Abstract
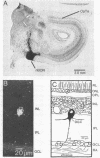
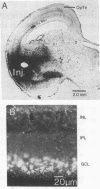
In the pigeon, the nucleus of the basal optic root, a component of the accessory optic system, projects directly upon the vestibulo-cerebellum. This nucleus receives a prominent projection composed of large-diameter retinal axons, known as the basal optic root. The cells of origin of this tract were identified using horseradish peroxidase (donor:hydrogen-peroxide oxidoreductase, EC 1.11.1.7) as a retrograde marker. Injections of horseradish peroxidase confined primarily to the basal optic root nucleus labeled displaced ganglion cells of the contralateral retina. Cell sizes were 18-30 micronm and the dendrites of these cells were confined to the first stratum of the inner plexiform layer. Approximately 3700 displaced ganglion cells were labeled after injections of horseradish peroxidase into basal optic root. In contrast, no displaced ganglion cells were labeled after injections of horseradish peroxidase into the optic tectum, which labeled only cells in the ganglion cell layer proper. These findings indicate that displaced ganglion cells constitute a unique population of retinal neurons that give rise to a bisynaptic pathway directed to the cerebellum via the nucleus of the basal optic root. These displaced ganglion cells may play a major role inoculomotor reflexes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binggeli R. L., Paule W. J. The pigeon retina: quantitative aspects of the optic nerve and ganglion cell layer. J Comp Neurol. 1969 Sep;137(1):1–18. doi: 10.1002/cne.901370102. [DOI] [PubMed] [Google Scholar]
- Bunt A. H., Hendrickson A. E., Lund J. S., Lund R. D., Fuchs A. F. Monkey retinal ganglion cells: morphometric analysis and tracing of axonal projections, with a consideration of the peroxidase technique. J Comp Neurol. 1975 Dec 1;164(3):265–285. doi: 10.1002/cne.901640302. [DOI] [PubMed] [Google Scholar]
- Bunt A. H., Lund R. D., Lund J. S. Retrograde axonal transport of horseradish peroxidase by ganglion cells of the albino rat retina. Brain Res. 1974 Jun 20;73(2):215–228. doi: 10.1016/0006-8993(74)91045-2. [DOI] [PubMed] [Google Scholar]
- Bunt A. H., Minckler D. S. Displaced ganglion cells in the retina of the monkey. Invest Ophthalmol Vis Sci. 1977 Jan;16(1):95–98. [PubMed] [Google Scholar]
- Chan R. Y., Naka K. The amacrine cell. Vision Res. 1976;16(10):1119–1129. doi: 10.1016/0042-6989(76)90252-2. [DOI] [PubMed] [Google Scholar]
- Galifret Y. Les diverses aires fonctionnelles de la rétine du pigeon. Z Zellforsch Mikrosk Anat. 1968;86(4):535–545. [PubMed] [Google Scholar]
- HAMDI F. A., WHITTERIDGE D. The representation of the retina on the optic tectum of the pigeon. Q J Exp Physiol Cogn Med Sci. 1954;39(2):111–119. doi: 10.1113/expphysiol.1954.sp001053. [DOI] [PubMed] [Google Scholar]
- Kelly J. P., Gilbert C. D. The projections of different morphological types of ganglion cells in the cat retina. J Comp Neurol. 1975 Sep;163(1):65–80. doi: 10.1002/cne.901630105. [DOI] [PubMed] [Google Scholar]
- LaVail J. H., LaVail M. M. The retrograde intraaxonal transport of horseradish peroxidase in the chick visual system: a light and electron microscopic study. J Comp Neurol. 1974 Oct 1;157(3):303–357. doi: 10.1002/cne.901570304. [DOI] [PubMed] [Google Scholar]
- Lázár G. Role of the accessory optic system in the optokinetic nystagmus of the frog. Brain Behav Evol. 1972;5(6):443–460. doi: 10.1159/000123761. [DOI] [PubMed] [Google Scholar]
- Magalhães-Castro H. H., Murata L. A., Magalhães-Castro B. Cat retinal ganglion cells projecting to the superior colliculus as shown by the horseradish peroxidase method. Exp Brain Res. 1976 Jul 28;25:541–549. doi: 10.1007/BF00239786. [DOI] [PubMed] [Google Scholar]
- Stell W. K., Witkovsky P. Retinal structure in the smooth dogfish, Mustelus canis: general description and light microscopy of giant ganglion cells. J Comp Neurol. 1973 Mar 1;148(1):1–31. doi: 10.1002/cne.901480102. [DOI] [PubMed] [Google Scholar]
- Werblin F. S., Dowling J. E. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969 May;32(3):339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]
- West R. W., Dowling J. E. Synapses onto different morphological types of retinal ganglion cells. Science. 1972 Nov 3;178(4060):510–512. doi: 10.1126/science.178.4060.510. [DOI] [PubMed] [Google Scholar]
- West R. W. Light and electron microscopy of the ground squirrel retina: functional considerations. J Comp Neurol. 1976 Aug 1;168(3):355–377. doi: 10.1002/cne.901680304. [DOI] [PubMed] [Google Scholar]