Abstract
Purpose
Determine the effects of glucose and exogenous TGFβ2 on viability and VEGF release by human retinal pericytes (HRP).
Methods
Human retinal pericytes (HRP) were cultured in 5 mM (physiologic) or high (18 mM) glucose with or without added TGFβ2. Viable cells were counted; TGFβ2 and VEGF in the conditioned media (CM) were measured by ELISA.
Results
High glucose significantly reduced viable cell number and increased the levels of TGFβ2 and VEGF. TGFβ2 caused a significant dose-dependent effect on viable cell number and on the level of VEGF secreted into the CM by HRP in physiologic glucose, decreasing viable cell number, and increasing VEGF release per 1000 cells at a low concentration (0.1 ng/ml) and increasing viable cell number and decreasing VEGF release per 1000 cells at higher concentrations (1.0 and 10 ng/ml). TGFβ2 affected neither parameter in high glucose.
Conclusions
Elevated glucose decreased HRP viability and modulated changes in TGFβ2 and VEGF release. This suggests a novel mechanism for HRP dropout in diabetic retinopathy.
Keywords: diabetic retinopathy, human retinal pericytes, hyperglycemia, TGFβ2, VEGF
INTRODUCTION
Diabetic retinopathy, the leading cause of blindness in Americans 20–74 years of age and the leading cause of new cases of blindness,1 is preceded by the selective loss of retinal capillary pericytes2 and is associated with increased levels of vascular endothelial growth factor, VEGF,3,4 and transforming growth factor beta-2, TGFβ2,5,6 in the aqueous and vitreous humors of affected eyes.
VEGF, a 45 kDa homodimeric glycoprotein with potent vascular permeability and angiogenic effects,7 has proven to be a major mediator of intraocular neovascularization and is produced by many cell types within the eye, including retinal pigment epithelium (RPE) cells, retinal capillary pericytes, endothelial cells, glial cells, Muller cells, and ganglion cells.8–10
TGFβ2 is a member of a large superfamily of growth factors whose receptors are found in ocular tissues, including the cornea, ciliary epithelium, lens epithelium, retina, and blood vessels;11 of the three TGFβ isoforms, TGFβ2 is the predominant isoform found in the monkey eye.12 The in vitro biologic effects of TGFβ are diverse. Depending on cell type, TGFβ may stimulate or inhibit proliferation, block or affect entry into a differentiation pathway, stimulate extracellular matrix formation, and promote or inhibit cell migration. But, for most cell types, TGFβ is known as a potent polypeptide growth inhibitor.13
There have been several studies suggesting that TGFβ can induce VEGF,14–19 but no studies have reported on its effect in human retinal pericytes.
The aim of the study is to examine the effects of glucose on pericyte viability and their VEGF and TGFβ2 release, and to examine how glucose and TGFβ2 affect VEGF release and pericyte viability. To this end, we treated human retinal pericytes with several concentrations of glucose and then quantified viable cell number and quantified their release of VEGF and TGFβ2. We then cultured the cells in a normal and high glucose level with several concentrations of exogenously added TGFβ2, and quantified the viable cell number as well as their release of VEGF. Results of these studies provide important information on possible factors affecting the development of diabetic retinopathy in humans.
MATERIALS AND METHODS
Cell Culture
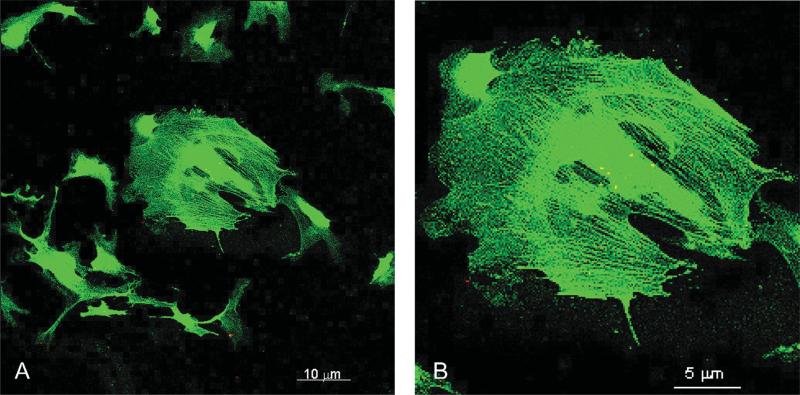
Human retinal pericytes (HRP) were isolated, purified, and cultured from donor eyes at the Medical University of South Carolina (MUSC) by Stephen Gee, using the standard method of Gitlin and D'Amore.20 They were characterized by their immunoreactivity with antibodies to alpha-smooth muscle actin (anti-αSMA)21 (Sigma F3777, St. Louis, MO) (Fig. 1). Frozen HRP, received at passage 6 from MUSC, were revived in a T-25 culture flask (BD Falcon 35-3109, Bedford, MA) containing Dulbecco's Modified Eagle's Medium (DMEM) (Gibco 23800-022, Long Island, NY)/F12 (Gibco 21700-075), 5.5 mM glucose (euglycemic), with 15% fetal bovine serum (FBS) (Gibco 26140-079), 200 μl/l antibiotic/antimycotic (Gibco 15240-096), and HEPES buffer 10 ml/l (GibcoBRL 15630-80). The pH was adjusted to 7.2, and cells were cultured at 37°C with 5% CO2. Cells were grown to confluence, a term used here to mean a degree of growth attained when a cell's processes were in contact with the processes of its neighbors, roughly equivalent to a total cell density of approximately 1.0–1.2 × 105 cells/ml. When the cells reached confluence, they were trypsinized (Mediatech 025-050-CI, Herndon, VA) and reseeded into 24-well plates with DMEM/F12, 5.5 mM glucose, 15% FBS, and incubated under standard conditions, i.e., 37°C with 5% CO2. When these cells reached confluence, they were used in experiments. For high glucose levels, we used 18, 33, and 40 mM levels representative of plasma glucose found in diabetics and compared them to a physiologic level (5 mM).
Figure 1.
Fluorescence microscopy of human retinal pericytes (HRP) shows labeling of α-smooth muscle actin fibers. Pericytes were immunoreacted with anti-α- smooth muscle actin conjugated with FITC. Labeled fibers illustrate the identity and phenotype of HRP. (A) Field of labeled HRP. Bar is 10 μm. (B) Close-up of a single HRP clearly showing α-smooth muscle actin fibers. Bar is 5 μm.
Counting Cells and Assessing Viability
Pericyte number and viability were determined by counting trypsinized pericytes in a hemacytometer using Trypan blue (Mediatech 25-900-CI) to label the nonviable cells. This was done by removing the spent media, rinsing each well twice with HBSS, and then adding 200 μl trypsin, which was allowed to react for 3 min at 37°C. The trypsin was neutralized by the addition of 800 μl media and was mixed with a pipette to make a uniform suspension. Two hundred microliters of this suspension were placed in a 0.5-ml Eppendorf with an equal volume of Trypan blue and allowed to incubate for 1.5 min. Aliquots of 20 μl were counted on the hemacytometer. A minimum of six aliquots from each well were counted. All cells, both viable and nonviable, were counted. Nonviable cells were identified as cells that had taken in any Trypan blue, while viable cells were identified by their exclusion of the blue dye.
ELISA Assay of VEGF
The total amount of VEGF secreted by cultured human retinal pericytes was determined by enzyme-linked immunosorbent assay (ELISA). First, confluent HRP at passage 8 were seeded into 24-well plates (Costar 3521, Corning, NY), as described above, at 5.5 mM and 18 mM glucose in serum-free (SF) media or SF media with added TGFβ2 purchased from R&D Systems (Cat #302-B2, Minneapolis, MN). These cells were cultured under standard conditions for 48 hr, at which time the conditioned media (CM) was collected and frozen at 4°C if assays were not done immediately. The cells in the wells were rinsed twice with 1-ml aliquots of HBSS, trypsinized, and then counted. VEGF assays were performed using R&D Quantikine VEGF ELISA kit (Cat #DVE00) according to manufacturer's instructions, and read at 450 nm by a Dynex Technologies plate-reader with Revelation software, λ correction at 540 nm. The concentration was determined by comparing the experimental absorbance against an assay standard curve for VEGF, which ranged from 15.6 pg/ml to 1000 pg/ml, the minimum detectable amount of VEGF typically being between 5.0–9.0 pg/ml. ELISA results were given in pg/ml.
ELISA Assay of TGFβ2
The amount of TGFβ2 in the CM was determined by ELISA assays. First, confluent HRP at passage 8 were seeded into 24-well plates, as described above, 5.5 mM and 18 mM glucose, in SF media or SF media with added TGFβ2. These cells were cultured under standard conditions for 48 hr, at which time the CM was collected and frozen at 4°C if assays were not done immediately. Cells were rinsed twice with 1-ml aliquots of HBSS, trypsinized, and then counted. The TGFβ2 assays (R&D Quantikine TGFβ2 ELISA kit #DB250) were performed according to manufacturer's instructions, and the absorbance read at 450 nm by a Dynex Technologies plate-reader with Revelation software, λ correction at 540 nm. The concentration was determined by comparing the experimental absorbance against an assay standard curve for TGFβ2, which ranged from 31.2 pg/ml to 1000 pg/ml, the minimum detectable amount of TGFβ2 typically being less than 7.0 pg/ml. ELISA results were given in pg/ml.
Statistical Methods
Statistical significance was calculated using Student's t-test with p ≤ 0.05 and ANOVA with α ≤ 0.05. Each data point represents at least three samples.
RESULTS
Effects of High Glucose on Cultured Human Retinal Pericytes
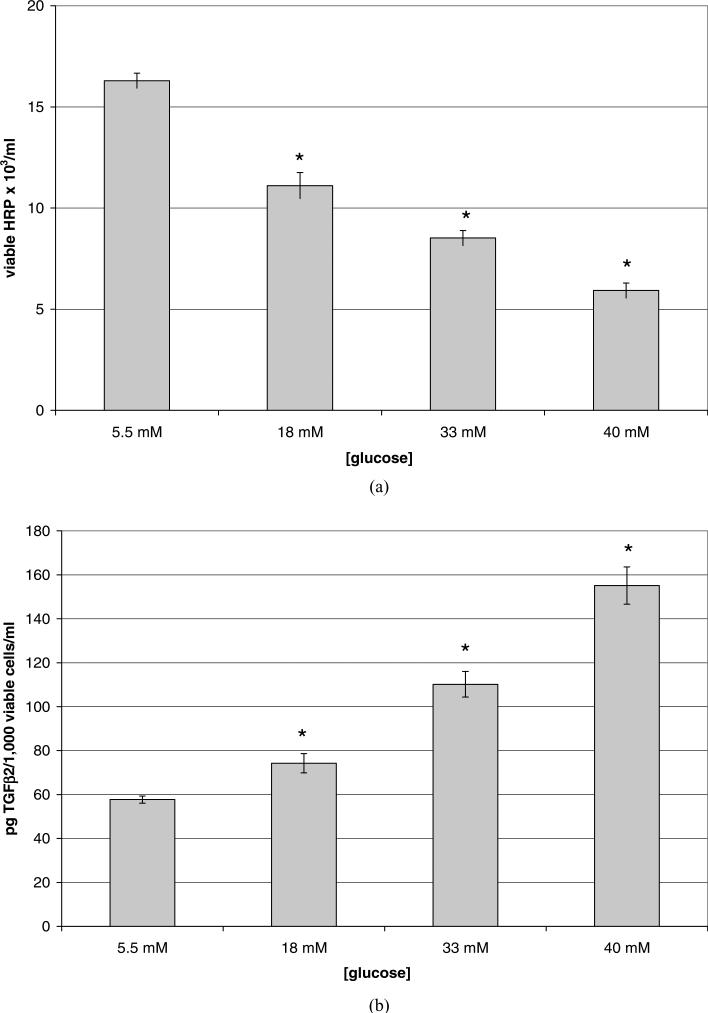
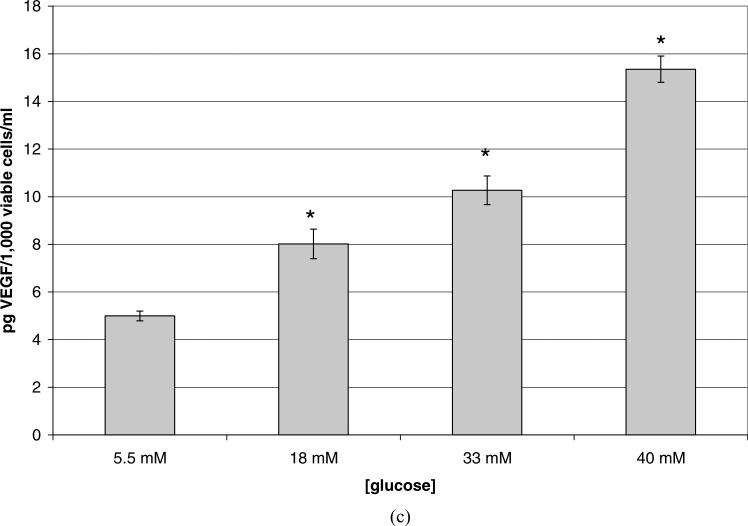
To examine the effects of high glucose on HRP viability and their secretion of TGFβ2 and VEGF, confluent cells were treated for 5 days with SF media containing high glucose (18, 33, or 40 mM), with the media being changed after the third day. As shown in Figure 2A, HRP exposed to hyperglycemic (18 mM) media for 5 days experienced a 32% reduction in viable cell number as compared with euglycemic controls (p = 0.002). Cells exposed to higher glucose (33 mM and=40 mM) showed a further decrease in viable cell number of 47% (p = 0.0001) and 64% (p = 3.84 × 10−5), respectively. The release of TGFβ2 per population of viable cells was also affected by high glucose. Each 1000 viable cells exposed to 18 mM glucose for 48 hr released 29% more TGFβ2 (Fig. 2B) than in the euglycemic condition (p = 0.024); each 1000 viable cells cultured in 33 mM glucose released 91% more (p 0.001), and each 1000 viable cells cultured in 40 mM glucose released 169% more (p = 0.0004) TGFβ2 than did cells in 5.5 mM glucose. Such increases in TGFβ2 release were also mirrored by similarly large increases in VEGF release per 1000 viable cells (Fig. 2C), i.e., 61% (p = 0.01) in the 18 mM condition, 106% (p = 0.001) in the 33 mM condition, and 207% (p = 6.06 × 10−5) in the 40 mM, compared to cells grown in 5.5 mM glucose. HRP cultured in 40 mM mannitol as an osmotic control provided results similar to 5.5 mM glucose (results not shown.)
Figure 2.
Confluent HRP grown in euglycemic (5.5 mM) media were serum-starved and then cultured with serum-free, high glucose (18 mM, 33 mM, or 40 mM) media for 5 days. Data shown represents the mean ± SE. N = 3. (A) Reduction in viable cell number of human retinal pericytes (HRP) that were exposed to hyperglycemic media for 5 days. Viable cells were counted using a hemacytometer and trypan blue. Cells grown in normal glucose were typically comprised of ~90% viable cells and those grown in high glucose ~85%. There was a 32% decrease in viable cell number due to high glucose (p = 0.022), 47% decrease in 33 mM (p = 0.001), and 64% decrease in 40 mM (p = 3.84 × 10−5). (B) Increase in TGFβ2 release per 1000 viable HRP exposed to 18 mM, 33 mM, or 40 mM glucose for 5 days. ELISA was performed on the conditioned media; results given in pg/ml were divided by the number of 1000 viable cells. There was a 29% increase in the release of TGFβ2 from each 1000 viable cells grown in 18 mM glucose (p = 0.02), 91% increase per 1000 viable cells grown in 33 mM (p = 0.001), and a 169% increase per 1000 viable cells grown in 40 mM glucose (p = 0.0004). (C) Increase in VEGF release per 1000 viable HRP exposed to 18 mM, 33 mM, or 40 mM glucose for 5 days. ELISA was performed on the conditioned media; results given in pg/ml were divided by the number of 1000 viable cells. There was a 61% increase in the release of VEGF from each 1000 viable HRP grown in 18 mM (p = 0.01), a 106% increase per 1000 viable cells grown in 33 mM (p = 0.001), and a 207% increase per 1000 viable cells grown in 40 mM (p = 6.1 × 10−5). (Continued )
Effect of Exogenous TGFβ2 on HRP Viability in Physiologic and High Glucose
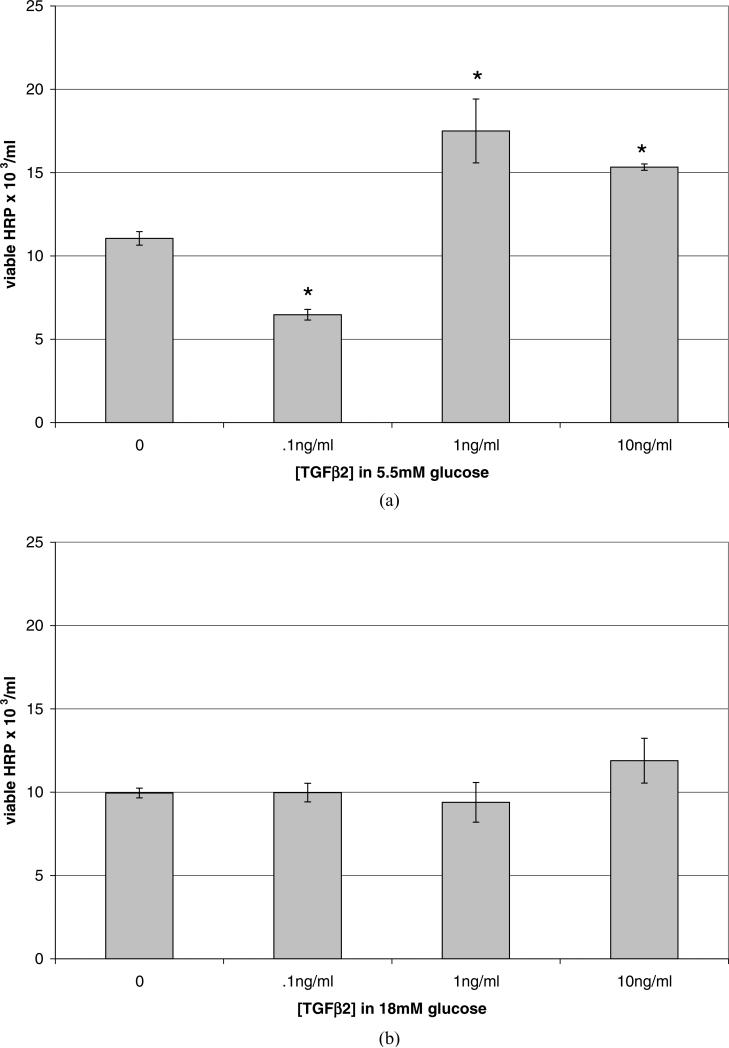
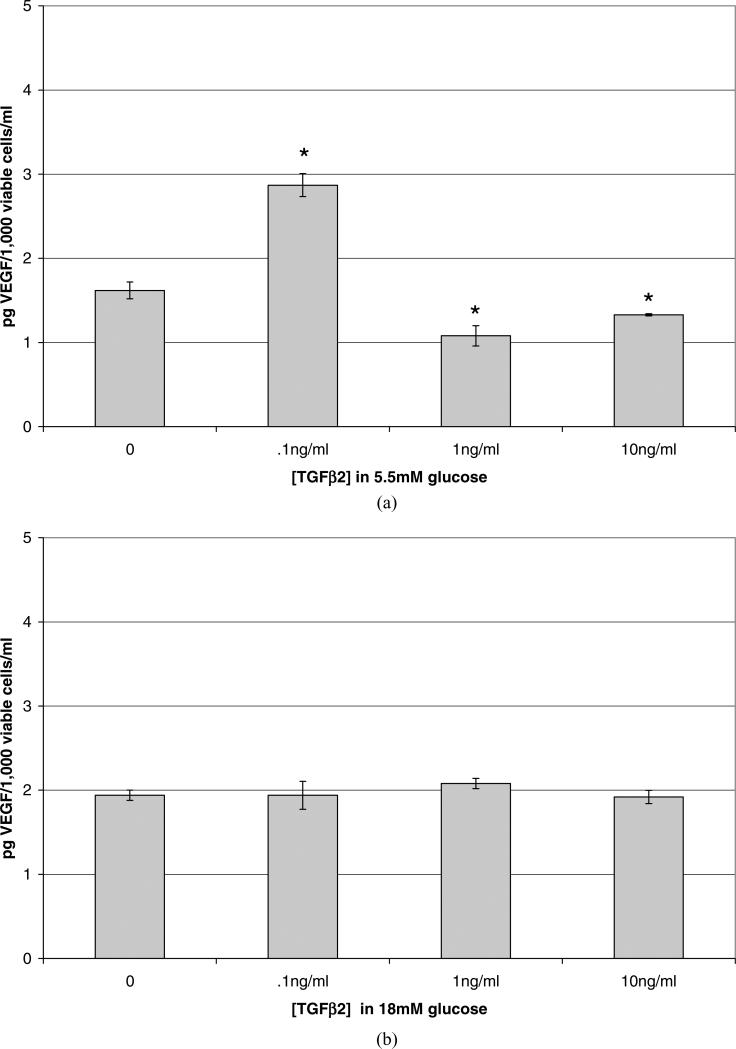
To investigate the effects of elevated levels of TGFβ2 on the viability of HRP both in normal and high glucose conditions, the 18 mM glucose level was selected to represent the high concentration. Confluent HRP were cultured in SF media containing normal or high glucose concentrations, with added exogenous TGFβ2 at concentrations of 0.1, 1.0, or 10 ng/ml for 48 hr. Figure 3A shows the effects of TGFβ2 on HRP cultured in a normal glucose concentration. In physiologic glucose, HRP treated with TGFβ2 at 0.1 ng/ml experienced a 41% reduction in viable cell number, as compared to control (p = 0.0001). However, TGFβ2 at both 1.0 and 10 ng/ml caused an increase in viable cell number, 58% and 39%, respectively (p = 0.017, p < 0.0001). Treatment with exogenous TGFβ2 at these levels also caused HRPs to lose their stellate shape and to adopt a more rounded, compact morphology.
Figure 3.
Changes in HRP viability due to high glucose (18 mM) and exogenous TGFβ2. Viable cells were counted using a hemacytometer and trypan blue. Data shown represent the mean ± SE. N = 3. (A) Confluent HRP cultured in euglycemic media (5.5 mM) were treated with serum-free media containing TGFβ2 at 0.1, 1.0, or 10 ng/ml for 48 hr. Cells in euglycemic media treated with 0.1 ng/ml TGFβ2, a physiologic level, experienced a 41% reduction in viable cell number (p = 0.0002), while cells treated with 1.0 or 10 ng/ml TGFβ2 showed increases in viable cell number, 58% (p = 0.02) and 39% (p =0.0001), respectively, as well as during counting decreased cell size and rounder morphology with smaller, fewer cell processes. (B) Confluent HRP cultured in hyperglycemic media (18 mM) were treated with serum-free media containing TGFβ2 at 0.1, 1.0, or 10 ng/ml for 48 hr. In hyperglycemic media, there was no change in viable cell number attributable to any concentration of TGFβ2 tested (p = 0.49).
The effect of the combination of high glucose and exogenous TGFβ2 on viable cell population is shown in Figure 3B. When HRP were cultured in high glucose, the addition of TGFβ2 had no significant effect on cell number at any concentration tested (p = 0.966, 0.763, and 0.09 for 0.1, 1.0, and 10 ng/ml TGFβ2, respectively).
Effect of Exogenous TGFβ2 on VEGF Release by HRP in Physiologic and High Glucose
The influence of TGFβ2 on VEGF release by HRP grown in normal and high glucose concentrations was examined. HRP grown in euglycemic media with the addition of exogenous TGFβ2 (Fig. 4A) responded to 0.1 ng/ml by increasing the release of VEGF by 78% per 1000 viable cells (p = 0.002). TGFβ2 added at 1.0 and 10 ng/ml decreased VEGF release per 1000 viable cells by 33% and 18%, respectively (p = 0.026, p = 0.042).
Figure 4.
Changes in VEGF release per 1000 viable cells due to high glucose and exogenous TGFβ2. Data shown represent the mean ± SE. N = 3. (A) Confluent HRP cultured in euglycemic media (5.5 mM) were treated with serum-free media containing TGFβ2 at 0.1, 1.0, or 10 ng/ml for 48 hr, after which the conditioned media was assayed for VEGF using ELISA; results given in pg/ml were divided by the number of 1000 viable cells. Cells treated with.01 ng/ml TGFβ2 released 78% more VEGF per 1000 viable cells (p = 0.002) than did controls. Cells treated with TGFβ2 at 1.0 or 10 ng/ml released less VEGF per 1000 cells, 33% (p = 0.03) and 18% less (p = 0.04), respectively. (B) Confluent HRP cultured in hyperglycemic media (18 mM) were treated with serum-free media containing TGFβ2 at 0.1, 1.0, or 10 ng/ml for 48 hr. In hyperglycemic media, there was no change in the amount of VEGF released per viable cell attributable to any concentration of TGFβ2 tested (p = 0.67).
When TGFβ2 was added to HRP grown in hyper-glycemic media, there was no significant effect on VEGF release per cell population at any TGFβ2 concentration tested (Fig. 4B) (p = 0.983, 0.171, and 0.897 for 0.1, 1.0, and 10 ng/ml TGF β2, respectively).
DISCUSSION
This is the first study to provide data describing the effects of elevated glucose and exogenous TGFβ2 on the viability or the VEGF release of cultured human retinal capillary pericytes. We show that high glucose led to decreased pericyte viability; this is consistent with results using bovine retinal capillary pericytes.22 In physiologic glucose, HRP secreted VEGF and TGFβ2 into the CM. When challenged with high glucose, they secreted significantly more VEGF and TGFβ2. These novel findings suggest that in hyperglycemic diabetics, the retinal pericytes can contribute to the VEGF and TGFβ2 load that can be observed in the ocular fluid compartments.3–6 We show for the first time that TGFβ2 exerts a dose-dependent effect on human retinal pericytes cultured in euglycemic conditions; viable cell number is decreased at a physiologic concentration, and the viable cell number is increased at higher concentrations of TGFβ2, while VEGF release per population of viable cells is increased at a physiologic concentration of TGFβ2 and reduced at higher concentrations.
Human retinal pericytes cultured in hyperglycemic media experienced effects due merely to increased glucose. Their viability was decreased, and the release of TGFβ2 and VEGF was increased. Reduced viable cell number is consistent with results from in vitro cell culture studies using several cell types.22–27
Our growth factor data are consistent with results from researchers who found elevated VEGF levels in response to hyperglycemia in ARPE-19 cells,28 human RPE cells,29 and others.30–32 Elevated glucose upregulates TGFβ1 in several cell types,33–35 and upregulates TGFβ2 by human Tenon's capsule fibroblasts.25 Although the mechanism for these actions of hyper-glycemia on cell viability and cytokine release remain unclear, we show that increasing concentrations of glucose demonstrated a dose-response effect on HRP cell viability and TGFβ2 and VEGF release per viable cell population.
Reports of dose-dependent effects due to the action of TGFβ2 in different cell types include the following observations: the effect on the proliferation of mesangial cells,36 LSK cells,37 smooth muscle cells,38 bovine adrenal microvascular endothelial cells,39 and collagen synthesis by osteoblast-like cells,40 as well as on the differentiation of pre-osteoclasts and osteoclasts.41 Furthermore, results from previous studies show that the dose-dependent action of TGFβ2 can be dependent on many variables, including on its combined action with other growth factors42 and their concentration in the cell environment,39 and on the expression of different TGFβ receptor phenotypes.43 Although dose-dependent modulation of VEGF due to TGFβ has not been previously noted in HRP, TGFβ upregulation of VEGF has been observed in several cell types.16,44–50 Furthermore, BMP-4, a member of the TGFβ super-family, is also known to upregulate VEGF secretion by ARPE-19 cells.51
When HRPs were cultured in high glucose with the addition of exogenous TGFβ2, the dose-dependent effects on viability and VEGF release per population of viable cells were lost. This would suggest a disruption either in ligand binding or signaling, possibly via Smad and MAPK signaling pathways.16,50,52,53 Some researchers suggest that pericyte growth and differentiation are inversely related,54 and that the activation of p38 has an opposing effect on the proliferation and migration of endothelial cells.55
Another possible modulator of the effect of TGFβ in hyperglycemic media is interference with TGFβ binding to its receptors. Known proteoglycans that can reduce levels of active TGFβ56 are expressed by bovine retinal pericytes,47 and are upregulated by TGFβ48,57 and by hyperglycemia.58 Decorin, in particular, is upregulated 20-fold under hyperglycemic conditions in human mesangial cells,59,60 as are bovine retinal pericyte-associated proteoglycans.61
One proposed mechanism of action is that decorin sequesters TGFβ in the extracellular matrix, away from its targeted receptor, and thereby modulates the activity of this growth factor.56
This study is also the first to note that TGFβ2 modulates the release of VEGF from human pericytes. VEGF and TGFβ2 are both important cytokines involved with the development and maintenance of microvessels,54,62,63 and VEGF is hypothesized to act in a juxtacrine/paracrine manner on the endothelial cells to stabilize microvessels.63
Our results suggest that the action of hyperglycemia on the growth of human retinal pericytes causes them to be refractory to the stimulation of TGFβ2. In physiologic glucose, TGFβ2 at concentrations of 1 and 10 ng/ml can lead to proliferation of human retinal pericytes; therefore, normalization of glucose levels with an increased level of active TGFβ2 might lead to an increased number of retinal pericytes, and this could possibly be enhanced due to the decreased production of decorin, biglycan, or other proteoglycans.
Our results also suggest that in human diabetic retinopathy, the observed pericyte drop-out could be due to the inability of increasing levels of TGFβ2 in the vitreous to exert a proliferative effect on the retinal pericytes. The ability of TGFβ2 to modulate and reduce VEGF levels is also lost, thereby enhancing the angiogenic effect of VEGF. Experiments exploring the signaling pathways and the roles of proteoglycans like decorin on the actions of TGFβ2 on HRP in normal and hyperglycemic conditions are pending, in hopes of elucidating the mechanisms involved in the observations made herein.
ACKNOWLEDGEMENTS
The authors would like to thank the National Institutes of Health MBRS/MARC, The Kronkosky Charitable Foundation, and The San Antonio Area Foundation for their support.
Footnotes
Declaration of interest: The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
Contributor Information
Eileen K. Vidro, Department of Biology, University of Texas at San Antonio, San Antonio, Texas, USA
Stephen Gee, Department of Ophthalmology, Medical University of South Carolina, Charleston, South Carolina, USA.
Richard Unda, Department of Biology, University of Texas at San Antonio, San Antonio, Texas, USA.
Jian-xing Ma, Department of Ophthalmology, Medical University of South Carolina, Charleston, South Carolina, USA.
Andrew Tsin, Department of Biology, University of Texas at San Antonio, San Antonio, Texas, USA.
REFERENCES
- 1.Pickup JC, Williams G. Textbook of Diabetes. 2nd ed. Blackwell Science; Oxford/Cambridge, MA: 1997. p. 48. [Google Scholar]
- 2.Li WY, Zhou Q, Tang L, Qin M, Hu TS. Intramural pericyte degeneration in early diabetic retinopathy study in vitro. Chin Med J (Engl) 1990;103:7–13. [PubMed] [Google Scholar]
- 3.Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 4.Pe'er J, Folberg R, Itin A, Gnessin H, Hemo I, Keshet E. Upregulated expression of vascular endothelial growth factor in proliferative diabetic retinopathy. Br J Ophthalmol. 1996;80:241–245. doi: 10.1136/bjo.80.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirase K, Ikeda T, Sotozono C, Nishida K, Sawa H, Kinoshita S. Transforming growth factor beta2 in the vitreous in proliferative diabetic retinopathy. Arch Ophthalmol. 1998;116:738–741. doi: 10.1001/archopht.116.6.738. [DOI] [PubMed] [Google Scholar]
- 6.Ochiai Y, Ochiai H. Higher concentration of transforming growth factor-beta in aqueous humor of glaucomatous eyes and diabetic eyes. Jpn J Ophthalmol. 2002;46:249–253. doi: 10.1016/s0021-5155(01)00523-8. [DOI] [PubMed] [Google Scholar]
- 7.Ferrara N. Vascular endothelial growth factor and the regulation of angiogenesis. Recent Prog Horm Res. 2000;55:15–35. discussion 15–36. [PubMed] [Google Scholar]
- 8.Aiello LP, Northrup JM, Keyt BA, Takagi H, Iwamoto MA. Hypoxic regulation of vascular endothelial growth factor in retinal cells. Arch Ophthalmol. 1995;113:1538–1544. doi: 10.1001/archopht.1995.01100120068012. [DOI] [PubMed] [Google Scholar]
- 9.Adamis AP, Shima DT, Yeo KT, et al. Synthesis and secretion of vascular permeability factor/vascular endothelial growth factor by human retinal pigment epithelial cells. Biochem Biophys Res Commun. 1993;193:631–638. doi: 10.1006/bbrc.1993.1671. [DOI] [PubMed] [Google Scholar]
- 10.Simorre-Pinatel V, Guerrin M, Chollet P, et al. Vasculotropin-VEGF stimulates retinal capillary endothelial cells through an autocrine pathway. Invest Ophthalmol Vis Sci. 1994;35:3393–3400. [PubMed] [Google Scholar]
- 11.Yamashita H, Tobari I, Sawa M, et al. [Functions of the transforming growth factor-beta superfamily in eyes]. Nippon Ganka Gakkai Zasshi. 1997;101:927–947. [PubMed] [Google Scholar]
- 12.Pfeffer BA, Flanders KC, Guerin CJ, Danielpour D, Anderson DH. Transforming growth factor beta 2 is the predominant isoform in the neural retina, retinal pigment epithelium-choroid, and vitreous of the monkey eye. Exp Eye Res. 1994;59:323–333. doi: 10.1006/exer.1994.1114. [DOI] [PubMed] [Google Scholar]
- 13.Barnard JA, Lyons RM, Moses HL. The cell biology of transforming growth factor beta. Biochim Biophys Acta. 1990;1032:79–87. doi: 10.1016/0304-419x(90)90013-q. [DOI] [PubMed] [Google Scholar]
- 14.Gary Lee YC, Melkerneker D, Thompson PJ, Light RW, Lane KB. Transforming growth factor beta induces vascular endothelial growth factor elaboration from pleural mesothelial cells in vivo and in vitro. Am J Respir Crit Care Med. 2002;165:88–94. doi: 10.1164/ajrccm.165.1.2104006. [DOI] [PubMed] [Google Scholar]
- 15.Malecaze F, Clamens S, Simorre-Pinatel V, et al. Detection of vascular endothelial growth factor messenger RNA and vascular endothelial growth factor-like activity in proliferative diabetic retinopathy. Arch Ophthalmol. 1994;112:1476–1482. doi: 10.1001/archopht.1994.01090230090028. [DOI] [PubMed] [Google Scholar]
- 16.Nagineni CN, Samuel W, Nagineni S, et al. Transforming growth factor-beta induces expression of vascular endothelial growth factor in human retinal pigment epithelial cells: Involvement of mitogen-activated protein kinases. J Cell Physiol. 2003;197:453–462. doi: 10.1002/jcp.10378. [DOI] [PubMed] [Google Scholar]
- 17.Renner U, Lohrer P, Schaaf L, et al. Transforming growth factor-beta stimulates vascular endothelial growth factor production by folliculostellate pituitary cells. Endocrinology. 2002;143:3759–3765. doi: 10.1210/en.2002-220283. [DOI] [PubMed] [Google Scholar]
- 18.Saadeh PB, Mehrara BJ, Steinbrech DS, et al. Transforming growth factor-beta1 modulates the expression of vascular endothelial growth factor by osteoblasts. Am J Physiol. 1999;277:C628–637. doi: 10.1152/ajpcell.1999.277.4.C628. [DOI] [PubMed] [Google Scholar]
- 19.Bian ZM, Elner SG, Elner VM. Regulation of VEGF mRNA expression and protein secretion by TGF-beta2 in human retinal pigment epithelial cells. Exp Eye Res. 2007;84:812–822. doi: 10.1016/j.exer.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gitlin JD, D'Amore PA. Culture of retinal capillary cells using selective growth media. Microvasc Res. 1983;26:74–80. doi: 10.1016/0026-2862(83)90056-0. [DOI] [PubMed] [Google Scholar]
- 21.Herman IM, D'Amore PA. Microvascular pericytes contain muscle and non-muscle actins. J Cell Biol. 1985;101:43–52. doi: 10.1083/jcb.101.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beltramo E, Berrone E, Giunti S, Gruden G, Perin PC, Porta M. Effects of mechanical stress and high glucose on pericyte proliferation, apoptosis, and contractile phenotype. Exp Eye Res. 2006;83:989–994. doi: 10.1016/j.exer.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Pomero F, Allione A, Beltramo E, et al. Effects of protein kinase C inhibition and activation on proliferation and apoptosis of bovine retinal pericytes. Diabetologia. 2003;46:416–419. doi: 10.1007/s00125-003-1044-5. [DOI] [PubMed] [Google Scholar]
- 24.Du Y, Sarthy VP, Kern TS. Interaction between NO and COX pathways in retinal cells exposed to elevated glucose and retina of diabetic rats. Am J Physiol Regul Integr Comp Physiol. 2004;287:R735–741. doi: 10.1152/ajpregu.00080.2003. [DOI] [PubMed] [Google Scholar]
- 25.Browning AC, Alibhai A, McIntosh RS, Rotchford AP, Bhan A, Amoaku WM. Effect of diabetes mellitus and hyperglycemia on the proliferation of human Tenon's capsule fibroblasts: Implications for wound healing after glaucoma drainage surgery. Wound Repair Regen. 2005;13:295–302. doi: 10.1111/j.1067-1927.2005.00130312.x. [DOI] [PubMed] [Google Scholar]
- 26.Rojas S, Rojas R, Lamperti L, Casanello P, Sobrevia L. Hyper-glycaemia inhibits thymidine incorporation and cell growth via protein kinase C, mitogen-activated protein kinases, and nitric oxide in human umbilical vein endothelium. Exp Physiol. 2003;88:209–219. doi: 10.1113/eph8802515. [DOI] [PubMed] [Google Scholar]
- 27.Duffy A, Liew A, O'Sullivan J, Avalos G, Samali A, O'Brien T. Distinct effects of high-glucose conditions on endothelial cells of macrovascular and microvascular origins. Endothelium. 2006;13:9–16. doi: 10.1080/10623320600659997. [DOI] [PubMed] [Google Scholar]
- 28.Heimsath EG, Jr, Unda R, Vidro E, Muniz A, Villazana-Espinoza ET, Tsin A. ARPE-19 cell growth and cell functions in euglycemic culture media. Curr Eye Res. 2006;31:1073–1080. doi: 10.1080/02713680601052320. [DOI] [PubMed] [Google Scholar]
- 29.Yao Y, Guan M, Zhao XQ, Huang YF. [Downregulation of the pigment epithelium derived factor by hypoxia and elevated glucose concentration in cultured human retinal pigment epithelial cells]. Zhonghua Yi Xue Za Zhi. 2003;83:1989–1992. [PubMed] [Google Scholar]
- 30.Kim NH, Jung HH, Cha DR, Choi DS. Expression of vascular endothelial growth factor in response to high glucose in rat mesangial cells. J Endocrinol. 2000;165:617–624. doi: 10.1677/joe.0.1650617. [DOI] [PubMed] [Google Scholar]
- 31.Iglesias-de la Cruz MC, Ziyadeh FN, Isono M, et al. Effects of high glucose and TGF-beta1 on the expression of collagen IV and vascular endothelial growth factor in mouse podocytes. Kidney Int. 2002;62:901–913. doi: 10.1046/j.1523-1755.2002.00528.x. [DOI] [PubMed] [Google Scholar]
- 32.Natarajan R, Bai W, Lanting L, Gonzales N, Nadler J. Effects of high glucose on vascular endothelial growth factor expression in vascular smooth muscle cells. Am J Physiol. 1997;273:H2224–2231. doi: 10.1152/ajpheart.1997.273.5.H2224. [DOI] [PubMed] [Google Scholar]
- 33.Kim YS, Jung DH, Kim NH, Lee YM, Kim JS. Effect of magnolol on TGF-beta1 and fibronectin expression in human retinal pigment epithelial cells under diabetic conditions. Eur J Pharmacol. 2007;562:12–19. doi: 10.1016/j.ejphar.2007.01.048. [DOI] [PubMed] [Google Scholar]
- 34.Panchapakesan U, Sumual S, Pollock CA, Chen X. PPARgamma agonists exert antifibrotic effects in renal tubular cells exposed to high glucose. Am J Physiol Renal Physiol. 2005;289:F1153–1158. doi: 10.1152/ajprenal.00097.2005. [DOI] [PubMed] [Google Scholar]
- 35.Wang S, Wu X, Lincoln TM, Murphy-Ullrich JE. Expression of constitutively active cGMP-dependent protein kinase prevents glucose stimulation of thrombospondin 1 expression and TGF-beta activity. Diabetes. 2003;52:2144–2150. doi: 10.2337/diabetes.52.8.2144. [DOI] [PubMed] [Google Scholar]
- 36.MacKay K, Striker LJ, Stauffer JW, Doi T, Agodoa LY, Striker GE. Transforming growth factor-beta. Murine glomerular receptors and responses of isolated glomerular cells. J Clin Invest. 1989;83:1160–1167. doi: 10.1172/JCI113996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henckaerts E, Langer JC, Orenstein J, Snoeck HW. The positive regulatory effect of TGF-beta2 on primitive murine hemopoietic stem and progenitor cells is dependent on age, genetic background, and serum factors. J Immunol. 2004;173:2486–2493. doi: 10.4049/jimmunol.173.4.2486. [DOI] [PubMed] [Google Scholar]
- 38.Majack RA. Beta-type transforming growth factor specifies organizational behavior in vascular smooth muscle cell cultures. J Cell Biol. 1987;105:465–471. doi: 10.1083/jcb.105.1.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pepper MS, Vassalli JD, Orci L, Montesano R. Biphasic effect of transforming growth factor-beta 1 on in vitro angiogenesis. Exp Cell Res. 1993;204:356–363. doi: 10.1006/excr.1993.1043. [DOI] [PubMed] [Google Scholar]
- 40.Ibbotson KJ, Orcutt CM, Anglin AM, D'Souza SM. Effects of transforming growth factors beta 1 and beta 2 on a mouse clonal, osteoblast-like cell line MC3T3-E1. J Bone Miner Res. 1989;4:37–45. doi: 10.1002/jbmr.5650040107. [DOI] [PubMed] [Google Scholar]
- 41.Berghuis HM, Dieudonne SC, Goei W, Veldhuijzen JP. Effects of TGF-beta 2 on mineral resorption in cultured embryonic mouse long bones; 45Ca release and osteoclast differentiation and migration. Eur J Orthod. 1994;16:130–137. doi: 10.1093/ejo/16.2.130. [DOI] [PubMed] [Google Scholar]
- 42.Roberts AB, Anzano MA, Wakefield LM, Roche NS, Stern DF, Sporn MB. Type beta transforming growth factor: A bifunctional regulator of cellular growth. Proc Natl Acad Sci USA. 1985;82:119–123. doi: 10.1073/pnas.82.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goodman LV, Majack RA. Vascular smooth muscle cells express distinct transforming growth factor-beta receptor phenotypes as a function of cell density in culture. J Biol Chem. 1989;264:5241–5244. [PubMed] [Google Scholar]
- 44.Wang XJ, Dong Z, Zhong XH, et al. Transforming growth factor-beta1 enhanced vascular endothelial growth factor synthesis in mesenchymal stem cells. Biochem Biophys Res Commun. 2008;365:548–554. doi: 10.1016/j.bbrc.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 45.Qian D, Lin HY, Wang HM, et al. Involvement of ERK1/2 pathway in TGF-beta1-induced VEGF secretion in normal human cytotrophoblast cells. Mol Reprod Dev. 2004;68:198–204. doi: 10.1002/mrd.20061. [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto T, Kozawa O, Tanabe K, et al. Involvement of p38 MAP kinase in TGF-beta-stimulated VEGF synthesis in aortic smooth muscle cells. J Cell Biochem. 2001;82:591–598. doi: 10.1002/jcb.1179. [DOI] [PubMed] [Google Scholar]
- 47.Kaji T, Sakurai S, Yamamoto C, et al. Characterization of chondroitin/dermatan sulfate proteoglycans synthesized by bovine retinal pericytes in culture. Biol Pharm Bull. 2004;27:1763–1768. doi: 10.1248/bpb.27.1763. [DOI] [PubMed] [Google Scholar]
- 48.Border WA, Okuda S, Languino LR, Ruoslahti E. Transforming growth factor-beta regulates production of proteoglycans by mesangial cells. Kidney Int. 1990;37:689–695. doi: 10.1038/ki.1990.35. [DOI] [PubMed] [Google Scholar]
- 49.Tokuda H, Hatakeyama D, Akamatsu S, et al. Involvement of MAP kinases in TGF-beta-stimulated vascular endothelial growth factor synthesis in osteoblasts. Arch Biochem Biophys. 2003;415:117–125. doi: 10.1016/s0003-9861(03)00225-x. [DOI] [PubMed] [Google Scholar]
- 50.Wang L, Kwak JH, Kim SI, He Y, Choi ME. Transforming growth factor-β1 stimulates vascular endothelial growth factor 164 via mitogen-activated protein kinase 3-p38α and p38 mitogen-activated protein kinase-dependent pathway in murine mesangial cells. J Biol Chem. 2004;279:33213–33219. doi: 10.1074/jbc.M403758200. [DOI] [PubMed] [Google Scholar]
- 51.Vogt RR, Unda R, Yeh LC, Vidro EK, Lee JC, Tsin AT. Bone morphogenetic protein-4 enhances vascular endothelial growth factor secretion by human retinal pigment epithelial cells. J Cell Biochem. 2006;98:1196–1202. doi: 10.1002/jcb.20831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Igarashi M, Wakasaki H, Takahara N, et al. Glucose or diabetes activates p38 mitogen-activated protein kinase via different pathways. J Clin Invest. 1999;103:185–195. doi: 10.1172/JCI3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: A unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23:599–622. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- 54.Papetti M, Shujath J, Riley KN, Herman IM. FGF-2 antagonizes the TGF-β1-mediated induction of pericyte alpha-smooth muscle actin expression: A role for myf-5 and Smad-mediated signaling pathways. Invest Ophthalmol Vis Sci. 2003;44:4994–5005. doi: 10.1167/iovs.03-0291. [DOI] [PubMed] [Google Scholar]
- 55.McMullen ME, Bryant PW, Glembotski CC, Vincent PA, Pumiglia KM. Activation of p38 has opposing effects on the proliferation and migration of endothelial cells. J Biol Chem. 2005;280:20995–21003. doi: 10.1074/jbc.M407060200. [DOI] [PubMed] [Google Scholar]
- 56.Yamaguchi Y, Mann DM, Ruoslahti E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature. 1990;346:281–284. doi: 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]
- 57.Heimer R, Bashey RI, Kyle J, Jimenez SA. TGF-β modulates the synthesis of proteoglycans by myocardial fibroblasts in culture. J Mol Cell Cardiol. 1995;27:2191–2198. doi: 10.1016/s0022-2828(95)91479-x. [DOI] [PubMed] [Google Scholar]
- 58.Chen CP, Chang SC, Vivian Yang WC. High glucose alters proteoglycan expression and the glycosaminoglycan composition in placentas of women with gestational diabetes mellitus and in cultured trophoblasts. Placenta. 2007;28:97–106. doi: 10.1016/j.placenta.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 59.Wahab NA, Harper K, Mason RM. Expression of extracellular matrix molecules in human mesangial cells in response to prolonged hyperglycaemia. Biochem J. 1996;316(Pt 3):985–992. doi: 10.1042/bj3160985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wahab NA, Parker S, Sraer JD, Mason RM. The decorin high glucose response element and mechanism of its activation in human mesangial cells. J Am Soc Nephrol. 2000;11:1607–1619. doi: 10.1681/ASN.V1191607. [DOI] [PubMed] [Google Scholar]
- 61.Fisher EJ, McLennan SV, Yue DK, Turtle JR. Cell-associated proteoglycans of retinal pericytes and endothelial cells: Modulation by glucose and ascorbic acid. Microvasc Res. 1994;48:179–189. doi: 10.1006/mvre.1994.1048. [DOI] [PubMed] [Google Scholar]
- 62.Deissler H, Lang GK, Lang GE. TGFβ induces transdifferentiation of iBREC to αSMA-expressing cells. Int J Mol Med. 2006;18:577–582. [PubMed] [Google Scholar]
- 63.Darland DC, Massingham LJ, Smith SR, Piek E, Saint-Geniez M, D'Amore PA. Pericyte production of cell-associated VEGF is differentiation dependent and is associated with endothelial survival. Dev Biol. 2003;264:275–288. doi: 10.1016/j.ydbio.2003.08.015. [DOI] [PubMed] [Google Scholar]