Abstract
Glutathione, γ-glutamylcysteinylglycine, exists abundantly in nearly all organisms. Glutathione participates in various physiological processes involved in redox reactions by serving as an electron donor/acceptor. We found that the abundance of total glutathione increased up to 60% in resistant wheat plants within 72 hours following attack by the gall midge Mayetiola destructor, the Hessian fly. The increase in total glutathione abundance, however, is coupled with an unbalanced activation of glutathione metabolic pathways. The activity and transcript abundance of glutathione peroxidases, which convert reduced glutathione (GSH) to oxidized glutathione (GSSG), increased in infested resistant plants. However, the enzymatic activity and transcript abundance of glutathione reductases, which convert GSSG back to GSH, did not change. This unbalanced regulation of the glutathione oxidation/reduction cycle indicates the existence of an alternative pathway to regenerate GSH from GSSG to maintain a stable GSSG/GSH ratio. Our data suggest the possibility that GSSG is transported from cytosol to apoplast to serve as an oxidant for class III peroxidases to generate reactive oxygen species for plant defense against Hessian fly larvae. Our results provide a foundation for elucidating the molecular processes involved in glutathione-mediated plant resistance to Hessian fly and potentially other pests as well.
Glutathione, a thiol tripeptide of γ-glutamylcysteinylglycine, is widely distributed in both prokaryotic and eukaryotic organisms1,2. The ubiquitous and abundant tripeptide is synthesized through two sequential, ATP-dependent reactions3. The first reaction is catalyzed by γ-glutamylcysteine synthetase, joining L-glutamate and L-cysteine into γ-glutamylcysteine. The second reaction is catalyzed by glutathione synthetase, joining γ-glutamylcysteine and L-glycine to produce glutathione. Glutathione can be recycled through the oxidation/reduction cycle of its reduced (GSH) and oxidized (GSSG) forms1. GSH serves as a nucleophilic co-substrate of detoxification enzymes such as glutathione S-transferases (GST) in detoxifying xenobiotics5,6. GSH also serves as an essential electron donor to glutathione peroxidases (GPx) in the reduction of hydroperoxides7. The oxidized GSSG can be converted back to GSH through the action of glutathione reductase. Like other small peptides, glutathione is degraded by various peptidases including tripeptide aminopeptidases and γ-glutamyl transpeptidases1,3.
Glutathione is involved in various physiological functions in different organisms. In prokaryotes, glutathione maintains the proper oxidation states of protein thiols and protects cells from damage under abnormal conditions such as low pH, chlorine compounds, oxidative and osmotic stresses8. In mammals, glutathione serves as a major antioxidant that helps to prevent or even reduce the effect of certain human diseases including cancer, inflammation, and various other disorders9. In plants, glutathione participates in detoxification as well as signaling in plant defense against biotic and abiotic stresses10. The abundance of glutathione increases in plants exposed to oxidative stimuli11,12. Glutathione abundance also increases in plants infected with avirulent pathogens during incompatible interactions, but decreases in plants during compatible interactions12,13. Plants with γ-glutamylcysteine synthetase-deficient mutations have reduced levels of glutathione and exhibit a more susceptible phenotype to pathogens4,14,15 and to generalist insects16,17.
The molecular mechanism of glutathione involvement in parasite resistance/susceptibility in plants is not quite clear at present and may vary in different systems under different conditions. One possible pathway for glutathione involvement in plant resistance to insects is through increased production of secondary metabolites17. Glutathione serves as a thiol donor in this process. Glutathione may also affect the levels of reactive oxygen species (ROS), and thus participate in the hypersensitive reaction launched by resistant plants following pathogen attack4,18.
The Hessian fly, Mayetiola destructor, is a gall midge that causes destructive damage to wheat plants. Hessian fly interacts with wheat in a way similar to many plant-pathogen interactions, including a fixed feeding site and a typical gene-for-gene relationship19. During compatible interactions, susceptible plants are manipulated by Hessian fly larvae, including the suppression of plant defense, inhibition of wheat growth, the reprogramming of wheat metabolic pathways, and formation of nutritive cells at the feeding site20,21,22. During incompatible interactions, resistant plants defend themselves effectively against Hessian fly larval attack and grow normally after some initial growth deficit23. As a result of plant defense, Hessian fly larvae die within 3–5 days after the initial attack. The exact mechanism of the larval death in resistant plants is not known. Numerous genes encoding toxic chemicals including proteinaceous inhibitors, lectins, and various enzymes that participate in the synthesis of secondary metabolites are induced in resistant plants after Hessian fly attack21,24,25. Membrane remodeling and cell-wall strengthening may also play a crucial role in wheat resistance to Hessian fly26,27. In addition, there is a rapid ROS accumulation at the site of Hessian fly attack in resistant wheat (Liu et al., 2010). ROS are toxic to Hessian fly larvae and can also participate in cell wall strengthening27,28. The toxic chemicals, strengthening of cell wall, and elevated levels of ROS may collectively lead to Hessian fly larval death.
During our previous characterization of molecular pathways leading to wheat resistance to Hessian fly using microarrays, we have found that the transcript levels of a large number of genes encoding various enzymes involved in glutathione metabolism are strongly affected in resistant plants after Hessian fly attack21,27, indicating that glutathione may play an important role in wheat defense against Hessian fly attack. The objective of this study was to determine if the level of glutathione increases in wheat tissue following Hessian fly infestation, the molecular pathways leading to a glutathione increase, and the potential molecular mechanisms for glutathione involvement in wheat resistance to this insect pest.
Results
Hessian fly induces higher levels of glutathione in resistant plants
The levels of total glutathione (GSH+GSSG) increased steadily in tissues at the feeding site in resistant plants after Hessian fly infestation (Figure 1). The increase became apparent as early as three hours after Hessian fly larvae reached the feeding site, and at 72 hours, the abundance of total glutathione increased by about 60%. The increase in glutathione abundance was due to a proportional rise in both the reduced (GSH) and oxidized (GSSG) forms of glutathione. The GSH/GSSG ratio did not change significantly in infested plants in comparison with that in uninfested control plants (Figure 1, bottom panel). In contrast, the level of total glutathione did not change significantly in infested susceptible plants except for a slight, transient increase in the abundance of GSSG. The level of glutathione did not change significantly in tissues corresponding to the feeding site of uninfested resistant and susceptible plants during the same period when the experiment was carried out (Figure S1).
Figure 1. Hessian fly induces higher levels of glutathione in wheat tissues at the feeding site in resistant plants, but did not affect the ratio of GSH/GSSG.
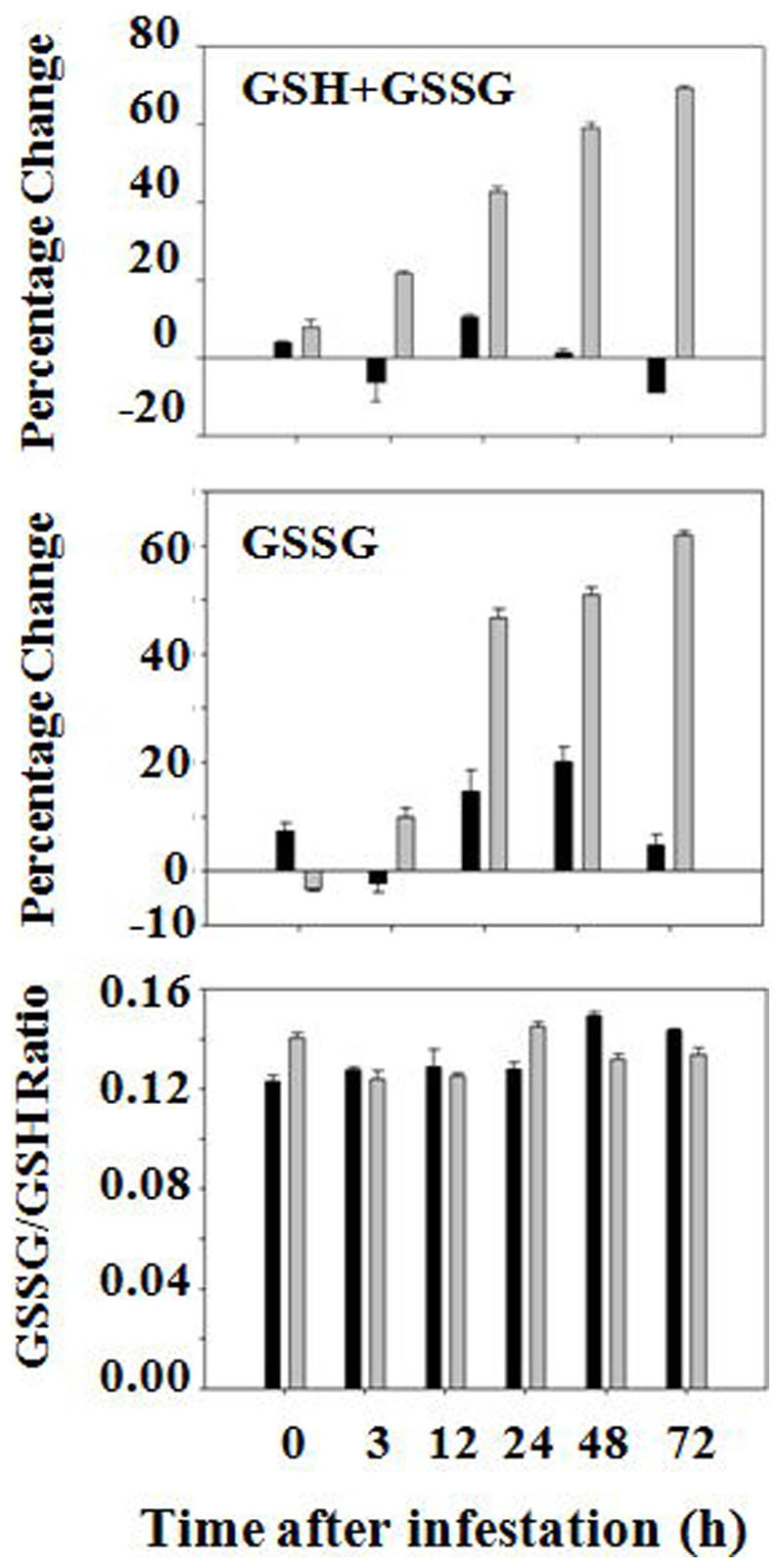
Black and grey bars represent data from susceptible (Newton) and resistant (Molly) plants, respectively. GSH+GSSG represent changes in the abundance of total glutathione, whereas GSSG represents changes in abundance of oxidized glutathione. The bottom panel represents the ratio of GSSG/GSH.
Hessian fly induces higher levels of glutathione synthetase activity in resistant plants
To determine if the increase in glutathione abundance is due to increased synthesis, we determined the activity of γ-glutamylcysteine synthetase and glutathione synthetase in wheat tissue at the feeding site. The enzymatic activities of these two enzymes were differentially regulated. The enzymatic activity of γ-glutamylcysteine synthetase did not change significantly in infested resistant plants (Figure S2). Consistent with the finding that there is no apparent increase in enzymatic activity, the transcript levels of genes encoding γ-glutamylcysteine synthetases did not change significantly either. On the other hand, the enzymatic activity of glutathione synthetase increased steadily in wheat tissue at the feeding site in resistant plants starting from three hours after Hessian fly infestation (Figure 2a). The increase in glutathione synthetase activity reached a maximum at 48 hours, and started to decrease at 72 hours after Hessian fly infestation. In susceptible plants, the enzymatic activity of glutathione synthetase decreased slightly in wheat tissue at the feeding site at three and 12 hours following Hessian fly infestation. At 24, 48, and 72 hours, glutathione synthetase activity increased in susceptible plants, but the increase was of a much smaller magnitude in comparison with that observed in resistant plants.
Figure 2. Hessian fly induces higher levels of enzymatic activity and transcript abundance of glutathione synthetases.
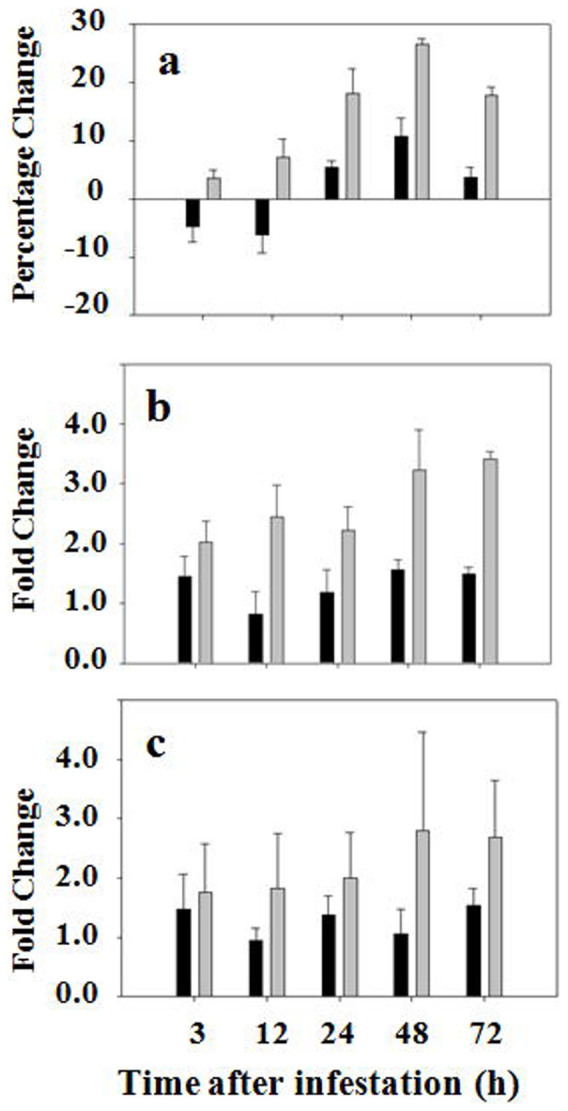
Black and grey bars represent data from susceptible (Newton) and resistant (Molly) plants, respectively. (a) Percentage change in enzymatic activity of glutathione synthetases in plants at different time points after Hessian fly infestation. (b) Fold changes of transcript abundance determined by qPCR using the primer pair common to CK156077, AJ579381 and AJ579382. (c) Fold changes of transcript abundance determined by qPCR using the primer pair specific to AJ579380.
To determine if the increased activity of glutathione synthetase in resistant plants was due to increased gene expression, qPCR was carried out to determine the abundance of transcripts of genes encoding glutathione synthetases. A search of Genbank and EST databases revealed four unique sequences that encode glutathione synthetases. Three of the sequences are very similar to each other (~96% sequence identity) and may represent transcripts derived from different homeologous alleles of the same gene (Figure S4). Accordingly, a common (Common) pair of primers was synthesized in the conserved regions for qPCR to determine the levels of transcripts corresponding to these three sequences. The other sequence of wheat glutathione synthetase is quite different with only 79% sequence identity to the three sequences mentioned earlier at the nucleotide level. A unique (Unique) pair of primers was designed for PCR to determine the abundance of transcript corresponding to this unique glutathione synthetase gene. As shown in Figure 2b, the levels of transcript abundance increased two to three fold in resistant plants after Hessian fly infestation when the common primer pair was used for qPCR analysis. No significant change in transcript abundance was observed in infested susceptible plants under the same conditions. Transcript abundance also increased in infested resistant plants when the unique primer pair was used for qPCR analysis even though the variation was bigger than that when PCR was carried with the primer pair common to three different sequences among different replicates (Figure 2c). Again, no significant change in transcript abundance was observed in infested susceptible plants when the same pair of primers unique to the genes was used for PCR. No significant change in transcript abundance of the genes encoding glutathione synthetases was observed in uninfested control plants during the same time period (Figure S5).
Hessian fly induces higher levels of glutathione peroxidase activity in both resistant and susceptible plants
Glutathione peroxidases detoxify ROS such as hydrogen peroxides using glutathione as an electron donor2,7. During this reaction, GSH is oxidized into GSSG. As shown in Figure 3a, the enzymatic activity of glutathione peroxidases increased steadily in both infested resistant and susceptible plants. The increase in glutathione peroxidase activity became significant at 12 hours after Hessian fly infestation, and reached maximum (~30% increase) at 48 hours.
Figure 3. Hessian fly induces higher levels of enzymatic activity and transcript abundance of glutathione peroxidases in both resistant and susceptible plants.
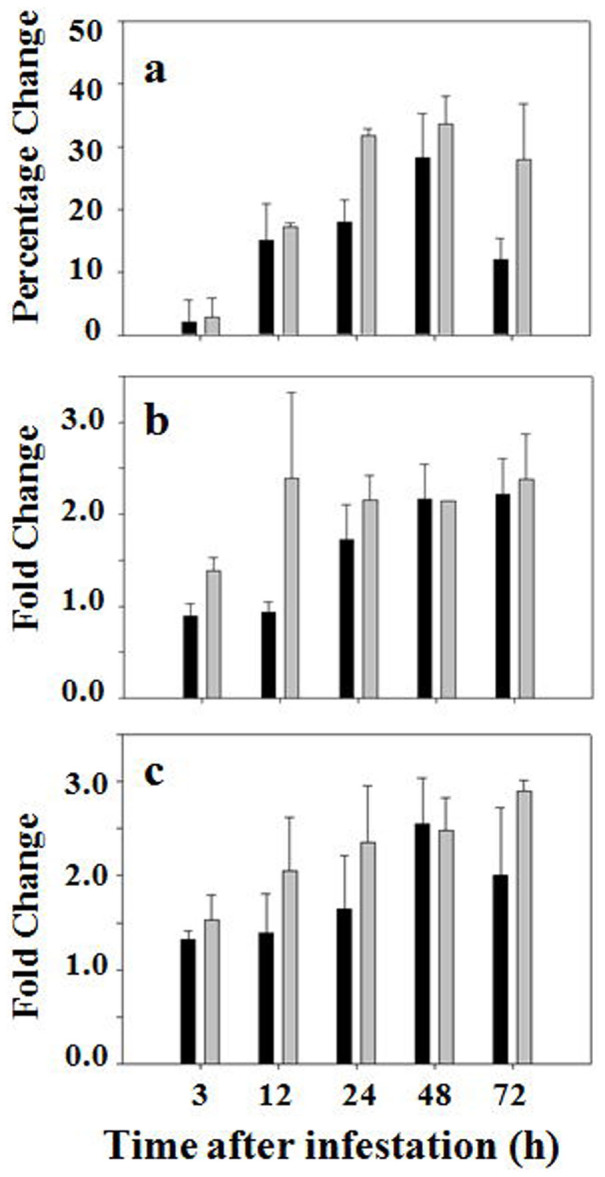
Black and grey bars represent data from susceptible (Newton) and resistant (Molly) plants, respectively. (a) Percentage change in enzymatic activity of glutathione peroxidases in plants at different time points after Hessian fly infestation. (b) Fold changes of transcript abundance determined by qPCR using the primer pair specific to BJ254939. (c) Fold changes of transcript abundance determined by qPCR using the primer pair specific to AY364468.
To determine if the increase in enzymatic activity of glutathione peroxidases was due to increased gene expression, qPCR was again conducted to determine if there was any change in abundance of glutathione peroxidase transcripts. A search of Genbank and EST databases identified two different glutathione peroxidase genes (Figure S6). Gene-specific primer pairs were designed to determine the levels of transcripts from these two genes. The abundance of transcripts corresponding to both genes increased significantly in both resistant and susceptible plants after Hessian fly infestation (Figures 3b, 3c).
Hessian fly induces higher levels of glutathione reductase activity in susceptible plants, but not in resistant plants
Glutathione reductases convert GSSG back into GSH using NADPH as an electron donor so that GSH can be reused for detoxification. Enzymatic activity of glutathione reductases increased steadily in susceptible plants after Hessian fly infestation (Figure 4a). However, no significant change was observed in the level of glutathione reductase activity in infested resistant plants.
Figure 4. Hessian fly induces higher levels of enzymatic activity and transcript abundance of glutathione reductases in susceptible plants, but not in resistant plants.
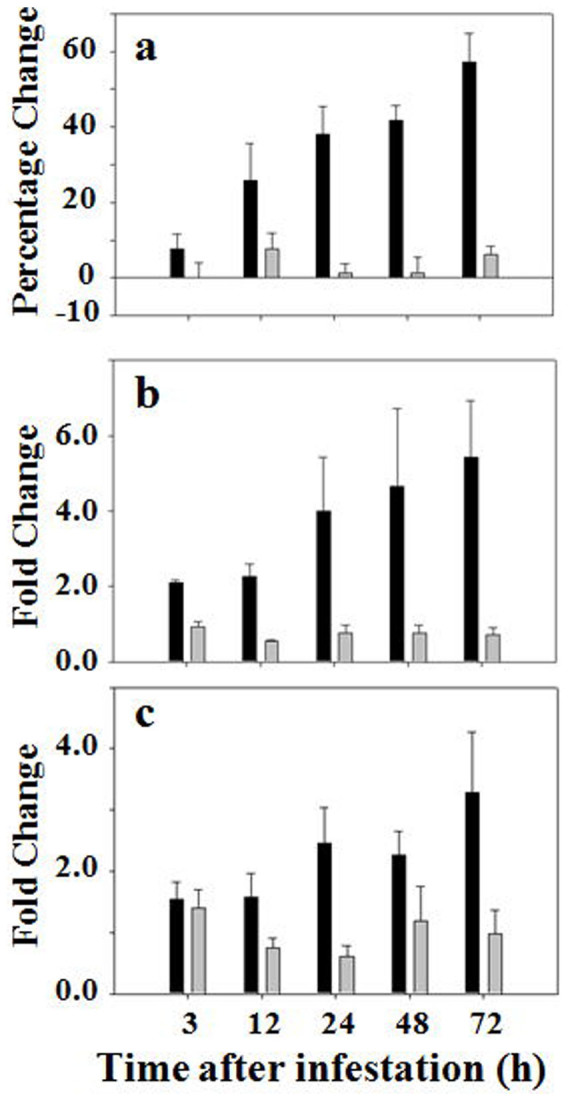
Black and grey bars represent data from susceptible (Newton) and resistant (Molly) plants, respectively. (a) Percentage change in enzymatic activity of glutathione reductases in plants at different time points after Hessian fly infestation. (b) Fold changes of transcript abundance determined by qPCR using the primer pair specific to AY364467. (c) Fold changes of transcript abundance determined by qPCR using the primer pair specific to FK827496.
To determine if the increase in enzymatic activity in infested susceptible plants was due to increased gene expression, qPCR was again carried out to determine the levels of transcripts encoding glutathione reductases. A search of Genbank and EST databases identified two distinct glutathione reductase genes (Figure S7). Unique primer pairs were designed for PCR analysis for each gene. qPCR analysis revealed that the abundance of transcripts increased significantly for both genes in infested susceptible plants (Figures 4b, 4c). No significant change in the levels of transcripts for either one of the genes was detected in infested resistant plants.
Hessian fly induces high levels of transcripts encoding glutathione degradation enzymes in susceptible plants, but not in resistant plants
Glutathione is degraded by various types of peptidases (Noctor et al., 1998, 2012). Therefore, the levels of peptidase activity can affect the turnover rates of glutathione, thus affecting the glutathione abundance in wheat cells. We analyzed the transcript abundance of two types of peptidases, γ-glutamyltransferases (γ-glutamyl transpeptidase, GT) and tripeptide aminopeptidases (TPA) by qPCR. γ-glutamyltransferases catalyze the transfer of the γ-glutamyl moiety of glutathione to an acceptor, whereas tripeptide aminopeptidases can also remove glutamyl group from glutathione1,3.
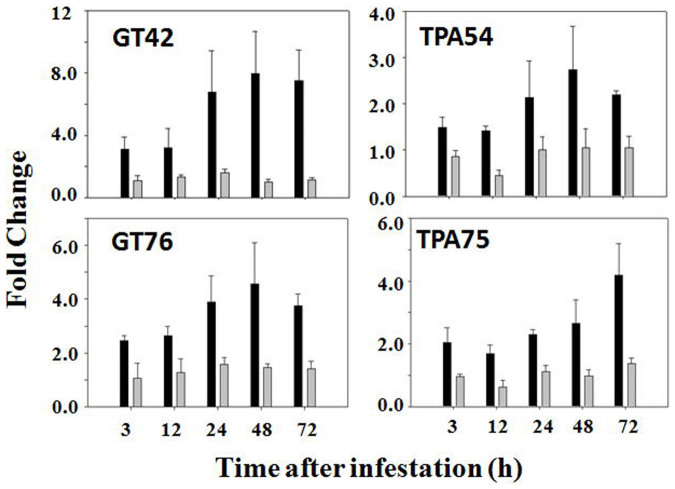
A search of Genbank and EST databases identified two different cDNAs (GT42 and GT76) encoding γ-glutamyltransferases (Figure S8). Primer pairs specific to the two sequences were designed for qPCR analysis. As shown in the left panel of Figures 5, both primer pairs detected significant increases in the abundance of transcripts encoding γ-glutamyltransferases in infested susceptible plants. However, no significant change was detected for both genes in infested resistant plants.
Figure 5. Hessian fly induces higher levels of transcript abundance of genes encoding glutathione degradation enzymes in susceptible plants, but not in resistant plants.
Black and grey bars represent data from susceptible (Newton) and resistant (Molly) plants, respectively. qPCR was carried out using primers specific to the γ-glutamyltransferase genes BU100842 (GT42) and AK333876 (GT76), and to the tripeptide aminopeptidase genes CJ717454 (TPA54) and HX136475 (TPA75), respectively.
A search of Genbank and EST databases identified three sequences that encode tripeptide aminopeptidases (Figure S9). Two of the three sequences are nearly identical and therefore were considered as the products of the same gene. Accordingly two primer pairs specific to each gene (TPA54 and TPA75) were designed for qPCR analysis. As shown in the right panel of Figures 5, significant increases were observed in the PCR result in samples derived from infested susceptible plants. Again no significant change was detected in infested resistant plants.
Discussion
The pathways for glutathione synthesis, recycling, and metabolism are well established (Figure 6). Glutathione synthesis is a two-step process involving the enzymes γ-glutamylcysteine synthetase (γ-GCS) and glutathione synthetase (GS). In this study, we found that the two enzymes for glutathione synthesis were unevenly regulated in resistant plants after Hessian fly infestation. Specifically, the enzymatic activity and transcript abundance of glutathione synthetase, the enzyme for the second reaction, was upregulated rapidly and significantly in infested resistant plants. However, no significant changes were detected for the enzymatic activity and transcript abundance of γ-glutamylcysteine synthetase, the enzyme for the first reaction, in infested resistant plants under the same condition. γ-Glutamylcysteine synthetase was reported to be the rate-limiting step for the overall glutathione biosynthesis process1,29. This unbalanced regulation of the glutathione synthetic pathway makes it hard to explain the rapid and steady increase of glutathione abundance in infested resistant plants. The finding that there is essentially no change in activity of the rate-limiting enzyme would suggest that there will be no increase in glutathione synthesis. However, the up to 60% increase in total glutathione in infested resistant plants could not be simply explained by increased stability of glutathione, since Hessian fly did not downregulate genes encoding the putative glutathione degradation enzymes (Figure 5). Further research will have to be carried out to explain this dilemma. The unbalanced regulation of the glutathione synthesis coupled with the rapid and steady increase of glutathione abundance in the wheat tissue of the infested resistant plants suggested that the γ-glutamylcysteine synthetase might be either inhibited by a negative feedback from GSH, or it is not the rate-limiting enzyme in the glutathione synthetic pathway in wheat plants under our experimental conditions. In mammalian cells, γ-glutamylcysteine synthetase is effectively inhibited by a GSH feedback, whereas glutathione synthetase is not subject to the negative feedback9,30. It has also been reported that the γ-glutamylcysteine synthetase is not the limiting step in plants under heavy metal stress conditions, and overexpression of a bacterial glutathione synthetase gene alone in Indian mustard results in higher concentrations of glutathione in the transgenic plant31.
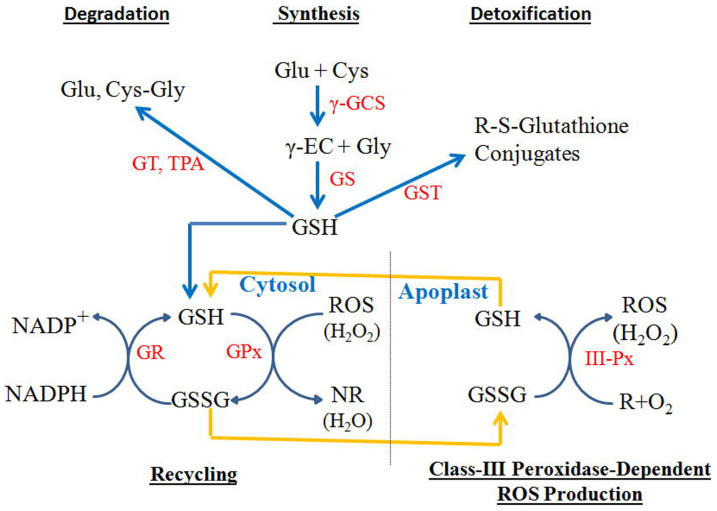
Figure 6. Metabolic pathways of glutathione synthesis, recycling, detoxification, and degradation; and a model for glutathione to serve as an oxidant for class III peroxidases during generation of ROS such as hydrogen peroxides.
Glu, Cys, γ-EC, Gly, and Cys-Gly represent glutamate, cysteine, γ-glutamylcysteine, glycine, and cysteinylglycine, respectively. γ-GCS, GS, GT, TPA, GST, GR, GPx, and III-Px represent γ-glutamylcysteine synthetases, glutathione synthetases, γ-glutamyltransferases, tripeptide aminopeptidases, glutathione S-transferases, glutathione reductases, glutathione peroxidases, and class III peroxidases, respectively. Blue arrows indicate direction of metabolite flow, whereas yellow arrows indicate transport of GSSG and GSH between cytosol and apoplast, where GSSG serves as an oxidant for class III peroxidases during ROS generation.
Not only was the regulation of the two step glutathione-synthetic pathway unevenly regulated, but the oxidation/reduction cycle of GSH/GSSG was also unbalanced in infested resistant plants. The enzymatic activity and transcript abundance of glutathione peroxidases, which convert GSH to GSSG when the enzyme detoxifies ROS, increased in infested resistant plants. However, the enzymatic activity and transcript abundance of glutathione reductases, which convert GSSG back to GSH, did not change significantly in infested resistant plants. The unbalanced activation of the two enzymes in the same cycle would suggest a net accumulation of GSSG in infested resistant plants. However, the ratio of GSSG/GSH did not change significantly in these plants, indicating that there is an alternative pathway(s) to convert GSSG back to GSH in infested resistant plants to maintain a stable GSSG/GSH ratio.
One possible alternative pathway to convert GSSG back to GSH is through class III peroxidases. Class III peroxidases are a large group of enzymes that are unique to plants15. Class III peroxidases are located in extracellular space and are able to produce ROS in plants under certain conditions7,32,33. Hessian fly infestation induces elevated levels of ROS at the feeding site in resistant plants28. The ROS burst in infested resistant wheat, however, is not produced by NADPH-dependent oxidases28,34, which are responsible for ROS production in many other plant-pest systems35,36. Instead, a large number of genes encoding class III peroxidases are rapidly and greatly upregulated, and peroxidase activity increases in apoplastic space28.
In order for class III peroxidases to produce ROS, two conditions must be satisfied: 1) ion fluxes that lead to extracellular alkalization and 2) an abundant and recyclable oxidant as electron acceptor37. Ion fluxes leading to an increase in local pH have been observed in several systems and could be induced by elicitation from avirulent Hessian fly larval elicitors. The abundant oxidant, however, has not been identified in any plant – pest system. The high abundance and rapid and steady increase of glutathione observed in this study indicate that oxidized GSSG can serve as the oxidant for class III peroxidases for ROS production. The conversion of GSSG back to GSH during ROS production would explain the unbalanced activation of the glutathione oxidation/reduction cycle. We hypothesize that the excess GSSG in cytosol were transported to extracellular space where class III peroxidases locate, and served as the oxidant either directly or indirectly during ROS production by class III peroxidases (Figure 6). The reduced GSH could be transported back to cytosol where they could be re-oxidized. The two independent pathways via glutathione reductases in cytosol and class III peroxidases in apoplast could maintain the balance of the GSH/GSSG cycle.
The hypothesis that GSSG functions as the oxidant for class III peroxidases during ROS production in apoplast, however, is not consistent with previous observations that a GSH peak was not observed in a bean apoplastic fluid before and after an oxidative burst, and glutathione was less effective for the production of reactive oxygen species than free cysteine in an in vitro assay37,38. The lack of GSH detection can be explained by the possibility that free GSH is maintained in apoplast at a minimum level and is transported back to cytosol after GSSG is reduced. The addition of glutathione into an in vitro solution may not be active as an oxidant in the absence of a system to generate the oxidized form of glutathione, which exists in different compartments in a living cell.
The rapid and steady increase in glutathione abundance in infested resistant plants has also been observed in other plant-pathogen systems32,39. For example, the abundance of glutathione increases in plants infected with avirulent pathogens during incompatible interactions13,40. Mutants with a disrupted synthetic pathway contain lower levels of glutathione and are more susceptible to both pathogens and generalist insects4,14,15,16,17. These data strongly suggest that glutathione plays a role in plant defense against a wide range of pests.
In comparison to what has been observed in infested resistant plants, the abundance of glutathione did not significantly change or even slightly decreased in susceptible plants following Hessian fly attack. Despite the relative stable abundance of glutathione in infested susceptible plants, there were, however, significant changes in metabolic pathways of glutathione in these plants. Specifically, the enzymatic activity of glutathione synthetases decreased slightly at earlier time points, but increased slightly at later time points. The enzymatic activities of both glutathione peroxidases and glutathione reductases increased significantly in infested susceptible plants. The coordinate increase of the two enzymes in the glutathione oxidation/reduction cycle indicates that there was increased usage and recycling of glutathione in susceptible plants even though the total amount of glutathione remained stable. A possible impact of the increased recycling of glutathione could be increased detoxification of ROS in infested susceptible plants, thus creating a more favorable diet for Hessian fly larvae. Interestingly, several genes encoding putative glutathione degradation enzymes were significantly upregulated in infested susceptible plants. The fact that the increase in gene expression of glutathione degradation enzymes did not significantly reduce the abundance of glutathione indicates that these peptidases might be induced for other functions. The enzymes that can degrade glutathione can also degrade other small peptides. Indeed, dipeptides and free amino acids increases in susceptible plants following Hessian fly attack22.
In summary, Hessian fly induces a rapid and steady accumulation of glutathione in resistant plants, but not in susceptible plants. The increase in glutathione abundance in infested resistant plants is at least partially achieved by increased synthesis via increased enzymatic activity and transcript abundance of genes encoding glutathione synthetases. Even though the abundance of total glutathione increased in infested resistant plants, the GSH/GSSG ratio remained the same. On the other hand, the enzymatic activity and transcript abundance of enzymes in the GSH/GSSG cycle are unevenly regulated. Specifically, the activity and transcript abundance of glutathione peroxidases, which convert GSH to GSSG, increased in infested resistant plants, but the enzymatic activity and transcript abundance of glutathione reductases, which converts GSSG back to GSH, did not change. The unbalanced regulation of two enzymes in the same cycle suggests that an alternative pathway exists to convert GSSG back to GSH. We hypothesize that GSSG produced by glutathione peroxidases in cytosol is transported into apoplastic spaces where class III peroxidases are located, and serve as an oxidant to class III peroxidases during the production of ROS for plant defense. Extensive evidence has been obtained that class III peroxidases are one of the mechanisms for launching oxidative defense in plants, but no oxidant for these enzymes has been identified in any plant – parasite systems. Our results could stimulate the elucidation of the molecular pathways involved in glutathione that lead to oxidative defense in plants.
Methods
Hessian fly population
Hessian fly (Mayetiola destructor) biotype GP was used in this study41. This biotype is virulent to wheat (Triticum aestivum) Newton (with no R gene), but avirulent to wheat Molly (containing R gene H13).
Plant materials and sample collection
Two near-isogenic wheat lines ‘Molly' (H13; resistant to biotype GP) and ‘Newton' (susceptible to biotype GP) were planted in groups of 20 seeds per pot (4-inch in diameter) containing Promix Professional growing medium (Premier Horticulture Inc., Quakertown, PA). After germination, 15 plants were kept for experiments and weak or extra plants were removed from the pot. Wheat plants were maintained in a growth chamber (L-41L2, Percival, Perry, IA) setting at 20 ± 1°C (day time) and 18 ± 1°C (night time) with a 14 h:10 h (L:D) photoperiod with light intensity of 1000 µmol m−2 s−1. Wheat seedlings in a pot were infested at 1.5-leaf stage with 20~25 eggs/plant by confining flies in the pot with a mesh screen cage. The initial infestation time point was defined as the time when the newly hatched larvae (neonates) just reached the feeding site between leaf sheaths at the base of the plant (monitored by dissecting extra infested plants). Each experiment consisted of three independent biological replicates, and each replicate was carried out at different times. Samples were harvested at 3, 12, 24, 48, and 72 hours after Hessian fly (Mayetiola destructor) infestation. Leaf-sheath tissue of 10–15 mm at the feeding site (crown tissue above the root-shoot junction) was cut out, insects removed, and used for sample extraction for various analyses. Each sample was handled in a 1.7 ml microtube that contained a pool of tissues collected from 10–15 plants. Harvested tissue samples were frozen immediately in liquid nitrogen and stored at −80°C.
RNA extraction and cDNA synthesis
Total RNA was extracted from frozen tissues using TRI reagent (Molecular Research Inc. Cincinnati, OH), according to the manufacturer's instruction. RNA samples were treated with the TURBO DNA-free kit (Applied Biosystems, Foster City, CA), further purified through an RNease kit (Qiagen), and quantified with a NanoDrop-1000 (NanoDrop Technologies Inc., Wilmington, DE). All purified RNA samples were diluted and adjusted to 400 ng/µl to ensure equal amounts of cDNA template for quantification of mRNA abundance. cDNA was synthesized using SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen, Grand Island, NY), according to the manufacturer's guidelines.
qPCR analysis
qPCR for selected wheat genes was conducted with the iQ SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA) on a BioRad-iCycler detection system as described previously (Liu et al., 2010). Target-specific PCR primers were designed using the software package Beacon Designer 8.01 and listed in Table S1. Relative fold-changes for transcripts were calculated using the comparative 2-ΔΔCT method42 (Livak and Schmittgen, 2001) and normalized to an actin control. Fold changes were calculated comparing transcript abundance of a gene in infested plants with that for the same gene in uninfested control samples.
Bioassays
Abundance of total glutathione (GSH + GSSG) and glutathione disulfide (GSSG) in wheat plants was determined using a Glutathione Assay Kit # 703002 (Cayman Chemical, Ann Arbor, MI, USA) according to the manufacturer's instruction. Briefly, 0.18 g frozen wheat tissue of each sample was homogenized in 1.1 ml cold homogenate buffer in a 1.7 ml tube, and 800 μl of supernatant was obtained after centrifuge. To determine the abundance of GSSG, GSH in the sample was first removed by a treatment with 2-vinylpyridine. The assay was performed in an 8 × 12-well microplate. Absorbance at 410 nm was measured once every minute at 7 time points using Microplate Spectrophotometer PowerWave XS(BioTek Instruments, Winooski, VT, USA). Data collection and analysis were done using the Microplate Data Collection & Analysis Software Gen5 (BioTek).
The enzymatic activities of γ-glutamylcysteine synthetase and gluthione synthetase were determined using the method described previously with a slight modification43. The assay for γ-glutamylcysteine synthetase activity is based on the formation of GSH from cysteine, glutamate, and glycine in the presence of the excess of glutathione synthetase. The assay for glutathione synthetase is based on the formation of GSH from γ-glutamylcysteine and glycine. In each case, the newly produced GSH was quantified using the Glutathione Assay Kit # 703002 (Cayman Chemical) described as previously.
The enzymatic activities of Glutathione Peroxidase and Glutathione Reductase were determined with the following kits (from Cayman Chemical): Glutathione Peroxidase Assay Kit # 703102 and Glutathione Reductase Assay Kit# 703202, according to the manufacturer's instruction. All the absorbance was read at 340 nm.
Statistical test
Data were subjected to analysis of variance (ANOVA), and Tukey's honestly significant difference (HSD) multiple comparisons were conducted using ProStat software Version 5.5(Poly Software International Inc., Pearl River, NY). Tukey's 95% simultaneous confidence intervals were used to separate data into groups.
Author Contributions
X.L. and S.Z. conducted the experimental work. M.S.C. conceived the study. X.L., S.Z., R.J.W., J.J.S. and M.C. analyzed the data and wrote the paper.
Supplementary Material
Supplementary Information
Acknowledgments
This paper is a joint contribution from the United States Department of Agriculture-Agriculture Research Service at Manhattan, Kansas, and the Kansas Agricultural Experiment Station. We thank Dr. Subbaratnam Muthukrishnan for reviewing an early version of the manuscript. Mention of a commercial or proprietary product does not constitute an endorsement or recommendation for its use by the USDA. The research was partially supported by a grant from the U.S. Department of Agriculture (USDA NIFA 2010-03741).
References
- Noctor G. et al. Glutathione in plants: an integrated overview. Plant, Cell Environ. 35, 454–484 (2012). [DOI] [PubMed] [Google Scholar]
- Dubreuil-Maurizi C. & Poinssot B. Role of glutathione in plant signaling under biotic stress. Plant Signal. Behav. 7, 210–212 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G. et al. Glutathione: biosynthesis, metabolism and relationship to stress tolerance explored in transformed plants. J. Exp. Bot. 49, 623–647 (1998). [Google Scholar]
- Dubreuil-Maurizi C. et al. Glutathione deficiency of the Arabidopsis mutant pad2-1 affects oxidative stress-related events, defense gene expression, and the hypersensitive response. Plant Physiol. 157, 2000–2012 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs K. A. The functions and regulation of glutathione S-transferases in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47, 127–158 (1996). [DOI] [PubMed] [Google Scholar]
- Öztetik E. A tale of plant glutathione S-transferases: Since 1970. Botanical Rev. 74, 419–437 (2008). [Google Scholar]
- Dixon D. P., Cummins I., Cole D. J. & Edwards R. Glutathione-mediated detoxification systems in plants. Curr. Opin. Plant Biol. 1, 258–266 (1998). [DOI] [PubMed] [Google Scholar]
- Masip L., Veeravalli K. & Georgiou G. The many faces of glutathione in bacteria. Antioxid. Redox Signal. 8, 753–762 (2006). [DOI] [PubMed] [Google Scholar]
- Lushchak V. I. Glutathione homeostasis and functions: Potential targets for medical interventions. J. Amino Acid. 2012, 1–26 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanta S. & Chattopadhyay S. Glutathione as a signaling molecule: Another challenge to pathogens. Plant Signal. Behav. 6, 783–788 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I. K. Stimulation of glutathione synthesis in photore-spiring plants by catalase inhibitors. Plant Physiol. 79, 1044–1047 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- May M. J., Hammond-Kosack K. E. & Jones J. D. G. Involvement of reactive oxygen species, glutathione metabolism and lipid peroxidation in the Cf-gene-dependent defence response of tomato cotyledons induced by race-specific elicitors of Cladosporium fulvum. Plant Physiol. 110, 1367–1379 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanacker H., Carver T. L. W. & Foyer C. H. Pathogen-induced changes in the antioxidant status of the apoplast in barley leaves. Plant Physiol. 117, 1103–1114 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisy V. et al. Identification of PAD2 as a γ-glutamylcysteine synthetase highlights the importance of glutathione in disease resistance of Arabidopsis. Plant J. 49, 159–172 (2007). [DOI] [PubMed] [Google Scholar]
- Hiraga S., Sasaki K., Ito H., Ohashi Y. & Matsui H. A large family of class III plant peroxidases. Plant Cell Physiol. 42, 462–468 (2001). [DOI] [PubMed] [Google Scholar]
- Schlaeppi K., Bodenhausen N., Buchala A., Mauch F. & Reymond P. The glutathione-deficient mutant pad2-1 accumulates lower amounts of glucosinolates and is more susceptible to the insect herbivore Spodoptera littoralis. Plant J. 55, 774–786 (2008). [DOI] [PubMed] [Google Scholar]
- Bednarek P. et al. A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science 323, 101–106 (2009). [DOI] [PubMed] [Google Scholar]
- Hiruma K. et al. Glutathione and tryptophan metabolism are required for Arabidopsis immunity during the hypersensitive response to hemibiotrophs. Proc. Natl. Acad. Sci. USA 110, 9589–9594 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart J. J., Chen M. S., Shukle R. & Harris M. O. Gall midges (Hessian flies) as plant pathogens. Annu. Rev. Phytopath. 50, 339–358 (2012). [DOI] [PubMed] [Google Scholar]
- Harris M. O. et al. Virulent Hessian fly (Diptera: Cecidomyiidae) larvae induce a nutritive tissue during compatible interactions with wheat. Ann. Entomol. Soc. Amer. 99, 305–316 (2006). [Google Scholar]
- Liu X. M. et al. Gene expression of different wheat genotypes during attack by virulent and avirulent Hessian fly (Mayetiola destructor) larvae. J. Chem. Ecol. 33, 2171–2194 (2007). [DOI] [PubMed] [Google Scholar]
- Zhu L. et al. Hessian fly (Mayetiola destructor) attack causes dramatic shift in carbon and nitrogen metabolism in wheat. Mol. Plant-Microb. Interact. 21, 70–78 (2008). [DOI] [PubMed] [Google Scholar]
- Anderson K. G. & Harris M. O. Does R gene resistance allow wheat to prevent plant growth effects associated with Hessian fly (Diptera: Cecidomyiidae) attack? J. Econ. Entomol. 99, 1842–1853 (2006). [DOI] [PubMed] [Google Scholar]
- Williams C. E., Collier C. C., Nemacheck J. A., Liang C. & Cambron S. E. A lectin-like wheat gene responds systematically to attempted feeding by avirulent first-instar Hessian fly larvae. J. Chem. Ecol. 28, 1411–1428 (2002). [DOI] [PubMed] [Google Scholar]
- Subramanyam S. et al. Functional characterization of HFR1, a high-mannose N-glycan-specific wheat lectin induced by Hessian fly larvae. Plant Physiol. 147, 1412–1426 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L. et al. Rapid mobilization of membrane lipids in wheat leaf sheathes during incompatible interactions with Hessian fly. Mol. Plant-Microb. Interact. 25, 920–930 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khajuria C. et al. Mobilization of lipids and fortification of cell wall and cuticle are important in host defense against Hessian fly. BMC Genomics 14, 423 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. M. et al. Reactive oxygen species are involved in plant defense against a gall midge. Plant Physiol. 152, 985–999 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G., Gomez L., Vanacker H. & Foyer C. H. Interactions between biosynthesis, compartmentation and transport in the control of glutathione homeostasis and signaling. J. Ex. Bot. 53, 1283–1304 (2002). [DOI] [PubMed] [Google Scholar]
- Wang W. & Ballatori N. Endogenous glutathione conjugates: Occurrence and biological functions. Pharmocol. Rev. 50, 335–355 (1998). [PubMed] [Google Scholar]
- Zhu Y. L., Pilon-Smits E. A. H., Jouanin L. & Terry N. Overexpression of glutathione synthetase in Indian mustard enhances Cadmium accumulation and tolerance. Plant Physiol. 119, 73–80 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwell G. P., Bindschedler L. V., Blee K. A., Butt V. & Davies D. R. The apoplastic oxidative burst in response to biotic stress in plants: a three-component system. J. Exp. Bot. 53, 1367–1376 (2002). [PubMed] [Google Scholar]
- Mika A., Minibayeva F., Beckett R. & Luthje S. Possible functions of extracellular peroxidases in stress-induced generation and detoxification of active oxygen species. Phytochem. Rev. 3, 173–193 (2004). [Google Scholar]
- Giovanini M. P. et al. Gene-for-gene defense of wheat against the Hessian fly lacks a classical oxidative burst. Mol. Plant-Microb. Interact. 19, 1023–1033 (2006). [DOI] [PubMed] [Google Scholar]
- Lamb C. & Dixon R. A. The oxidative burst in plant disease resistance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48, 251–275 (1997). [DOI] [PubMed] [Google Scholar]
- Moloi M. J. & van der Westhuizen A. The reactive oxygen species are involved in resistance response of wheat to the Russian wheat aphid. J. Plant Physiol. 163, 1118–1125 (2006). [DOI] [PubMed] [Google Scholar]
- Bolwell G. P. et al. Recent advances in understanding the origin of the apoplastic oxidative burst in plant cells. Free Rad. Res. 32, S137–145 (1999). [DOI] [PubMed] [Google Scholar]
- Bolwell G. P., Davies D. R., Gerrish C., Auh C.-K. & Murphy T. M. Comparative biochemistry of the oxidative burst produced by rose and French bean cells reveals two distinct mechanisms. Plant Physiol. 116, 1379–1385 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres M. A., Jonathan D. G. & Dangl J. L. Reactive oxygen species signaling in response to pathogens. Plant Physiol. 141, 373–378 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- May M. J. & Leaver C. J. Oxidative stimulation of glutathione synthesis in Arabidopsis thaliana suspension cultures. Plant Physiol. 103, 621–627 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. S. et al. Virulence analysis of Hessian fly (Mayetiola destructor) populations from Texas, Oklahoma, and Kansas. J. Econ. Entomol. 102, 774–780 (2009). [DOI] [PubMed] [Google Scholar]
- Livak K. J. & Schmittgen T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408 (2001). [DOI] [PubMed] [Google Scholar]
- Volohonsky G. et al. A spectrophotmetric assay of γ-glutamylcysteine synthetase and glutathione synthetase in crude extracts from tissues and cultured mammalian cells. Chemico-Biol. Interact. 140, 49–65 (2002). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information