Abstract
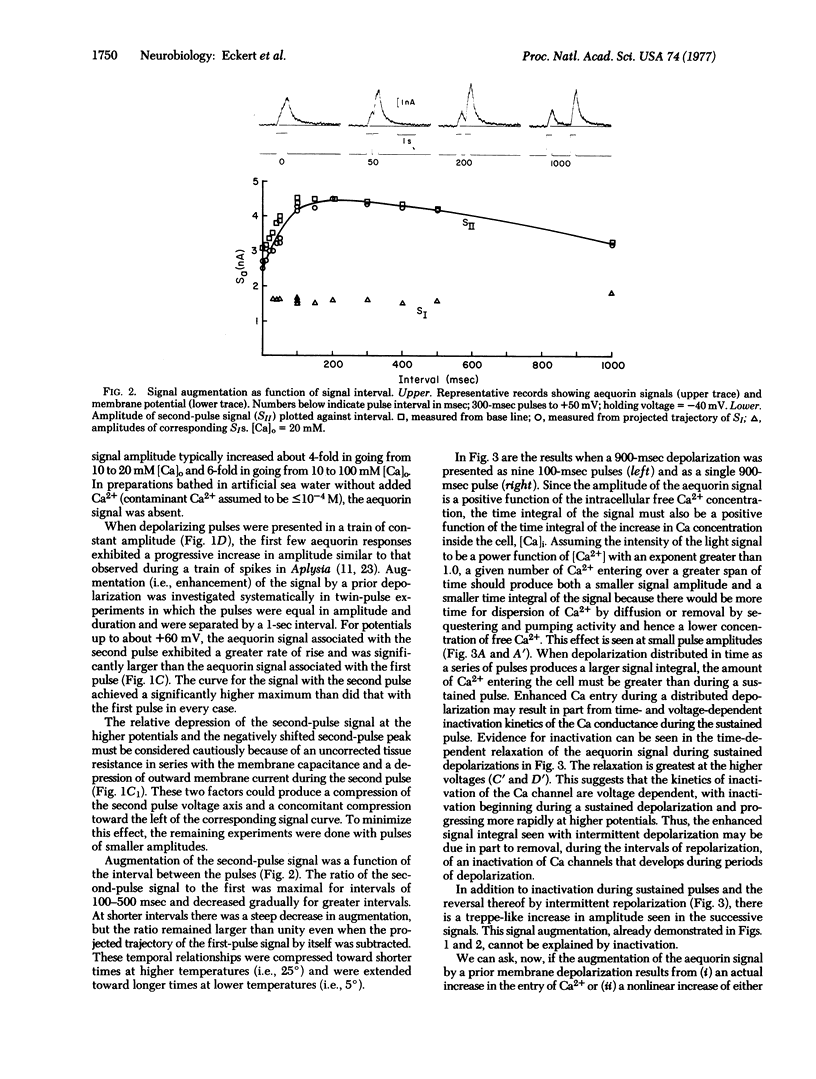
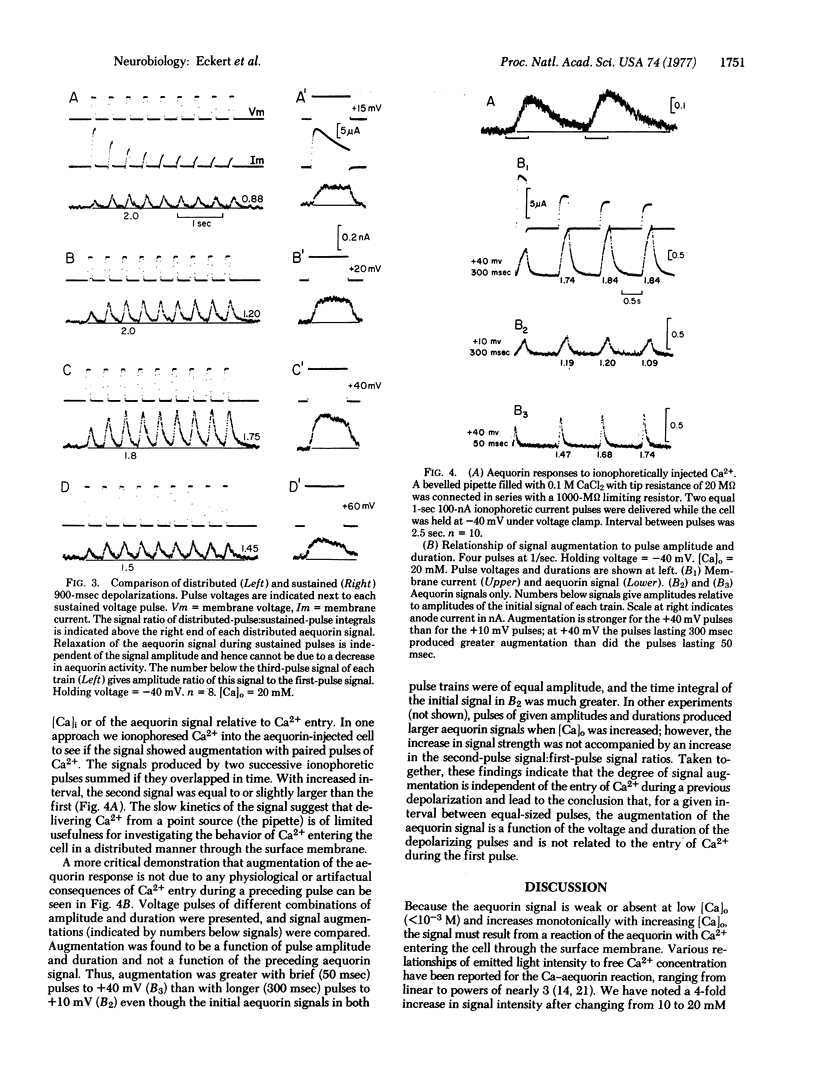
Voltage-clamp experiments were performed on giant neurons of the nudibranch Anisodoris nobilis injected with the Ca-sensitive photoprotein, aequorin. Depolarization beyond -10 to +5 m V produced an aequorin signal, the amplitude of which depended on the extracellular Ca2+ concentration, the amplitude of the depolarization, and its duration. In paired pulse experiments, the amplitude of the aequorin signal produced in response to the second of two identical depolarizing pulses was larger than that produced during the first, resulting from an increased entry of Ca2+ during the second pulse. The increment in Ca conductance inferred from the augmented signal during the second pulse was independent of Ca2+ influx during the first pulse but, instead, was related to the amplitude and duration of the first pulse.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., Hodgkin A. L., Ridgway E. B. Depolarization and calcium entry in squid giant axons. J Physiol. 1971 Nov;218(3):709–755. doi: 10.1113/jphysiol.1971.sp009641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinks J. R., Prendergast F. G., Allen D. G. Photoproteins as biological calcium indicators. Pharmacol Rev. 1976 Mar;28(1):1–93. [PubMed] [Google Scholar]
- Eckert R., Lux H. D. A non-inactivating inward current recorded during small depolarizing voltage steps in snail pacemaker neurons. Brain Res. 1975 Jan 17;83(3):486–489. doi: 10.1016/0006-8993(75)90840-9. [DOI] [PubMed] [Google Scholar]
- Eckert R., Lux H. D. A voltage-sensitive persistent calcium conductance in neuronal somata of Helix. J Physiol. 1976 Jan;254(1):129–151. doi: 10.1113/jphysiol.1976.sp011225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B. Quantitative description of sodium currents in myelinated nerve fibres of Xenopus laevis. J Physiol. 1960 Jun;151:491–501. doi: 10.1113/jphysiol.1960.sp006455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelperin A. Rapid food-aversion learning by a terrestrial mollusk. Science. 1975 Aug 15;189(4202):567–570. doi: 10.1126/science.1145215. [DOI] [PubMed] [Google Scholar]
- Hagiwara S. Ca spike. Adv Biophys. 1973;4:71–102. [PubMed] [Google Scholar]
- Heyer C. B., Lux H. D. Control of the delayed outward potassium currents in bursting pace-maker neurones of the snail, Helix pomatia. J Physiol. 1976 Nov;262(2):349–382. doi: 10.1113/jphysiol.1976.sp011599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer C. B., Lux H. D. Properties of a facilitating calcium current in pace-maker neurones of the snail, Helix pomatia. J Physiol. 1976 Nov;262(2):319–348. doi: 10.1113/jphysiol.1976.sp011598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The effect of prolonged depolarization on synaptic transfer in the stellate ganglion of the squid. J Physiol. 1971 Jul;216(2):503–512. doi: 10.1113/jphysiol.1971.sp009537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Hess R. Tetrodotoxin-resistant dendritic spikes in avian Purkinje cells. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2520–2523. doi: 10.1073/pnas.73.7.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Nicholson C. Calcium role in depolarization-secretion coupling: an aequorin study in squid giant synapse. Proc Natl Acad Sci U S A. 1975 Jan;72(1):187–190. doi: 10.1073/pnas.72.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Presynaptic calcium currents and their relation to synaptic transmission: voltage clamp study in squid giant synapse and theoretical model for the calcium gate. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2918–2922. doi: 10.1073/pnas.73.8.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux H. D., Eckert R. Inferred slow inward current in snail neurones. Nature. 1974 Aug 16;250(467):574–576. doi: 10.1038/250574a0. [DOI] [PubMed] [Google Scholar]
- Meech R. W., Standen N. B. Potassium activation in Helix aspersa neurones under voltage clamp: a component mediated by calcium influx. J Physiol. 1975 Jul;249(2):211–239. doi: 10.1113/jphysiol.1975.sp011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgway E. B., Ashley C. C. Calcium transients in single muscle fibers. Biochem Biophys Res Commun. 1967 Oct 26;29(2):229–234. doi: 10.1016/0006-291x(67)90592-x. [DOI] [PubMed] [Google Scholar]
- SHIMOMURA O., JOHNSON F. H., SAIGA Y. Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. J Cell Comp Physiol. 1962 Jun;59:223–239. doi: 10.1002/jcp.1030590302. [DOI] [PubMed] [Google Scholar]
- Shimomura O., Johnson F. H. Calcium binding, quantum yield, and emitting molecule in aequorin bioluminescence. Nature. 1970 Sep 26;227(5265):1356–1357. doi: 10.1038/2271356a0. [DOI] [PubMed] [Google Scholar]
- Shimomura O., Johnson F. H. Properties of the bioluminescent protein aequorin. Biochemistry. 1969 Oct;8(10):3991–3997. doi: 10.1021/bi00838a015. [DOI] [PubMed] [Google Scholar]
- Stinnakre J., Tauc L. Calcium influx in active Aplysia neurones detected by injected aequorin. Nat New Biol. 1973 Mar 28;242(117):113–115. doi: 10.1038/newbio242113b0. [DOI] [PubMed] [Google Scholar]
- Zucker R. S. Characteristics of crayfish neuromuscular facilitation and their calcium dependence. J Physiol. 1974 Aug;241(1):91–110. doi: 10.1113/jphysiol.1974.sp010642. [DOI] [PMC free article] [PubMed] [Google Scholar]



