Abstract
Background
Exposure to ethanol during central nervous system (CNS) development can lead to a wide array of neuroanatomical, behavioral and cognitive abnormalities, broadly subsumed under the Fetal Alcohol Spectrum Disorder (FASD) classification. One mode of ethanol-induced interference in the normal developmental program appears to be through induction of apoptotic processes mediated by the Bcl-2 family of survival-regulatory proteins. The present series of studies investigated the role of the Bcl-2-related, pro-apoptotic Bid protein, and its truncated, apoptotically active fragment, tBid, in developmental ethanol neurotoxicity.
Methods
Protein analyses were made via enzyme-linked immunosorbent assays (ELISA) in neonatal rat cerebellum, of basal Bid, and of Bid and tBid, following ethanol exposure via vapor inhalation, at an age of peak ethanol sensitivity in this region (postnatal day 4 [P4]) and a later age of relative resistance (P7). ELISA analyses were also made of Bax:tBid heterodimers, a process which activates Bax, essential for its apoptotic functioning. Finally, in vitro assessments of the importance of tBid to ethanol neurotoxicity were made in cultured cerebellar granule cells, using a specific tBid inhibitor.
Results
Basal levels of Bid were higher at P4 compared to P7, possibly contributing to the differential sensitivity. Ethanol exposure elicited further increases in cytosolic Bid and mitochondrial tBid when administration was at P4, but not at P7. Bax:tBid heterodimers were markedly increased by ethanol exposure on P4, an increase which persisted even two-hours after termination of treatment. Similar effects were not seen at P7. The in vitro analyses revealed that tBid inhibition provided complete protection against ethanol-induced cell death, and depressed ethanol-mediated cytochrome-c release.
Conclusions
These results suggest that Bid/tBid may be important elements in ethanol-mediated neurotoxicity during CNS development. The molecular processes and interactions revealed may represent critical points which can be targeted in studies concerned with designing possible therapeutic strategies for minimizing these devastative effects.
Keywords: Ethanol, Bax, Bid/tBid, fetal alcohol syndrome, cerebellum
INTRODUCTION
Exposure to ethanol during the development of the central nervous system (CNS) can lead to a wide array of neuroanatomical, behavioral and cognitive abnormalities, broadly subsumed under the Fetal Alcohol Spectrum Disorder (FASD) classification. Such exposure during these sensitive early periods is acknowledged to be a leading cause of intellectual disability worldwide (Mattson et al., 2011). Despite concerted efforts to increase awareness of the dangers of developmental alcohol exposure, the incidence of alcohol-related developmental disorders has continued to escalate since it was first characterized 40 years ago (Jones and Smith, 1973; May and Gossage, 2001). The developing nervous system is not uniformly sensitive to ethanol neurotoxicity. Both regional and temporal differences in vulnerability have been defined, using animal models of FASD. The developing cerebellum, for example, is particularly susceptible to ethanol-induced neurodegenerative changes, with substantial loss of both Purkinje and granule cells produced by even brief exposure during the early neonatal period in the rodent FASD model, a developmental phase corresponding to the human third trimester (Bonthius and West, 1991; Hamre and West, 1993). The peak period of such susceptibility has been defined during the first postnatal week, at postnatal days 4–5 [P4–5]), while this region becomes relatively resistant to similar insult only slightly later in the neonatal period (P7–8; Goodlett and Eilers, 1997; Moore et al., 1999; Pierce et al., 1999).
A central mechanism contributing to ethanol-induced neuronal loss appears to be an induction of apoptotic processes. The Bax protein, a pro-apoptotic member of the Bcl-2 survival-regulatory gene family, has been demonstrated to be a major factor in ethanol-mediated cell death, with cell loss in Bax gene-deleted animals being markedly reduced in many developing CNS regions following developmental ethanol exposure (Young et al., 2003; Heaton et al., 2006). When ethanol is administered at the age of maximal vulnerability in developing rat cerebellum, Bax is released from its cytosolic 14-3-3 anchoring proteins, and translocates to the mitochondrial membrane, where it interacts with proteins of the mitochondrial permeability transition pore (PTP) complex, leading to collapse of the mitochondrial membrane potential, and initiation of apoptosis (Marzo et al, 1998; Narita et al., 1998; Heaton et al., 2012; 2013). The manner in which the Bax protein becomes activated, allowing it to integrate into the mitochondrial membrane, has not yet been determined, however.
The present study investigated the role of BH3-only pro-apoptotic Bid in ethanol-induced developmental cell death. Bid can be cleaved following CNS trauma, by caspases 3, 8 or 9, producing a 15kD C-terminal fragment (Hayakawa et al., 2008). The truncated Bid (tBid) binds to Bax, eliciting Bax conformational change and activation, a process critical to its subsequent apoptotic function (Desagher et al., 1999; Eskes et al., 2000). For this investigation, in vivo assessments were made of the following, at the age of peak ethanol sensitivity in neonatal cerebellum (P4), and the later age of relative resistance (P7): (1) Basal levels of Bid, to determine whether disparate levels of the protein at the two ages may contribute to differential ethanol sensitivity; (2) comparative effects of ethanol on Bid expression and truncation at the two ages; and (3) comparative effects of ethanol on the formation of Bax:tBid heterodimers at the two ages. In addition, the role of tBid in ethanol-mediated cell death was further assessed in vitro, by direct inhibition of tBid activation in cultured cerebellar granule cells. Since Bax is a critical mediator of ethanol-induced cell death, and since cleaved Bid is an important Bax activator, we hypothesized that ethanol would produce alterations in the Bid protein conducive to Bax activation, and that these alterations would predominate at the age of heightened cerebellar sensitivity, compared to the later, ethanol-resistant age.
MATERIALS AND METHODS
Ethanol Vapor Inhalation Exposure Paradigm
All procedures used in this study were carried out in compliance with the rules and regulations for the experimental use and care of laboratory animals at the University of Florida, and were approved by the Institutional Animal Care and Use Committee. For ethanol inhalation treatment, neonatal Long Evans male and female rats produced from timed-pregnant dams (Charles River Co., Portage MI), were acutely exposed to ethanol via the vapor inhalation procedure, as previously described (e.g., Heaton et al. 2000). Briefly, litters were culled to 10 male and female pups, and at P4 or P7, pups, in their home cage along with the nursing dam, were placed in a Plexiglas inhalation chamber, fitted with intake and exhaust hoses. Air flow was provided by a pump delivering 0.8–1.0 liter/minute. For the ethanol groups, air flowed through an air stone submerged in a flask containing 95% ethanol. The ethanol-laden vapor flowed into the chamber, and the exhaust hose led ethanol vapor into a fume hood. For the controls, air was pumped into the chamber without exposure to ethanol. Ethanol and control exposure periods were 2.5 hours each day. This acute exposure protocol mimics a “binge” pattern of ethanol consumption, a common consumption pattern in women who drink during pregnancy (Stephens, 1985).
For the molecular analyses, pups were sacrificed via decapitation either immediately after exposure to ethanol or control conditions (for Bid, tBid, and Bax:tBid analyses), or two hours after termination of exposure (Bax:tBid analyses). The initial time point was selected because ethanol-mediated changes in molecular events in the developing CNS often occur very rapidly (Ge et al., 2004). The later sampling point for the Bax:tBid analyses was chosen to determine the degree to which the effects produced were sustained. Following sacrifice, the brains were removed, and the cerebellae taken for protein analyses. Blood ethanol concentrations (BECs) were determined 2 hours following completion of exposure, using trunk blood from animals exposed in parallel to those used for the various assays, via a kit from BioAssay Systems (Hayward, CA; catalog #ECET-100). We have previously found that BECs are at peak levels at this time point (Heaton et al., 2003a).
ELISA Analyses of Basal Levels of Bid Protein
Quantitative analyses of basal levels of cerebellar Bid were performed in control P4 and P7 animals to determine whether developmental regulation of these proteins may contribute to differential ethanol sensitivity. This investigation utilized ELISA analyses. For each ELISA plate, cerebellar tissues from P4 and P7 control litters were used, so that comparative analyses of protein content at the two ages could be made. To avoid possible litter bias, only two animals from a given litter were used for a given plate, and this tissue was pooled to constitute one sample. Since gender-related differences in the effects of ethanol on rat cerebellum at this developmental stage have not been found (Andrews et al., 1999; Bonthius and West, 1991), tissues from male and female pups were pooled. On each plate, five-six P4 and five-six P7 samples of pooled tissue were added, and four replicate plates were run. For these analyses, a kit from TSZ ELISA (Framingham, MA; catalog #R6817) was used, following the kit protocol. After incubation, absorbance was read on a microplate reader at 450nm, and values were normalized per gram of tissue assayed.
Subcellular Fractionation
For the analyses of the relative amounts of Bid and tBid in P4 vs. P7 cerebellum following exposure to experimental conditions, tissue was fractionated, to separate cytosolic and mitochondrial contents. Full-length Bid is a cytosolic protein, and was assessed in cytosolic fractions, while tBid moves to the mitochondrial membrane and was accessed in mitochondrial fractions. For this procedure, cerebellae were harvested immediately after termination of experimental treatment. Tissues were homogenized and separated into fractions using the Mitochondrial/Cytosol Fractionation Kit and protocol provided by BioVision Inc. (Mountain View, CA; catalog #K256), as described in detail previously (e.g., Heaton et al., 2011; 2013).
ELISA Analyses of Cytosolic Bid and Mitochondrial tBid Proteins Following Exposure to Experimental Conditions
Analyses of cytosolic Bid and mitochondrial tBid were performed via ELISA assessment. Ethanol effects on full-length Bid were examined via the TSZ ELISA kit, as described above for basal Bid assessments. For this and the following analyses, each ELISA plate contained cerebellar tissues from only one neonatal age, unlike the quantitative assesements of basal protein levels, described above, when tissues from the two ages were analyzed concurrently. Thus, these analyses do not represent absolute protein values, but rather relative responsiveness to the experimental conditions at the two ages. Only two animals from a given litter were used for a given plate, and this tissue was pooled to constitute one sample, as above. Each plate contained four-five ethanol and four-five control samples of pooled tissue, from P4 or P7 animals, plated in triplicate. Two-three replicate plates were run per protein for each age.
The analyses of cerebellar tBid were made via the Express ELISA Kit from GenScript (Piscataway, NJ; catalog #ABIN769931), according to the kit protocol. For these analyses, a primary tBid rabbit polyclonal antibody from Calbiochem (San Diego, CA; catalog #PC645) was used. This antibody was chosen for its purity and specificity, as evidenced by tests conducted by the vendor (Calbiochem), using positive controls (lysates from TNFα-treated L929 cells). In these tests, the antibody exhibited no cross-reactivity with full-length Bid or other Bcl-2-related proteins. After incubations were complete, absorbance was read and the values normalized as above.
ELISA Assessment of Bax:tBid Interactions
Changes in native protein-protein interactions between Bax and tBid following ethanol exposure were determined using an ELISA-based approach, as described in detail previously (Siler-Marsiglio et al., 2005), using a rabbit polyclonal Bax antibody as the capture antibody (Cell Signaling, Danvers, MA; catalog #2772), coupled with a goat polyclonal anti-tBid (Biovision; catalog #650-428). Samples from male and females were pooled and plated as above, with two-five samples per condition per age. Two-three replicate plates were run.
Cerebellar Granule Cell Cultures
Cerebellar granule cell cultures derived from P8 pups were used to further assess the role of tBid in ethanol neurotoxicity. It should be noted that in vivo, this population is maximally vulnerable to ethanol at the same age as Purkinje cells, P4, with relative resistance found by P7-8 (e.g., Hamre and West, 1993). When older granule cells are isolated in cell culture, however, they are separated from their normal supportive environment, including their projection field, afferent input, and other sources of protective support, and thus become ethanol vulnerable. This vulnerability can be countered by replacement of supportive substances (e.g., Luo et al., 1997; Heaton et al., 2011). Cultures were established in Dulbecco’s modified Eagle’s medium (DMEM; Cellgro, from Fisher Scientific, Pittsburgh, PA; catalog #MT10101CV) with 15% fetal bovine serum (Invitrogen; Camarillo, CA; catalog #16000-044), penicillin/streptomycin (Fisher, catalog #SV30010), and trypsin inhibitor (Sigma; St. Louis, MO; catalog #T6522), as described in detail previously (e.g., Heaton et al., 2011). For the assessments of neuronal survival via MTT assay, aliquots of the cell suspension were pipetted into 96-well plates coated with poly-L-lysine (0.05mg/ml; Sigma; catalog #P4707). For the cytochrome-c assays, cells were plated in poly-L-lysine-coated 100mm culture dishes. All cultures were established for 24 hours in a tissue culture incubator at 37°C, with 5% CO2-95% air, in the serum-containing medium. We have found cells prepared in this manner to be ~95% neuronal, as determined by immunochemical detection of glial fibrillary acidic protein (GFAP) and type III β-tubulin, with densities averaging 35,000–39,000 neurons/cm2 (Siler-Marsiglio et al., 2004).
Application of Experimental Conditions
After the initial 24-hour plating period, the serum-containing medium was replaced with serum-free, modified N2 medium, as described previously (e.g., Heaton et al., 2011). This medium eliminates contamination from unknown serum components, and discourages proliferation of non-neuronal cells (Muller at al., 1997). The experimental conditions then applied included 400mg/dl ethanol, which represents a relatively high, challenging level, but is well within the range seen both in awake humans following binge consumption, and in animal studies of ethanol toxicity (e.g., Lindblad et al., 1976; Young et al., 2003). We previously found this ethanol concentration elicits cell death in this neuronal population at this age, preceded by Bax mitochondrial translocation (Heaton et al., 2011). Ethanol was also coupled with the tBid inhibitor BI-6C9, a selective small molecule 4-phenylsulfanyl-phenylamine derivative that functions by occupying a deep hydrophobic crevice on the surface of Bid/tBid, and interferes with exposure of the BH3 domain, locking the protein in an inactive configuration, and blocking tBid interactions; it does not bind to other Bcl-2 family proteins (Becattini et al., 2004; Sigma; catalog #BO186). The inhibitor was applied at a concentration of 20μM (Becattini et al., 2004). The following experimental conditions were applied, for 24 hours: (1) Control, with N2 medium alone; (2) N2 medium plus 400mg/dl ethanol; (3); N2 medium plus tBid inhibitor; and (4) N2 medium with tBid inhibitor + 400mg/dl ethanol. The dishes were sealed with sterile breathable plate sealer, to minimize ethanol evaporation. Ethanol concentrations were confirmed by the QuantiChrom Ethanol microenzymatic assay (BioAssay Systems, Hayward, CA; catalog #BH-DIET500), and evaporation during this exposure time was minimal.
MTT Neuronal Survival Assay
To assess neuronal viability, the MTT assay was used. For this assay, MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide; Sigma; catalog #M5655) was prepared at 5mg/ml in serum-free, phenol red-free Eagle’s Minimum Essential Medium, as in our previous studies (e.g., Mitchell et al., 1998). The assay was initiated 24 hours after application of experimental conditions. Granule cell cultures were incubated in the MTT solution for 5 hours at 37°C, producing the formazan product as a result of the cleavage of the tetrazolium salt MTT by the mitochondrial enzyme succinate-dehydrogenase. Only living cells can make the conversion, and therefore the amount of blue formazan production is directly proportional to the number of viable cells present. This procedure produces highly accurate measurements of neuronal number in non-proliferative preparations, with quantification comparable to those achieved by light microscopic observations, or other measures of cell viability (e.g., Annexin V, Propidium Iodide staining; Manthorpe et al., 1986; Ankarcrona et al., 1995; Siler-Marsiglio et al., 2004). Following incubation, the plates were centrifuged, and the untransformed MTT solution removed. Propanol (50μl) was added to each well and the plates were shaken 60 seconds to uniformly solubilize the blue formazan. The optical density (OD) of each well was measured using an automated plate reader with a test wavelength of 560nm and a reference wavelength of 690nm. Each MTT plate contained multiple wells of two experimental conditions: Ethanol, and ethanol + tBid inhibitor (8–12 wells each); or Control, and tBid inhibitor alone (8–12 wells each). This procedure was replicated on a total of three plates.
Cytochrome-c Assay
Analyses of cytochrome-c (cyto-c) release were made as a measure of apoptosis initiation in the cultured granule cells, using an ELISA kit from Invitrogen (catalog #KH01051), according to kit instructions. For this assay, each plate contained three wells from each of the four conditions, and two replicate plates were run.
Statistical Analyses
Statistical comparisons of basal levels of Bid, and tBid proteins in the P4 and P7 cerebellum were made via the Student’s t-test. The effects of ethanol on P4 and P7 Bid, tBid and on Bax:tBid protein-protein interactions were made via the two-way analysis of variance (ANOVA) using StatView software. Posthoc comparisons were made with the Bonferroni/Dunn test, where appropriate. Analyses of survival of and cyto-c release in cerebellar granule cells following experimental treatments were made via a one-way ANOVA, with post hoc testing as above.
RESULTS
Blood Ethanol Concentrations
The blood ethanol concentrations (BECs) of P4 and P7 animals, measured two hours following termination of ethanol exposure averaged 273.27±3.92 mg/dl (mean/SEM) in the P4 pups (n=33), and 279.04±3.42 mg/dl in the P7 pups (n=28). BECs measured at this interval following vapor inhalation are within the peak period, which extends from 1.5 to 2.5 hours post exposure (Heaton et al., 2003a).
ELISA Analysis of Basal Expression of Bid Protein in P4 and P7 Cerebellum
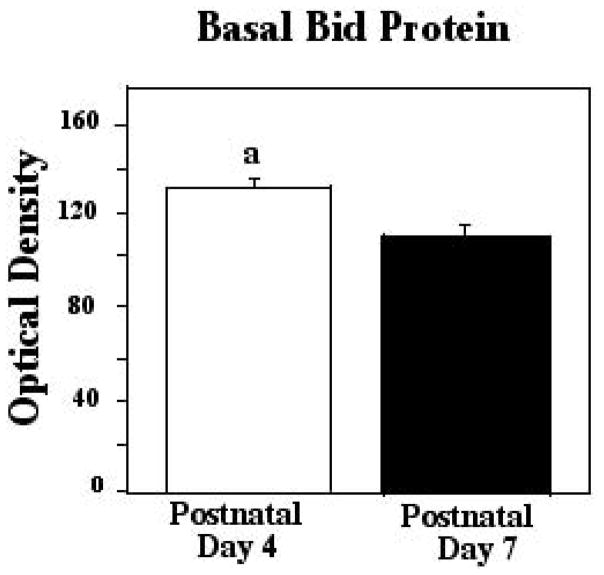
For the analyses of baseline levels of Bid, P4 and P7 tissues from control animals were assessed in the same ELISA plates, so that comparisons of absolute values could be made, to determine whether differential expression at the two ages may contribute to their differential ethanol sensitivity. These analyses revealed that baseline Bid was slightly higher at P4 compared to P7 (+14%), as measured by the Student’s t-test (p= 0.0062). These data are depicted in Figure 1.
Figure 1.
Basal levels of Bid were measured via ELISA assay in postnatal day 4 (P4) and P7 neonatal rats. Error bars represent standard error of the mean (SEM). a=Significantly greater than P7, Student’s t-test, p=0.0062.
ELISA Analyses of Cytosolic Bid and Mitochondrial tBid Proteins in Neonatal Cerebellum Following Ethanol Exposure at P4 and P7
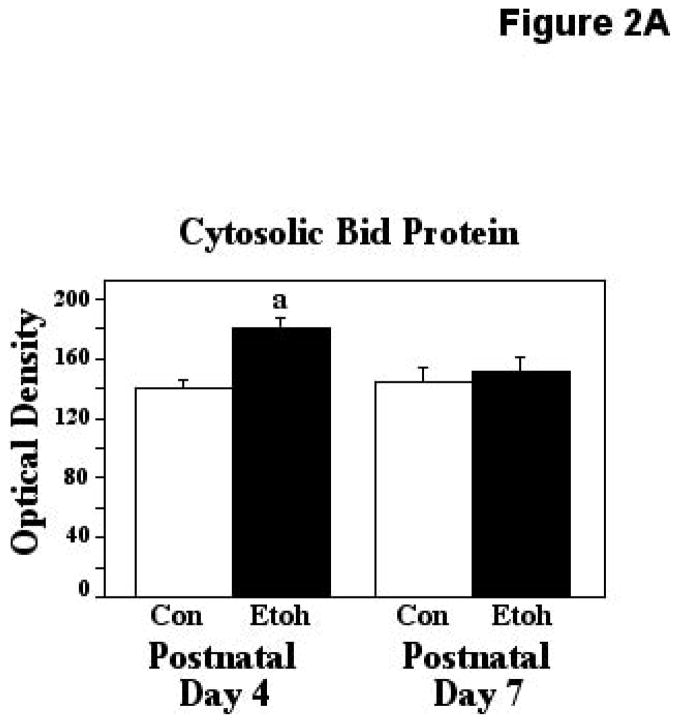
Analyses of alterations in cytosolic Bid and mitochondrially-migrated tBid were made immediately following exposure to ethanol or control conditions at P4 and P7. For these analyses, each ELISA plate contained tissue from ethanol and control animals of only one age. Thus, these analyses do not represent absolute protein values, but rather relative responsiveness to the experimental conditions at the two ages. The two-way ANOVA applied to the analyses of ethanol influences on cytosolic Bid indicated a significant effect of treatment (F [1,33] = 8.42, p=0.0065), and a significant age X treatment interaction (F [1,33] = 4.39, p=0.0438). The Bonferroni/Dunn posthoc test indicated that ethanol treatment resulted in a significant 28% increase in Bid when administered on P4 (P=0.0004), but not on P7. These results are presented in Figure 2A. Note that in this and the following figures, since only relative comparisons could be made at a given age, control optical density values were normalized.
Figure 2.
Figure 2A. Cytosolic Bid protein was measured using ELISA assays in P4 and P7 neonatal rats immediately following exposure to ethanol via vapor inhalation (Etoh), or to control conditions (Con). Note that the optical density values in this figure are arbitrary units, not absolute values, and control data from the two ages have been normalized. Error bars represent standard error of the mean (SEM). The ANOVA revealed a significant effect of treatment (F [1,33] = 8.42, p=0.0065), and a significant age X treatment interaction (F [1,33] = 4.39, p=0.0438). a=Significantly greater than P4 controls (Bonferroni/Dunn posthoc test), p=0.0004.
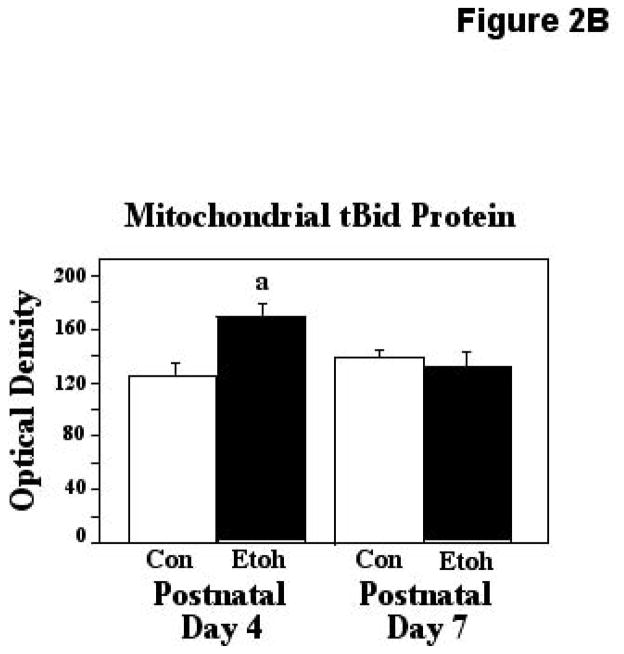
Figure 2B. Mitochondrial truncated Bid (tBid) protein was measured via ELISA assays of isolated mitochondrial fractions derived from P4 and P7 neonatal rats following exposure to ethanol via vapor inhalation (Etoh), or to control conditions (Con). Optical density values in this figure are arbitrary units, not absolute values, and control data from the two ages have been normalized. Error bars represent standard error of the mean (SEM). The ANOVA indicated a significant age X treatment interaction (F [1,42] = 6.42, p=0.0151), a=Significantly greater than P4 controls, p=0.0057.
Similar ELISA analyses were made of ethanol effects on mitochondrial tBid. These analyses at the two ages indicated a significant age X treatment interaction (F [1,42] = 6.42, p=0.0151), and posthoc testing indicated that ethanol treatment resulted in a 35% increase in mitochondrial tBid, but only at the earlier, ethanol sensitive age (P4; p=0.0057). These results are presented in Figure 2B.
ELISA Analyses of Bax:tBid Interactions in Neonatal Cerebellum Following Ethanol Exposure at P4 and P7
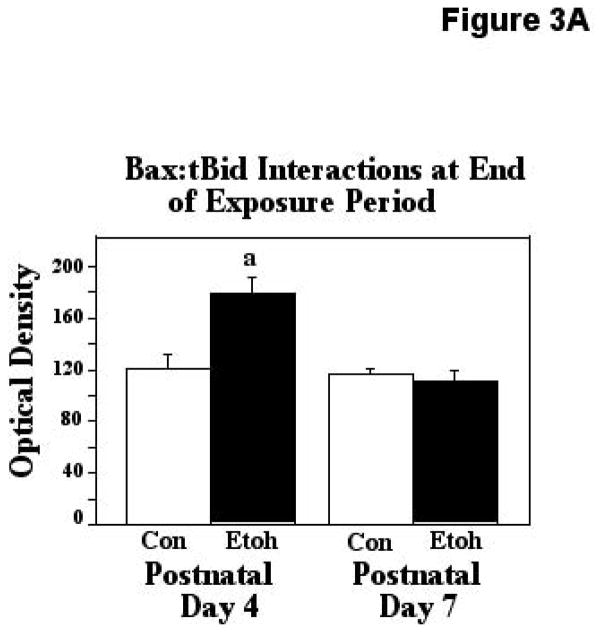
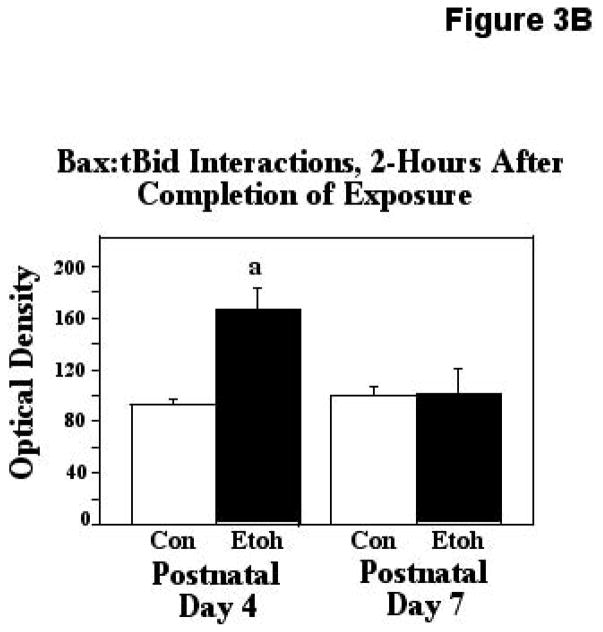
Truncated Bid (tBid)-Bax interactions, a step essential for Bax activation, were assessed via our ELISA-based approach, immediately after ethanol exposure, and two hours following termination of exposure. The first analytical point was chosen to examine the more rapid molecular changes elicited by the ethanol exposure, and the later sampling point was chosen to determine the degree to which the effects produced were sustained. The two-way ANOVA applied to the observations made immediately after treatment revealed a significant effect of age (F [1,45] = 18.18, p=0.0001), treatment (F [1,45] = 9.40, p=0.0037), and an age X treatment interaction (F [1,45] = 13.79, p=0.0006). The post hoc test showed that these heterodimeric Bax:tBid associations were increased by 48% in the P4 cerebellum following ethanol treatment (p=0.0021), but were unaffected by similar treatment in the P7 animals. These results are depicted in Figure 3A. When Bax:tBid interactions were analyzed two-hours after termination of treatment, the ANOVA again detected significant effects of age (F [1,25] = 4.24, p=0.0499), treatment (F [1,25] = 7.41, p=0.0116), and an age X treatment interaction (F [1,25] = 6.69, p=0.0159). The posthoc test revealed a significant, 80% increase in these associations induced by ethanol at the earlier age (P4; p=0.0009), but these interactions were not affected by ethanol treatment at the later age of relative ethanol resistance (P7). These results are presented in Figure 3B.
Figure 3.
Figure 3A. Bax:tBid heterodimers were measured by ELISA analysis in tissue derived from P4 and P7 neonatal rats immediately after completion of two-hours exposure to ethanol via vapor inhalation (Etoh), or to control conditions (Con). Error bars represent standard error of the mean (SEM). The ANOVA revealed a significant effect of age (F [1,45] = 18.18, p=0.0001), treatment (F [1,45] = 9.40, p=0.0037), and an age X treatment interaction (F [1,45] = 13.79, p=0.0006). a=Significantly greater than P4 controls, p=0.0021.
Figure 3B. Bax:tBid heterodimers were measured by ELISA analysis in tissue derived from P4 and P7 neonatal rats two hours after completion of exposure to ethanol (Etoh), or to control conditions (Con). Error bars represent standard error of the mean (SEM). The ANOVA indicated significant effects of age (F [1,25] = 4.24, p=0.0499), treatment (F [1,25] = 7.41, p=0.0116), and an age X treatment interaction (F [1,25] = 6.69, p=0.0159). a=Significantly greater than P4 controls, p=0.0009.
Analyses of Neuronal Survival and Cytochrome-c Release in Cerebellar Granule Cells Co-cultured with Ethanol and tBid Inhibitor
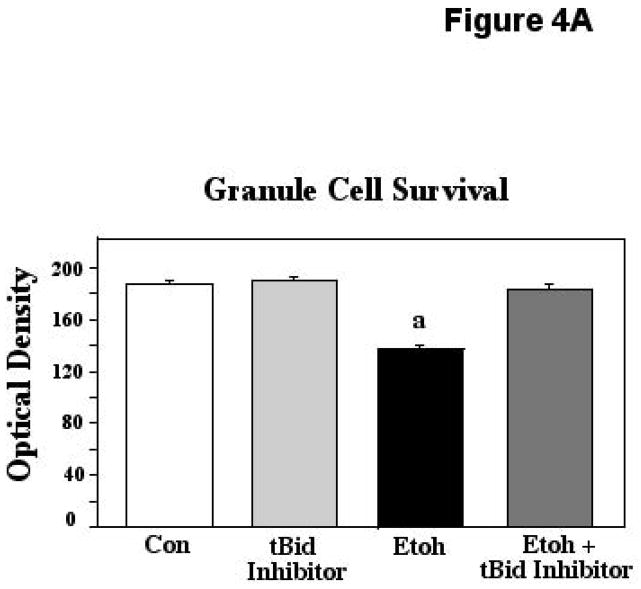
Postnatal day 8 cerebellar granule cell preparations were used to further assess the importance of Bid/tBid to ethanol-induced neurotoxicity and cell death. For these analyses, granule cells were cultured with 400mg/dl ethanol, a concentration we have previously found to elicit cell death in this population at this age, preceded by robust Bax mitochondrial translocation (Heaton et al., 2011). Ethanol was also combined with tBid inhibitor, BI-6C9, a specific blocker of tBid interactions (Becattini et al, 2004). Cultures were exposed to experimental conditions for 24 hours, followed by analyses of survival via the MTT assay, and assessment of cytochrome-c (cyto-c) release via ELISA quantification. The ANOVA applied to the neuronal survival data indicated a significant effect of treatment (F [3,113] = 90.72; p<0.0001). Posthoc assessments showed that while ethanol depressed survival in these preparations compared to controls and cultures with tBid inhibitor alone (p<0.0001 in each instance), this cell loss was completely prevented by inhibition of tBid activities, with survival in the ethanol + tBid inhibitor cultures differing significantly from ethanol alone (p<0.0001), but being equivalent to that seen in both controls and in cultures with tBid inhibitor alone. These results are depicted in Figure 4A.
Figure 4.
Figure 4A. Postnatal day 8 cerebellar granule cells were cultured in control medium (Con), in medium supplemented with tBid inhibitor (BI-6C9), in medium supplemented with 400mg/dl ethanol (Etoh), and in medium with ethanol plus the tBid inhibitor. Neuronal survival was measured via the MTT assay. Error bars represent standard error of the mean (SEM). The ANOVA applied to these data indicated a significant effect of treatment (F [3,113] = 90.72; p<0.0001). a=Significantly less than controls, tBid inhibitor alone, and ethanol plus tBid inhibitor, p<0.0001 in each instance.
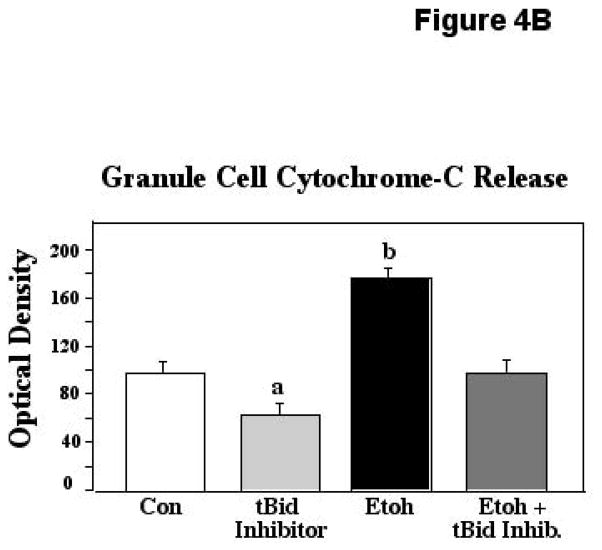
Figure 4B. Postnatal day 8 cerebellar granule cells were cultured in control medium (Con), in medium supplemented with tBid inhibitor (BI-6C9), in medium supplemented with 400mg/dl ethanol (Etoh), and in medium with ethanol plus the tBid inhibitor. Cytochrome-c release was measured via an ELISA assay. Error bars represent standard error of the mean (SEM). The ANOVA applied to these data indicated a significant effect of treatment (F [3,20] = 21.60; p<0.0001). a=Significantly less than controls (p=0.0290), ethanol (p<0.0001), and ethanol plus tBid inhibitor (p=0.,0325); b=significantly greater than controls, tBid inhibitor alone, and ethanol plus tBid inhibitor, p<0.0001 in each instance.
When cytochrome-c was measured in cultures with ethanol with and without the tBid inhibitor, similar protective effects were found. The ANOVA applied to these data revealed a significant effect of treatment (F [3,20] = 21.60; p<0.0001). Posthoc assessments showed that ethanol elicited marked increases in cyto-c in these cultures, with levels in these preparations differing significantly from all other conditions (p<0.0001 in all instances). In ethanol cultures containing the tBid inhibitor, however, this spike was prevented, and cyto-c detected in these preparations differed significantly from ethanol-only cultures (P<0.0001), but was equivalent to that measured in controls. Cyto-c release in the cultures containing tBid inhibitor alone was significantly depressed compared to controls (p= 0.0290) and ethanol + inhibitor (p=0.0325), although this decrease did not correspond to an increase in survival, at least within the constraints of our experimental paradigm. The lower cyto-c in these preparations suggests that the tBid inhibitor affords some protection against the attrition normally seen in cultured cells. These results are depicted in Figure 4B.
DISCUSSION
Ethanol-induced disruption of nervous system development appears to rely to a considerable extent on the induction of apoptotic processes. This has been demonstrated in a number of developing regions in which ethanol exposure was found to upregulate apoptosis-promoting proteins, particularly those of the Bcl-2 survival-regulatory gene family (e.g., Bax, Bad), with concomitant down-regulation of pro-survival proteins (e.g., Bcl-2; Bcl-xl; Moore et al., 1999; Heaton et al., 2003a,b; Ge et al., 2004; Nowoslawski, 2005; Lee et al., 2008). The apoptosis effector, Bax, appears to be critical to ethanol-induced neurotoxicity in many populations, with Bax gene-deleted animals exhibiting marked resistance to such effects (Young et al., 2003; Heaton et al., 2006). In studies of the sequence of events occurring as a consequence of ethanol exposure, we found that developmental ethanol exposure, particularly at periods of peak sensitivity, leads to release of Bax from its cytosolic 14-3-3 anchor, followed by Bax mitochondrial translocation, interaction with mitochondrial membrane proteins, resultant loss of the mitochondrial membrane potential, release of apoptogenic contents, and initiation of the apoptosis cascade (Heaton et al., 2012; 2013). In order for Bax to form these associations at the mitochondria and fulfill its death function, however, it must undergo a conformational change. One key mode of Bax activation is through direct interaction with other pro-apoptotic proteins such as truncated Bid (tBid; Desagher et al., 1999; Eskes et al., 2000; Lovell et al., 2008). Therefore, we investigated the role of Bid in ethanol-mediated apoptosis in developing cerebellum. This investigation revealed the following: (1) basal levels of cerebellar Bid are greater at the ethanol-sensitive age (P4) compared to the ethanol-resistant age (P7); (2) ethanol exposure enhances Bid expression at P4, but not at P7; (3) ethanol treatment enhances truncated Bid (tBid) at P4, but not at P7; (4) heterodimerization of Bax with tBid is also differentially affected at the two ages, with such interactions sustained even two hours after termination of exposure in the P4 animals; and finally (5) inhibition of tBid activities blocks ethanol-induced death of cultured cerebellar granule cells, along with significant reductions of cytochrome-c release. In the following sections, these results will be considered in the context of prior investigations.
The Role of Bid in Neuronal Apoptosis
Bid, a member of the BH3-only subgroup of the Bcl-2 family, is considered an “activator” protein. These proteins share their only sequence homology within the BH3 domain, which is essential for apoptotic activation and interactions with other Bcl-2 family members (Korsmeyer et al., 2000). Full-length Bid is localized to the cytosol (Korsmeyer et al., 2000). Following an apoptotic stimulus, Bid is cleaved by caspases, eliminating the N-terminus, which contains a regulatory sequence that ensures the cytosolic locus of the full-length protein, and negatively regulates membrane binding (Billen et al., 2009). The cleaved C-terminal p15 Bid fragment, containing the BH3 domain, migrates to the mitochondria where it can bind to other Bcl-2-related proteins. Only the truncated, tBid, has this capacity (Eskes et al., 2000). Bid can be cleaved by caspase-8, caspase-3 or caspase-9 (Hayakawa et al., 2008). It is notable that while developmental ethanol exposure does not appear to induce caspase-8 activation, caspase-3 is robustly activated following even brief exposure, both in vitro and in vivo (Oberdoerster et al., 1999; Olney et al., 2002; Young et al, 2003; Siler-Marsiglio et al., 2004). Consistent with the present study, Bid cleavage occurs rapidly following CNS trauma, and is thought to play an important role in certain neurodegenerative diseases (e.g., amyotrophic lateral sclerosis; Plesnila et al., 2001; Guegan et al., 2002; Becattini et al., 2004). The differential temporal effects of ethanol on Bid expression and truncation seen in the present study, correlating with differential temporal ethanol sensitivity, suggest that Bid may play a major role in ethanol-mediated cell death in this developing region.
Bax-tBid Interactions
In many forms of apoptosis, Bax is activated by direct interaction with BH3-only proteins such as tBid (Lovell et al., 2008; Ren et al, 2010). The Bax-tBid interaction occurs at the mitochondrial membrane, and induces a Bax conformational change, exposing the N-terminal domain (Lovell et al, 2008). Bax, thus activated, integrates into the mitochondrial membrane, where it can oligomerize, or heterodimerize with proteins of the mitochondrial PTP complex, triggering loss of mitochondrial membrane potential, release of cytochrome-c, and initiation of the apoptosis cascade (Desagher et al. 1999; Billen et al., 2009). In the present study we found ethanol exposure markedly increased Bax:tBid heterodimers, but only at the age of peak ethanol sensitivity in developing cerebellum. These interactions were sustained for at least two hours after termination of the exposure. This sustained elevation may be related to the fact that peak blood ethanol concentrations achieved with the inhalation procedure persist for two-three hours following treatment (Heaton et al., 2003a). Future studies should determine whether these sustained apoptotic events are dependent on the BEC, whether they subside as blood ethanol declines, or whether the apoptotic process may continue for a considerable time, even in the absence of the proximate stimulus.
The Role of Bid/tBid in Ethanol Neurotoxicity
To assess the importance of Bax-tBid interactions to ethanol-induced cerebellar apoptosis, cultured cerebellar granule cells were exposed to ethanol in the presence of a specific inhibitor of tBid activities. In these preparations, tBid inhibition blocked ethanol effects, and prevented cytochrome-c release, with survival and cytochrome-c levels in the inhibitor-supplemented ethanol cultures being restored to control levels. These results, together with our in vivo observations, suggest that the ethanol-mediated changes in Bid are critical to ethanol-mediated cell death in this developing CNS region. The sequence of events preceding this activation is not clear, however: Apoptosis mediated by tBid is often viewed as an “amplifying” process, since the Bid protein must be cleaved by caspase-3, -8 or -9 to become active (Korsmeyer et al., 2000; Hayakawa et al., 2008). Thus, caspase activation must occur prior to tBid activities. The stimulus for initial caspase activation could be Bax, activated by other means (e.g., Bim interactions), triggering the apoptosis cascade and caspase activation. This would not explain the complete protection afforded by tBid inhibition, however. Therefore, we propose that Bid cleavage following ethanol insult may be mediated by ethanol-induced generation of reactive oxygen species (ROS). ROS generation is a well-known concomitant of ethanol exposure, both in vivo and in vitro (Heaton et al., 2003a; b; 2011), and oxidative stress/ROS have been shown to activate caspase-3, -8 and -9, in a variety of cell types, including neurons, even in the absence of cytochrome-c release (Kobayashi et al., 2001; Kim and Park, 2003; Kim and Chung, 2007; Carvour et al., 2008; Choudhary and Wang, 2009). Consistent with this hypothesis are the observations that both ROS generation and caspase activation occur very rapidly after acute ethanol exposure in cultured cells, with robust activity seen within 5–10 minutes post-exposure (Ramachandran et al., 2003; Siler-Marsiglio et al., 2004; Gonzalez et al, 2007). Further studies should be designed to clarify this sequence of events.
CONCLUSIONS
In summary, Bid/tBid appears to play an important role in developmental ethanol neurotoxicity. The molecular processes and interactions involved may represent critical points which can be targeted in studies concerned with designing possible therapeutic strategies for minimizing the devastative effects of developmental ethanol exposure.
Acknowledgments
This research was supported by NIAAA grants AA012151 and AA016327.
References
- Andrews DL, Williams GS, Mahoney JC, West JR. DNA fragmentation during exposure of rat cerebella to ethanol under hypoxia imposed in vitro. J Neurobiol. 1999;8:82–92. doi: 10.1002/(sici)1097-4695(199901)38:1<82::aid-neu6>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Ankarcrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA, Nicotera P. Glutamate-induced neuronal death: A succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15:961–973. doi: 10.1016/0896-6273(95)90186-8. [DOI] [PubMed] [Google Scholar]
- Becattini B, Sareth S, Zhai D, Crowell KJ, Leone M, Reed JC, Pellecchia M. Targeting apoptosis via chemical design: inhibition of Bid-induced cell death by small organic molecules. Chem Biol. 2004;11:1107–1117. doi: 10.1016/j.chembiol.2004.05.022. [DOI] [PubMed] [Google Scholar]
- Billen LP, Shamas-Din A, Andrews DW. Bid: A Bax-like BH3 protein. Oncogene. 2009;27:93–104. doi: 10.1038/onc.2009.47. [DOI] [PubMed] [Google Scholar]
- Bonthius DJ, West JR. Permanent neuronal deficits in rats exposed to alcohol during the brain growth spurt. Teratology. 1991;44:147–163. doi: 10.1002/tera.1420440203. [DOI] [PubMed] [Google Scholar]
- Carvour M, Song C, Kaul S, Anantharam V, Kanthasamy A, Kanthasamy A. Chronic low-dose oxidative stress induces caspase-3-dependent PKCdelta proteolytic activation and apoptosis in a cell culture model of dopaminergic neurodegeneration. Ann NY Acad Sci. 2008;1139:197–205. doi: 10.1196/annals.1432.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary S, Wang HR. Role of reactive oxygen species in proapoptotic ability of oncogenic H-Ras to increase human bladder cancer cell susceptibility to histone deacetylase inhibitor for caspase induction. J Cancer Res Clin Oncol. 2009;135:1601–1613. doi: 10.1007/s00432-009-0608-2. [DOI] [PubMed] [Google Scholar]
- Desagher S, Osen-Sand A, Nichols A, Eskes R, Montessuit S, Lauper S, Maundrell K, Antonsson B, Martinou JC. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J Cell Biol. 1999;144:891–901. doi: 10.1083/jcb.144.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskes R, Desagher S, Antonsson B, Martinou JC. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol Cell Biol. 2000;20:929–935. doi: 10.1128/mcb.20.3.929-935.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y, Belcher SM, Pierce DR, Light KE. Altered expression of Bcl-2, Bad and Bax mRNA occurs in the rat cerebellum within hours after ethanol exposure on postnatal day 4 but not on postnatal day 9. Mol Brain Res. 2004;129:124–134. doi: 10.1016/j.molbrainres.2004.06.034. [DOI] [PubMed] [Google Scholar]
- González A, Pariente JA, Salido GM. Ethanol stimulates ROS generation by mitochondria through CA+2 mobilization and increases GFAP content in rat hippocampal astrocytes. Brain Res. 2007;1178:28–37. doi: 10.1016/j.brainres.2007.08.040. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Eilers AT. Alcohol-induced Purkinje cell loss with a single binge exposure in neonatal rats: a stereological study of temporal windows of vulnerability. Alcohol Clin Exp Res. 1997;2:738–744. [PubMed] [Google Scholar]
- Guegan C, Vila M, Teisman P, Chen C, Onteniente B, Li M, Friedlander RM, Przedborski S. Instrumental activation of Bid by caspase-1 in transgenic mouse model of ALS. Mol Cell Neurosci. 2002;20:553–562. doi: 10.1006/mcne.2002.1136. [DOI] [PubMed] [Google Scholar]
- Hamre KM, West JR. The effects of the timing of ethanol exposure during the brain growth spurt on the number of cerebellar Purkinje and granule cell nuclear profiles. Alcohol Clin Exp Res. 1993;17:610–622. doi: 10.1111/j.1530-0277.1993.tb00808.x. [DOI] [PubMed] [Google Scholar]
- Hayakawa A, Kawamoto Y, Nakajima H, Sakai J, Takasawa R, Nakashima I, Magae J, Tanuma S. Bid truncation mediated by caspases-3 and -9 in vinorelbine-induced apoptosis. Apoptosis. 2008;13:523–530. doi: 10.1007/s10495-008-0184-y. [DOI] [PubMed] [Google Scholar]
- Heaton MB, Mitchell JJ, Paiva M, Walker DW. Ethanol-induced alterations in the expression of neurotrophic factors in the developing rat central nervous system. Devel Brain Res. 2000;121:97–107. doi: 10.1016/s0165-3806(00)00032-8. [DOI] [PubMed] [Google Scholar]
- Heaton MB, Moore DB, Paiva M, Madorsky I, Mayer J, Shaw G. The role of neurotrophic factors, apoptosis-related proteins, and endogenous antioxidants in the differential temporal vulnerability of neonatal cerebellum to ethanol. Alcohol Clin Exp Res. 2003a;27:657–669. doi: 10.1097/01.ALC.0000060527.55252.71. [DOI] [PubMed] [Google Scholar]
- Heaton MB, Paiva M, Madorsky I, Shaw G. Ethanol effects on neonatal rat cortex: comparative analyses of neurotrophic factors, apoptosis-related proteins, and oxidative processes during vulnerable and resistant periods. Devel Brain Res. 2003b;145:249–262. doi: 10.1016/j.devbrainres.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Heaton MB, Paiva M, Madorsky I, Siler-Marsiglio K, Shaw G. The effect of Bax deletion on ethanol sensitivity in the neonatal rat cerebellum. J Neurobiol. 2006;66:95–101. doi: 10.1002/neu.20208. [DOI] [PubMed] [Google Scholar]
- Heaton MB, Paiva M, Siler-Marsiglio K. Ethanol influences on Bax translocation, mitochondrial membrane potential, and reactive oxygen species generation are modulated by vitamin E and brain-derived neurotrophic factor. Alcohol Clin Exp Res. 2011;35:1122–1133. doi: 10.1111/j.1530-0277.2011.01445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton MB, Paiva M, Kubovec S, Kotler A, Rogozinski J, Swanson E, Madorsky V, Posados M. Differential effects of ethanol on c-jun N-terminal kinase, 14-3-3 proteins, and Bax in postnatal day 4 and postnatal day 7 rat cerebellum. Brain Res. 2012;1432:15–27. doi: 10.1016/j.brainres.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton MB, Siler-Marsiglio K, Paiva M, Kotler A, Rogozinski J, Kubovec S, Coursen M, Madorsky V. Ethanol influences on Bax association with mitochondrial membrane proteins in neonatal rat cerebellum. Devel Neurobiol. 2013;73:127–141. doi: 10.1002/dneu.22042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. The Lancet. 1973:999–1000. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- Kim BM, Chung HW. Hypoxia/reoxygenation induces apoptosis through a ROS mediated caspase-8/Bid/Bax pathway in human lymphocytes. Biochem Biophys Res Comm. 2007;363:745–750. doi: 10.1016/j.bbrc.2007.09.024. [DOI] [PubMed] [Google Scholar]
- Kim JY, Park JH. ROS-dependent caspase-9 activation in hypoxic cell death. FEBS Lett. 2003;549:94–98. doi: 10.1016/s0014-5793(03)00795-6. [DOI] [PubMed] [Google Scholar]
- Kobayashi D, Sasaki M, Watanabe N. Caspase-3 activation downstream from reactive oxygen species in heat-induced apoptosis of pancreatic carcinoma cells carrying a mutant p53 gene. Pancreas. 2001;22:255–260. doi: 10.1097/00006676-200104000-00005. [DOI] [PubMed] [Google Scholar]
- Korsmeyer SJ, Wei MC, Saito M, Weiler S, Oh KJ, Schlesinger PH. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Diff. 2000;7:1166–1173. doi: 10.1038/sj.cdd.4400783. [DOI] [PubMed] [Google Scholar]
- Lee HY, Naha N, Kim JH, Jo MJ, Min KS, Seong HH, Shin DH, Kim MO. Age- and area-dependent distinct effects of ethanol on Bax and Bcl-2 expression in prenatal rat brain. J Microbiol Biotech. 2008;18:1590–1598. [PubMed] [Google Scholar]
- Lindblad B, Olsson R. Unusually high levels of blood alcohol? JAMA. 1976;236:1600–1602. [PubMed] [Google Scholar]
- Lovell JF, Lieven PB, Binder S, Shamas-Din A, Fradin C, Leber B, Andrews DW. Membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax. Cell. 2008;135:1074–1084. doi: 10.1016/j.cell.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Luo J, West JR, Pantazis N. Nerve growth factor and basic fibroblast growth factor protect rat cerebellar granule cells in culture against ethanol-induced cell death. Alcohol Clin Exp Res. 1997;21:1108–1120. [PubMed] [Google Scholar]
- Manthorpe M, Fagnani R, Skaper SD, Varon S. An automated colorimetric microassay for neuronotrophic factors. Brain Res. 1986;390:191–198. doi: 10.1016/s0006-8993(86)80227-x. [DOI] [PubMed] [Google Scholar]
- Marzo I, Brenner C, Zamzami N, Jurgensmeier JM, Susin SA, Vieira HLA, Prevost M-C, Xie Z, Matsuyama S, Reed JC, Kroemer G. Bax and adenine nucleotide translocator cooperate in the mitochondrial control of apoptosis. Science. 1998;281:2027–2031. doi: 10.1126/science.281.5385.2027. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Crocker N, Nguyen TT. Fetal alcohol spectrum disorders: Neuropsychological and behavioral features. Neuropsychol Rev. 2011;21:81–101. doi: 10.1007/s11065-011-9167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP. Estimating the prevalence of fetal alcohol syndrome. A summary. Alcohol Res Health. 2001;25:159–167. [PMC free article] [PubMed] [Google Scholar]
- Mitchell JJ, Paiva M, Heaton MB. Optimal 96-well plate set up to avoid ethanol volatility when assessing ethanol cytotoxicity. Alcohol. 1998;15:137–139. doi: 10.1016/s0741-8329(97)00124-9. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid calorimetric assay for cellular growth and survival: Applications to proliferation and cytotoxicity assays. J Immunol Meth. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Moore DB, Walker DW, Heaton MB. Neonatal ethanol exposure alters Bcl-2 family mRNA levels in the rat cerebellar vermis. Alcohol Clin Exp Res. 1999;23:1251–1261. doi: 10.1111/j.1530-0277.1999.tb04286.x. [DOI] [PubMed] [Google Scholar]
- Muller Y, Tangre K, Clos J. Autocrine regulation of apoptosis and Bcl-2 expression by nerve growth factor in early differentiating cerebellar granule neurons involves low affinity neurotrophin receptor. Neurochem Internatl. 1997;31:177–191. doi: 10.1016/s0197-0186(96)00147-7. [DOI] [PubMed] [Google Scholar]
- Narita M, Shimizu S, Ito T, Chittenden T, Lutz RJ, Matsuda H, Tsujimoto Y. Bax interacts with the permeability transition pore to induce permeability transition and cytochrome c release in isolated mitochondria. Proc Natl Acad Sci USA. 1998;95:14681–14686. doi: 10.1073/pnas.95.25.14681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdoerster J, Rabin RA. Enhanced caspase activity during ethanol-induced apoptosis in rat cerebellar granule cells. Eur J Pharmacol. 1999;385:273–282. doi: 10.1016/s0014-2999(99)00714-1. [DOI] [PubMed] [Google Scholar]
- Olney JW, Tenkova T, Dikranian K, Muglia LJ, Jermakowicz WJ, D’Sa C, Roth A. Ethanol-induced caspase-3 activation in the in vivo developing mouse brain. Neurobiol Dis. 2002;9:205–219. doi: 10.1006/nbdi.2001.0475. [DOI] [PubMed] [Google Scholar]
- Nowoslawski L, Klocke BJ, Roth KA. Molecular regulation of acute ethanol-induced neuron apoptosis. J Neuropathol Exp Neurol. 2005;64:490–497. doi: 10.1093/jnen/64.6.490. [DOI] [PubMed] [Google Scholar]
- Pierce DR, Williams DK, Light KE. Purkinje cell vulnerability to developmental ethanol exposure in the rat cerebellum. Alcohol Clin Exp Res. 1999;23:1650–1659. [PubMed] [Google Scholar]
- Plesnila N, Zinkel S, Le DA, Amin-Hanjani S, Wu Y, Qiu J, Chiarugi A, Thomas SS, Kohane DS, Korsmeyer SJ, Moskowitz MA. BID mediates neuronal cell death after oxygen/glucose deprivation and focal cerebral ischemia. Proc Natl Acad Sci USA. 2001;98:15318–15323. doi: 10.1073/pnas.261323298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran V, Watts LT, Maffi SK, Chen J, Schenker S, Henderson G. Ethanol-induced oxidative stress precedes mitochondrially mediated apoptotic death of cultured fetal cortical neurons. J Neurosci Res. 2003;74:577–588. doi: 10.1002/jnr.10767. [DOI] [PubMed] [Google Scholar]
- Ren D, Tu HC, Kim H, Wang GX, Bean GR, Takeuchi O, Jeffers JR, Zambetti GP, Hsieh JJ-D, Cheng EH-Y. BID, BIM, and PUMA are essential for activation of the BAX- and BAK-dependent cell death program. Science. 2010;330:1390–1393. doi: 10.1126/science.1190217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siler-Marsiglio KI, Shaw G, Heaton MB. Pycnogenol and vitamin E inhibit ethanol-induced apoptosis in rat cerebellar granule cells. J Neurobiol. 2004;59:261–271. doi: 10.1002/neu.10311. [DOI] [PubMed] [Google Scholar]
- Siler-Marsiglio KI, Paiva M, Madorsky I, Pan Q, Shaw G, Heaton MB. Functional mechanisms of apoptosis-related proteins in neonatal rat cerebellum are differentially influenced by ethanol at postnatal day 4 and 7. J Neurosci Res. 2005;81:632–643. doi: 10.1002/jnr.20591. [DOI] [PubMed] [Google Scholar]
- Stephens CJ. Alcohol consumption during pregnancy among southern city women. Drug Alcohol Depend. 1985;16:19–29. doi: 10.1016/0376-8716(85)90078-x. [DOI] [PubMed] [Google Scholar]
- Young C, Klocke BJ, Tenkova T, Choi J, Labruyere J, Qin YQ, Holtzman DM, Roth KA, Olney JW. Ethanol-induced neuronal apoptosis in vivo requires Bax in the developing mouse brain. Cell Death Differen. 2003;10:1148–1155. doi: 10.1038/sj.cdd.4401277. [DOI] [PubMed] [Google Scholar]