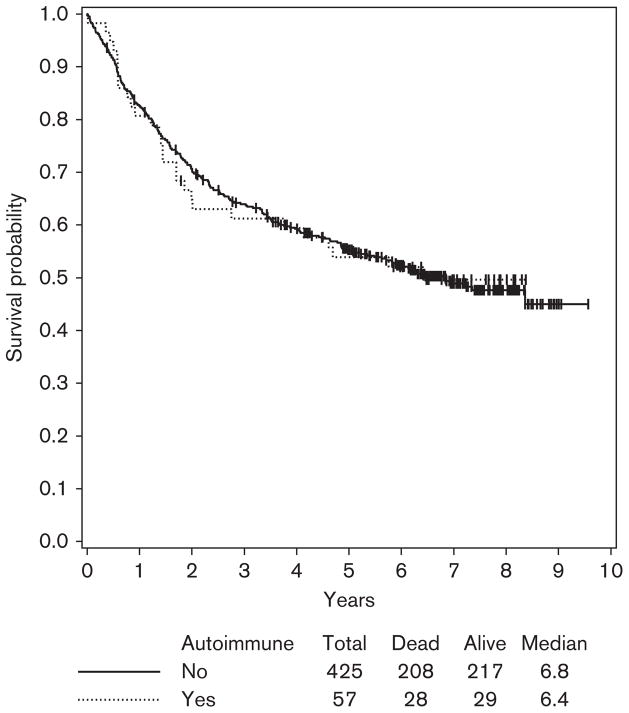
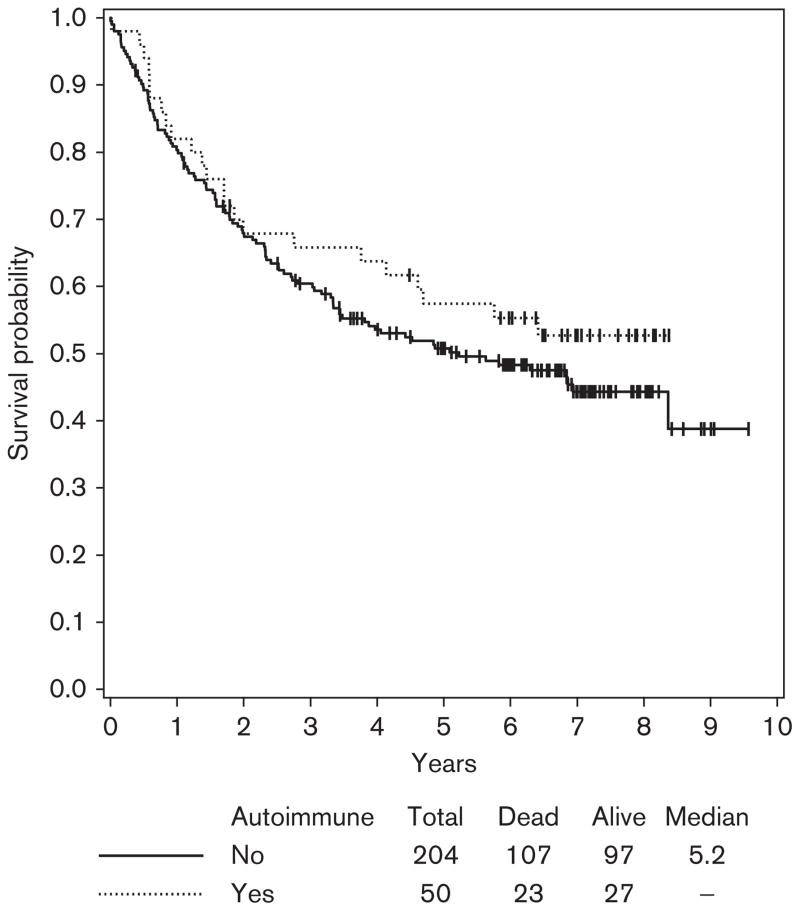
Given the larger numbers of patients in E1694, we could assess the correlation between HDI-induced autoimmunity and survival as this was initially shown in the Hellenic Oncology group He13A/98 study. However, unlike the Hellenic study, we had no information on the clinical manifestations of autoimmunity. Similar to the Hellenic study, we excluded patients with the baseline presence of serum autoantibodies (unless they developed different autoantibodies during treatment with HDI) and also excluded stage IIB patients (given their negligible numbers in the Hellenic trial). In addition, as two-thirds of all patients with induced autoantibodies had these detected at >12–14 weeks after initiation of therapy, we carried out a 1-year landmark analysis to avoid lead time bias. Adjusting for treatment in the multivariate Cox regression model, patients who developed autoimmunity showed no significant improvement in survival within the stage III population of patients studied here (both HDI and GMK groups), (HR=0.92; 95% CI: 0.72–1.39; P=0.92) (Fig. 5). However, among the stage III patients treated with HDI analyzed in the univariate Cox model, there was a trend toward improved OS in patients who developed auto-immunity (HR=0.80; 95% CI: 0.50–1.98; P=0.33) that did not reach statistical significance (Fig. 6). No statistically significant associations were found between the induction of autoimmunity and RFS.
Fig. 5.
Overall survival (OS) by autoimmunity at 1-year landmark time among stage III patients in E1694. Including both study arms (high-dose interferon and vaccine) and adjusting for treatment, there was no significant association with OS (hazard ratio=0.92, 95% confidence interval: 0.72–1.39; P=0.92).
Fig. 6.
Overall survival (OS) by autoimmunity at 1-year landmark time among stage III patients treated with high-dose interferon in E1694. There was a trend toward improved OS in patients who developed autoimmunity (hazard ratio=0.80, 95% confidence interval: 0.50–1.98; P=0.33).
Discussion
Autoimmunity appearing spontaneously in melanoma patients or induced by immunomodulatory agents such as IFNα-2b, IL-2, and anti-CTLA4 monoclonal antibodies, appears to be associated with increased resistance to melanoma progression and mortality. Spontaneous regression has been reported in melanoma, suggesting a role for host immunity. The presence of lymphoid infiltrates at the site of primary melanoma that have been associated with tumor regression provides histopathological support for this concept. Paraneoplastic depigmentation of patients with melanoma has also been reported as a sign of improved prognosis [26–28]. An association between the development of clinical or serological evidence of autoimmunity and favorable antitumor effects has been reported for several forms of immunotherapy including IFN-α2b, IL-2, and anti-CTLA4 blocking antibodies in patients with melanoma [11–22]. The benefits of high-dose IL-2 on progression-free survival and OS of melanoma have been associated with the development of autoimmune responses, and in particular, patients who have developed autoimmune thyroiditis [11–18]. More recent studies of immunotherapy for melanoma with anti-CTLA4 blocking antibodies have shown a correlation of antitumor effects and autoimmune phenomena of a broad array that includes colitis, thyroiditis, hypophysitis, enteritis, hepatitis, and dermatitis [19–22,29,30]. IFN-α therapy of hematologic malignancies and chronic viral hepatitis has also been reported to be associated with the induction of high levels of autoantibodies, including antithyroid microsomal and antithyroglobulin antibodies, antinuclear antibodies, anti-DNA, islet cell antigen, or platelet-associated antibodies persisting for months [31–36]. The Hellenic Oncology group reported that high-risk melanoma patients participating in a trial of 200 patients evaluating 4 versus 52 weeks of a modified (reduced dose) high-dose IFN-α2b regimen who developed serological or clinical manifestations of autoimmunity following treatment showed markedly improved clinical outcomes measured in terms of time to progression or to death compared with patients who did not develop these manifestations of autoimmunity [23]. Similarly, in a study of melanoma patients receiving adjuvant low-dose IFNα, the occurrence of autoimmune thyroid disease was associated with improved RFS [37]. However, no control group has been evaluated for autoimmunity to date. In our current evaluation of E2696 and E1694, we show that HDI is associated with a significantly higher incidence of serologic conversion or evidence of induced autoimmunity compared with the control group receiving GMK vaccination. The incidence of autoimmunity is comparable with that seen in the Hellenic study (25% in E2696 and 19.3% in E1694 compared with 26% in Hellenic study). In E2696, seven of 17 patients who developed autoantibodies following HDI were first detected at the 12–14-week time points. The remaining 10 patients were first detected at the next time point tested (week 52). Similarly, in E1694, four patients had induced autoantibodies detected at the 4–6 week time point, 20 at weeks 12–14, and 43 (2/3 of patients) at the next time points at which blood serum was collected (≥ week 48). Therefore, the induction of autoimmunity develops over the course of year, and cannot be used to guide the application nor to alter the course of this therapy.
The prognostic impact of the development of three autoantibodies (anticardiolipin antibodies, antithyroglobulin antibodies, and antinuclear antibodies) on prognosis was explored in patients with melanoma receiving intermediate doses of IFN-α or no treatment in a subgroup of patients participating in the EORTC 18952 trial [38] and the Nordic IFN trial [39]. The appearance of these three autoantibodies was not found to have a strong prognostic nor a strong predictive value after adjusting for lead time bias [40,41]. The same group of investigators sought to determine the prognostic or the predictive significance of the same three autoantibodies in patients assigned randomly to receive pegylated interferon (PEG-IFN) or no treatment (EORTC 18991) [42]. Among 220 patients with negative baseline antibodies, the analysis showed a significantly higher occurrence of induced autoantibodies during follow-up in the PEG-IFN-treated patients (18% in the observation arm vs. 52% in the PEG-IFN arm). The appearance of autoantibody was of prognostic importance by a model that considered the appearance of autoantibody as a time-independent variable (HR=0.56; 95% CI: 0.36–0.87; P = 0.01). However, when guarantee-time bias was taken into account, statistical significance was lost.
For the autoantibody analyses, a subset of patients of 187/1388 in EORTC 18952 and 356/855 in the Nordic trial was included. In EORTC 18991, only 296/1256 patients were included. Here also, unlike the Hellenic study, there were no data on the clinical manifestations of autoimmunity. Moreover, the autoimmunity analysis in these studies was limited to three autoantibodies. The fact that there was a difference in the proportion of induced autoantibodies and a demonstrable prognostic importance using model 1 is notable in EORTC 18991. In contrast, although all 220 patients in EORTC 18991 were autoantibody negative at baseline, the high proportion of patients who went on to develop any one of only three autoantibodies is higher than would be expected, even using a smaller panel of three specificities.
In E1694, there is a trend toward improved OS in patients treated with HDI who developed autoantibodies that is not statistically significant. It is important to note that in the Hellenic study, data on induced autoimmunity were collected prospectively, including clinical manifestations of autoimmunity (such as vitiligo) and not only serologic evidence. In addition, the laboratory testing was conducted prospectively in a certified clinical laboratory. In E2696 and E1694, we were limited to autoantibodies tested in banked blood sera in a research laboratory. No data on the clinical manifestations of autoimmunity were available. This may account for the lower incidence of HDI-induced autoimmunity in E1694 compared with the Hellenic study and the lesser impact in terms of survival.
One major issue that remains to be explored is the therapeutic predictive value of induced autoimmunity. In other words, are patients who develop autoimmunity likely to benefit from immunotherapy with HDI (and by extension IL-2 and anti-CTLA4 antibodies)? There is evidence that immunogenetic markers might help identify patients who may benefit from IFN-α2b therapy including the human leukocyte antigen (HLA) genotype, which is clearly associated with certain autoimmune diseases. There is also evidence for an association between specific HLA antigen expression of melanoma patients and response to immunotherapy and survival [43]. Serological testing for HLA class I and class II antigen expression has shown an association between specific HLA antigen expression and OS and clinical response to IL-2 therapy in patients with metastatic melanoma treated with IL-2 (e.g. HLA-DQ1) [44,45] or combination therapy with IFN-α and IL-2 [46]. One study reported an association of homozygosity of HLA-DR and decreased chance of response to treatment with IL-2 [44]. Gogas et al. [47] have recently reported an analysis exploring the influence of the HLA genotype on the likelihood of a favorable clinical outcome in 284 high-risk melanoma patients participating in the Hellenic randomize IFN-α trial and 246 healthy controls who were molecularly typed for HLA class I and II. The presence of the HLA–Cw*06 allele was related to better RFS and OS and the appearance of autoimmunity [47]. Other potential predictors include polymorphisms in the CTLA-4 gene and the FOXP3 transcription factor gene that are associated with certain autoimmune diseases as well as interferon pathway SNPs related to autoimmunity. These and other biomarker studies are ongoing in the context of the adjuvant study E1697 that compares 1-month intravenous high-dose IFN-α2b versus observation. Future analyses should include antitumor immune responses in patients who have developed autoimmunity that may ultimately allow us to define new target antigens of melanoma in the context of the MHC of these patients. These tumor antigens may yield more effective vaccine candidates for immunotherapy of melanoma.
Conclusion
The impact of HDI upon melanoma relapse and mortality is associated with immunomodulation and induction of autoimmunity to endocrine and other targets. Induction of autoimmunity should be further investigated as a surrogate biomarker of adjuvant immunotherapy therapeutic benefit. This biomarker develops over the course of a year, and cannot be used to alter the course of HDI therapy at this time. Our observation of autoimmunity among vaccine recipients who did not receive HDI indicates that beyond the presence of autoimmunity, further characterization of the kinetic and in-depth characteristics of autoimmunity as well as the genetic determinants of the capacity for induction of autoimmunity are needed to better identify those patients most likely to respond to HDI.
Acknowledgments
This investigator-initiated study was supported by the ECOG and by NIH award P50CA121973 and in part by DiaSorin Inc. UPCI shared resources that are supported in part by NIH/NCI award P30CA047904 were used for this project.
Grantee: Robert L. Comis, MD and Mitchell D. Schnall, MD, PhD, Group Co-Chairs
Grant/project #: ECOG-ACRIN Cancer Research Group
Title: Supported in part by Public Health Service Grants CA180820, CA180794, CA180844, and from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services.
Rationale: The original studies (E2696 and E1694) in which this correlative study was nested were conducted by the Eastern Cooperative Oncology Group (ECOG). ECOG was acknowledged in the manuscript.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manola J, Atkins M, Ibrahim J, Kirkwood J. Prognostic factors in metastatic melanoma: a pooled analysis of Eastern Cooperative Oncology Group trials. J Clin Oncol. 2000;18:3782–3793. doi: 10.1200/JCO.2000.18.22.3782. [DOI] [PubMed] [Google Scholar]
- 4.Tarhini AA, Agarwala SS. Cutaneous melanoma: available therapy for metastatic disease. Dermatol The. 2006;19:19–25. doi: 10.1111/j.1529-8019.2005.00052.x. [DOI] [PubMed] [Google Scholar]
- 5.Kirkwood JM, Strawderman MH, Ernstoff MS, Smith TJ, Borden EC, Blum RH. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol. 1996;14:7–17. doi: 10.1200/JCO.1996.14.1.7. [DOI] [PubMed] [Google Scholar]
- 6.Kirkwood JM, Ibrahim JG, Sondak VK, Richards J, Flaherty LE, Ernstoff MS, et al. High- and low-dose interferon alfa-2b in high-risk melanoma: first analysis of intergroup trial E1690/S9111/C9190. J Clin Oncol. 2000;18:2444–2458. doi: 10.1200/JCO.2000.18.12.2444. [DOI] [PubMed] [Google Scholar]
- 7.Kirkwood JM, Ibrahim JG, Sosman JA, Sondak VK, Agarwala SS, Ernstoff MS, et al. High-dose interferon alfa-2b significantly prolongs relapse-free and overall survival compared with the GM2-KLH/QS-21 vaccine in patients with resected stage IIB-III melanoma: results of intergroup trial E1694/S9512/C509801. J Clin Oncol. 2001;19:2370–2380. doi: 10.1200/JCO.2001.19.9.2370. [DOI] [PubMed] [Google Scholar]
- 8.Kirkwood JM, Manola J, Ibrahim J, Sondak V, Ernstoff MS, Rao U. A pooled analysis of eastern cooperative oncology group and intergroup trials of adjuvant high-dose interferon for melanoma. Clin Cancer Res. 2004;10:1670–1677. doi: 10.1158/1078-0432.ccr-1103-3. [DOI] [PubMed] [Google Scholar]
- 9.Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635–3648. doi: 10.1200/JCO.2001.19.16.3635. [DOI] [PubMed] [Google Scholar]
- 10.Kirkwood JM, Ibrahim J, Lawson DH, Atkins MB, Agarwala SS, Collins K, et al. High-dose interferon alfa-2b does not diminish antibody response to GM2 vaccination in patients with resected melanoma: results of the Multicenter Eastern Cooperative Oncology Group Phase II Trial E2696. J Clin Oncol. 2001;19:1430–1436. doi: 10.1200/JCO.2001.19.5.1430. [DOI] [PubMed] [Google Scholar]
- 11.Atkins MB, Mier JW, Parkinson DR, Gould JA, Berkman EM, Kaplan MM. Hypothyroidism after treatment with interleukin-2 and lymphokine-activated killer cells. N Engl J Med. 1988;318:1557–1563. doi: 10.1056/NEJM198806163182401. [DOI] [PubMed] [Google Scholar]
- 12.Weijl NI, Van der Harst D, Brand A, Kooy Y, Van Luxemburg S, Schroder J, et al. Hypothyroidism during immunotherapy with interleukin-2 is associated with antithyroid antibodies and response to treatment. J Clin Oncol. 1993;11:1376–1383. doi: 10.1200/JCO.1993.11.7.1376. [DOI] [PubMed] [Google Scholar]
- 13.Scalzo S, Gengaro A, Boccoli G, Masciulli R, Giannella G, Salvo G, et al. Primary hypothyroidism associated with interleukin-2 and interferon alpha-2 therapy of melanoma and renal carcinoma. Eur J Cancer. 1990;26:1152–1156. doi: 10.1016/0277-5379(90)90275-x. [DOI] [PubMed] [Google Scholar]
- 14.Krouse RS, Royal RE, Heywood G, Weintraub BD, White DE, Steinberg SM, et al. Thyroid dysfunction in 281 patients with metastatic melanoma or renal carcinoma treated with interleukin-2 alone. J Immunother Emphasis Tumor Immunol. 1995;18:272–278. doi: 10.1097/00002371-199511000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Phan GQ, Attia P, Steinberg SM, White DE, Rosenberg SA. Factors associated with response to high-dose interleukin-2 in patients with metastatic melanoma. J Clin Oncol. 2001;19:3477–3482. doi: 10.1200/JCO.2001.19.15.3477. [DOI] [PubMed] [Google Scholar]
- 16.Becker JC, Winkler B, Klingert S, Brocker EB. Antiphospholipid syndrome associated with immunotherapy for patients with melanoma. Cancer. 1994;73:1621–1624. doi: 10.1002/1097-0142(19940315)73:6<1621::aid-cncr2820730613>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 17.Rosenberg SA, White DE. Vitiligo in patients with melanoma: normal tissue antigens can be targets for cancer immunotherapy. J Immunother Emphasis Tumor Immunol. 1996;19:81–84. [PubMed] [Google Scholar]
- 18.Franzke A, Peest D, Probst-Kepper M, Buer J, Kirchner GI, Brabant G, et al. Autoimmunity resulting from cytokine treatment predicts long-term survival in patients with metastatic renal cell cancer. J Clin Oncol. 1999;17:529–533. doi: 10.1200/JCO.1999.17.2.529. [DOI] [PubMed] [Google Scholar]
- 19.Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanderson K, Scotland R, Lee P, Liu D, Groshen S, Snively J, et al. Autoimmunity in a phase I trial of a fully human anti-cytotoxic T-lymphocyte antigen-4 monoclonal antibody with multiple melanoma peptides and Montanide ISA 51 for patients with resected stages III and IV melanoma. J Clin Oncol. 2005;23:741–750. doi: 10.1200/JCO.2005.01.128. [DOI] [PubMed] [Google Scholar]
- 21.Dranoff G. CTLA-4 blockade: unveiling immune regulation. J Clin Oncol. 2005;23:662–664. doi: 10.1200/JCO.2005.09.923. [DOI] [PubMed] [Google Scholar]
- 22.Camacho LH, Antonia S, Sosman J, Kirkwood JM, Gajewski TF, Redman B, et al. Phase I/II trial of tremelimumab in patients with metastatic melanoma. J Clin Oncol. 2009;27:1075–1081. doi: 10.1200/JCO.2008.19.2435. [DOI] [PubMed] [Google Scholar]
- 23.Gogas H, Kirkwood JM. Prognostic significance of autoimmunity during treatment of melanoma with interferon. N Engl J Med. 2006;354:709–718. doi: 10.1056/NEJMoa053007. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 25.Cox DR. Regression models and life tables (with discussion) J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 26.Nordlund JJ, Kirkwood JM, Forget BM, Milton G, Albert DM, Lerner AB. Vitiligo in patients with metastatic melanoma: a good prognostic sign. J Am Acad Dermatol. 1983;9:689–696. doi: 10.1016/s0190-9622(83)70182-9. [DOI] [PubMed] [Google Scholar]
- 27.Bystryn JC, Rigel D, Friedman RJ, Kopf A. Prognostic significance of hypopigmentation in malignant melanoma. Arch Dermatol. 1987;123:1053–1055. [PubMed] [Google Scholar]
- 28.Schallreuter KU, Levenig C, Berger J. Vitiligo and cutaneous melanoma. A case study. Dermatologica. 1991;183:239–245. doi: 10.1159/000247693. [DOI] [PubMed] [Google Scholar]
- 29.Tarhini AA, Cherian J, Moschos SJ, Tawbi HA, Shuai Y, Gooding WE, et al. Safety and efficacy of combination immunotherapy with interferon alfa-2b and tremelimumab in patients with stage IV melanoma. J Clin Oncol. 2012;30:322–328. doi: 10.1200/JCO.2011.37.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Voskens CJ, Goldinger SM, Loquai C, Robert C, Kaehler KC, Berking C, et al. The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS One. 2013;8:e53745. doi: 10.1371/journal.pone.0053745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deutsch M, Dourakis S, Manesis EK, Gioustozi A, Hess G, Horsch A, et al. Thyroid abnormalities in chronic viral hepatitis and their relationship to interferon alfa therapy. Hepatology. 1997;26:206–210. doi: 10.1002/hep.510260127. [DOI] [PubMed] [Google Scholar]
- 32.Kalkner KM, Ronnblom L, Karlsson Parra AK, Bengtsson M, Olsson Y, Oberg K. Antibodies against double-stranded DNA and development of polymyositis during treatment with interferon. Qjm. 1998;91:393–399. doi: 10.1093/qjmed/91.6.393. [DOI] [PubMed] [Google Scholar]
- 33.Okanoue T, Sakamoto S, Itoh Y, Minami M, Yasui K, Sakamoto M, et al. Side effects of high-dose interferon therapy for chronic hepatitis C. J Hepatol. 1996;25:283–291. doi: 10.1016/s0168-8278(96)80113-9. [DOI] [PubMed] [Google Scholar]
- 34.Wesche B, Jaeckel E, Trautwein C, Wedemeyer H, Falorni A, Frank H, et al. Induction of autoantibodies to the adrenal cortex and pancreatic islet cells by interferon alpha therapy for chronic hepatitis C. Gut. 2001;48:378–383. doi: 10.1136/gut.48.3.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zuffa E, Vianelli N, Martinelli G, Tazzari P, Cavo M, Tura S. Autoimmune mediated thrombocytopenia associated with the use of interferon-alpha in chronic myeloid leukemia. Haematologica. 1996;81:533–535. [PubMed] [Google Scholar]
- 36.Vallisa D, Cavanna L, Berte R, Merli F, Ghisoni F, Buscarini L. Autoimmune thyroid dysfunctions in hematologic malignancies treated with alphainterferon. Acta Haematol. 1995;93:31–35. doi: 10.1159/000204086. [DOI] [PubMed] [Google Scholar]
- 37.Satzger I, Meier A, Schenck F, Kapp A, Hauschild A, Gutzmer R. Autoimmunity as a prognostic factor in melanoma patients treated with adjuvant low-dose interferon alpha. Int J Cancer. 2007;121:2562–2566. doi: 10.1002/ijc.22951. [DOI] [PubMed] [Google Scholar]
- 38.Eggermont AM, Suciu S, MacKie R, Ruka W, Testori A, Kruit W, et al. Post-surgery adjuvant therapy with intermediate doses of interferon alfa 2b versus observation in patients with stage IIb/III melanoma (EORTC 18952): randomised controlled trial. Lancet. 2005;366:1189–1196. doi: 10.1016/S0140-6736(05)67482-X. [DOI] [PubMed] [Google Scholar]
- 39.Hansson J, Aamdal S, Bastholt L, Brandberg Y, Hernberg M, Nilsson B, et al. Two different durations of adjuvant therapy with intermediate-dose interferon alfa-2b in patients with high-risk melanoma (Nordic IFN trial): a randomised phase 3 trial. Lancet Oncol. 2011;12:144–152. doi: 10.1016/S1470-2045(10)70288-6. [DOI] [PubMed] [Google Scholar]
- 40.Bouwhuis MG, Suciu S, Collette S, Aamdal S, Kruit WH, Bastholt L, et al. Autoimmune antibodies and recurrence-free interval in melanoma patients treated with adjuvant interferon. J Natl Cancer Inst. 2009;101:869–877. doi: 10.1093/jnci/djp132. [DOI] [PubMed] [Google Scholar]
- 41.Bouwhuis MG, Suciu S, Testori A, Kruit WH, Sales F, Patel P, et al. Phase III trial comparing adjuvant treatment with pegylated interferon Alfa-2b versus observation: prognostic significance of autoantibodies – EORTC 18991. J Clin Oncol. 2010;28:2460–2466. doi: 10.1200/JCO.2009.24.6264. [DOI] [PubMed] [Google Scholar]
- 42.Eggermont AM, Suciu S, Santinami M, Testori A, Kruit WH, Marsden J, et al. Adjuvant therapy with pegylated interferon alfa-2b versus observation alone in resected stage III melanoma: final results of EORTC 18991, a randomised phase III trial. Lancet. 2008;372:117–126. doi: 10.1016/S0140-6736(08)61033-8. [DOI] [PubMed] [Google Scholar]
- 43.Gogas H, Kirkwood JM, Falk CS, Dafni U, Sondak VK, Tsoutsos D, et al. Correlation of molecular human leukocyte antigen typing and outcome in high-risk melanoma patients receiving adjuvant interferon. Cancer. 2010;116:4326–4333. doi: 10.1002/cncr.25211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marincola FM, Venzon D, White D, Rubin JT, Lotze MT, Simonis TB, et al. HLA association with response and toxicity in melanoma patients treated with interleukin 2-based immunotherapy. Cancer Res. 1992;52:6561–6566. [PubMed] [Google Scholar]
- 45.Rubin JT, Day R, Duquesnoy R, Simonis B, Adams S, Lee J, et al. HLA-DQ1 is associated with clinical response and survival of patients with melanoma who are treated with interleukin-2. Ther Immunol. 1995;2:1–6. [PubMed] [Google Scholar]
- 46.Scheibenbogen C, Keilholz U, Mytilineos J, Suciu S, Manasterski M, Hunstein W. HLA class I alleles and responsiveness of melanoma to immunotherapy with interferon-alpha (IFN-alpha) and interleukin-2 (IL-2) Melanoma Res. 1994;4:191–194. doi: 10.1097/00008390-199406000-00008. [DOI] [PubMed] [Google Scholar]
- 47.Gogas H, Kirkwood JM, Falk CS, Dafni U, Sondak VK, Tsoutsos D, et al. Correlation of molecular human leukocyte antigen typing and outcome in high-risk melanoma patients receiving adjuvant interferon. Cancer. 2010;116:4326–4333. doi: 10.1002/cncr.25211. [DOI] [PMC free article] [PubMed] [Google Scholar]